Abstract
Early environmental conditions have been suggested to influence subsequent locomotor performance in a range of species, but most measurements have been of initial (baseline) performance. By manipulating early growth trajectories in green swordtail fish, we show that males that underwent compensatory growth as juveniles had a similar baseline swimming endurance when mature adults to ad libitum fed controls. However, they had a reduced capacity to increase endurance with training, which is more likely to relate to Darwinian fitness. Compensatory growth may thus result in important locomotor costs later in life.
Keywords: growth rate, exercise, endurance, resource allocation, swimming performance
1. Introduction
Changes in food availability during growth and development are likely to be common for many animal species. If food availability is initially low but then increases, individuals have the chance to compensate for their poor start in life, so that they catch-up and rejoin, or even exceed, their original growth trajectory. Such an increase in size may be beneficial for survival and reproduction, but growth compensation is also associated with numerous and varied long-term costs (Metcalfe & Monaghan 2001). One of the potential costs of rapid growth is a reduction in locomotor performance (Munch & Conover 2004), possibly mediated through altered muscle development (Galloway et al. 1999).
Most studies of locomotor performance have simply taken initial baseline measurements, often using laboratory-reared animals (reviewed by Kolok 1999; Irschick & Garland 2001). However, performance changes with nutritional and energetic status (Guderley 2004) and vertebrates usually improve their performance with repeated exposure (‘exercise training’, Davison 1997). Despite this, there has been little investigation of the factors that might influence this rate of improvement, especially as the response to exercise is arguably more important to survival than baseline performance, since it is a measure of how well the individual can match performance to current environmental conditions.
Here, we test the hypothesis that early growth trajectory affects the subsequent ability to improve locomotor performance, using green swordtail fish (Xiphophorus helleri). Males of this species virtually cease body growth at the onset of sexual maturation, but their adult body size is a strong predictor of dominance in interactions with other males (Beaugrand & Cotnoir 1996). Consequently, any reduction in early growth rate might be expected to induce a compensatory response prior to sexual maturation, which is predicted to have negative long-term effects on the ability to increase physical fitness.
2. Material and methods
(a) Rearing regimes
Fry of wild parentage were reared in the laboratory under controlled conditions (stable temperature of 23±1 °C, 16L : 8D light regime, ad libitum food). Individual fry were placed in one-half of an acrylic plastic rearing tank (320×170×180 mm; 10 l), which was divided longitudinally by a transparent Perspex partition. This ensured that fishes were physically, but not visually isolated from their neighbours. Each tank had a gravel substrate, a plastic plant for cover and was aerated and filtered. At two months of age, equal numbers of fishes from each brood were allocated to one of two treatment groups.
Good/Good (GG)—ad libitum food daily for the duration of the experimental period (two months of age onwards).
Poor/Good (PG)—fed three times a week from two to six months of age, then subsequently put onto the same daily ad libitum diet as GG fishes from six months onwards.
Details of the feeding protocol and basic husbandry are given in the electronic supplementary material. Measurements of baseline endurance were taken on 71 males from 19 different families at 12 months of age from both treatment groups. The response to exercise was assessed in a subsample of 13 size-matched pairs of sibling males to control for genetic and maternal effects on endurance (n=13 dams), one from each of treatment groups 1 and 2 (table 1). Thirteen size-matched pairs of non-sibling males (from different families to each other and to the sib pairs), one from each of treatment groups 1 and 2 (n=13), acted as untrained controls to assess change in endurance without exercise (see below).
Table 1.
Biometrics, when tested for swimming, of males used in the experimental response to exercise and controls. (Mean±1 s.e.)
| sib pairs (n=13) | non-sib controls (n=13) | |||||
|---|---|---|---|---|---|---|
| GG | PG | paired t-test | GG | PG | paired t-test | |
| SL (mm) | 42.07±0.93 | 42.19±0.94 | t=0.11, p=0.92 | 43.11±0.99 | 43.79±1.64 | t=0.48, p=0.64 |
| TL (mm) | 73.69±1.71 | 74.22±1.57 | t=0.23, p=0.82 | 73.61±1.66 | 77.24±3.13 | t=1.35, p=0.20 |
| mass (g) | 1.451±0.115 | 1.551±0.116 | t=0.75, p=0.47 | 1.739±0.144 | 1.819±0.234 | t=0.35, p=0.73 |
Fishes were weighed (±0.01 g) and measured initially at two months, and then subsequently every two weeks until 10 months of age. On each occasion, standard length (i.e. from tip of nose to tip of caudal peduncle), total length (standard length plus length of the caudal fin, including the ‘sword’ extension of the fin if present) and maximum body depth were recorded (±0.1 mm). Growth rate was quantified as the growth increment of standard length (SL, in mm) over successive growth periods (two to six months and six to ten months).
