Abstract
Dromaeosaurid theropod dinosaurs possess a strongly recurved, hypertrophied and hyperextensible ungual claw on pedal digit II. This feature is usually suggested to have functioned as a device for disembowelling herbivorous dinosaurs during predation. However, modelling of dromaeosaurid hindlimb function using a robotic model and comparison of pedal ungual morphology with extant analogue taxa both indicate that this distinctive claw did not function as a slashing weapon, but may have acted as an aid to prey capture.
Keywords: Dromaeosauridae, predation, functional morphology
1. Introduction
Dromaeosaurid theropod dinosaurs, such as Deinonychus, possess a strongly recurved, hypertrophied ungual claw on pedal digit II (Ostrom 1969; figure 1a). It is commonly suggested that, in combination with strong kicking/slashing actions of the hindlimb, this claw functioned to disembowel prey, in particular large herbivorous dinosaurs, such as the contemporaneous Tenontosaurus (Ostrom 1969, 1990). We have tested this hypothesis using evidence from comparative pedal ungual morphology and by construction of a robotic model (figure 1b) of a dromaeosaurid hindlimb that was used to simulate the forces acting at the ungual/flesh interface during attacks on prey. Our data suggest that, contrary to the existing consensus, dromaeosaurid claws were not designed for slashing through flesh, but were used to grip the hides of prey many times larger than themselves in an analogous fashion to climbing crampons.
Figure 1.
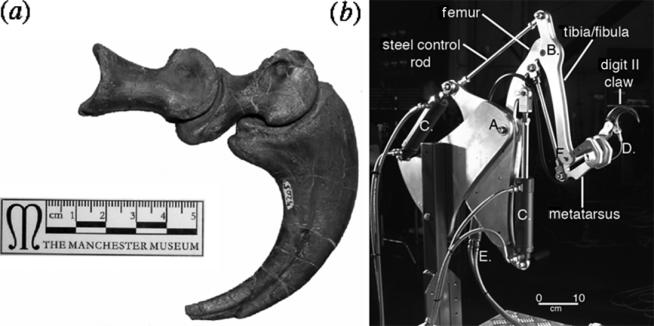
(a) Hypertrophied ungual claw on pedal digit II of Deinonychus antirrhopus (Yale Peabody Museum 5025). Scale bar, 5 cm. (b) Hydraulic dromaeosaur limb. Scale bar, 10 cm.
2. Material and Methods
The robotic limb built to test the disembowelling hypothesis was designed and constructed by Pennicott Payne Models and Special Effects (London) in connection with a BBC television production (‘The Truth About Killer Dinosaurs’). The dimensions for the hydraulic limb were constrained by using the limb dimensions, articulations and functional morphology of the dromaeosaurs Velociraptor mongoliensis (Osborn 1924; Norell & Makovicky 1997, 1999) and Deinonychus antirrhopus (Ostrom 1969, 1976, 1990; P.L.M. 2004, personal observation).
The limb consists of a hydraulic arm, braced by steel control rods, with the segments articulated via simple pinned joints whose degree of movement could be accurately controlled and powered by hydraulic rams: the hyperextensible movement of the ungual claw was produced by a control cable linked to a smaller hydraulic ram (figure 1b).
The degree of flexure of digit II and of the retracted claw was determined by reference to the articulated foot of D. antirrhopus (Ostrom 1969; P.L.M., personal observation). The movements of the limb were fixed by adjusting the position of each hydraulic unit controlling flexure, predetermined by the step (kick)-cycle for D. antirrhopus. The passive action of ligaments would have retracted the claw of dromaeosaurs, but the flexure of the claw was under the active control of flexor muscles and tendons. The proximal (hip) joint of the femur was constrained to articulate through an arc of 40° and the proximal (knee) joint of the tibia/fibula through a maximum arc of 80° on the basis of observations on both Velociraptor and D. antirrhopus fossils (Osborn 1924; Ostrom 1969, 1976, 1990; Norell & Makovicky 1997, 1999, 2004; P.L.M., personal observation). Movement of the limb was produced by two aviation-sourced hydraulic rams. The articulated digit II and recurved claw were flexed by a control cable, linked to a hydraulic ram smaller than those controlling upper limb movement.
