Abstract
House sparrow (Passer domesticus) numbers have declined rapidly in both rural and urban habitats across Western Europe over the last 30 years, leading to their inclusion on the UK conservation red list. The decline in farmland has been linked to a reduction in winter survival caused by reduced food supply. This reduction in food supply is associated with agricultural intensification that has led to the loss of seed-rich winter stubble and access to spilt grain. However, urban house sparrows have also declined, suggesting that reduced food supply in farmland is not the sole reason for the decline. Here, we show that changes in house sparrow mass and thus fat reserves are not regulated to minimize starvation risk, as would be expected if limited winter food were the only cause of population decline. Instead, the species appears to be responding to mass-dependent predation risk, with starvation risk and predation risk traded-off such that house sparrows may be particularly vulnerable to environmental change that reduces the predictability of the food supply.
Keywords: Passer domesticus, starvation–predation risk trade-off, farmland birds, body mass, starvation risk
1. Introduction
The house sparrow (Passer domesticus) is a small passerine bird (24–36 g) whose numbers are estimated to have declined by 60% in the UK between 1970 and 2000, a decrease of more than 12 million (Crick et al. 2002; Gregory et al. 2002). Many possible explanations have been suggested (Crick et al. 2002). Currently, the strongest evidence suggests that population declines are linked, at least in rural areas, to agricultural intensification resulting in lower winter survival caused by high starvation risk (Hole et al. 2002).
Small birds have been shown to respond to increased starvation risk by amassing fat reserves until the cost of increased predation risk due to reduced flight performance or foraging exposure equals the benefits of reduced starvation risk (Lima 1986; Witter & Cuthill 1993; Gosler et al. 1995). Body mass can, therefore, be a useful measure of the balance between starvation risk and predation risk (Lima 1986; Houston et al. 1993; Rogers & Smith 1993; Witter & Cuthill 1993). Theory predicts that, when faced with a high starvation risk, animals should compensate by carrying increased fat reserves so that starvation mortality is minimized and should build up daily fat reserves early to compensate for the possibility of an unpredictable food supply later in the day (Lima 1986; Houston et al. 1993; Bednekoff & Houston 1994b).
In this study, we use the mass-dependence of the starvation–predation risk trade-off to investigate if starvation risk is the main factor affecting winter energy reserves in house sparrows. We predicted that, if high starvation risk were the principle cause of house sparrows' declines, they would substantially increase energy reserves in winter and gain most mass early in the day.
2. Material and methods
We analysed mass data for 10 203 individual house sparrows caught between 1996 and 2001 as part of the British and Irish Ringing Scheme (Clark et al. 2004). We then compared change in mass between the non-breeding season of August to November and winter (defined as the three coldest months of the year, December to February, based on monthly average temperatures (BADC 2002)). Preliminary results showed that young birds leave the nest before they reach adult body mass, so initially they are significantly lighter than adult birds for reasons other than starvation risk. This difference disappears rapidly and by October first year birds have reached a stage where they are no longer significantly lighter than adults due to incomplete growth (mean difference=−0.09±0.48 g, t134=0.2, p=0.846). To ensure only fully grown birds appeared in the analysis, we excluded juvenile birds captured before October from the data. Day length between sunrise and sunset was divided into five equal periods to give a good balance between the sample sizes needed to quantify mass gain accurately and the resolution needed to identify when mass gain was occurring. An independent t-test was used to examine the mean gain in mass between the first and second parts of the day.
The results were compared to similar analyses for five other passerine species selected on the basis that they shared the sparrows' ground-feeding foraging guild and were also among Britain's top 10 most common garden feeding birds (Toms 2003). Unlike the house sparrow their populations were not declining, due to high starvation risk or any other cause, during the study (Gregory et al. 2002). British populations of some species include some migrants so as a result data from March and April, when some individuals in Britain might be experiencing pre-migratory fattening, were excluded from the analysis. In any case, as size was also controlled for, it is unlikely that partial migration in some species could influence the comparison. Location and time of capture were also controlled for where appropriate.
To examine if house sparrows were physiologically capable of changing mass gain patterns, we compared mean residual mass on six islands without resident sparrowhawks Accipiter nisus (South Uist, Benbecula, St Agnes, Tresco, St Mary's and Fair Isle) to mass on the six islands with sparrowhawks present (Isle of Man, South Ronaldsay, Hoy, Orkney Mainland, Sanday and North Ronaldsay) (Gibbons et al. 1993). Use of residual mass in the analysis, calculated from a general linear model based on known causes of mass variation helped control for the possible confounding effects of body size, sex, temperature, year, month, time, longitude and latitude of capture (Cresswell 1998; Macleod et al. 2005a,b). The islands are also distributed over a wide geographical and climatic range. Sparrowhawks are the main avian predator of sparrows in Britain so general linear modelling using sparrowhawk abundance data for the whole of country (Gibbons et al. 1993) was then used to examine the effect of predator abundance on mass more generally.
