Abstract
Marine organisms have evolved defence mechanisms to prevent epibiosis. This study investigated the anti-settlement properties of natural periostracal microtopographies of two mytilid species, Mytilus edulis (from North, Baltic and White Seas) and Perna perna (from the SW Atlantic). Resin replicas of shells were exposed to cyprids of the barnacle Semibalanus balanoides. Replicas with intact isotropic microtopographies and smooth controls were much less fouled than roughened anisotropic surfaces. This indicates that in both M. edulis and P. perna the periostracum possesses a generic anti-settlement property, at least against S. balanoides cyprids, which is not regionally adapted. Such a potential globally effective anti-settlement mechanism possibly contributes to the invasive success of Mytilidae.
Keywords: mytilidae, microtopography, epibiosis, mussels
1. Introduction
Settlement of marine larvae is not a stochastic process and many species show some degree of choice (Keough & Downes 1982). Choices are made in response to diverse physical and chemical cues and may be highly specific (Rittschof & Costlow 1989), causing intense settlement pressure on any substratum in the sea, including other organisms. Epibiosis is a major problem for marine organisms, and yet the surfaces of most species are rarely completely overgrown. Since epibiosis may have harmful effects (Wahl 1997a), many marine species have evolved chemical, physical or mechanical defence mechanisms (Wahl 1989). The shells of epibenthic bivalves offer substantial space for larvae; however, the shells of mytilids often appear less fouled than adjacent non-biological substrata (Wahl et al. 1998; Bers & Wahl 2004).
Given that epibiosis reduces fitness (Wahl 1997b), then we might expect that surfaces of endemic species might be adapted for preventing settlement of the local epibiota. Conversely, more cosmopolitan species should exhibit a generalized antifouling activity. As the antifouling capacity of the shells of the mussels Mytilus edulis (Wahl et al. 1998; Bers & Wahl 2004) and M. galloprovincialis (Scardino et al. 2003) is related to the texture of the periostracum, then there is the possibility that periostracal textures may be adapted to locally abundant epibionts. Hence, the aim of this study was to investigate the anti-settlement properties of natural microtopographies of shells from different populations of mytilids and species with regard to one epibiont, the cyprid of the acorn barnacle Semibalanus balanoides. Shells from three different populations of the blue mussel M. edulis, and from one population of the brown mussel Perna perna were used in this study. Both of these species are cosmopolitan in northern and southern hemispheres, respectively. P. perna was chosen in order to include a closely related, but non-Mytilus, mytilid in the study as a reference point.
2. Material and methods
M. edulis valves were obtained from the Baltic Sea (Kiel Fiord, Germany, 54°22′N, 10°9′E), White Sea (Matryonin Island, Russia, 66°18′N, 33°40′E), and North Sea (Hartlepool Marina, UK, 54°41′N, 1°11′W). P. perna valves were obtained from the south-west Atlantic (Itaipu beach, Brazil, 22°56′S, 43°03′W). All sites were sheltered bays.
To quantify the effect of periostracal microtopographies on cyprid settlement separately from other possible surface properties, resin replicas of valves were made. This method gives sub-micrometre replication and is non-toxic (Marrs et al. 1995). Microfouling on the shells was removed with a soft toothbrush and sterile filtered seawater (Sartorius 0.8 μm cellulose nitrate filter). Casts of shells were made using Kerr's Extrude Wash Type 3 (Kerr, USA) and high resolution resin replicas were made using Devcon 2-TON epoxy resin (Devcon, UK). Replicas were coloured uniform grey using Coelan Farbpaste (Coelan, Germany) and cured for 12–18 h at room temperature. Smooth control surfaces were made by sealing a second resin replica of the same individual shell with resin to give a glassy smooth surface. Rough control surfaces were made by standardized roughening of a third resin replica (Grade 70/Grit M2 abrasive paper, English Abrasives & Chemical Limited, UK). An experimental triplet comprised a smooth, natural and rough replica and three triplets were made for each provenance, each from a different individual's right or left valve.
Field experiments were performed from 5th to 18th April 2003 at Keppel Pier, Millport, Clyde Sea, UK (55°45′N, 4°54′W). No other species of barnacle settle at this time and the large numbers of cyprids exclude all other non-barnacle epibionts (Hills & Thomason 1998; Hansson et al. 2003).
Replicas in each triplet were fixed 2 cm apart on grey PVC sheet using non-toxic silicone adhesive. Triplets were randomized across the PVC sheet and the sheet attached intertidally (2.05 m above chart datum) in the middle of the S. balanoides settlement zone using 8 mm bolts. Digital photographs of each replica were taken every low tide for 25 tides (Canon D30 camera, 100 mm lens). Cyprids and metamorphs were counted using ImageTool 3.0 only in the central 1 cm2 of each replica to reduce edge effects.