The hip height of the hydraulic limb (when in a neutral support pose) was 0.5 m, giving an estimated dromaeosaur body length of approximately 2 m, consistent with estimates based upon fossil remains (Ostrom 1969, 1990; Norell & Makovicky 1999, 2004). The body mass of a 2 m long dromaeosaur has been calculated by several authors as approximately 20–80 kg (Ostrom 1969, 1990; Norell & Makovicky 2004; Hutchinson 2004). The forces (f) applied to the hydraulic limb were therefore based upon a running dromaeosaur of mass 40 kg. Force was calculated as product of the mass (m) of the animal and acceleration due to gravity (g=9.81 m s−2), then multiplying this result by 2.5, following an empirically derived index of the increased forces generated during running, relative to standing values (Alexander 1981; Hutchinson 2004). Modelled forces transmitted through the limb were based on those known to be exerted during high-speed running, as this locomotory regime results in the highest resultant forces on the limb elements (Alexander 1981; Hutchinson 2004). Unfortunately, comparative data on kicking forces in extant analogue taxa are lacking. As a result, our experimental procedure represents the maximum forces that would habitually be acting upon the limb, which are an overestimate of the necessarily lower forces that would be involved in kicking behaviour. This calculation produced a resultant f of 981 N, which was applied to the claw via the robotic limb. Calculations of f from the limb of a running ostrich (Alexander et al. 1979), gave 1100 N for a 41.5 kg bird, comparable to the forces used in this study. The forces applied were within the safe limits (safety factor) for the biological materials expected for a dromaeosaur limb (Alexander 1981).
The foot was constructed of aluminium plate. Metatarsals had a maximum degree of rotation at the proximal joint of 90° and a rotation at the distal joint of 90° with the phalanges. Digits III, IV and I were static components, positioned fully flexed. Digit II was articulated, with 90° rotation in the distal joint of phalanx I and 40° in the joint connecting the distal phalanx II to the recurved claw. Steel gears controlled the movement of digit II, which was flexed by the cable, driven by the smallest hydraulic ram. The combined degree of flexure of the foot gave an arc of 220° for the recurved claw (Norell & Makovicky 2004) (not including the upper limb components).
Claw form and function vary widely among vertebrates, but claw sheath composition does not. Claws, nails and hooves are composed of keratin, a strong, fibrous protein (e.g. Raven & Johnson 1992). Keratin protects the bone of the terminal phalanx and assists many species of bird, reptile and mammal in providing traction during climbing, prey capture and, occasionally, killing. The morphology of the fossil ungual claw cores of dromaeosaurs combined with rare soft tissue preservation (Clark et al. 1999) indicates that it would have been protected by a similar keratin sheath. Extant Phylogenetic Bracketing (EPB: Witmer 1995) can be used to reconstruct the structure and properties of the keratin claws possessed by non-avian dinosaurs. Mammal claws are composed of an α-keratin (helical), but bird and reptile claws are composed of β-keratins (pleated-sheet) (e.g. Fraser & MacRae 1980). Given that dinosaurs fall within the EPB of birds and crocodilians, it is likely that the claws of dinosaurs were composed of β-keratin. Experiments to determine the Young modulus of ostrich claw β-keratin indicated that they are mechanically anisotropic, a property arising from the orientation of the keratin fibrils (Bonser 2000). The Young modulus of elasticity for the β-keratin along the length of an ostrich claw is 1.84 GPa and perpendicular to this, 1.33 GPa (Bonser 2000). The mechanical strength runs preferentially along the axis of the keratinous claw (Vincent & Owers 1986; Bonser 2000). A requirement of all biological materials subject to loading is that stresses are kept within safe limits (Alexander 1981). It is likely that the keratin claw and bony core (ungual phalanx) of dromaeosaur claws would have had similar safety limits to those of extant cursorial bipeds.
