Abstract
Productive areas are patchily distributed at sea and represent important feeding grounds for many marine organisms. Although pinnipeds are known to travel on direct routes and return regularly to particular feeding sites, the environmental information seals use to perform this navigation is as yet unknown. As atmospheric dimethyl sulphide (DMS) has been demonstrated to be a reliable indicator for profitable foraging areas, we tested seals for their ability to smell DMS at concentrations typical for the marine environment. Using a go/no-go response paradigm we determined the DMS detection threshold in two harbour seals (Phoca vitulina vitulina). DMS stimuli from 8.05×108 to 8 pmol (DMS) m−3(air) were tested against a control stimulus using a custom-made olfactometer. DMS-thresholds determined for both seals (20 and 13 pmol m−3) indicate that seals can detect ambient concentrations associated with high primary productivity, e.g. in the North Atlantic. Thus, seals possess an extraordinarily high olfactory sensitivity for DMS, which could provide a sensory basis for identifying or orienting to profitable foraging grounds.
Keywords: olfaction, seal, dimethyl sulphide, orientation
1. Introduction
Pinnipeds are known to leave the coastline during their foraging trips and to spend several days at sea. Based on data from telemetry studies, northern fur seals (Callorhinus ursinus) (Loughlin et al. 1987) and harbour seals (P. vitulina L.) (Thompson & Miller 1990) are reported to swim on direct routes to their feeding grounds and back to their haul out-sites at the coast. For both pinniped species, it has also been shown that they return regularly to particular feeding areas, indicating a directed orientation rather than a random search biased away from the coastline. However, as food resources at open sea are patchily distributed, it has remained unclear which environmental information seals use to identify attractive feeding grounds.
Foraging grounds for harbour seals have been described as areas of high marine productivity, where prey is likely to be found (Thompson & Miller 1990). A reliable indicator for such productive zones is an elevated atmospheric concentration of dimethyl sulphide (DMS) (C2H6S) (Bürgermeister et al. 1990; Andreae et al. 1994). As DMS is cleaved from DMSP produced by phytoplankton in response to zooplankton grazing (Dacey & Wakeham 1986), its occurrence is linked to the dynamics of the marine pelagic food web (Gabric et al. 1993). Thus, the local patchiness and distribution of DMS at sea reflect the pattern of primary production (Bürgermeister et al. 1990; Andreae et al. 1994) and—because it is linked to the pelagic food web—also the abundance of zooplankton and commercially important shoaling fish (Sims & Quayle 1998), which are food resources for seals (Riedman 1990). Correlated to its concentration in seawater, DMS is transferred across the water/air interface into the atmospheric boundary layer, generating elevated atmospheric DMS concentrations in productive areas (Bürgermeister et al. 1990; Andreae et al. 1994). In the Atlantic (north of 40°N) and English Channel (north of 48°N), DMS concentrations of 8×103 pmol (DMS) m−3 (air) were measured in productive areas (Bürgermeister et al. 1990). DMS is suggested to play a role in basking sharks when orienting along most profitable plankton patches in the English Channel, while it has been demonstrated that procellariiform seabirds use atmospheric DMS as an olfactory foraging cue (Nevitt et al. 1995).
Although olfaction has drawn little attention in research on marine mammals, and the few early anatomical studies lead to contradictory results regarding the significance of the sense of smell in pinnipeds (Harrison & Kooyman 1968; Kuzin & Sobolevsky 1976), atmospheric odours like DMS could provide essential environmental information for these air-breathing predators. Therefore, we tested whether harbour seals are able to smell DMS at concentrations typical of what they might encounter in their natural foraging habitat.
2. Material and methods
The olfactory detection threshold for DMS was determined in two male harbour seals (P. vitulina vitulina; Nick and Bill). Both seals were born and raised in captivity, had fully reached sexual maturity (Nick: five years, Bill: six years) and were kept under the same feeding regime during the experimental period. Experiments were performed with the two seals over a total period of 18 months.
Stimuli were prepared using 100 ml sealable glass syringes, each containing a 2 cm2 filter paper. Filter papers were moisturized inside the syringes with either 2 μl DMS solution (solvent: distilled water) as olfactory stimulus or 2 μl distilled water as control stimulus. Solutions were vaporized for 1 h at the same temperatures at which experiments were conducted. Different gaseous DMS concentrations were obtained by vaporizing different dilutions of DMS-solution inside syringes. The high volatility (645 hPa at 25 °C) and saturation vapour pressure of DMS in air led to a total vaporization of the DMS solution in a syringe and allowed the calculation of the application concentrations (see Electronic Appendix). To reduce trace odour contamination, syringes and the entire apparatus were cleaned thoroughly with ethanol and water after each experimental session and afterwards syringes were incubated for at least 12 h at 60 °C.
