Abstract
The viral dynamics in human immunodeficiency virus type 1 (HIV-1) infection have been studied extensively using mathematical modeling, but data from other primate lentivirus systems are scarce. This study was initiated to increase the understanding of the differences and similarities between the different primate lentiviruses. Four cynomolgus macaques were infected with SIVmac251. Six months after infection the monkeys received a 7-day course of subcutaneous, quadruple antiretroviral therapy with zidovudine, lamivudine, tenofovir, and ritonavir-boosted lopinavir. Plasma virus levels were determined before therapy, daily during the first 10 days of therapy, and after 14 days using a sensitive commercial reverse transcriptase assay. All four monkeys showed a rapid and uniform decline in plasma virus load between day 1 and day 4 of treatment (first-phase decay). Two mathematical models, a piecewise linear regression analysis and a nonlinear model, were used to estimate the rate of viral decay in plasma and gave similar results. The mean half-life for plasma virus was 0.47 days (range, 0.37 to 0.50) and reflects the underlying decline of virus-producing CD4+ lymphocytes. This is the fastest primate lentivirus decay described hitherto. The rapid decay may be due to the high antiviral potency of the therapy or to intrinsic differences between simian immunodeficiency virus (SIV) infection in macaques and HIV-1 infection in humans.
Over the last decade, detailed studies of the viral dynamics have provided important information about the pathogenesis of human immunodeficiency virus type 1 (HIV-1) and the activity of antiretroviral drugs (6, 9, 12-14). These studies have allowed estimation of the life span of free virus particles and different populations of virus-infected cells. Thus, virus-producing CD4+ T lymphocytes (CD4 cells) have a very short life span, which is reflected in the rapid first-phase decline in plasma virus levels after introduction of antiretroviral therapy (ART). Other HIV-1-infected cells, in particular resting CD4+ T lymphocytes, have very long half-lives, which makes it unrealistic that HIV-1 infection can be cured by ART alone (3, 4, 15). Initial studies indicated that the half-life of virus-producing CD4 cells is approximately 1.5 days (6, 13, 14), but recent studies, in which more-efficient drug combinations and more-intense samplings were used, have shown the true half-life is likely to be considerably shorter (0.7 days) (9). Thus, Markowitz et al. concluded that standard triple-combination ART is not maximally suppressive and can be augmented 25% to 30% by a novel quadruple therapy consisting of lamivudine, tenofovir, efavirenz, and ritonavir-boosted lopinavir (8, 9). At present it is not known if the potency of ART can be further enhanced. However, it is important to stress that increased antiretroviral potency does not automatically lead to better treatment outcome, because standard triple combination treatment may be sufficient to durably suppress virus production and because treatment outcome is strongly influenced by nonvirological factors, such as adherence and tolerability of treatment (8, 16).
Comparisons of the dynamics of different primate lentivirus infections may give information about similarities and differences in the interaction between lentiviruses and their hosts. Recently, we reported that virus-producing CD4 cells appear to have similar half-lives in HIV-1 and HIV-2 infection (2), despite the fact that the rate of disease progression and transmission is much slower in HIV-2 compared to HIV-1 infection (7, 10). Here we have gone on to study viral dynamics in cynomolgus monkeys (Macaca fascicularis) that received highly active quadruple ART during chronic, experimental SIVsm infection. Asian macaques that have been experimentally infected with SIV are valuable models of HIV-1 infection, although disease progression is generally faster and dependent on the SIV strain (5). There is only one previous report of the viral dynamics in experimental SIV infection (11). In that study, Nowak et al. used monotherapy [with (R)-9-(2-phosphonylmethoxypropyl)adenine (tenofovir, TDF, or PMPA)] to block SIVsmE660 infection in a Macaca nemestrina model. Their estimate of a half-life of 0.7 to 1.4 days for SIV-producing cells is likely to be an underestimation, because the monkeys received monotherapy instead of combination ART. Here we provide support for this assumption by showing that SIV-producing cells have a half-life of approximately 0.5 days in monkeys that have received quadruple ART. This is the fastest primate lentivirus decay hitherto reported and has interesting implications for viral pathogenesis and the suppressive effect of combinational ART.
MATERIALS AND METHODS
Animal infections.
