Abstract
An increasing number of studies in a variety of taxa demonstrate the role of maternal sex steroids on offspring development. In avian species, mothers deposit substantial amounts of androgens in their eggs, and experimental evidence indicates that these maternal androgens influence the chick's early development. Despite the well-known organizing role of sex steroids on brain and behaviour, studies on avian maternal egg hormones almost exclusively focus on the chick phase. Here, we show experimentally that in Black-headed gulls maternal androgens in the egg enhance the development of the nuptial plumage and the frequency of aggressive and sexual displays almost 1 year after hatching. We conclude that maternal sex steroids may be a key factor for the determination of subtle but important individual differences within the same sex and species, which may have important consequences for Darwinian fitness and evolutionary processes.
Keywords: maternal effects, life-history traits, testosterone, social dominance, plumage development
1. Introduction
Individuals of the same species and sex may differ consistently in morphology, physiology and behaviour. Understanding this variation is a key to unravelling both causal mechanisms and evolutionary consequences of developmental plasticity. Sex steroid hormones are likely a major factor underlying such inter-individual differences. Early exposure to these hormones not only organizes sex differences in brain, behaviour and morphology (Balthazart & Adkins-Regan 2002), but also affects individual differentiation within the same sex (Clark & Galef 1995; Crews 1998; Drea et al. 1998; Moore et al. 1998). The presence of substantial levels of maternal androgens in eggs of vertebrate oviparous species (see Groothuis et al. 2005a for a review) opens the intriguing possibility that mothers may control individual differentiation in their offspring by differential allocation of these hormones.
A rapidly increasing number of both descriptive and experimental studies report systematic between- and within-clutch variation in levels of androgens in avian eggs and its effects on the offspring. This hormone allocation has been interpreted as a tool for the mother to manipulate sibling competition in her brood (Schwabl 1993; Schwabl et al. 1997). Interestingly, social rank-order in juvenile canaries was correlated with maternal yolk androgen levels in the eggs from which these birds hatched, suggesting longer-lasting effects too (Schwabl 1993). However, this effect could have been caused by other aspects of the egg that are related to yolk androgen levels. The only study so far that has addressed long-term effects experimentally is that of Strasser & Schwabl (2004). They injected testosterone in House sparrow eggs, resulting in enlarged badge size in adult males. Furthermore, in staged dyadic encounters, birds from testosterone-treated eggs showed shorter latencies to approach a food resource and monopolize it. This exciting result suggests a long-term effect of yolk androgens on aggressive behaviour, but this was not statistically demonstrated.
We manipulated yolk hormone levels of gull eggs within the physiological range and studied the effect on nuptial plumage, territorial and aggressive behaviour under semi-natural conditions when the birds were 10 months of age.
2. Material and Methods
(a) Animals
We used the Black-headed gull (Larus ridibundus) as study species. Eggs of this species contain high levels of androgens that vary systematically within and between clutches, suggestive for adaptive maternal hormone allocation (Eising et al. 2001; Groothuis & Schwabl 2002). Furthermore, experimental elevation of yolk androgen levels within the physiological range showed that these hormones are biologically relevant (see above).
(b) Egg sequence and egg injections
We determined egg sequence by marking eggs during daily checks in two colonies near Westernieland (53°24′ N, 06°28′ E). At the day of clutch completion (three eggs), we injected first laid eggs, which contain naturally low levels of maternal androgens, with either a combination of 0.12 μg testosterone and 10.0 μg androstanedione (for details see Eising et al. 2001) dissolved in 50 μl sesame oil (androgen egg), or with 50 μl sesame oil only (oil egg). The androgen dose mimics the higher levels of maternal androgens found in third laid eggs (Eising et al. 2001). All injected eggs were cross-fostered to other nests so that each nest received at least one androgen and one oil egg, matched for mass. Chicks from these eggs were reared by their foster parents. All original eggs of foster nests were fostered to other incomplete nests of similar age, in order to have only experimental eggs in our focal nests.
(c) Housing and data collection
The experiment was carried out in two batches, in 1999 and 2001. At fledging, the young were collected in the field and housed under semi-natural conditions in a large outdoor aviary (15×35×6 m) at our laboratory. In the aviary, birds had access to continuously refreshed water and ad libitum food. All birds were individually recognizable by unique wing tags and were molecularly sexed. Data collection was carried out blindly with respect to the treatment of the birds. In April 2000 (18 Oil: 10 males, 8 females; 10 T: 7 males, 3 females) and April 2002 (7 Oil: 3 males, 4 females; 8 T: 6 males, 2 females), we observed the behaviour of these birds for 15 h over a period of 8 days from a hide attached to the aviary, using tape recorders. We recorded all social interactions of identifiable dyads.