(b) Measurement of endurance
Swimming endurance was measured in a modified Bläzka respirometer (see electronic supplementary material), which pumped a steady flow of water through a central chamber containing a single fish. The fish was placed into the chamber for 5 min to settle at an initial flow rate of 21 cm s−1, then allowed to acclimatize for 1 min at a flow rate of 27 cm s−1, before the pump was turned up to the experimental running speed (50 cm s−1) and maintained until fatigue. Fishes were deemed to be exhausted when they were forced back against the back fine mesh grid for more than 5 s (Ryan 1988) and were no longer able to continue swimming, despite tapping of the side of the chamber (Ojanguren & Braña 2000). Once exhausted, the pump was turned off and the fish allowed 5 min recuperation time before being placed back in its rearing tank. Endurance is defined as the amount of time that a fish swam at the highest flow rate (50 cm s−1).
(c) Exercise protocol
After initial screening of endurance, sib pairs were put through the following training regime over a three-week period. Each week involved a single 25 min session of exercise as follows: Week 1—15 min at a low flow rate (21 cm s−1) then 5 min at a medium flow rate (27 cm s−1), finishing with a further 5 min at the low rate. Week 2—10 min low/10 min medium/5 min low. Week 3—5 min low/15 min medium/5 min low. Control pairs of fishes did not go through the exercise regime. Both sib pairs and (untrained) controls were again tested for endurance at the end of the three-week period.
(d) Statistical analysis
Endurance was log transformed to normalize the data before analysis. We used the penalized log likelihood (Akaike Information Criteria, AIC) in mixed-effects general linear models (GLMs) to compare the fit of different models of endurance, following sequential dropping of non-significant terms from a full model (Crawley 2002). The smaller the AIC, the better the model fit. The log-likelihood ratio test was used to compare the fit of successive models (Crawley 2002). A two-factor repeated measures ANOVA with standard length as the response variable and age (2, 6 and 10 months) and treatment (GG versus PG) as the factors was used to analyse growth. All statistical tests were conducted using S-Plus 6 for Windows or SPSS 10 for Windows.
3. Results
(a) Growth rate: sib males
There was, unsurprisingly, a significant effect of age on body size for sib pairs (F2,10=210.17, p<0.0005; figure 1). There was no overall effect of treatment on growth (F1,11=0.84, p=0.38), but there was a significant age×treatment interaction (F2,10=14.27, p=0.001; figure 1). This was because PG males, although slightly larger at two months, were significantly smaller than their GG brothers at six months of age, but then (as a result of compensatory growth) were no different in size by 10 months of age (quadratic term: F1,11=25.81, p<0.0005; figure 1).
Figure 1.
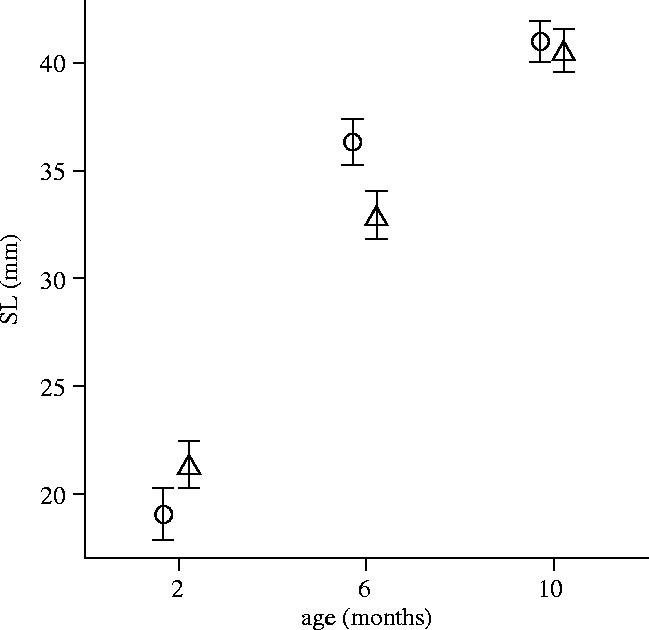
Standard length (SL; mm) in relation to age (months) for male sib pairs (n=13). Mean±1 s.e. Circular symbols represent GG males and triangles PG males.
(b) Endurance
Baseline endurance levels were not related to age at maturation (t44=0.31, p=0.76), treatment (t50=1.03, p=0.31), standard length at two months of age (t49=0.53, p=0.60) or sword length (t45=0.48, p=0.63). Body size (SL) was positively, but non-significantly related to baseline endurance (t51=1.37, p=0.18).
