Abstract
Steller's sea cow, a giant sirenian discovered in 1741 and extinct by 1768, is one of the few megafaunal mammal species to have died out during the historical period. The species is traditionally considered to have been exterminated by ‘blitzkrieg’-style direct overharvesting for food, but it has also been proposed that its extinction resulted from a sea urchin population explosion triggered by extirpation of local sea otter populations that eliminated the shallow-water kelps on which sea cows fed. Hunting records from eighteenth century Russian expeditions to the Commander Islands, in conjunction with life-history data extrapolated from dugongs, permit modelling of sea cow extinction dynamics. Sea cows were massively and wastefully overexploited, being hunted at over seven times the sustainable limit, and suggesting that the initial Bering Island sea cow population must have been higher than suggested by previous researchers to allow the species to survive even until 1768. Environmental changes caused by sea otter declines are unlikely to have contributed to this extinction event. This indicates that megafaunal extinctions can be effected by small bands of hunters using pre-industrial technologies, and highlights the catastrophic impact of wastefulness when overexploiting resources mistakenly perceived as ‘infinite’.
Keywords: Bering Island, historical extinction, Hydrodamalis gigas, megafauna, overhunting, population viability analysis
1. Introduction
Few studies are able to address the full course of an extinction event, from first population decline to death of the last individual of a species, because an epistemological gap exists between neontological and palaeontological data. Extant threatened species can reveal factors responsible for declines and general patterns of range collapse, but are typically uninformative about decline duration or potential persistence of remnant populations. Prehistoric extinctions are instead informative about temporal, geographical and taxonomic patterns of extinction, rather than the build-up to these events. Historical data alone can potentially reveal both last-occurrence dates for recently extinct species and information about drivers, speed and magnitude of declines, and permit evaluation of the efficacy of primitive hunting practices in driving a species to extinction.
Steller's sea cow (Hydrodamalis gigas) was a giant dugongid sirenian, and possibly the largest Recent non-cetacean mammal (ca 750 cm, 4500–5900 kg; Anderson & Domning 2002). It was the only Quaternary representative of a North Pacific Neogene sirenian radiation, and fossil material indicates a wide distribution across this region during the Pleistocene (Domning 1978). However, by the late Holocene it was restricted to shallow waters around the uninhabited Commander Islands (Bering and Copper Islands), probably because of prehistoric human hunting elsewhere across its range (Domning 1978). These relict populations were discovered in 1741 and studied by a single scientist, Georg Steller, before the islands became regular stopping-off points for Russian fur hunters until 1762–1763 (Steller 1751). The last sea cow was killed ca 1768 on Bering Island (Stejneger 1887; Domning 1978), making it one of the few truly megafaunal mammals (≥44 kg; sensu Martin 1984) to have become extinct during the historical period.
It is often considered that the Commander Islands sea cow populations were rapidly exterminated by direct overharvesting (Stejneger 1887; Domning 1978), following the ‘blitzkrieg’ model for prehistoric Quaternary megafaunal extinctions (Martin 1984). However, fur hunters also overexploited local populations of sea otters (Domning 1978), a keystone species responsible for structuring nearshore communities (Estes et al. 1978). Sea otter extirpation generates an alternate stable nearshore community dominated by Strongylocentrotus sea urchins, which largely eliminate shallow-water kelps (Simenstad et al. 1978). Several authors have proposed that hunting alone was insufficient to wipe out sea cows, and their extinction was instead driven largely by this removal of their food source (Haley 1980; Anderson 1995; Anderson & Domning 2002).
Although Steller provided no numerical population or life-history data on the sea cows he observed, a 1741 population of less than or equal to 1500 animals was suggested by Leonhard Stejneger (1887), who visited Bering Island and collected records of all Russian hunting and trading expeditions known to have overwintered there, including crew numbers and duration of stay (table 1; figure 1). One report, reproduced by Domning (1978), states that one sea cow could feed 33 men for a month, and it was customary to store sea cow meat to provision ongoing voyages of at least 12 months. Such provisioning also helped eradicate other insular vertebrates, notably the dodo and several giant tortoise species (Strickland & Melville 1848; Townsend 1925). These records also suggest that five times as many animals were killed as were actually utilized, through wasteful hunting methods. Using Stejneger's historical data, and life-history data extrapolated from the dugong, the sea cow's closest living relative (Anderson 2002), sea cow extinction dynamics can be investigated using Population Viability Analysis (PVA) in Vortex 9.42 (Lacy et al. 2005), to examine whether recorded hunting levels were actually sufficient to drive the species to extinction.