Female cynomolgus macaques (Macaca fascicularis) of Chinese origin were housed in the Astrid Fagraeus laboratory at the Swedish Institute for Infectious Disease Control. Housing and care procedures were in compliance with the provisions and general guidelines of the Swedish Animal Welfare Agency, and the Local Ethical Committee on Animal Experiments approved all procedures. Four macaques were challenged intrarectally with 50 units of the 50% monkey infectious dose of SIVmac251 and were given antiretroviral therapy after 6 months of infection. None of the animals had any clinical sign of immunodeficiency at onset of therapy, but their percentages of CD4+ T cells were reduced in comparison with preinfection levels (data not shown).
Antiretroviral therapy and samplings.
Subcutaneous quadruple antiretroviral therapy was given with zidovudine (AZT) at 4.5 mg/kg of body weight twice daily, lamivudine (3TC) at 2.5 mg/kg twice daily, TDF at 3.0 mg/kg once daily, and lopinavir-ritonavir (LPV/r) at 45 (LPV) and 10 (ritonavir) mg/kg twice daily. The therapy was started on day 0 and terminated on day 7 due to injection site reactions. SIV viral load in plasma (stored at −70°C) was measured with ExaVir Load version 2 using version 1.61 of the kit computer software (Cavidi Tech, Uppsala, Sweden) according to the recommendations of the manufacturer. ExaVir is a sensitive reverse transcriptase (RT) activity test that detects HIV-1, HIV-2, and SIV. RT activity was measured in a spectrometer, and the values were converted into the equivalent RNA copies/ml using the software provided by the manufacturer. The dynamic range of the assay was 260 to 680,000 RNA copy equivalents/ml in our experiments. All samples were measured for viral load in the same experiment.
Mathematical modeling.
We used both a piecewise linear regression model and a nonlinear mathematical model to explore our data. For the piecewise linear regression, we assumed that ARV treatment has no effect during the first 24 h. Between days 1 and 4 the log virus load declines linearly with rate a (first-phase decay), between days 4 and 7 the log virus load declines with another rate, b (second-phase decay), and after day 7 the log virus load increases with rate c.
The nonlinear model had the form v(t) = va + vb for t ≤ 1, v(t) = vaa(t − 1) + vbb(t − 1) for 1 < t ≤ 7, and v(t) = v(7)c(t − 7) for t > 7, where v(t) is the virus load at time t (in days) and va and vb are the initial cell loads in the two different compartments with different decay rates.
The viral load and the time when samples were taken are used to estimate the rate parameters a, b, and c in the nonlinear model.
RESULTS
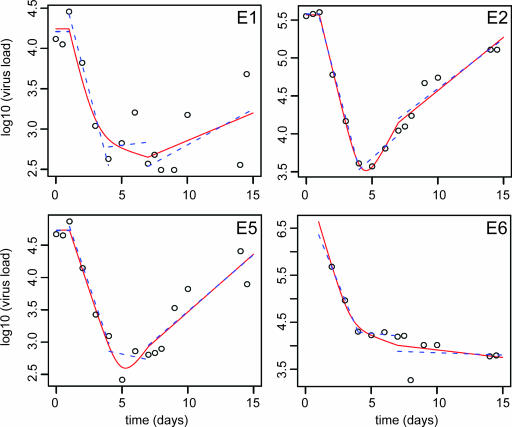
To obtain detailed information about the early viral dynamics in SIV-infected cynomolgus macaques, we studied four animals that received quadruple ART with AZT, 3TC, TDF, and LPV/r. The viral load was measured in blood samples drawn every day during the first 10 days and twice daily on the day of treatment start (day 0), on the day of treatment termination (day 7), and on the last day of follow-up (day 14). Pretreatment plasma SIV RNA levels in the four animals ranged from 13,000 to >680,000 SIV RNA copy equivalents/ml (Fig. 1). Thus, the pretreatment SIV RNA levels spanned at least 1.5 logs. The stability of the pretreatment virus levels shows that the plasma virus measurements had good reproducibility.
FIG. 1.
Dynamics of plasma SIV RNA levels in four (E1, E2, E5, and E6) SIV-infected monkeys who where given antiretroviral therapy on days 0 to 7. The results from the piecewise linear regression analysis are shown as a blue dashed line for each phase (days 1 to 4, days 4 to 7, and days 7 to 14). The results from the nonlinear model are shown as a red solid line. Note that the pretreatment plasma virus level in monkey E6 exceeded the upper quantification limit of the assay, i.e., 680,000 SIV RNA copies/ml.