Experiments were conducted using a go/no-go response paradigm (Dehnhardt et al. 1998). Seals were trained to place their muzzle in an opening of a PVC-board, which was lined with latex closely fitting the muzzle (figure 1). This way, visual cues were excluded and the seals were prevented from opening their mouths for potential gustatory sampling. The animal's chin was positioned on a little knob (jaw-station) such that the nose of the experimental animal was 2 cm from the aperture of the syringe. Syringes were discharged at a constant flow (4 ml s−1) using an electronically controlled discharging apparatus. DMS and control stimuli were presented pseudorandomly (Gellerman 1933). Discharging a syringe was announced by an acoustic signal and the seal indicated the detection of DMS by immediately leaving its position at the board (go response). A single DMS stimulus was presented for a maximum of 10 s. However, go responses usually occurred within the first three seconds after a DMS presentation started. During control trials, the seal was required to keep its position at the jaw station for 10 s (no-go response). Correct responses were rewarded with a piece of cut herring. The inter-trial interval was always 1 min.
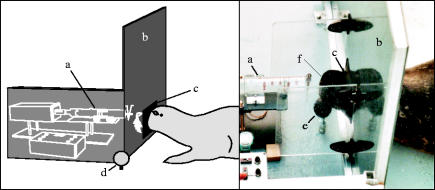
Figure 1.
The experimental set-up for the determination of olfactory detection thresholds of harbour seals for dimethyl sulphide (DMS) (left, schematic; right, photograph). (a) Discharging apparatus with syringe. (b) Board. (c) Latex lining closely fitting the muzzle. (d) Response target. (e) Jaw-station. The photograph shows the seal Nick sniffing with nostrils (f) wide open.
This experimental procedure was trained using first fish odour and then eucalyptus odour to check whether seals actually are able to detect olfactory stimuli. Each of the two odours was tested in at least 100 stimulus-present trials versus 100 control trials (no odour), but no thresholds were determined. For DMS detection threshold determination, one experimental session was conducted per day consisting of 15 stimulus-present trials with the same DMS concentration and 15 control trials. Each DMS concentration was tested in a block of four sessions. DMS concentration was reduced from block to block from 8.05×108 to 8 pmol (DMS) m−3 (air). Detection thresholds were determined using the psychophysical method of constant stimuli, defining the threshold as the interpolated stimulus value associated with 50% correct detections involving only stimulus-present trials (see Electronic Appendix). As a measure of a subject's response bias, the false-alarm rate is calculated for each stimulus intensity from trials in which a subject shows a go-response to a control stimulus.
Employing the same DMS stimuli, olfactometer and procedure as used for the seal experiments, we roughly determined the olfactory detection threshold for six students (10 odour present trials versus 10 control trials) to compare it with the human threshold reported in the literature.
3. Results
Once seals were trained to the experimental procedure, seals snuffled with their nostrils wide open as soon as they got into the correct position at the test apparatus (figure 1). When highly concentrated fish odour and eucalyptus odour were used as stimuli, both seals showed a go-response in almost 100% of the odour present trials, but reliably stayed at the jaw-station during control trials (2.2–11% false-alarm rate). Although no thresholds were determined, these results convinced us that seals are able to respond to olfactory stimuli.
Both seals also showed a spontaneous go-response when presented for the first time with a DMS stimulus of 8.05×108 pmol m−3. At the human threshold concentration of ∼1.6×107 pmol m−3 (figure 2), seals' detection performance was 100% correct, while the concentration of 8×103 pmol m−3, typical for productive zones (range 1–8×103 pmol m−3, Bürgermeister et al. 1990) was still detected in ≥85% of DMS presentations. Interpolated detection thresholds were 20 pmol m−3 (Nick) and 13 pmol m−3 (Bill), respectively. These detection thresholds show that a seal's olfactory sensitivity to DMS is several orders of magnitude higher than that of humans. With regard to DMS concentrations that have been measured in association with productivity, seals' detection thresholds are three orders of magnitude lower than the DMS concentration measured in productive areas of the English Channel (Bürgermeister et al. 1990). False-alarm rates of both animals were relatively low throughout the experiments (figure 2). Only at the first DMS concentration below threshold did Nick's false-alarm rate increase to 50%, indicating that his decisions were based on chance as soon as he was presented with DMS concentrations below his detection threshold. Bill's detection behaviour was more conservative, as his false-alarm rate increased only slightly with decreasing DMS concentration (see Electronic Appendix).
Figure 2.