Table 1.
Details of expeditions that visited Bering Island, 1743–1762 (from Stejneger 1887).
| year | men | months | provisioning | estimated sea cows hunted |
|---|---|---|---|---|
| 1743 | 30 | 8 | no | 37 |
| 1745 | 30 | 12 | yes | 164 |
| 1747 | 50 | 9 | yes | 250 |
| 1748 | 30 | 11 | no | 50 |
| 1749 | 50 | 8.5 | yes | 247 |
| 1751 | 26 | 16 | yes | 158 |
| 1753 | 34 | 10 | no | 52 |
| 1754 | 30 | 8 | yes | 146 |
| 1754 | 40 | 8 | yes | 194 |
| 1754 | 30 | 9.5 | no | 44 |
| 1754 | 33 | 6 | yes | 150 |
| 1755 | 4 | 12 | no | 8 |
| 1756 | 34 | 8 | no | 42 |
| 1756 | 38 | 9 | yes | 190 |
| 1757 | 39 | 8 | yes | 190 |
| 1758 | 45 | 9.5 | no | 65 |
| 1760 | 38 | 8.5 | yes | 188 |
| 1762 | 45 | 9.5 | yes | 229 |
| 1762 | 45 | 9 | no | 62 |
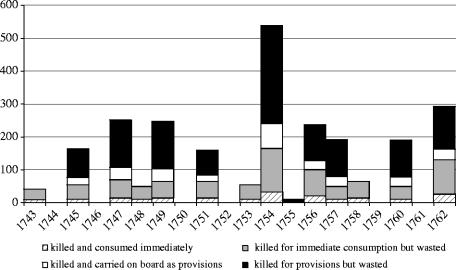
Figure 1.
Estimated numbers of sea cows hunted off Bering Island, 1743–1762 (from Stejneger 1887).
2. Material and methods
Three approaches were taken: historical data were used to compare three alternative hunting regimes; the maximum sustainable hunt was calculated; and a sensitivity analysis was undertaken (see electronic supplementary material). Each simulation entailed 10 000 iterations of the model. The first approach compared the estimated real level of hunting and two counterfactual regimes, one with efficient (non-wasteful) hunting, the other without provisioning for ongoing voyages but with wasteful hunting. Mean annual hunt was calculated per regime, and hunting was continued beyond the period of historical data at this mean rate until extinction if it had not already occurred. Maximum sustainable sea cow catch was calculated by simulating 11 scenarios, where hunting varied in steps of one from 15 to 25 animals per year, and outputting extinction probability (i.e. what proportion of the 10 000 populations was extinct) each year.
Exponential growth is only checked in the Vortex default model by reducing the population to K (carrying capacity) each year it is exceeded. As natural populations conversely usually increase more slowly as K is approached, we entered a density-dependent function of breeding based on the logistic (Miller & Lacy 2005). Percentage animals breeding per year (B) was given by
where γ is the interbirth interval and α is the adjustment factor. The α was chosen by simulating populations in the absence of hunting and varying α until the average equilibrium value equalled K. Note that in these simulations extinction never occurred (i.e. P[extinction]<0.0001). A fixed annual hunt may be specified in Vortex, but as our hunt estimates varied between years a survival-only catastrophe function was created for each scenario to simulate required hunting level.
Vortex outputs the mean population size per simulation, when the median is preferable for exponential processes and information on percentiles is useful. The 10 000 iterations were therefore simulated as different Vortex scenarios and subsequently manipulated by Excel macros to obtain the median population size and 2.5 and 97.5% percentiles.
