Abstract
Wandering albatrosses (Diomedea exulans) nest on Southern Ocean islands, building elevated nests upon which they incubate eggs and raise chicks, and which the chicks occupy through winter. The nests support high invertebrate biomass, including larvae of the flightless moth Pringleophaga marioni. Here we argue that high biomass of P. marioni in the nests is not associated with nutrient loading as previously suspected, but that higher temperatures in the nests increase growth and feeding rate, and decrease deleterious repeated cold exposure, providing fitness advantages for P. marioni. Thus, wandering albatrosses may be serving as thermal engineers, modifying temperature and therefore enabling better resource use by P. marioni.
Keywords: wandering albatross, ecosystem engineering, development thresholds
1. Introduction
Ecosystem engineers are the organisms that modify the resources available to other organisms through their action or presence (Jones et al. 1994). This can be by modifying or creating a resource or habitat (as in the case of beaver dams), or by altering the impact of an abiotic factor on resource use or availability (as in the case of mussel beds modifying wave action and therefore erosion) (examples from Jones et al. 1994). On oceanic islands, like those of the Southern Ocean, the dominant marine–terrestrial links are via seabirds, which transfer considerable quantities of marine-sourced nutrients to shore (Sánchez-Piñero & Polis 2000), and in the case of burrowing species also displace vast quantities of soil, modifying habitats and changing soil properties which in turn alter the terrestrial community structure (Walls 1978; Bancroft et al. 2004). Under Jones et al.'s (1994) scheme, nutrient input is probably not ecosystem engineering (since the seabirds are directly providing a resource), but the effects of nesting activities do constitute ecosystem engineering.
The largest flying seabird is the Wandering albatross (Diomedea exulans), which nests exclusively on Southern Ocean islands. They build large (ca 0.1–0.25 m3), elevated nests from the surrounding vegetation, upon which they lay their eggs in summer and incubate chicks which remain on the nests for the entire winter, before fledging in November (Warham 1997). Albatross nests have a higher density of invertebrates than the surrounding peat and vegetation, and this has been assumed to be a response of the invertebrate assemblage to the increased nutrients from spilled food and faeces of the adult and nestling albatrosses (Joly et al. 1987), and hence not an example of ecosystem engineering. We address this assumption by examining the biomass of larvae of the flightless moth Pringleophaga marioni (Lepidoptera: Tineidae) with respect to the nutrient and physical environment of the nests. Larvae of P. marioni are key decomposers on Marion Island (Smith & Steenkamp 1992) and have been the subject of both ecological (e.g. Crafford 1990) and physiological (e.g. Klok & Chown 1997) studies, and are readily collected from abandoned albatross nests for these studies. We test the hypothesis that caterpillar biomass is higher in nest habitats than in other habitats on the island, and propose that this higher biomass is a result of a hitherto undescribed form of ecosystem engineering.
2. Material and methods
(a) Caterpillar biomass and nest nutrients
Fifteen abandoned albatross nests, located in wet or dry mire communities (Gremmen 1981), were identified within 3–4 h walk of the Meteorological station on Marion Island's east coast. The location of each nest was recorded, its diameter and height were measured, and it was scored as ‘new’ (from the current season, abandoned within the past 6 weeks, n=5) or ‘old’ (from the previous or earlier seasons, n=10), based on its condition and the plant growth on and around it. The nest material was searched twice by hand for caterpillars in situ. Caterpillars were returned to the laboratory on the island in a bag with nest material. Three samples of nest material (50 g each) were taken during collection of the caterpillars from central portions of the nests (which are typically and most densely occupied by caterpillars), mixed, air-dried and a 50 g sub-sample taken for nutrient analysis (conducted by Bemlab, South Africa). In the laboratory, caterpillars were wiped clean of nest material with a soft paper towel and weighed wet (Mettler AE163 balance, ±0.1 mg). The dry mass was estimated from a linear regression of dry on wet mass obtained from a sub-sample of 150 animals used for a physiological study (Sinclair & Chown 2005) and dried to a constant mass at 60 °C. The remaining caterpillars were returned within 24 h to the nests from which they were collected. The volume of the nest was approximated to the volume of a cylinder, and caterpillar biomass was expressed as mg dry mass m−3 to allow comparison with previously collected data. Since the nest samples were sorted in the field, it is likely that we missed early-instar caterpillars. Together with the central depression in the nest, this means that we slightly overestimated nest volume and hence underestimate the density of caterpillars.