Microtopographies were examined by SEM. Three replicas from each provenance were sputter coated with a 20 nm thick gold—palladium alloy and viewed with a Zeiss DSM 940 SEM. Additionally, a 4 mm profile of each replica in one triplet was quantified with a Uniscan OSP100a laser profilometer.
Data for the last tide (25) were tested for normality, transformed ((count+1)0.07) and analysed using ANOVA (SPSS v.11): within-subjects factor was surface (natural, smooth, rough), between subjects factor was provenance, and the response was count. Sphericity of the data was assessed using Mauchly's test and where they were aspherical, Greenhouse–Geisser adjustments of F were used. All variances were homogeneous (Levene's test, p>0.05).
3. Results
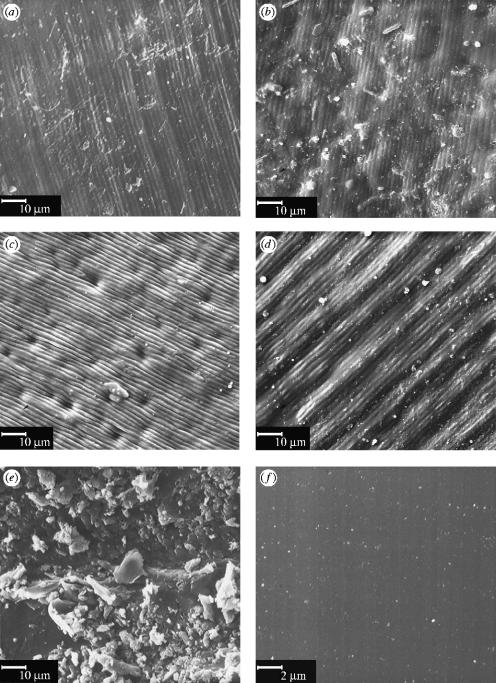
Valves from each provenance featured a rippled periostracal microtopography, with a wavelength of 1.5–2.0 μm running orthogonally to the growth rings of the shell (figure 1). There was no significant difference in arithmetic mean roughness (Ra) between natural and roughened replicas (all provenances combined, ANOVA, n=8, F1,6=0.097, p=0.77, Ra,natural=14.01±4.26 μm, Ra,rough=14.74±3.08 μm). Smooth replicas could not be scanned in the laser profilometer due to their high reflectance.
Figure 1.
Scanning electron micrographs of: (a) Perna perna (Brazil); (b) Mytilus edulis (Russia); (c) Mytilus edulis (UK); (d) Mytilus edulis (Germany); (e) rough control; (f) smooth control.
The tide 25 data showed no significant interaction between country and surface (F6,16=1.38, p<0.28), and no significant main effects for country (F3,8=0.57, p=0.65). However, there were significant differences between surfaces (F1,2=33.79, p<0.001, figure 2). An a priori contrast test (natural surface=reference) indicated that this difference was attributable to a significant difference between rough and both natural and smooth surfaces (table 1). This suggests that the anti-settlement property of the valves is the same irrespective of species or regional provenance. We did not differentiate between metamorphs and newly settled cyprids and the data include both. Reduction of space due to permanent attachment is seen as the plateaus after tide 17 (figure 2). Analysis of differences in the rate of settlement was undertaken using another ANOVA. Data for all tides were transformed and analysed with both tide number and surface as within subjects factors, and country as the between subjects factor. Initially, a fully factorial model was run and then main effects and interactions excluded from the model if p>0.1. The final model was tide+surface+tide×surface. There were significant interactions between surface and tide (F6.04,66.4=9.3, p<0.001, table 1). An a priori contrast test (tide 1=reference) showed that significant differences (p<0.001) only occurred after tide 4 (figure 2). These results show that intense settlement leads to rapid differences between surfaces.
Figure 2.