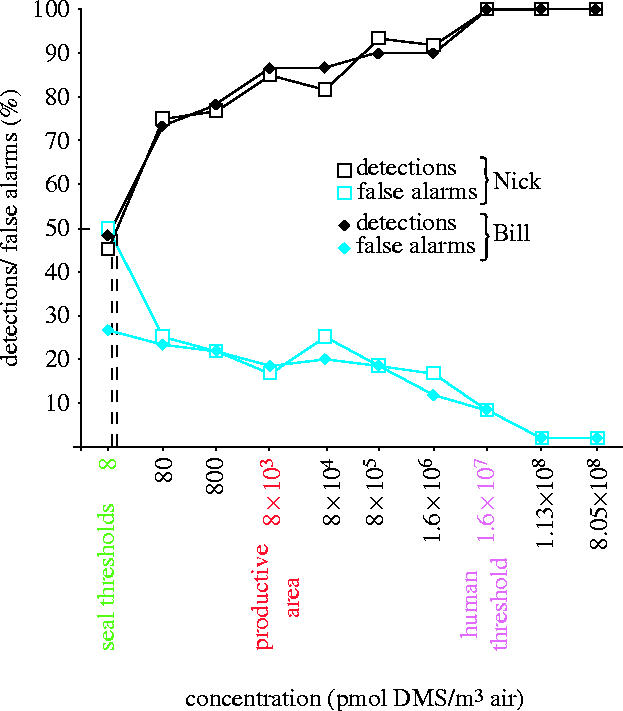
Psychometric functions of performance of two harbour seals detecting dimethyl sulphide (temperature range during data collection: 1–22 °C). Behavioural detection thresholds are defined as 50% detections in DMS-present trials (dashed lines). Each data point was calculated from 60 trials collected during four sessions. Black symbols represent percentage of correct go-responses (DMS stimuli present), blue symbols represent go-responses to the corresponding control trials (false-alarms). The DMS concentration found in the atmosphere of marine productive areas (red label) refers to measurements by Bürgermeister et al. (1990) in the Atlantic north of 40° and in the English Channel, where harbour seals and grey seals are abundant.
The DMS concentration detected by our human subjects in 50% of stimulus-present trials ranged between 1.3×107 and 2.0×107 pmol m−3 (air) (mean 1.6×107 pmol m−3), which is in the same order of magnitude as reported by the Material Safety Data Sheet of Gaylord Chemical Corporation as well as Selyuzhitskii (1972).
4. Discussion
Previous research on olfaction in pinnipeds did not reveal a consistent picture of their olfactory capacity (King 1983). Although detailed descriptions of peripheral and central olfactory structures in pinnipeds are not available, early anatomical examinations of pinniped brains suggest that the sense of smell is of little importance for these aquatic mammals. The ‘olfactory apparatus is variably reduced, but more reduced in phocids than in otariids and Odobenus’ (Harrison & Kooyman 1968). However, Kuzin & Sobolevsky (1976) described the nasal olfactory epithelium of fur seals as being of the typical mammalian structure. Correspondingly, anecdotal reports suggest that olfaction may be important for mother–pup recognition (Burton et al. 1975) as well as for pinniped orientation. Salter (1979), for example, speculated about olfactory site-recognition in walruses, and Sergeant (1970) reported how young harp seals orient into the wind during their solitary northward migrations, possibly maintaining their course by olfactory cues. However, a quantitative study of the olfactory capacity in pinnipeds has never been conducted. Thus, our results demonstrate for the first time an extraordinarily high olfactory sensitivity in a pinniped species for a substance potentially relevant to their sensory ecology.
As the DMS concentration typical for productive zones is detected by both seals in >85% of stimulus-present trials, the olfactory sensitivity of these animals to DMS is well tuned to the DMS concentration found in the marine habitat and could provide the sensory basis for the location or at least identification of patchily distributed foraging grounds.
Feeding grounds of harbour seals described by Thompson & Miller (1990) are associated with offshore sandbanks or rocky reefs. High DMS concentrations are often associated with such hydrographic features, where upwelling occurs and plankton is abundant. Linked by the pelagic food web to these plankton aggregations, fish abundance is also often high in these areas (Sims & Quayle 1998), thus making these regions interesting feeding sites for piscivorus predators like seals. Based on the hydrographic features often found at ocean sites of high biological productivity, Nevitt (2000) described a DMS landscape superimposed on the ocean surface. In the vertical column of the marine atmosphere, highest DMS concentrations were measured next to the water surface (Ferek et al. 1986), where it is easily detectable for a breathing seal. Navigating through a DMS landscape, a seal detecting a DMS concentration indicating a productive area could then switch to a small-scale foraging behaviour because the chance of encounters with prey is high. Although further experimentation is necessary to finally prove the use of their well-developed olfactory capacity for large-scale orientation, the high sensitivity for DMS found here demonstrates that olfaction may play a significant and hitherto underestimated role in pinnipeds.