Sea cow life-history parameters were taken from Anderson (2002), using estimate range mid-points where appropriate. In all simulations except sensitivity analyses, life expectancy was 90 years, based on extrapolation from living sirenians, with annual mortality rates (age) of 10% (0–1), 5% (1–2), 3% (2–10), 2.5% (10–16) and 1.37% (17+). Standard deviations were half these rates. Calves per birth=1. Other parameter values are given in the electronic supplementary material.
3. Results
PVA indicates that sea cow rmax is 1.35%, within the lower range of dugong rmax values given by Marsh et al. (2004), and similar to this range when it is scaled appropriately for body size (0.53–3.40%; cf. Savage et al. 2004, using dugong data from Anderson 2002). The maximum sustainable catch of sea cows, where P [extinction after 100 years]≤0.1 (i.e. maximum probability of extinction that does not qualify the species for any of the IUCN ‘threatened’ categories, according to Red List Criterion E; IUCN Species Survival Commission 2001), was 17 individuals per year. By Stejneger's estimates, a mean of 123.30 individuals per year were killed between 1743 and 1763, so that historical hunting was carried out at 7.25 times the sustainable limit for the species. Hunters would have killed 47.15 individuals per year (2.77×sustainable limit) with wasteful hunting but without provisioning, and 24.95 individuals per year (1.47×sustainable limit) with provisioning but without wastage.
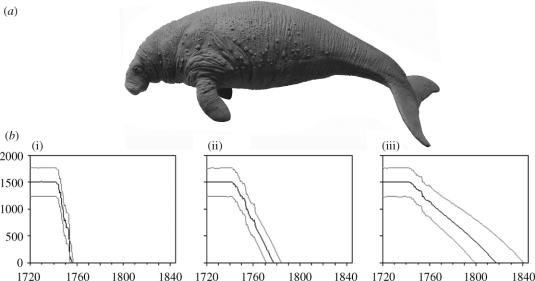
The species could not have survived the persecution levels of any of these possible hunting regimes (p<0.001; figure 2). Given a pre-human population of 1500 animals, median sea cow extinction date is 1756 (95% CI, 1754–1757); without provisioning, this terminal date is 1778 (95% CI, 1770–1786), and without wastage it is 1817 (95% CI, 1799–1841). The species' known extinction date (1768) falls later than the 95% CI for simulated maximum hunting; as hunters relied almost exclusively on sea cows for food, it is unlikely that hunting intensity could have decreased in response to declining sea cow numbers, and sensitivity analysis indicates that most variation in extinction date is explained by initial population density. A pre-human population of 2000 animals, suggested by some researchers (Domning 1978; Anderson & Domning 2002), gives an alternative median extinction date of 1760, with a 95% CI (1756–1763), including the date of the last major sea cow hunt. A starting population of 2900 animals is necessary for the species to persist until 1768. However, this surplus may represent the additional small population found around Copper Island, not considered by Stejneger or later researchers.
Figure 2.
(a) Steller's Sea Cow (image courtesy of the Natural History Museum). (b) Projections of Bering Island sea cow population following its initial discovery under different hunting regimes, based on 10 000 Vortex runs. Black line, median number of sea cows; grey lines, 2.5 and 97.5% percentiles. (i) Estimated real level of hunting; (ii) without provisioning for ongoing journeys; (iii) non-wasteful hunting.
4. Discussion
This quantification of the historical hunting regime of an extinct megafaunal mammal highlights the vulnerability of K-selected species, and the potential for their massive overexploitation using even pre-industrial hunting technologies—in this case, harpooning from the shore or small boats (Stejneger 1887; Domning 1978). Indeed, hunting pressures on the Commander Islands populations are likely to have been even stronger than estimated here, because Stejneger (1887) considered that his historical data were ‘very defective’, and additional unrecorded eighteenth-century expeditions may also have stopped at Bering Island. Extinction risk among other Quaternary mammals is directly related to body size (Lessa & Fariña 1996) or slow reproductive rate (Johnson 2002), characteristics positively associated in other vertebrates with extinction risk via human persecution, but negatively correlated with extinction risk via habitat loss (Owens & Bennett 2000). Studies on extant sirenians indicate that these species are declining primarily through unsustainable anthropogenic mortality from direct overharvesting (Heinsohn et al. 2004; Marsh et al. 2004) or accidental boat collisions (Marmontel et al. 1997), rather than diminishing food sources. This suggests that effects of sea otter removal on sea cow decline were minimal—hunting was more than sufficient to exterminate the sea cow without invoking a further collapse of offshore ecosystems.