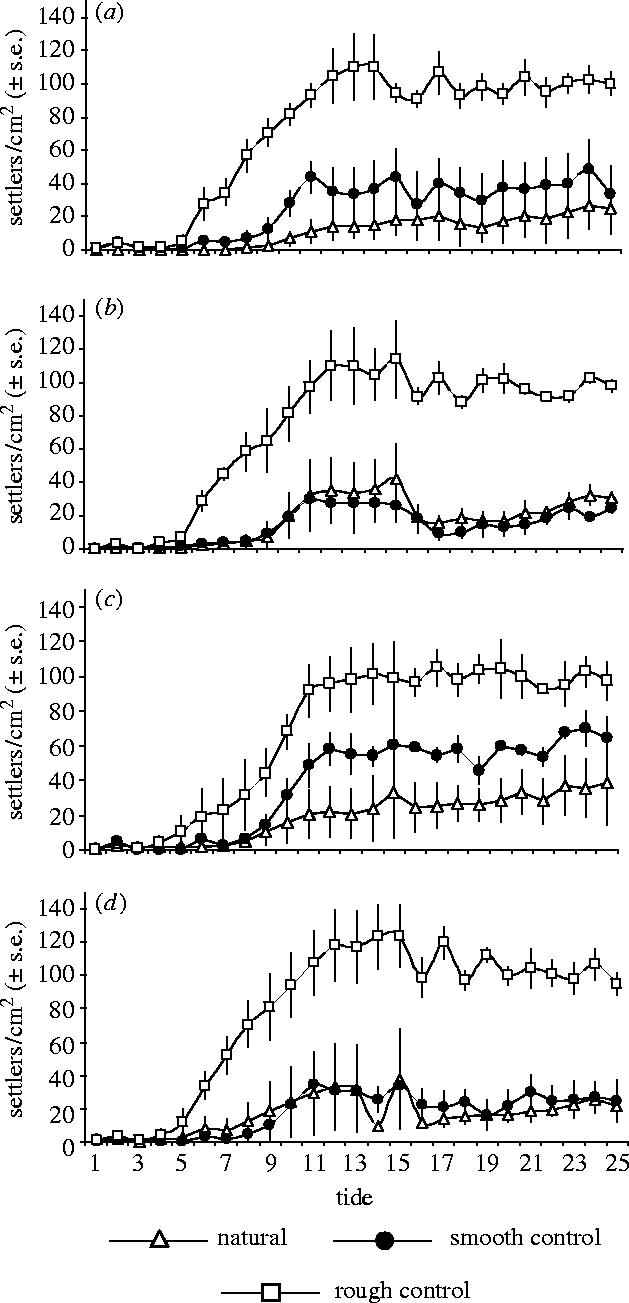
Barnacle settlement on natural, smooth and rough replicas of (a) Perna perna (Brazil), (b) Mytilus edulis (Russia), (c) Mytilus edulis (UK), (d) Mytilus edulis (Germany). Error bars indicate s.e.
Table 1.
Contrast test with natural surface as reference level.
| source | surface | d.f. | F-value | p-value |
|---|---|---|---|---|
| surface | natural versus smooth | 1 | 1.802 | 0.216 |
| smooth versus rough | 1 | 42.076 | <0.001 |
4. Discussion
This simple field assay-based study revealed remarkable similarities in the anti-settlement properties of mytilid shells against larvae of S. balanoides. There was no difference between smooth control and natural surfaces for both species, as well as no differences between M. edulis shells from the Baltic, North and White Seas. In all cases smooth controls and natural surfaces were much less fouled than the rough control surfaces, and although none remained completely unfouled, this difference was seen after only four tides. As the magnitude of the surface topographies was the same for both natural and roughed valves, then this result is attributable to the change from a natural isotropic microtopography to the anisotropic roughened surfaces. It is known that the periostracum prevents boring organisms damaging the shell structure (Harper & Skelton 1993; Kaehler 1999) and it is now also known that intact periostracal textures maintain general fitness by reducing fouling (Scardino & de Nys 2004). External surfaces of most mollusc shells are generally rough. This is probably the ancestral condition and is governed by the difficulty of producing and maintaining a very smooth surface without frequent maintenance by the mantle. Mytilids appear to have adapted to reduce epibiosis by evolving an isotropic periostracum, not a smooth one. The consistency of these results indicates that all populations of M. edulis may possess an anti-settlement property, at least against S. balanoides cyprids, even when this species is absent, i.e. in the Baltic, where the dominant barnacle is Balanus improvisus.
Hills & Thomason (1998) demonstrated that S. balanoides cyprids prefer surface roughness about the size of the larval body (0.5–1 mm), and do not like very smooth surfaces. They did not test rugosities at the scale presented here where the roughened control and natural shells both had roughness ∼14 μm, somewhere between 35–70 times smaller than those tested by Hills & Thomason (1998). These results clearly show that cyprids of S. balanoides will settle on topographies at the micrometre scale as long as they are anisotropic.
Thus, it can be cautiously inferred that the periostracal topography has anti-settlement activity that is not locally adapted. Additional support for this view comes from the similarity not only between M. edulis and P. perna as found by this study, but also from the similarity between these two species and M. galloprovincialis, which also has antifouling properties (Scardino et al. 2003; Scardino & de Nys 2004).
Artificial isotropic microtopographies have been shown to reduce barnacle settlement by reducing exploration time (Berntsson et al. 2000). It is therefore possible that the generalized anti-settlement property reported here is a reflection of this behaviour. It is possible that isotropic microtopographies will confer a general protection against the settlement of all barnacle larvae.