Acknowledgments
We are grateful to H.-U. Fried (Institute for Genetics, Cologne), Dr Stich and Dr Iffland (Institute for Toxicology, Cologne) for help with DMS stimuli preparation. The experimental animals were treated in accordance with the official German regulations for research on animals. This work has been funded by a grant of the VolkswagenStiftung to G. D.
Supplementary Material
References
- Andreae T.W, Andreae M.O, Schebeske G. Biogenic sulfur emissions and aerosols over the tropical south Atlantic 1. Dimethylsulfide in seawater and the atmosphere boundary layer. J. Geophys. Res. 1994;99:22 819–22 829. doi:10.1029/94JD01837 [Google Scholar]
- Bürgermeister S, Zimmermann R.L, Georgii H.W, Bingemer H.G, Kirst G.O, Janssen M, Ernst W. On the biogenic origin of dimethylsulfide: relation between chlorophyll, ATP, organismic DMSP, phytoplankton species, and DMS distribution in Atlantic surface water and atmosphere. J. Geophys. Res. 1990;95:20 607–20 615. [Google Scholar]
- Burton R.W, Andersen S.S, Summers C.F. Perinatal activities in the grey seal (Halichoerus grypus) J. Zool., Lond. 1975;177:197–201. [Google Scholar]
- Dacey J.W.H, Wakeham S.G. Oceanic dimethylsulfide: production during zooplankton grazing on phytoplankton. Science. 1986;233:1314–1315. doi: 10.1126/science.233.4770.1314. [DOI] [PubMed] [Google Scholar]
- Dehnhardt G, Mauck B, Bleckmann H. Seal whiskers detect water movements. Nature. 1998;394:235–236. doi:10.1038/28303 [Google Scholar]
- Ferek R.J, Chatfield R.B, Andreae M.O. Vertical distribution of dimethylsulphide in the marine atmosphere. Nature. 1986;320:514–516. doi:10.1038/320514a0 [Google Scholar]
- Gabric A, Murray N, Stone L, Kohl M. Modelling the production of dimethylsulfide during a phytoplankton bloom. J. Geophys. Res. 1993;98:22 805–22 816. [Google Scholar]
- Gellerman L.W. Chance orders of altering stimuli in visual discrimination experiments. J. Gen. Psychol. 1933;42:206–208. [Google Scholar]
- Harrison R.J, Kooyman G.L. General physiology of pinnipedia. In: Harrison R.J, editor. The behavior and physiology of pinnipeds. Appleton-Century-Crofts; New York: 1968. pp. 212–296. [Google Scholar]
- King J.E. Oxford University Press; Oxford, UK: 1983. Seals of the world. [Google Scholar]
- Kuzin A.Y, Sobolevsky Y.I. Proc. 6th All-Union Conf. on the study of Marine Mammals, Kiev, 1–3 October 1975. 1976. Morphological and functional characteristics of fur seal's respiratory system; pp. 168–170. [Google Scholar]
- Loughlin T.R, Bengtson J.I, Merrick R.L. Characteristics of feeding trips of female northern fur seals. Can. J. Zool. 1987;65:2079–2084. [Google Scholar]
- Nevitt G.A. Olfactory foraging by Antarctic procellariiform seabirds: life at high Reynolds numbers. Biol. Bull. 2000;198:245–253. doi: 10.2307/1542527. [DOI] [PubMed] [Google Scholar]
- Nevitt G.A, Veit R.R, Kareiva P. Dimethyl sulphide as a foraging cue for Antarctic procellariiform seabirds. Nature. 1995;376:680–682. doi:10.1038/376680ao [Google Scholar]
- Riedman M. University of California Press; Berkeley and Los Angeles: 1990. The pinnipeds: seals, sea lions, and walruses. [Google Scholar]
- Salter R.E. Site utilisation, activity budgets, and disturbance responses of Atlantic walruses during terrestrial haul-out. Can. J. Zool. 1979;57:1169–1180. [Google Scholar]
- Selyuzhitskii, G. V. 1972 Gig. Tr. Prof. Zabol 16, 46 (Russian). Ref. 1972, Chem. Abstr 77, 92439 r.
- Sergeant D.E. Proc. Seventh Ann. Conf. Biol. Sonar Diving Mamm. Stanford Research Institute Biological Sonar Laboratories; Menlo Park, CA: 1970. Migration and orientation in harp seals; pp. 123–131. [Google Scholar]
- Sims D.W, Quayle V.A. Selective foraging behaviour of basking sharks on zooplankton in a small-scale front. Nature. 1998;393:460–464. doi:10.1038/30959 [Google Scholar]
- Thompson P.M, Miller D. Summer foraging activity and movements of radio-tagged common seals (Phoca vitulina. L.) in the Moray Firth, Scotland. J. Appl. Ecol. 1990;27:492–501. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.