3. Results
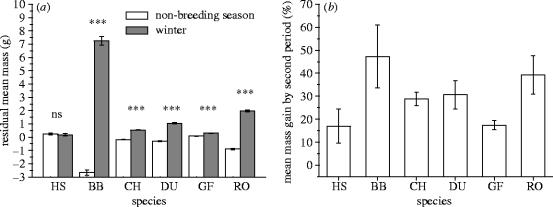
Our results show that, despite being the only species thought to be declining due to starvation, the house sparrow was the only species that was not significantly heavier in winter (figure 1a): house sparrows, therefore, respond as if predation risk rather than starvation risk is relatively important. House sparrow mean change in mass from non-breeding season to winter: −0.06±0.06 g, t4121=1.0, p=0.324; blackbird: 9.93±0.19 g, t5916=51.6, p<0.001; chaffinch: 0.72±0.02 g, t19 773=36.0, p<0.001; dunnock: 1.35±0.04 g, t4811=33.6, p<0.001; greenfinch: 0.22±0.02 g, t32 127=11.3, p<0.001; robin: 2.86±0.04 g, t5630=70.5, p<0.001. The percentage change in mean autumn body mass from non-breeding season to winter was significantly lower for the house sparrow compared to all of the five other species (p<0.001 in all post hoc pair wise comparisons).
Figure 1.
Seasonal and diurnal mass change in house sparrows and comparable species. (HS, house sparrow; BB, blackbird Turdus merula; CH, chaffinch Fringilla coelebs; DU, dunnock Prunella modularis; GF, greenfinch Carduelis chloris; RO, robin Erithacus rubecula; ns, no significant difference; ***, very highly significant difference.) Residual mass controls for size (measured by wing length), location of capture (longitude and latitude within Britain) and for part (a) time of capture (since dawn). (a) Inter-specific comparison of residual mean mass (±95% CI) in non-breeding and winter seasons. House sparrows do not show any significant mass gain in winter consistent with compensation for high predation risk, while all five other species do. (b) Inter-specific comparison of percentage diurnal mass gain (±95% CI) at start of day in winter. House sparrows show a low mass gain pattern early in the day consistent with compensation for high predation risk. Mass gain between the first and second parts of the day is expressed as the proportion of mass gained over the whole day by that species.
Additionally, house sparrows gained a relatively low amount of mass early in the day consistent with predation risk being relatively important (figure1b). House sparrow percentage of daily mass change gained by second period of day: 17.0±7.4%, t714=2.3, p=0.021; blackbird: 47.3±13.6%, t1618=3.47, p<0.001; chaffinch: 28.8±3.0%, t6346=9.5, p<0.001; dunnock: 30.6±6.2%, t1407=4.9, p<0.001; greenfinch: 17.4±2.0%, t7033=8.05, p=0.001; robin: 39.2±8.4%, t1633=4.6, p<0.001). In absolute terms house sparrows gained significantly less mass early in the day than all other species apart from greenfinch: examination of the t-test 95% CI for each species shows that those for greenfinch lie entirely within those for house sparrow (figure 1b).
Figure 2 shows that, in the absence of their main avian predator, house sparrows are physiologically capable of gaining greater mass during the winter. The mean residual mass of house sparrows on six UK islands, where sparrowhawks were absent was 1.7±0.3 g compared to 0.3±0.2 g on six UK islands, where sparrowhawks are present (mean difference=1.3±0.3 g, t10=3.9, p=0.003) and −0.1±0.02 g on the mainland, where sparrowhawks were widespread. Table 1 shows that across the whole of Britain predator abundance is a significant predictor of house sparrow mass.
Figure 2.
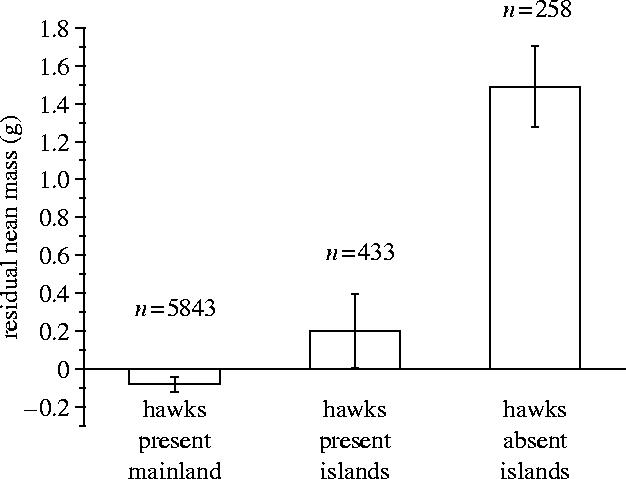
House sparrow mass in the presence and absence of sparrowhawks. The significantly higher mass of individuals living in areas without sparrowhawks demonstrates that house sparrows are not phenotypically constrained from maintaining greater body mass. Error bars represent ±95% CI.