Quadruple ART with AZT, 3TC, TDF, and LPV/r was started on day zero. In all animals we observed an initial lag phase of approximately 1 day before plasma SIV levels started to decline. This lag phase corresponds to the time it takes for the drugs to absorb, distribute, and penetrate into target cells (13).
Starting after approximately 24 h, all animals showed a rapid decline in plasma SIV levels which lasted approximately 3 days, i.e., up until day 4 of treatment. The decline ranged from 1.5 to 2.0 log virus levels. After this initial uniform and rapid decline of plasma viral levels, the animals displayed a much more variable treatment response characterized by stable or increasing plasma SIV levels.
The dynamics of SIV infection were investigated by calculating the rate of plasma SIV decline and rebound using piecewise linear regression as well as by a nonlinear model (Table 1). The estimate for the first-phase decay agreed very well between the two models and was remarkably similar between animals. By piecewise linear regression the slope of the regression line for the first-phase decay (i.e., during days 1 to 4) had a mean of −1.49 (range, −1.39 to −1.59), corresponding to a mean half-life for plasma virus of 0.47 days (range, 0.37 to 0.50). The nonlinear model gave a steeper slope, with a mean of −1.73 (range, −1.42 to −2.2), and consequently a shorter viral half-life, with a mean of 0.41 days (range, 0.31 to 0.49). The difference between the two estimates was mainly due to differences in how the inflection point between the first- and second-phase decline was obtained. In the piecewise linear regression the inflection point was fixed to day 4, whereas it was estimated to a mean of 3.93 days (range, 3.44 to 4.23) from the data in the nonlinear model.
TABLE 1.
Summary of slopes and half-lives in first-phase decay in all four animals (E1, E2, E5, and E6)a
| Decay phase and animal no. | Linear regression analysis
|
Nonlinear model
|
||
|---|---|---|---|---|
| Slope ± SE | Half-life | Slope ± SE | Half-life | |
| First-phase decay (days 1 to 4) | ||||
| E1 | −1.44 ± 0.12 | 0.48 | −1.63 ± 1.07 | 0.42 |
| E2 | −1.52 ± 0.10 | 0.46 | −1.67 ± 0.13 | 0.42 |
| E5 | −1.39 ± 0.16 | 0.50 | −1.43 ± 0.24 | 0.49 |
| E6 | −1.59 ± 0.04 | 0.44 | −2.20 ± 1.50 | 0.31 |
| Mean | −1.49 | 0.47 | −1.73 | 0.41 |
| Median | −1.48 | 0.47 | −1.65 | 0.42 |
| Second-phase decay (days 4 to 7) | ||||
| E1 | 0.045 ± 0.36 | −15.44 | −0.11 ± 0.40 | 6.17 |
| E2 | 0.35 ± 0.11 | −1.97 | 0.74 ± 0.16 | −0.93 |
| E5 | −0.010 ± 0.34 | 6.98 | 0.65 ± 0.54 | −1.06 |
| E6 | −0.062 ± 0.048 | 11.11 | −0.25 ± 0.28 | 2.75 |
| Rebound (days 7 to 14) | ||||
| E1 | 0.20 ± 0.15 | −3.39 | 0.16 ± 0.11 | −4.38 |
| E2 | 0.30 ± 0.56 | −2.29 | 0.32 ± 0.034 | −2.14 |
| E5 | 0.40 ± 0.11 | −1.72 | 0.41 ± 0.074 | −1.68 |
| E6 | −0.023 ± 0.12 | 30.33 | −0.073 ± 0.086 | 9.45 |
Both the linear regression analysis and the nonlinear model results are shown. Note that the day 1 sample from animal E6 was excluded from these calculations because its virus level exceeded the dynamic range of the virus load assay.
The estimates of second and third phases of change in virus load varied considerably between the animals (Fig. 1 and Table 1). In animal E2 the virus load appeared to have already started to increase before treatment was stopped on day 7. The difference between the animals makes it very difficult to provide a realistic estimate of the second and third phases of virus decay.