(d) Statistics
Percentage brown plumage on head and folded wings was scored with respect to the percentage coverage in adult birds (head 100%, wings 0%). C.E. and T.G. scored this independently and the data were averaged per bird. These percentages were arcsine-transformed and behavioural scores log-transformed to normalize the data. We used general linear models in SPSS for the analyses with alpha set to 0.05. We used treatment of the egg, sex and year as fixed factors. Year, nor its interaction with treatment and sex, significantly contributed to any of the models (all p>0.21).
3. Results
Body mass at the time of fledging, when birds were re-housed to the aviary, did not differ between the treatments (generalized linear model, effect of treatment: F1,39=0.49, p=0.49, treatment by sex: F1,39=0.13, p=0.72), although there was a significant sex effect (F1,39=4.63, p=0.04).
We scored the social behaviour and nuptial plumage development of birds from androgen and oil eggs in the outside aviary during the first half of the subsequent reproductive season. During this period both sexes very frequently perform stereotyped and conspicuous postural and vocal displays, which have an important function in both aggressive and sexual contexts (e.g. territorial defence; pair bonding: Van Rhijn & Groothuis 1987). Just before or during this period, both sexes develop the characteristic nuptial plumage, consisting of a dark brown mask on the head. Birds in their first spring, initially show light brown feathers on the head and upper wing coverts which are gradually moulted into an (often incomplete) dark brown adult mask and the adult-like light grey upper wing coverts in the course of the following months.
The incidence of the conspicuous oblique and forward postures was substantially higher in birds from androgen eggs (figure 1). Further, birds from androgen eggs performed more charges (although only marginally significant) and more aggressive pecks than those from oil eggs, and were more successful in displacing opponents during aggressive interactions when competing for mates, territories or access to food (figure 1). Thus, enhanced levels of androgens in the egg enhance later sexual and aggressive behaviour, and success in aggressive interactions in a period when birds are physiologically able to reproduce (Glutz von Blotzheim & Bauer 1982). Birds from androgen eggs also had a more developed brown mask and fewer brown feathers on the wing than birds from oil eggs (figure 2). As a result, the former looked more adult-like both in behaviour and in plumage.
Figure 1.
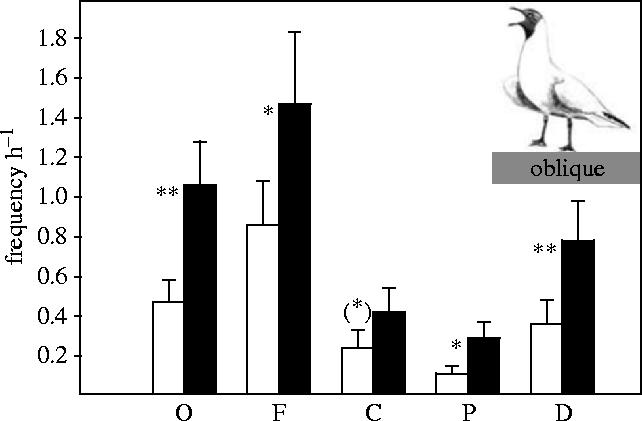
Enhanced social behaviours (frequency h−1) and dominance in 10-month-old gulls from eggs injected with androgens (filled bars) compared to birds from eggs injected with oil (open bars). O, oblique display: F1,41=7.79, p=0.008; F, forward display: F1,41=4.22, p=0.046; C, charge: F1,41=3.88, p=0.056; P, aggressive peck: F1,41=5.99, p=0.019; D, displacement: F1,41=9.19, p=0.004. Sex did not affect behaviour (all p>0.11), nor were there any sex×treatment interactions (all p>0.42).
Figure 2.