Very little is known about biogeographical differences in defences of marine invertebrates (Becerro et al. 2003). Since the prevention of epibiosis, and hence maintenance of fitness, is essential, anti-settlement defences with global effectiveness would allow the invasion of new areas and may have contributed to the evolutionary and invasive capability of the mytilids. Understanding how these microtopographies function is attracting increasing attention given the urgent need to find alternatives to chemically active antifouling coatings (Andersson et al. 1999).
Acknowledgments
Special thanks to Bernardo A. P. da Gama and Sergey Dobretsov for collecting mussels and Alan Henderson for access to Hartlepool Marina.
References
- Andersson M, Berntsson K, Jonsson P, Gateholm P. Microtextured surfaces: towards macrofouling resistant coatings. Biofouling. 1999;14:167–178. [Google Scholar]
- Becerro M, Thacker R, Turon X, Uriz M, Paul V. Biogeography of sponge chemical ecology: comparisons of tropical and temperate defenses. Oecologia. 2003;135:91–101. doi: 10.1007/s00442-002-1138-7. [DOI] [PubMed] [Google Scholar]
- Berntsson K, Andreasson H, Jonsson P, Larsson L, Ring K, Petronis K, Gatenholm P. Reduction of barnacle recruitment on micro-textured surfaces: analysis of effective topographic characteristics and evaluation of skin friction. Biofouling. 2000;16:245–261. [Google Scholar]
- Bers A, Wahl M. The influence of natural surface microtopographies on fouling. Biofouling. 2004;20:43–51. doi: 10.1080/08927010410001655533. doi:10.1080/08927010410001655533 [DOI] [PubMed] [Google Scholar]
- Hansson L, Hudson I, Seddon R, Shaw O, Thomason J. Massive recruitment of the barnacle Semibalanus balanoides in the Clyde Sea (Scotland, UK) in the spring of 2000. J. Mar. Biol. Assoc. UK. 2003;83:923–924. doi:10.1017/S0025315403008063h [Google Scholar]
- Harper E, Skelton P. A defensive value of the thickened periostracum in the Mytiloidea. Veliger. 1993;36:36–42. [Google Scholar]
- Hills J, Thomason J. The effect of scales of surface roughness on the settlement of barnacle (Semibalanus balanoides) cyprids. Biofouling. 1998;12:57–69. [Google Scholar]
- Kaehler S. Incidence and distribution of phototrophic shell-degrading endoliths of the brown mussel Perna perna. Mar. Biol. 1999;135:505–514. doi:10.1007/s002270050651 [Google Scholar]
- Keough M, Downes B. Recruitment of marine invertebrates: the role of active larval choices and early mortality. Oecologia. 1982;54:346–352. doi: 10.1007/BF00380003. doi:10.1007/BF00380003 [DOI] [PubMed] [Google Scholar]
- Marrs S, Thomason J, Cowling M, Hodgkiess T. A replica method for the study of marine biofilms. J. Mar. Biol. Assoc. UK. 1995;75:759–762. [Google Scholar]
- Rittschof D, Costlow J. Surface determination of macroinvertebrate larval settlement. In: Klekowski R, Styczynska-Jurewicz E, Falkowsky L, editors. Twenty first European marine biology symposium. Polish Academy of Sciences, Institute for Oceanology; Gdansk, Poland: 1989. pp. 155–163. [Google Scholar]
- Scardino A, de Nys R. Fouling deterrence on the bivalve shell Mytilus galloprovincialis: a physical phenomenon? Biofouling. 2004;20:249–257. doi: 10.1080/08927010400016608. doi:10.1080/08927010400016608 [DOI] [PubMed] [Google Scholar]
- Scardino A, de Nys R, Ison O, O'Connor W, Steinberg P. Microtopography and antifouling properties of the shell surface of the bivalve molluscs Mytilus galloprovinvialis and Pinctata imbricata. Biofouling. 2003;19:221–230. doi: 10.1080/0892701021000057882. doi:10.1080/0892701021000057882 [DOI] [PubMed] [Google Scholar]
- Wahl M. Marine epibiosis I. Fouling and antifouling. Some basic aspects. Mar. Ecol. Prog. Ser. 1989;58:175–189. [Google Scholar]
- Wahl M. Living attached: aufwuchs, fouling, epibiosis. In: Nogabushanam R, Thompson M, editors. Fouling organisms of the Indian Ocean: biology and control technology. Oxford & IBH Publishing Company; New Delhi: 1997a. pp. 31–83. [Google Scholar]
- Wahl M. Increased drag reduces growth of snails: comparison of flume and in situ experiments. Mar. Ecol. Prog. Ser. 1997b;151:291–293. [Google Scholar]
- Wahl M, Kröger K, Lenz M. Non-toxic protection against epibiosis. Biofouling. 1998;12:205–226. [Google Scholar]