DISCUSSION
In this study we provide the first realistic estimate of the dynamics of experimental SIV infection. We report that the first-phase decay of SIV virions in plasma has a slope of −1.49, which corresponds to a half-life of 0.47 days. This is the fastest primate lentivirus decay hitherto reported. As in HIV-1 infection, the first-phase decay probably reflects the underlying decline of virus-producing CD4+ lymphocytes (13). It is likely that the short half-life is due to the high potency of the ART that was given, but we cannot exclude the possibility that it may also be due to in vivo differences in the dynamics of HIV-1 and SIV infection.
We used a sensitive RT assay to estimate SIV levels in plasma, whereas earlier studies on HIV-1, HIV-2, and SIV have quantified viral RNA in plasma by different commercial and in-house techniques. The RT assay was chosen because it is a standardized, commercial assay with good reproducibility and a dynamic range. Furthermore, the assay quantifies all primate lentiviruses, including SIV, with similar efficiency (G. Corrigan et al., unpublished data). We did not have access to an RNA-based assay with these specifications. The stability of the SIV levels in plasma during the first 24 h of treatment shows that the RT assay had a good reproducibility in our hands. Thus, we feel that the results from our study can be directly compared with the results from earlier studies that used RNA-based assays.
We used both linear and nonlinear methods to estimate the different phases of SIV decline in plasma. Theoretically, a nonlinear model should be more accurate, but this applies only if the model is correct and if there are enough data points to accurately estimate the different parameters of the model. In our experiments the linear and nonlinear estimates for the first-phase decay showed very good agreement. However, the standard error of estimate was greater for the nonlinear model. For this reason we have chosen to rely primarily on the estimate of the first-phase SIV decay in plasma provided by the linear regression model. The second and third phases of viral change varied considerably between animals and methods. Furthermore, the standard error of the estimates was high. Thus, our study does not provide a realistic or accurate estimate of these phases of SIV decline. In animal E2 we observed an increase in viral load before treatment termination (Fig. 1). We do not have a good explanation for this phenomenon, but it is unlikely that it is due to development of antiretroviral drug resistance. It is highly unlikely that resistance would develop to quadruple therapy during a single week of therapy.
Our estimate for the SIV-infected cells is considerably shorter than the earlier estimate of half-life of SIV in experimentally infected monkeys (11). This difference is not surprising, because monotherapy with TDF was used in the previous study while we used a much more potent quadruple therapy. Thus, the earlier study is likely to have underestimated the true rate of CD4 cell elimination during the first phase of viral decay. In line with this finding, Markowitz and Louie showed that quadruple therapy of HIV-1-infected patients provided a more accurate assessment of HIV-1 dynamics and CD4 cell decay in vivo, i.e., a faster viral decay than earlier studies that used less potent regimens (8, 9). Interestingly, we have found that the rate of CD4 cell decay in the SIV monkey model is even faster than the HIV-1 decay reported by Markowitz and Louie. This finding may have at least three explanations. First, it is possible that the therapy that we provided (AZT, 3TC, TDF, and LPV/r) is more potent than the combination given by Markowitz and Louie (AZT, 3TC, EFV, and LPV/r). However, this appears unlikely, because EFV is considered at least as potent as TDF. Second, our subcutaneous administration may have been more effective than the oral administration used by Markowitz and Louie. Finally, the rate of elimination of virus-producing CD4 cells may be faster in cynomolgus monkeys infected with SIVmac251 than in humans infected with HIV-1. It is interesting that the four monkeys showed strikingly similar first-phase viral decay even though the baseline plasma virus levels differed by >1.5 log RNA copies. This is in agreement with earlier studies on HIV-1, which have shown that the rate of HIV-1 decay is unrelated to baseline virus levels (1, 6). In summary, our estimate of the half-life of virus-producing cells in experimental SIV infection is the shortest that has been reported for any primate lentivirus.
Acknowledgments
We thank E. Hanson and R. Benthin for valuable technical assistance.
The project was supported by grants from the Swedish Medical Research Council and the Research Foundation of Swedish Physicians Against AIDS.