Estimates of caterpillar biomass from nests were compared with those obtained from systematic sampling of five major vegetation communities on the lowlands of the eastern coastal plain of Marion Island conducted by Hänel (1999) in April 1997. At each of the five sites, 10 core samples (7 cm diameter, 10 cm deep) were taken in a randomized 5 sub-sites×2 samples design. The caterpillars were hand-sorted from the cores in the laboratory and dry mass was determined. For these five vegetation communities, the nutrient data presented by Gabriel et al. (2001) were used for comparison with the albatross nest nutrient data. The communities on Marion Island may be divided into those with high nutrient (coastal Cotula plumosa herbfield and Poa cookii tussock grassland) and low nutrient (Blepharidophyllum densifolium mire, Sanionia uncinata mire, Crassula moschata herbfield) communities (Smith & Steenkamp 2001; Smith et al. 2001). Comparisons of caterpillar biomass in newly abandoned nests, old nests and in each of the plant communities, were made independently for those with high- and low-nutrient contents. The comparisons of biomass were made using a generalized linear model (GLZ) with Poisson errors and a log-link function, corrected for overdispersion in Statistica v. 6.1 (Statsoft Inc., Tulsa, OK, USA). The comparisons of nutrient contents (P, Total N, Total C, NH4, NO3−) of old and new nests and other habitats (combined means) were made using analysis of variance (ANOVA) on Statistica.
(b) Microclimate measurements
Microclimate temperatures were measured for 17 days at 30-min intervals using calibrated thermochron iButtons (Model DS1920, Dallas Semiconductors, Dallas, TX, USA, accuracy 0.5 °C) mounted on 300 mm aluminium strips, and inserted into a pre-cut horizontal slot at mid-height on the side of unoccupied (n=5) or occupied (n=4) Wandering albatross nests. Only the nests occupied by experienced breeding birds in areas where they are habituated to human activity were used for occupied measurements. The unoccupied nests within 100 m of occupied nests were selected. A further iButton was placed 1 cm below the soil surface next to the Stevenson Screen at the Meteorological station. All of the monitored nests were within 1 km distance and 20 m elevation of this data logger.
3. Results and discussion
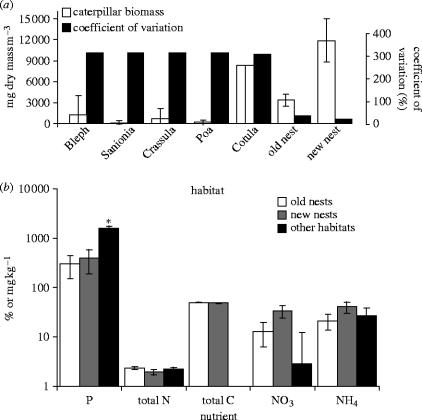
Biomass of P. marioni (Smith & Steenkamp 1992) was significantly higher in recently abandoned (within the past 6 weeks) nests than in ‘old’ (previous year's) nests (figure 1a), and these both harboured a higher biomass of caterpillars than all the other habitats on the island, with the exception of the highly variable (and often highly biotically enriched) C. plumosa herbfield. Furthermore, the coefficient of variation of caterpillar biomass is substantially lower in nests than in the other habitats (figure 1a), demonstrating that caterpillars are not only more abundant, but also distributed more evenly in nest habitats than in other habitats on the island. However, total nitrogen and several other nutrients did not differ significantly between the recently abandoned and old nests, or between the nests and other habitats on the island that have been enriched by nutrients (figure 1b). Indeed, the only significant difference in nutrients was between the nests and other habitats, which had higher total phosphorus than the nests. This suggests that the high caterpillar biomass in the nests is not explained by an increase in the nutrient content of the nest material compared to the other habitats, as might be expected from other observations of the impacts of nutrient input from seabirds into terrestrial environments (e.g. Sánchez-Piñero & Polis 2000). In addition, the higher biomass in recently abandoned nests, by comparison with older nests, indicates that the nest structure itself does not directly result in increased caterpillar biomass.
Figure 1.
(a) Mean (±95% CI) and coefficient of variation biomass of P. marioni larvae in seven types of habitat on Marion Island (Bleph, Blepharidophyllum densifolium mire; Sanionia, Sanionia uncinata mire; Crassula, Crassula moschata herbfield; Poa, Poa cookii tussock grassland; Cotula, Cotula plumosa herbfield; old nest, old abandoned albatross nests; new nest, newly abandoned nests). Confidence intervals for Cotula are very large, only lower bar shown. Caterpillar biomass in newly abandoned nests is significantly greater than biomass in all other habitats (χ2=25.4, p<0.00004) except for Cotula (χ2=2.5, p>0.47). (b) Comparison of mean+s.e.m. nutrients of new (abandoned in past 6 weeks) and old (previous year) albatross nests, and all other habitats on the island (no data for C). Total N and C measured as %, other nutrients measured in g kg−1. Total P is significantly lower in nests than other habitats (F2,17=13.93, p<0.003), but other nutrients do not differ between habitats (p>0.05).