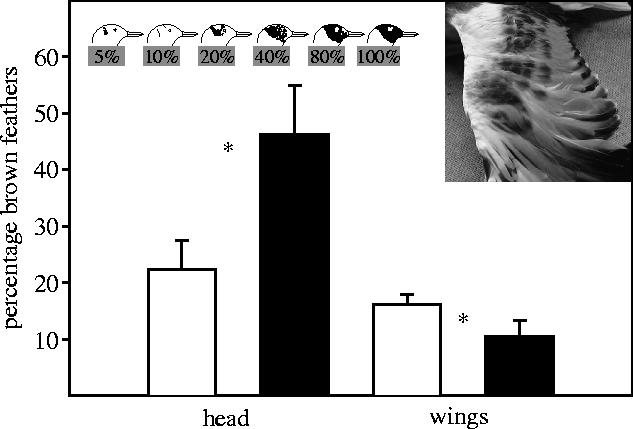
Stage of the nuptial brown head plumage and the juvenile characteristics of brown feathers on the wing in 10-month-old gulls from androgen injected eggs (filled bars) or oil injected eggs (open bars). Androgen injections in ovo enhanced the development of the nuptial plumage (F1,41=6.88, p=0.012) and the change from juvenile to adult plumage on the wing (F1,41=6.54, p=0.014). Sex did not affect plumage development (both p>0.14, sex×treatment both p>0.10).
4. Discussion
As there was no effect of treatment on body mass at the start of the captive period, it is unlikely that the long-term effect of yolk androgens which we observed is indirectly caused by the enhancing effect of androgens on growth early in life (Eising et al. 2001). All three traits that were affected by egg androgens (the frequencies of the behaviours, the development of the brown mask and the disappearance of the brown wing covers) are strongly dependent on androgens in adult life (Van Oordt & Junge 1933; Boss 1943; Groothuis & Meeuwissen 1992). In the Black-headed gull, early post-natal exposure to androgens increases the sensitivity to these hormones later in life (Ros et al. 2002), consistent with their organizing role in the development of brain and behaviour. Therefore, the long-lasting effects may be mediated by an upregulation of androgen receptors later in life. Alternatively, the early hormone exposure may have influenced the hypothalamus–pituitary–gonad axis, resulting in higher androgen production later in life.
Although most organizing effects of sex steroids are known to affect sexual differentiation, we did not find any indication for sex-specific effects of yolk hormones. This is not surprising since: (i) the Black-headed gull is monomorphic with respect to behaviour and nuptial plumage (Van Rhijn 1985); (ii) normal sexual differentiation in a species with genetic sex differentiation could be altered or interrupted by maternal hormones and even lead to infertility; (iii) sexual differentiation in birds is mainly under the control of oestrogens and not of androgens, which may also explain why avian eggs contain very low levels of this hormone (Schwabl 1993; Groothuis & Schwabl 2002).
The long-lasting effects of egg androgens are almost certainly beneficial for Darwinian fitness. Successful territory establishment and defence by means of aggressive interactions are essential for reproductive success in this colonial breeder. In addition, the displays are important for mate selection (Van Rhijn 1985; Van Rhijn & Groothuis 1987). Moreover, the timing of development of the brown mask in this species has been shown to correlate with important fitness parameters. In both sexes, earlier nuptial moult is related to stronger pair bonds, earlier egg-laying and a higher number of eggs in that season (Van Rhijn & Groothuis 1987). Interestingly, the timing of nuptial moult is highly consistent within an individual over many years (Van Rhijn & Groothuis 1987). Therefore, the effects of yolk hormones on the timing of the development of the nuptial plumage may have lifetime consequences for fitness components. It is tempting to speculate that the large variation in timing of nuptial moult and reproduction within a season (Van Rhijn & Groothuis 1987) and in the age of the first reproduction (Glutz von Blotzheim & Bauer 1982) is a consequence of differences in early exposure to maternal androgens.
Clearly, in birds, maternal hormone deposition in eggs may profoundly influence individual differentiation of fitness related traits. Since these hormones suppress early immune function of the chick (Groothuis et al. 2005b) and reduce long-term survival (Eising et al. submitted), mothers may be faced with a trade-off between producing offspring with lower survival prospects but higher reproductive success per year, or with higher chances of survival and lower annual reproductive output. By producing eggs that differ in levels of maternal hormones, mothers seem to produce a variety of phenotypes, perhaps an adaptive strategy in unpredictable environmental conditions. Since natural selection acts upon such phenotypic variation, shaping a population's demography, the role of maternal androgens in this selective process may be much greater than anticipated until now.
Our results are consistent with those of Strasser & Schwabl (2004) and extend the findings to both territorial and sexual behaviour in a natural setting. Both studies indicate that the current predominant approach of studying avian maternal hormone allocation as a maternal strategy to influence the degree of sibling competition early in life can only give an incomplete picture of its possible adaptive value.
Acknowledgments
We are grateful to Cor Dijkstra, Serge Daan and Leo Bruinzeel for helpful discussions and suggestions for improving the manuscript.