The claw in this study was constructed of an aluminium core and a thin (less than 2 mm) composite sheath comprising Kevlar and carbon fibre strands set in an epoxy resin base. The carbon fibre and Kevlar strands ran longitudinally along the claw, as this orientation supports the loading regime expected for such a structure, mimicking the mechanical properties described for reptile and bird β-keratin (Vincent & Owers 1986; Bonser 2000).
The composite claw was finished by shaping and polishing the surface, terminating in a sharp point, with the darker carbon fibre strands still visible through the epoxy resin (figure 2a). Calculation showed, however, that the thin sheath would not have significantly altered the mechanical properties of the model claw compared with uncoated aluminium. This has a Young modulus of around 73 GPa (Gordon 1968), making the reconstructed claw 40 times as stiff longitudinally and 55 times as stiff perpendicularly as ostrich claw keratin (Bonser 2000). If this much stronger reconstructed claw could not cut or tear through flesh, it would indicate strongly that a keratin claw would be much less effective. The external morphology of the composite claw was based upon the dimensions of the digit II ungual phalanx of D. antirrhopus (Yale Peabody Museum 5025). The shape of the reconstructed claw, in cross-section, defines the ‘sharpness’ of its ventral curvature. Personal observations by one of us (P.L.M.) on over 200 species of extant reptiles, mammals and birds (housed in the Zoology Collection, The Manchester Museum), and data gathered from the literature (Feduccia 1993; Raikow 1994; Bonser 2000; Zani 2000), indicate that the shape of reptile and bird claws in cross-section is remarkably conservative. The cross-section of bird claws describes a convex arch, with a shallowly convex ventral surface that is delineated by medial and lateral ridges. The only exceptions to this general rule were the owls (Strigiformes), which possess more cylindrical keratin sheaths. Notably, none of the bird or reptile keratin sheaths studied possessed a ‘sharp’ (i.e. ventrally keeled, rather than ventrally convex) inside curvature. It seems very unlikely that dromaeosaurs possessed sharp, ventrally keeled claws, given that this morphology is not present in any of the extant taxa that form the EPB of this extinct clade.
Figure 2.
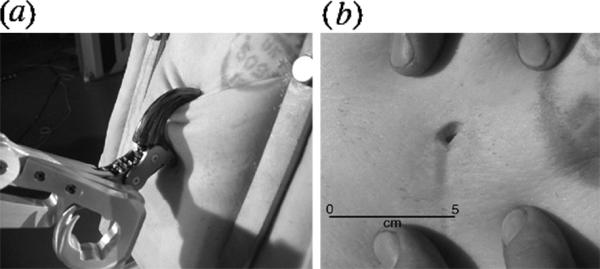
(a) Claw caused flesh ventral to the impact to bunch together, preventing the claw from sliding out of the wound. (b) Entry/exit wound produced in fleshy substrate by composite claw. Scale bar, 5 cm.
The length of the keratin sheath and the ungual phalanx was measured for 10 species of birds of prey (Accipitriformes and Falconiformes) as potentially suitable analogues for a raptorial dinosaur. In all cases, the keratin sheath protruded beyond the tip of the ungual phalanx by a maximum of 15% of total claw length. Given the extreme curvature of ungual phalanx II in dromaeosaurs (Ostrom 1969, 1990), the keratin claw might have seriously impeded function if it extended beyond 15–20% of the bone core.
The claw was tested at both low (2 m s−2) and high (11 m s−2) speed, mimicking a kicking motion, into a fleshy substrate (fresh pig carcass) mounted in the test frame (figure 2a). The claw was also tested against crocodile flesh, but the dermal armour did not allow penetration of the claw and in one test run, broke the tip of the reconstructed claw. Moreover, the large iguanodontian ornithopods thought to form the usual prey of Deinonychus (Ostrom 1969) did not possess dermal armour (Norman 2004).