This study thus supports the hypothesis that earlier Quaternary megafaunal extinctions could have been effected by small bands of hunters with primitive hunting technologies (Martin 1984; Fiedel & Haynes 2004). This hypothesis is increasingly plausible if, like Commander Islands sea cows, target species were periodically restricted to environmental refugia or had patchy distributions in habitat mosaics. There is considerable evidence that this was indeed the case for many Quaternary megafaunal species, especially immediately prior to their extinction (Stuart 1999; Fiedel & Haynes 2004; Stuart et al. 2004).
Modelling sea cow extinction also highlights the catastrophic impact of wastefulness when overexploiting resources mistakenly perceived as ‘infinite’. Similar short-term intensive slaughter with abundant wastage also characterizes historical overexploitation of other extinct species, such as the passenger pigeon (Schorger 1955), and exploitation by Polynesian settlers of New Zealand's moa, the only extinct megafaunal species for which extensive archaeological information on human hunting is available (Trotter & McCulloch 1984; Holdaway & Jacomb 2000). Wasteful hunting practices may therefore constitute a general model for prehistoric human exploitation of vertebrate resources, as well as remaining a pervasive problem in marine and terrestrial systems today. However, sea cows had such a low maximum population growth rate and sustained yield that the species would unfortunately have become extinct even under a careful and conservative hunting regime.
Acknowledgments
We thank P. Anderson, B. Collen, G. Mace, P. White and W. Hanage for useful discussion.
Supplementary Material
References
- Anderson P. Competition, predation and the evolution and extinction of Steller's Sea Cow, Hydrodamalis gigas. Mar. Mammal Sci. 1995;11:391–394. [Google Scholar]
- Anderson P.K. Habitat, niche, and evolution of sirenian mating systems. J. Mamm. Evol. 2002;9:55–98. doi:10.1023/A:1021383827946 [Google Scholar]
- Anderson P, Domning D.P. Steller's sea cow. In: Perrin W.F, Würsig B, Thewissen J.G.M, editors. Encyclopedia of marine mammals. Academic Press; San Diego: 2002. pp. 1178–1181. [Google Scholar]
- Domning D.P. Sirenian evolution in the North Pacific Ocean. Univ. Calif. Publ. Geol. Sci. 1978;118:1–176. [Google Scholar]
- Estes J.A, Smith N.S, Palmisano J.F. Sea otter predation and community organization in the western Aleutian Islands, Alaska. Ecology. 1978;59:822–833. [Google Scholar]
- Fiedel S, Haynes G.J. A premature burial: comments on Grayson and Meltzer's “Requiem for overkill”. J. Archaeol. Sci. 2004;31:121–131. doi:10.1016/j.jas.2003.06.004 [Google Scholar]
- Haley D. The great northern sea cow. Oceans. 1980;13:7–11. [Google Scholar]
- Heinsohn R, Lacy R.C, Lindenmayer D.B, Marsh H, Kwan D, Lawler I. Unsustainable harvest of dugongs in Torres Strait and Cape York (Australia) waters: two case studies using population viability analysis. Anim. Conserv. 2004;7:1–9. doi:10.1017/S1367943004001593 [Google Scholar]
- Holdaway R.N, Jacomb C. Rapid extinction of the moas (Aves: Dinornithiformes): model, test and implications. Science. 2000;287:2250–2254. doi: 10.1126/science.287.5461.2250. doi:10.1126/science.287.5461.2250 [DOI] [PubMed] [Google Scholar]
- IUCN Species Survival Commission . IUCN; Gland/Cambridge, UK: 2001. IUCN red list categories and criteria: version 3.1. [Google Scholar]