References
- Balthazart J, Adkins-Regan E.A. Sexual differentiation of brain and behaviour in birds. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Rabin R, editors. Hormones, brain and behavior. vol. IV. Elsevier; Netherlands: 2002. pp. 223–301. [Google Scholar]
- Boss W.R. Hormonal determination of adult characteristics and sex behavior in Herring gulls. J. Exp. Zool. 1943;94:181–210. doi:10.1002/jez.1400940203 [Google Scholar]
- Clark M.M, Galef B.G., Jr Prenatal influences on reproductive life history strategies. TREE. 1995;10:151–153. doi: 10.1016/s0169-5347(00)89025-4. [DOI] [PubMed] [Google Scholar]
- Crews D. On the organization of individual differences in sexual behaviour. Am. Zool. 1998;38:118.132. [Google Scholar]
- Drea C.M, Weldele M.L, Forger N.G, Coscia E.M, Frank L.G, Licht P, Glickman S.E. Androgens and masculinization of genitalia in the spotted hyaena. J. Reprod. Fertil. 1998;113:117–127. doi: 10.1530/jrf.0.1130117. [DOI] [PubMed] [Google Scholar]
- Eising C.M, Eikenaar C, Schwabl H, Groothuis T.G.G. Maternal androgens in black-headed gull eggs. Proc. R. Soc. B. 2001;268:839–846. doi: 10.1098/rspb.2001.1594. doi:10.1098/rspb.2001.1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eising, C. M., Van der Jeugd, H. P., Müller, W. & Groothuis, T. G. G. Submitted. Maternal androgens in avian eggs reduce long-term survival probabilities.
- Glutz von Blotzheim, U. N. & Bauer, K. M. (eds) 1982 Handbuch der Voegel Mitteleuropas, Band 8, pp. 273–360. Wiesbaden: Academische Verlaggesellschaft.
- Groothuis T.G.G, Meeuwissen G. The influence of testosterone on the development and fixation of the form of displays in two age classes of young Black-headed gulls. Anim. Behav. 1992;43:189–208. [Google Scholar]
- Groothuis T.G.G, Schwabl H. The influence of laying sequence and habitat characteristics on maternal yolk hormone levels. Funct. Ecol. 2002;16:281–289. doi:10.1046/j.1365-2435.2002.00623.x [Google Scholar]
- Groothuis T.G.G, Müller W, von Engelhardt N, Carere C, Eising C.M. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 2005a;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. doi:10.1016/j.neubiorev.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Groothuis T.G.G, Eising C.M, Dijkstra C, Müller W. Balancing between costs and benefits of maternal hormone deposition in avian eggs. Biol. Lett. 2005b;1:78–81. doi: 10.1098/rsbl.2004.0233. doi:10.1098/rsbl.2004.0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C.M, Hews D.A, Knapp R. Hormonal control and evolution of alternative male phenotypes: generalizations of models for sexual differentiation. Am. Zool. 1998;38:133–151. [Google Scholar]
- Ros A.F.H, Dieleman S.J, Groothuis T.G.G. Social stimuli, testosterone, and aggression in gull chicks: support for the challenge hypothesis. Horm. Behav. 2002;41:334–342. doi: 10.1006/hbeh.2002.1768. doi:10.1006/hbeh.2002.1768 [DOI] [PubMed] [Google Scholar]
- Schwabl H. Yolk is a source of maternal testosterone for developing birds. Proc. Natl Acad. Sci. USA. 1993;90:11 446–11 450. doi: 10.1073/pnas.90.24.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabl H, Mock D.W, Gieg J.A. A hormonal mechanism for parental favouritism. Nature. 1997;386:231. doi:10.1038/386231a0 [Google Scholar]
- Strasser R, Schwabl H. Yolk testosterone organizes behaviour and male plumage coloration in house sparrows. Behav. Ecol. Soc. 2004;56:491–497. [Google Scholar]
- Van Oordt G.J, Junge G.G.A. Der Einfluss der Kastration bei männlichen Lachmöwen. Wilhelm Roux' Archiv für Entwicklungsmechanik der Organismen. 1933;128:165–180. doi: 10.1007/BF00578947. [DOI] [PubMed] [Google Scholar]
- Van Rhijn J. Black-headed gull or Black-headed girl? Behaviour. 1985;100:134–169. [Google Scholar]
- Van Rhijn J, Groothuis T.G.G. On the mechanism of mate selection in Black-headed gulls. Behaviour. 1987;74:284–293. [Google Scholar]