3. Results
Impact of the hydraulically powered claw at both low- and high-speed contact produced small, round puncture wounds (figure 2b) that reached maximal depths of 30–40 mm, with minimal trauma to surrounding tissues: no slashing/cutting occurred, even with a reconstructed claw that was at least 40 times stiffer than β-keratin (Vincent & Owers 1986; Bonser 2000). The extreme curvature of the claw caused flesh ventral to the impact to bunch together, preventing the claw from sliding out of the wound (figure 2a). It seems highly unlikely that wounds of this depth would have posed a danger to the vital organs of a large herbivorous dinosaur, though they would obviously be fatal to small prey. Moreover, the geometry of the dromaeosaur claw would have caused the claw to rotate ventrally as it was pushed into the prey, resulting in a maximum depth of trauma equal to the radius of the claw arc. Maximum penetration of such highly recurved claws may have reached only 40–50 mm, a function of ungual size and geometry. Hence, these claws do not appear to have been suitable for producing slashing wounds when employed on large animals.
4. Discussion
The extreme size and curvature of dromaeosaur unguals indicate that they could have used these structures to pierce and grip flesh, which suggests an alternative prey-capture strategy for dromaeosaurs. Instead of using the unguals as slashing cutlasses, they may have been used as climbing crampons. The claw geometry of mammals and birds correlates strongly with arboreal versus terrestrial habits (Feduccia 1993). Among extant birds, inner claw arc measurements range from 52.2 to 77.6° (ground-dwellers), 101.8 to 125.3° (perching) and 129.5 to 161.6° (trunk-climbers) (Feduccia 1993). The pedal digit II ungual of Deinonychus possesses an inner arc measurement of 160°, supporting a climbing function for this structure.
We envisage dromaeosaurs leaping onto live prey and establishing footholds on the latter's flanks using the piercing/gripping functions of their pedal digits in combination with the grasping recurved claws on the manus. The sharp, finely serrated teeth of dromaeosaurs (Ostrom 1969, 1990; Norell & Makovicky 2004) could then have inflicted many wounds on their prey, while firmly locked onto the latter's flanks. If the prey turned to defend itself from attack, its attacker would be turned by and with the prey, given that the former was ‘hooked’ on to the latter. This fatal embrace is analogous to the hunting technique used by many species of big cat that use their protracted claws to cling onto to their prey, as powerful jaws crush the windpipe of their prey (Kiltie 1991; Antón & Turner 1997).
Acknowledgments
We thank the BBC for funding the construction of the hydraulic limb, especially Peter Leonard, Penny Palmer and Alice Harper. Thanks also to Professors R. A. D. Pattrick, C. McGowan and J. N. W. Prag, Mr H. McGhie, Miss R. Smith, Mrs B. T. Loudon, Drs L. Anderson, J. MacQuaker, D. L. Brinkman, W. Joyce, D. Yalden and P. Bienkowski for their valuable comments on this manuscript and access to collections.