- Johnson C.N. Determinants of loss of mammal species during the Late Quaternary ‘megafauna’ extinctions: life history and ecology, but not body size. Proc. R. Soc. B. 2002;269:2221–2227. doi: 10.1098/rspb.2002.2130. doi:10.1098/rspb.2002.2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy R.C, Hughes K.A, Miller P.S. Vortex: a stochastic simulation of the extinction process. Version 9.42. Chicago Zoological Society; Brookfield: 2005. [Google Scholar]
- Lessa E.P, Fariña R.A. Reassessment of extinction patterns among the Late Pleistocene mammals of South America. Palaeontology. 1996;39:651–662. [Google Scholar]
- Marmontel M, Humphrey S.R, O'Shea T.J. Population viability analysis of the Florida manatee (Trichechus manatus latirostris), 1976–1991. Conserv. Biol. 1997;11:467–481. doi:10.1046/j.1523-1739.1997.96019.x [Google Scholar]
- Marsh H, Lawler I.R, Kwan D, Delean S, Pollock K, Alldredge M. Aerial surveys and the potential biological removal technique indicate that the Torres Strait dugong fishery is unsustainable. Anim. Conserv. 2004;7:435–443. doi:10.1017/S1367943004001635 [Google Scholar]
- Martin P.S. Prehistoric overkill: the global model. In: Martin P.S, Klein R.G, editors. Quaternary extinctions. Arizona University Press; Tucson, AZ: 1984. pp. 354–403. [Google Scholar]
- Miller P.S, Lacy R.C. Vortex: a stochastic simulation of the extinction process. Version 9.50 user's manual. Conservation Breeding Specialist Group (SSC/IUCN); Apple Valley, MN: 2005. [Google Scholar]
- Owens I.P.F, Bennett P.M. Ecological basis of extinction risk in birds: habitat loss versus human persecution and introduced predators. Proc. Natl Acad. Sci. USA. 2000;97:12 144–12 148. doi: 10.1073/pnas.200223397. doi:10.1073/pnas.200223397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage V.M, Gillooly J.F, Brown J.H, West G.B, Charnov E.L. Effects of body size and temperature on population growth. Am. Nat. 2004;163:429–441. doi: 10.1086/381872. doi:10.1086/381872 [DOI] [PubMed] [Google Scholar]
- Schorger A.W. The passenger pigeon. Wisconsin University Press; Madison, WI: 1955. [Google Scholar]
- Simenstad C.A, Estes J.A, Kenyon K.W. Aleuts, sea otters, and alternate stable-state communities. Science. 1978;200:403–411. doi: 10.1126/science.200.4340.403. [DOI] [PubMed] [Google Scholar]
- Stejneger L. How the great northern sea-cow (Rytina) became exterminated. Am. Nat. 1887;21:1047–1054. doi:10.1086/274607 [Google Scholar]
- Steller G.W. De bestiis marinis. Novi Comm. Acad. Sci. Petropolitanae. 1751;2:289–398. [Google Scholar]
- Strickland H.E, Melville A.G. The dodo and its kindred. Reeve, Benham & Reeve; London: 1848. [Google Scholar]
- Stuart A.J. Late Pleistocene megafaunal extinctions: a European perspective. In: MacPhee R.D.E, editor. Extinctions in near time. Kluwer Academic/Plenum Publishers; New York: 1999. pp. 257–270. [Google Scholar]
- Stuart A.J, Kosintsev P.A, Higham T.F.G, Lister A.M. Pleistocene to Holocene extinction dynamics in giant deer and woolly mammoth. Nature. 2004;431:684–689. doi: 10.1038/nature02890. doi:10.1038/nature02890 [DOI] [PubMed] [Google Scholar]
- Townsend C.H. The Galápagos tortoises in their relation to the whaling industry. Zoologica. 1925;4:55–135. [Google Scholar]
- Trotter M.M, McCulloch B. Moas, men and middens. In: Martin P.S, Klein R.G, editors. Quaternary extinctions. Arizona University Press; Tucson, AZ: 1984. pp. 708–727. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.