REFERENCES
- 1.Bonhoeffer, S., G. A. Funk, H. F. Gunthard, M. Fischer, and V. Muller. 2003. Glancing behind virus load variation in HIV-1 infection. Trends Microbiol. 11:499-504. [DOI] [PubMed] [Google Scholar]
- 2.Brandin, E., C. Brostrom, E. Gille, S. Bonhoeffer, and J. Albert. 2005. Short communication: HIV type 2 dynamics. AIDS Res. Hum. Retrovir. 21:608-610. [DOI] [PubMed] [Google Scholar]
- 3.Chun, T. W., L. Carruth, D. Finzi, X. Shen, J. A. DiGiuseppe, H. Taylor, M. Hermankova, K. Chadwick, J. Margolick, T. C. Quinn, Y. H. Kuo, R. Brookmeyer, M. A. Zeiger, P. Barditch-Crovo, and R. F. Siliciano. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183-188. [DOI] [PubMed] [Google Scholar]
- 4.Finzi, D., M. Hermankova, T. Pierson, L. M. Carruth, C. Buck, R. E. Chaisson, T. C. Quinn, K. Chadwick, J. Margolick, R. Brookmeyer, J. Gallant, M. Markowitz, D. D. Ho, D. D. Richman, and R. F. Siliciano. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295-1300. [DOI] [PubMed] [Google Scholar]
- 5.Gardner, M. B., and P. A. Luciw. 1989. Animal models of AIDS. FASEB J. 3:2593-2606. [DOI] [PubMed] [Google Scholar]
- 6.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123-126. [DOI] [PubMed] [Google Scholar]
- 7.Kanki, P. J., K. U. Travers, S. Mboup, C. C. Hsieh, R. G. Marlink, N. A. Gueye, T. Siby, I. Thior, M. Hernandez-Avila, J. L. Sankale, et al. 1994. Slower heterosexual spread of HIV-2 than HIV-1. Lancet 343:943-946. [DOI] [PubMed] [Google Scholar]
- 8.Louie, M., C. Hogan, M. Di Mascio, A. Hurley, V. Simon, J. Rooney, N. Ruiz, S. Brun, E. Sun, A. S. Perelson, D. D. Ho, and M. Markowitz. 2003. Determining the relative efficacy of highly active antiretroviral therapy. J. Infect. Dis. 187:896-900. [DOI] [PubMed] [Google Scholar]
- 9.Markowitz, M., M. Louie, A. Hurley, E. Sun, M. Di Mascio, A. S. Perelson, and D. D. Ho. 2003. A novel antiviral intervention results in more accurate assessment of human immunodeficiency virus type 1 replication dynamics and T-cell decay in vivo. J. Virol. 77:5037-5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marlink, R., P. Kanki, I. Thior, K. Travers, G. Eisen, T. Siby, I. Traore, C. C. Hsieh, M. C. Dia, E. H. Gueye, et al. 1994. Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science 265:1587-1590. [DOI] [PubMed] [Google Scholar]
- 11.Nowak, M. A., A. L. Lloyd, G. M. Vasquez, T. A. Wiltrout, L. M. Wahl, N. Bischofberger, J. Williams, A. Kinter, A. S. Fauci, V. M. Hirsch, and J. D. Lifson. 1997. Viral dynamics of primary viremia and antiretroviral therapy in simian immunodeficiency virus infection. J. Virol. 71:7518-7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perelson, A. S., P. Essunger, Y. Cao, M. Vesanen, A. Hurley, K. Saksela, M. Markowitz, and D. D. Ho. 1997. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387:188-191. [DOI] [PubMed] [Google Scholar]
- 13.Perelson, A. S., A. U. Neumann, M. Markowitz, J. M. Leonard, and D. D. Ho. 1996. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 271:1582-1586. [DOI] [PubMed] [Google Scholar]
- 14.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, B. H. Hahn, et al. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373:117-122. [DOI] [PubMed] [Google Scholar]
- 15.Wong, J. K., M. Hezareh, H. F. Gunthard, D. V. Havlir, C. C. Ignacio, C. A. Spina, and D. D. Richman. 1997. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278:1291-1295. [DOI] [PubMed] [Google Scholar]
- 16.Yeni, P. G., S. M. Hammer, C. C. Carpenter, D. A. Cooper, M. A. Fischl, J. M. Gatell, B. G. Gazzard, M. S. Hirsch, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, M. S. Saag, M. Schechter, R. T. Schooley, M. A. Thompson, S. Vella, and P. A. Volberding. 2002. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society—USA panel. JAMA 288:222-235. [DOI] [PubMed] [Google Scholar]