References
- Alexander R.McN. Factors of safety in the structure of animals. Sci. Prog. (Oxf.) 1981;67:109–130. [PubMed] [Google Scholar]
- Alexander R.McN, Maloiy G.M.O, Njau R, Jayes A.S. Mechanics of running of the ostrich (Struthio camelus) J. Zool. 1979;187:169–178. [Google Scholar]
- Antón M, Turner A. Columbia University Press; New York: 1997. The big cats and their fossil relatives: an illustrated guide to their evolution and natural history. [Google Scholar]
- Bonser R.H.C. The Young's modulus of ostrich claw keratin. J. Mater. Sci. Lett. 2000;19:1039–1040. doi:10.1023/A:1006786919376 [Google Scholar]
- Clark J.M, Norell M.A, Chiappe L.M. An oviraptorid skeleton from the Late Cretaceous of Ukhaa Tolgod, Mongolia, preserved in an avianlike brooding position over an oviraptorid nest. Am. Mus. Novit. 1999;3265:1–35. [Google Scholar]
- Feduccia A. Evidence from claw geometry indicating arboreal habits of Archaeopteryx. Science. 1993;259:790–793. doi: 10.1126/science.259.5096.790. [DOI] [PubMed] [Google Scholar]
- Fraser R.D.B, MacRae T.P. Molecular structure and mechanical properties of keratins. In: Vincent J.F.V, Currey J.D, editors. The mechanical properties of biological materials. Cambridge University Press; Cambridge: 1980. pp. 211–246. [PubMed] [Google Scholar]
- Gordon J.E. Penguin; London: 1968. The new science of strong materials. [Google Scholar]
- Hutchinson J.R. Biomechanical modelling and sensitivity analysis of bipedal running ability. II. Extant taxa. J. Morphol. 2004;262:441–461. doi: 10.1002/jmor.10241. doi:10.1002/jmor.10240 [DOI] [PubMed] [Google Scholar]
- Kiltie R.A. How cats work. In: Seidensticker J, Lumpkin S, editors. Great cats. Merehurst; London: 1991. pp. 54–67. [Google Scholar]
- Norell M.A, Makovicky P.J. Important features of the dromaeosaur skeleton: information from a new specimen. Am. Mus. Novit. 1997;3215:1–28. [Google Scholar]
- Norell M.A, Makovicky P.J. Important features of the dromaeosaur skeleton. II. Information from newly collected specimens of Velociraptor mongoliensis. Am. Mus. Novit. 1999;3282:1–45. [Google Scholar]
- Norell M.A, Makovicky P.J. Dromaeosauridae. In: Weishampel D.B, Dodson P, Osmólska H, editors. The Dinosauria. 2nd edn. University of California Press; 2004. pp. 196–209. [Google Scholar]
- Norman D.B. Basal iguanodontia. In: Weishampel D.B, Dodson P, Osmólska H, editors. The Dinosauria. 2nd edn. University of California Press; Berkeley, CA: 2004. pp. 413–417. [Google Scholar]
- Osborn H.F. Three new Theropoda, Protoceratops zone, central Mongolia. Am. Mus. Novit. 1924;144:1–12. [Google Scholar]
- Ostrom J.H. Osteology of Deinonychus antirrhopus, an unusual Theropod from the Lower Cretaceous of Montana. Bull. Pea. Mus. Nat. Hist. 1969;30:1–165. [Google Scholar]
- Ostrom J.H. On a new specimen of the Lower Cretaceous theropod dinosaur Deinonychus antirrhopus. Breviora. 1976;439:1–21. [Google Scholar]
- Ostrom J.H. Dromaeosauridae. In: Weishampel D.B, Dodson P, Osmólska H, editors. The Dinosauria. 1st edn. University of California Press; Berkeley: 1990. pp. 269–279. [Google Scholar]
- Raikow R.J. Climbing adaptations in the hindlimb musculature of Woodcreepers (Dendrocolaptinae) Condor. 1994;96:1103–1106. [Google Scholar]
- Raven P.H, Johnson G.B. 3rd edn. Mosby Year Book; St Louis: 1992. Biology. [Google Scholar]
- Vincent J.F.V, Owers P. Mechanical design of hedgehog spines and porcupine quills. J. Zool. 1986;210:55–75. [Google Scholar]
- Witmer L.M. The extant phylogenetic bracket and the importance of reconstructing soft tissue in fossils. In: Thomason J.J, editor. Functional morphology in vertebrate palaeontology. Cambridge University Press; Cambridge: 1995. pp. 19–33. [Google Scholar]
- Zani P.A. The comparative evolution of lizard claw and toe morphology and clinging performance. J. Evol. Biol. 2000;13:316–325. doi:10.1046/j.1420-9101.2000.00166.x [Google Scholar]