Table 1.
House sparrow mass is predicted by predator abundance. (GLM of factors predicting mass variation, produced by reverse stepwise regression. Dependent variable is mass, N=7580 individual house sparrows, adjusted R2=0.14.)
| source of variation | sum of squares | d.f. | F | sig. |
|---|---|---|---|---|
| size (wing length) | 1816 | 1 | 499.7 | <0.001 |
| time of day | 459 | 1 | 126.2 | <0.001 |
| sparrowhawk abundancea | 434 | 1 | 119.3 | <0.001 |
| latitude | 413 | 1 | 113.6 | <0.001 |
| sex | 99 | 1 | 27.1 | <0.001 |
| month | 599 | 11 | 15.0 | <0.001 |
| longitude | 44 | 1 | 12.0 | <0.001 |
| day length | 13 | 1 | 3.7 | 0.056 |
| sex×month | 281 | 11 | 7.0 | <0.001 |
| model | ||||
| explained | 4611 | 29 | 43.7 | <0.001 |
| residual | 27 447 | 7550 | ||
| total | 32 058 | 7579 |
Parameter estimate for hawk abundance =−1.74±0.159.
4. Discussion
The lack of winter fattening and low mass gain at the start of the day might have arisen if house sparrows did not face a heightened winter starvation risk. However, theory predicts all diurnal foraging birds should face an increased starvation risk in winter, irrespective of food supply changes, due to the increased energy reserves required to survive winter's longer overnight fast and colder temperatures (Witter & Cuthill 1993; Bednekoff & Houston 1994b; Houston et al. 1997). In addition, the experimental work of Hole et al. (2002) has already shown that, at least in farmland, reduced house sparrow survival is linked to winter starvation risk. Instead, we believe the results arise because house sparrows are constrained from depositing reserves that would reduce their starvation risk (Lima 1986; Witter & Cuthill 1993; Bednekoff & Houston 1994a).
Phenotypic limitation that prevents greater mass being acquired and maintained by the species can be discounted because house sparrows are capable of maintaining considerably greater mass than they actually do in winter (figure 2). Instead, the results can be explained by a mass-dependent predation risk constraint, where house sparrows have lower mass and delay mass gain until later in the day to be better able to escape from predators. The results are also consistent with existing knowledge about predator vulnerability in different species. Despite being less common than the five comparison species house sparrows are the most frequent bird-prey of domestic cats and the species most vulnerable to sparrowhawk predation (Gotmark & Post 1996; Toms 2003; Woods et al. 2003). In our comparison greenfinch, the second most vulnerable species to predation (Gotmark & Post 1996), adopts the most similar mass change patterns, supporting the conclusion that these patterns are due to the constraint of high predation risk.
We, therefore, suggest that, due to mass-dependent predation, house sparrows are unable to increase body mass to reduce their high starvation risk without substantially increased predation risk. In general, high mass-dependent predation risk will heighten the susceptibility of a species to population decline as a consequence of a lack of food and we suggest this provides a mechanism for the house sparrow population declines witnessed across western Europe in recent decades. In the future, this hypothesis can be explicitly tested because it predicts that declining populations of house sparrows will have a lower degree of winter fattening than stable populations, where predators are present and the reverse when they are absent. Meanwhile, we suggest that this hypothesis and its implications be explicitly considered in conservation planning for the species.
Acknowledgements
Many thanks to all the ringers who collected the original data. The BTO Ringing Scheme is supported by a partnership of the British Trust for Ornithology, the JNCC, The National Parks and Wildlife Service (Ireland) and the ringers themselves. Thanks to Richard Bradbury, John Quinn, Andy Gosler, Mark Whittingham, Ben Sheldon, Colin MacLeod, Humphrey Crick, Rob Robinson, Stephen Baillie, Steve Freeman, David Gibbons and Susanne Shultz for useful discussions and commenting on drafts of the manuscript. RM and PB were supported by NERC studentships at Oxford University, JAC by the BTO, and WC by a Royal Society University Research Fellowship.