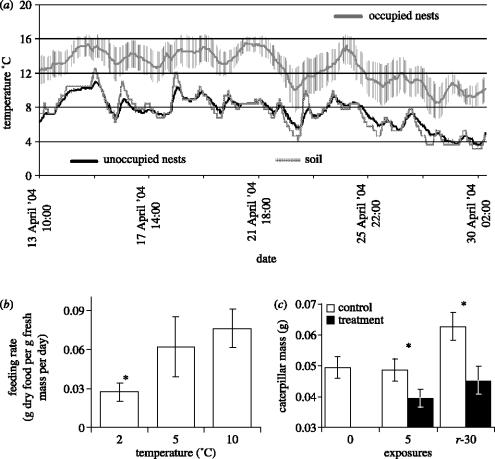
We assume that the recently abandoned nests represent a state similar to (and associated with) the recent presence of albatrosses on the nest. Smith (1978) published data on total organic nitrogen contents of occupied (3.0±0.32%, mean±s.d., n=3) and unoccupied nests (2.4±0.64%, n=15), and these values do not differ significantly (t-test, p=0.139), nor do Smith's (1978) unoccupied nest values differ from our values for unoccupied nests (2.36±0.22%, n=10, t-test, p=0.85). Smith (1978) did not provide data on carbon or phosphorus of occupied nests, but nitrogen is the most significant limiting nutrient for insect growth and development (Scriber & Slansky 1981). As well as inputting nutrients, albatrosses also heat the nest material while incubating and occupying the nest. Incubating adults, and chicks that have left the guard stage, maintain a body temperature of ca 38 °C (Warham 1997). The data loggers inserted into four occupied nests recorded temperatures more than 5 °C higher than temperatures measured concurrently in abandoned nests, which were nearly identical to nearby soil temperatures (figure 2a). Albatross heat input elevates nest temperatures to the near optimum for P. marioni feeding (figure 2b), and will probably keep winter microhabitat temperatures above the chill coma temperature of −0.6 °C (Klok & Chown 1997), thus increasing growth rates by allowing caterpillars to spend more of the winter period foraging. Furthermore, there are also likely to be strong fitness advantages for caterpillars that are able to avoid cold exposure. Repeated exposure to sub-zero temperatures is detrimental to P. marioni, resulting in cessation of feeding, decline in mass (figure 2c), decrease in relative gut mass and consequent reduction in growth rate, and presumably fitness, even after a period of recovery (Sinclair & Chown 2005). Caterpillars should not only grow better under warm nest conditions, leading to elevated biomass, but either the larvae or ovipositing female moths should preferentially seek out albatross nests, which would explain the observed reduction in biomass variation between nests compared with variation between sites within other habitats (figure 1a).
Figure 2.
(a) Mean±s.e.m. microclimate temperature of occupied wandering albatross nests (n=4), unoccupied nests (n=5, standard error smaller than plot line) and soil (n=1). Mean difference between occupied and unoccupied nests=5.0 °C; mean minimum difference=2.0 °C; mean maximum difference=9.5 °C. (b) Mean±s.e.m. feeding rate of larvae of P. marioni larvae held at constant temperatures, n=29, data from Crafford (1990). Asterisks indicate that feeding rate is significantly lower (t-test, p<0.05) at 2 °C than at 5 °C or 10 °C (which do not differ). (c) Least-squares mean mass±95% CI of P. marioni larvae exposed to five consecutive freeze–thaw cycles (filled bars, treatment; open bars, handling control). 0, no exposure; 5, exposed to freeze–thaw; r–30, with 30 days recovery after final exposure. Asterisks indicate control–treatment pairs that differ significantly (p<0.05) (for details, see Sinclair & Chown 2005).
Conventional examples of ecosystem engineering involve an organism altering a physical attribute of the ecosystem in a way that makes resources available for other organisms. The thermal environment is an important determinant of distribution at small and large scales (Gaston 2003), and on Marion Island (mean annual temperature of 6 °C; Pakhomov & Chown 2003), temperature is an important limiting factor for P. marioni feeding, growth and development (figure 2b). By heating the nest material, albatrosses are presumably altering the thermal environment in such a way that the caterpillars will be able to utilise food resources more effectively, and do so for the duration of the winter. Although the nests themselves provide an obvious and long-lasting effect on the landscape, caterpillar biomass is highest if there has recently been an albatross on the nest. It seems that the physical and nutrient structure of the nest is not as important as the island of heat that the albatross creates. Since the heat island appears to enable better survival and greater exploitation of the food resources in the nest by P. marioni, wandering albatrosses are likely to be thermal ecosystem engineers. Manipulative field experiments are required to confirm this finding, though they may prove to be problematic given that wandering albatrosses are IUCN red-listed as vulnerable.