After training, GG reared males had significantly greater endurance capacity than their PG sibs (figure 2a). A mixed-effect GLM was fitted to these data, with the difference between final and initial endurance capacity (log) as the response variable, family as a random effect and initial endurance (log), standard length, standard length at two months of age and treatment as fixed effects. Standard length (t8=0.76, p=0.47) and standard length at two months (t9=1.13, p=0.29) were sequentially dropped from successive models without significantly increasing the AIC. The minimum adequate model included initial endurance (t10=5.79, p=0.0002), treatment (t10=2.33, p=0.042) and their interaction (t10=2.03, p=0.070). The interaction was retained in the model, as its removal resulted in a significant increase in the AIC (likelihood ratio=4.44, p=0.035). GG males with the lowest initial endurance showed the greatest change in endurance following training. PG males showed very little response to training (figure 2a).
Figure 2.
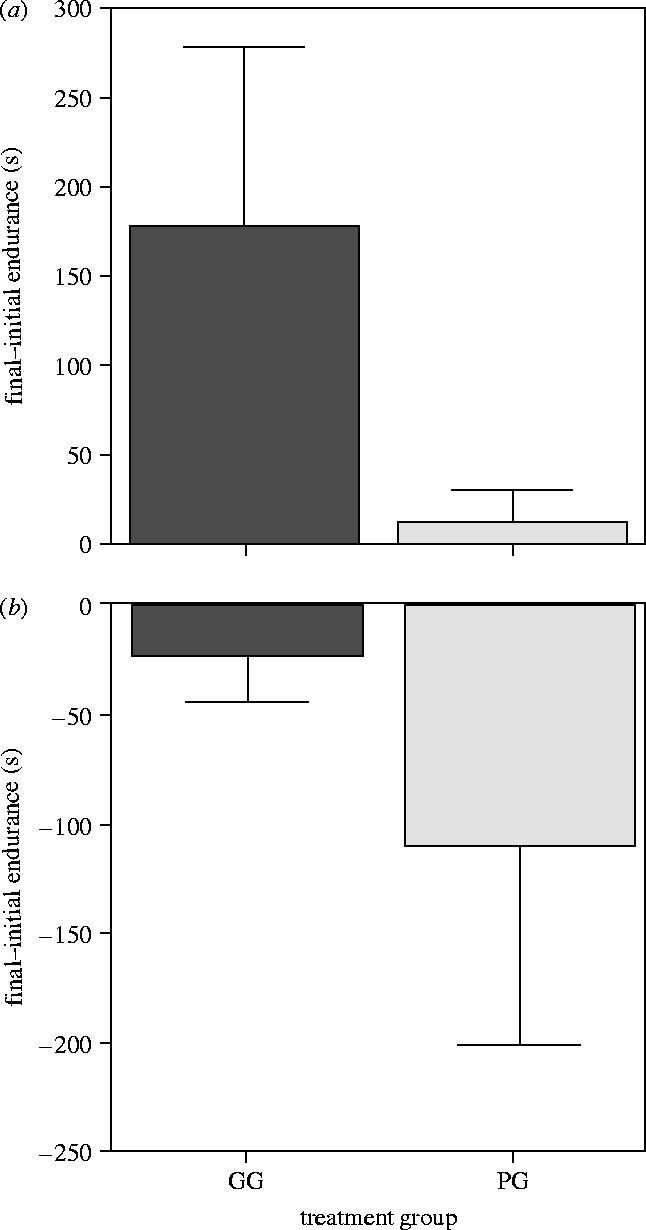
(a) Change in endurance in response to exercise for male sib pairs and (b) change in endurance for non-sib male controls (that were not exercised) over the same time period. Mean±1 s.e. Data presented using untransformed values for clarity. Statistics were performed on log values for endurance (see text).
In contrast, there was no effect of treatment on the change in endurance capacity between control pairs of males over the same period (GLM; F1,22=0.18, p=0.68). There was also no significant effect of initial endurance on endurance capacity (F1,22=3.84, p=0.063), although endurance decreased overall (figure 2b). However, there was a significant interaction between initial endurance and treatment (F1,22=12.12, p=0.002): GG males showing the lowest initial endurance had the biggest decrease in endurance over time, whereas the greatest decline in PG males was in those with the highest initial endurance.
4. Discussion
Male swordtails experiencing an improvement in food availability during development (PG males) were subsequently able to compensate fully in body and tail (sword) size compared to GG males, and had a similar baseline level of endurance. However, their capacity to improve their performance was significantly reduced. Since the fishes were not exposed to unidirectional currents in their rearing tanks, it is perhaps not surprising that differences in swimming capacity were only revealed once they were repeatedly forced to swim against a strong current. Nonetheless, baseline aerobic swimming performance is routinely used in laboratory studies as a measure of locomotor capacity (Kolok 1999), despite the problems of translating laboratory performance to ecological function (Irschick & Garland 2001). Exercise training more readily replicates the conditions found in the wild, where swordtails often court females feeding on algal-covered rocks in strong currents (Ryan 1988).