References
- BADC 2002 http://www.badc.rl.ac.uk/home/: British Atmospheric Data Centre.
- Bednekoff P.A, Houston A.I. Avian daily foraging patterns—effects of digestive constraints and variability. Evol. Ecol. 1994;8:36–52. doi:10.1007/BF01237664 [Google Scholar]
- Bednekoff P.A, Houston A.I. Optimizing fat reserves over the entire winter—a dynamic model. Oikos. 1994b;71:408–415. [Google Scholar]
- Clark J.A, Robinson R.A, Balmer D.E, Adams S.Y, Collier M.P, Grantham M.J, Blackburn J.R, Griffin B.M. Bird ringing in Britain and Ireland in 2003. Ring. Migr. 2004;22:95–127. [Google Scholar]
- Cresswell W. Diurnal and seasonal mass variation in blackbirds Turdus merula: consequences for mass-dependent predation risk. J. Anim. Ecol. 1998;67:78–90. doi:10.1046/j.1365-2656.1998.00174.x [Google Scholar]
- Crick H.Q.P, Robinson R.A, Appleton G.F, Clark N.A, Rickard A.D. Investigation into the causes of the decline of starlings and house sparrows in Great Britain: BTO Research Report No. 290. Department of Environment, Food and Rural Affairs; London: 2002. [Google Scholar]
- Gibbons D.W, Reid J.B, Chapman R.A. The new atlas of breeding birds in Britain and Ireland: 1988–91. T & A D Poyser; London: 1993. [Google Scholar]
- Gosler A.G, Greenwood J.J.D, Perrins C. Predation risk and the cost of being fat. Nature. 1995;377:621–623. doi:10.1038/377621a0 [Google Scholar]
- Gotmark F, Post P. Prey selection by sparrowhawks, Accipiter nisus: relative predation risk for breeding passerine birds in relation to their size, ecology and behaviour. Phil. Trans. R. Soc. B. 1996;351:1559–1577. [Google Scholar]
- Gregory R.D, Wilkinson N.I, Noble D.G, Robinson J.A, Brown J.A, Hughes J, Proctor D, Gibbons D.W, Galbraith C.A. The population status of birds in the United Kingdom, Channel Islands and the Isle of Man: an analysis of conservation concern 2002–2007. Brit. Birds. 2002;95:410–448. [Google Scholar]
- Hole D.G, Whittingham M.J, Bradbury R.B, Anderson G.Q.A, Lee P.L.M, Wilson J.D, Krebs J.R. Widespread local house-sparrow extinctions—agricultural intensification is blamed for the plummeting populations of these birds. Nature. 2002;418:931–932. doi: 10.1038/418931a. doi:10.1038/418931a [DOI] [PubMed] [Google Scholar]
- Houston A.I, McNamara J.M, Hutchinson J.M.C. General results concerning the trade-off between gaining energy and avoiding predation. Phil. Trans. R. Soc. B. 1993;341:375–397. [Google Scholar]
- Houston A.I, Welton N.J, McNamara J.M. Acquisition and maintenance costs in the long-term regulation of avian fat reserves. Oikos. 1997;78:331–340. [Google Scholar]
- Lima S.L. Predation risk and unpredictable feeding conditions—determinants of body-mass in birds. Ecology. 1986;67:377–385. [Google Scholar]
- Macleod R, Barnett P, Clark J.A, Cresswell W. Body mass change strategies in blackbirds Turdus merula: the starvation–predation risk trade-off. J. Anim. Ecol. 2005;74:292–302. doi:10.1111/j.1365-2656.2005.00923.x [Google Scholar]
- Macleod R, Gosler A.G, Cresswell W. Diurnal mass gain strategies and perceived predation risk in the great tit Parus major. J. Anim. Ecol. 2005;74:956–964. doi:10.1111/j.1365-2656.2005.00993.x [Google Scholar]
- Rogers C.M, Smith J.N.M. Life-history theory in the nonbreeding period—trade-offs in avian fat reserves. Ecology. 1993;74:419–426. [Google Scholar]
- Toms M.P. The BTO/CJ garden birdwatch book. British Trust for Ornithology; Thetford: 2003. [Google Scholar]
- Witter M.S, Cuthill I.C. The ecological costs of avian fat storage. Phil. Trans. R. Soc. B. 1993;340:73–92. doi: 10.1098/rstb.1993.0050. [DOI] [PubMed] [Google Scholar]
- Woods M, McDonald R.A, Harris S. Predation of wildlife by domestic cats Felis catus in Great Britain. Mammal Rev. 2003;33:174–188. doi:10.1046/j.1365-2907.2003.00017.x [Google Scholar]