Acknowledgments
Thanks to Shallin Abrahams, Thembile Koza, Bettine Jansen van Vuuren and Richard Mercer for field assistance, and Peter Le Roux and three anonymous referees for comments on an earlier manuscript version. This work was supported by National Research Foundation grant 2068305, with logistic support from the Department of Environmental Affairs and Tourism. B.J.S was supported by a New Zealand Science and Technology Postdoctoral Fellowship. Work close to albatrosses was undertaken with Nico de Bruyn and under permit from the Prince Edward Islands Management Committee via the albatross monitoring research at Marion Island.
References
- Bancroft W.J, Hill D, Roberts J.D. A new method for calculating volume of excavated burrows: the geomorphic impact of wedge-tailed shearwater burrows on Rottnest island. Funct. Ecol. 2004;18:752–759. doi:10.1111/j.0269-8463.2004.00898.x [Google Scholar]
- Crafford J.E. 1990. Patterns of energy flow in populations of the dominant insect consumers on Marion Island. MSc thesis, University of Pretoria, South Africa. [Google Scholar]
- Gabriel A.G.A, Chown S.L, Barendse J, Marshall D.J, Mercer R.D, Pugh P.J.A, Smith V.R. Biological invasions of southern ocean islands: the Collembola of Marion Island as a test of generalities. Ecography. 2001;24:421–430. doi:10.1034/j.1600-0587.2001.d01-198.x [Google Scholar]
- Gaston K.J. Oxford University Press; Oxford, UK: 2003. The structure and dynamics of geographic ranges. [Google Scholar]
- Gremmen N.J.M. W. Junk; The Hague: 1981. The vegetation of the subantarctic islands Marion and Prince Edward. [Google Scholar]
- Hänel C. 1999. The distribution and abundance of macro-invertebrates in the major vegetation communities of Marion Island and the impact of alien species. MSc thesis, University of Pretoria, South Africa. [Google Scholar]
- Joly Y, Frenot Y, Vernon P. Environmental modifications of a subantarctic peat-bog by the wandering albatross (Diomedea exulans): a preliminary study. Polar Biol. 1987;8:61–72. doi:10.1007/BF00297166 [Google Scholar]
- Jones C.G, Lawton J.H, Shachak M. Organisms as ecosystem engineers. Oikos. 1994;69:373–386. [Google Scholar]
- Klok C.J, Chown S.L. Critical thermal limits, temperature tolerance and water balance of a sub-Antarctic caterpillar, Pringleophaga marioni (Lepidoptera: Tineidae) J. Insect Physiol. 1997;43:685–694. doi: 10.1016/s0022-1910(97)00001-2. doi:10.1016/S0022-1910(97)00001-2 [DOI] [PubMed] [Google Scholar]
- Pakhomov E, Chown S.L. The Prince Edward islands: southern ocean oasis. Ocean Yearbook. 2003;17:348–379. [Google Scholar]
- Sánchez-Piñero F, Polis G.A. Bottom-up dynamics of allochthonous input: direct and indirect effects of seabirds on islands. Ecology. 2000;81:3117–3132. [Google Scholar]
- Scriber J.M, Slansky F. The nutritional ecology of immature insects. Annu. Rev. Entomol. 1981;26:183–211. doi:10.1146/annurev.en.26.010181.001151 [Google Scholar]
- Sinclair B.J, Chown S.L. Deleterious effects of repeated cold exposure in a freeze-tolerant sub-Antarctic caterpillar. J. Exp. Biol. 2005;208:869–879. doi: 10.1242/jeb.01455. doi:10.1242/jeb.01455 [DOI] [PubMed] [Google Scholar]
- Smith V.R. Animal–plant–soil relationships on Marion Island (subantarctic) Oecologia. 1978;32:239–253. doi: 10.1007/BF00366075. doi:10.1007/BF00366075 [DOI] [PubMed] [Google Scholar]
- Smith V.R, Steenkamp M. Soil macrofauna and nitrogen on a sub-Antarctic island. Oecologia. 1992;92:201–206. doi: 10.1007/BF00317365. doi:10.1007/BF00317365 [DOI] [PubMed] [Google Scholar]
- Smith V.R, Steenkamp M. Classification of the terrestrial habitats on Marion Island based on vegetation and soil chemistry. J. Veg. Sci. 2001;12:181–198. [Google Scholar]
- Smith V.R, Steenkamp M, Gremmen N.J.M. Terrestrial habitats on Marion Island: their vegetation, edaphic attributes, distribution and response to climate change. S. Afr. J. Bot. 2001;67:641–654. [Google Scholar]
- Walls G.Y. The influence of the tuatara on fairy prion breeding on Stephens island, cook strait. New. Zeal. J. Ecol. 1978;1:91–98. [Google Scholar]
- Warham J. Academic Press; London: 1997. The Petrels: their ecology and breeding systems. [Google Scholar]