In many cases, natural selection appears to increase physical performance. Despite this, physical performance in humans and other animals is often highly variable. Le Galliard et al. (2004) showed that initial endurance (at birth) in lizards was heritable, but selection in favour of increased endurance was weak. This was because environmental conditions following birth determined the expression of a genetic predisposition for high initial endurance (Le Galliard et al. 2004). Early developmental conditions may thus greatly influence the effect of locomotor performance on Darwinian fitness. The importance of endurance capacity for courtship (Ryan 1988), combined with the greater dominance of GG males in paired encounters with size-matched PG males (Royle et al. 2005), suggests that compensation following poor initial growth may reduce the mating success of male swordtails much later in life, and illustrates the importance of incorporating behaviour into a ‘performance paradigm’ of locomotor function (Irschick & Garland 2001). The challenge now is to identify the structural (e.g. Galloway et al. 1999) or metabolic (Davison 1997; Guderley 2004) causes for the growth-induced effects on muscular performance.
Acknowledgments
This work was supported by BBSRC grant 17/S15807. Thanks to John Laurie, June Freel, Graham Adam and Helicia Lepatik for help with fish husbandry, and two anonymous referees for comments on an earlier version of the paper.
Supplementary Material
References
- Beaugrand J.P, Cotnoir P.-A. The role of individual differences in the formation of triadic dominance orders of male green swordtail fish (Xiphophorus helleri) Behav. Process. 1996;38:287–296. doi: 10.1016/s0376-6357(96)00039-3. doi:10.1016/S0376-6357(96)00039-3 [DOI] [PubMed] [Google Scholar]
- Crawley M.J. Wiley; Chichester, UK: 2002. Statistical computing: an introduction to data analysis using S-Plus. [Google Scholar]
- Davison W. The effects of exercise training on teleost fish: a review of recent literature. Comp. Biochem. Physiol. A. 1997;117:67–75. doi:10.1016/S0300-9629(96)00284-8 [Google Scholar]
- Galloway T.F, Kjorsvik E, Kryvi H. Muscle growth and development in Atlantic cod (Gadus morhua L.) related to different somatic growth rates. J. Exp. Biol. 1999;202:2111–2120. doi: 10.1242/jeb.202.15.2111. [DOI] [PubMed] [Google Scholar]
- Guderley H. Locomotor performance and muscle metabolic capacities: impact of temperature and energetic status. Comp. Biochem. Physiol. B. 2004;139:371–382. doi: 10.1016/j.cbpc.2004.04.001. doi:10.1016/j.cbpc.2004.04.001 [DOI] [PubMed] [Google Scholar]
- Irschick D.J, Garland T. Integrating function and ecology in studies of adaptation: investigations of locomotor capacity as a model system. Annu. Rev. Ecol. Syst. 2001;32:367–396. doi:10.1146/annurev.ecolsys.32.081501.114048 [Google Scholar]
- Kolok A.S. Interindividual variation in the prolonged locomotor performance of ectothermic vertebrates: a comparison of fish and herpatofaunal methodologies and a brief review of the fish literature. Can. J. Fish. Aquat. Sci. 1999;56:700–710. doi:10.1139/cjfas-56-4-700 [Google Scholar]
- Le Galliard J.-F, Clobert J, Ferrière R. Physical performance and Darwinian fitness in lizards. Nature. 2004;432:502–505. doi: 10.1038/nature03057. doi:10.1038/nature03057 [DOI] [PubMed] [Google Scholar]
- Metcalfe N.B, Monaghan P. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. doi:10.1016/S0169-5347(01)02124-3 [DOI] [PubMed] [Google Scholar]
- Munch S.B, Conover D.O. Nonlinear growth cost in Menidia menidia: theory and empirical evidence. Evolution. 2004;58:661–664. [PubMed] [Google Scholar]
- Ojanguren A.F, Braña F. Thermal dependence of swimming endurance in juvenile brown trout. J. Fish Biol. 2000;56:1342–1347. doi:10.1111/j.1095-8649.2000.tb02147.x [Google Scholar]
- Royle N.J, Lindström J.E, Metcalfe N.B. A poor start in life affects dominance status in adulthood independent of body size in green swordtails Xiphophorus helleri. Proc. R. Soc. B. 2005;272:1917–1922. doi: 10.1098/rspb.2005.3190. doi:10.1098/rspb.2005.3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M.J. Phenotype, genotype, swimming endurance and sexual selection in a swordtail (Xiphophorus nigrensis) Copeia. 1988;2:484–487. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


