Abstract
Cholinesterase inhibitors improve cognition and behaviour in some patients with Alzheimer's disease (AD). Studies that have focused on methods to predict response to anticholinesterase therapy and markers for response are reviewed. Among the possible predictors of improvement in cognitive outcomes are apolipoprotein genotype, pretreatment postural blood pressure drop, quantitative electroencephalography (qEEG) and disease progression rate. Of these, qEEG profile after a single dose of an acetylcholinesterase inhibitor was consistently found to be a good predictor of cognitive response. Studies have assessed baseline behavioural profiles and baseline single-photon emission computed tomographic profiles as possible predictors of improvement of behavioural symptoms of AD, but these require further study. Possible markers of response during drug treatment include red blood cell cholinesterase inhibition, cerebrospinal fluid monoamine measurement, pupillary response and platelet amyloid precursor protein analyses. Although they, too, require further study, the analysis of platelet amyloid precursor protein may have value as a correlate of the putative disease-modifying effects of long-term treatment. Studying correlates of response may help to elucidate the mechanism of action of acetylcholinesterase inhibitors.
Medical subject headings: acetylcholine; acetylcholinesterase; Alzheimer disease; amyloid beta-protein precursor; apolipoproteins E; behavioral symptoms; cholinesterase inhibitors; dementia; electroencephalography; sex factors; tacrine; tomography, emission-computed, single-photon; treatment outcome.
Abstract
Les inhibiteurs de l'acétylcholinestérase améliorent la cognition et le comportement de certains patients atteints de la maladie d'Alzheimer (MA). Des études portant avant tout sur les méthodes de prévision de la réaction au traitement par anticholinestérase et sur les marqueurs de réaction sont examinées. Le génotype de l'apolipoprotéine, l'hypotension orthostatique avant le traitement, l'électroencéphalographie quantitative (EEGq) et le taux de progression de la maladie comptent parmi les prédicteurs possibles de l'amélioration des résultats cognitifs. Il a été établi clairement que le profil d'EEGq est un bon prédicteur de la réaction sur le plan cognitif suite à l'administration d'une dose d'un inhibiteur de l'acétylcholinestérase. Des études ont évalué les profils de comportement de base et les profils établis au départ par tomographie monophotonique d'émission comme prédicteurs possibles de l'amélioration des symptômes comportementaux de la MA. Ces profils devraient toutefois faire l'objet d'autres études. L'inhibition de la cholinestérase des érythrocytes, la mesure de la monoamine du liquide céphalorachidien, le réflexe pupillaire et les analyses de la protéine précurseur amyloïde plaquettaire sont au nombre des marqueurs possibles de la réaction au cours d'une pharmacothérapie. Même s'il faut aussi y consacrer d'autres études, l'analyse de la protéine précurseur amyloïde plaquettaire pourrait être un corrélat valable des effets présumés d'une pharmacothérapie à long terme comme traitement de fond. L'étude des corrélats de réaction pourrait aider à révéler le mécanisme d'action des inhibiteurs de l'acétylcholinestérase.
Introduction
Alzheimer's disease (AD) is a complex neurodegenerative disease characterized by impairment in cognitive function, behaviour and ability to perform activities of daily living. Both neurochemical and neurohistological alterations contribute to the clinical manifestations in patients with AD. The cholinergic system originates in the forebrain and projects diffusely to the cerebral cortex. In AD, there is a marked loss of neurons in the basal forebrain nuclei, which usually exceeds 75% of the total neuronal population at the time of an autopsy.1,2 The death of cholinergic neurons leads to reductions in choline acetyltransferase in the hippocampus and temporal cortex.1,3 There is also an overall loss in acetylcholinesterase (AChE) in the brains of patients with AD.3,4 The loss of central cholinergic activity has been correlated with severity of dementia on dementia rating scales.5 On the basis of these findings, it has been hypothesized that the deficit in cholinergic transmission plays a primary role in the pathogenesis of AD.6
Currently available AD-specific therapies include a symptomatic approach based on enhancement of cholinergic function. Initial attempts at improving cholinergic neurotransmission used the acetylcholine (ACh) precursors choline and lecithin, but neither improved performance on a wide variety of learning, memory and self-care tasks in AD.7,8,9,10
Cholinergic agonists act directly at the postsynaptic receptor, and several, such as arecoline11 and oxotremorine,12 have been tried in AD. Muscarinic receptors are G protein-coupled receptors in the parasympathetic nervous system that mediate their responses by activating a cascade of intracellular pathways. Muscarinic receptors play an important role in the regulation of movement, arousal, attention, learning and memory.13 However, peripheral side effects and lack of sensitivity for brain muscarinic receptors impeded the clinical use of cholinergic agonists.11,12
Cholinesterase inhibitors (ChEI) exert their beneficial effect by blocking AChE, the enzyme responsible for ACh breakdown, thus prolonging the action of ACh at the postsynaptic cholinergic receptor and enhancing cholinergic function. ChEIs can be either competitive or noncompetitive. Six ChEIs have been evaluated in randomized controlled trials. Cognitive improvement has been reported in studies with physostigmine.11,14 Improvement in verbal memory has also been reported with tacrine, a noncompetitive ChEI;15,16 however, the effect size was small, and side effects, including elevation of liver enzymes during treatment, were a major concern.17 In clinical trials, donepezil, a piperidine that has both competitive and noncompetitive properties,18 has also been shown to improve or reduce the rate of decline in both cognitive performance and global functioning with minimal side effects.19 Metrifonate, an irreversible ChEI, also had functional benefits in a 6-month study that evaluated its efficacy in patients with mild-to-moderate AD;20 however, metrifonate development has been halted because of a possible association with respiratory paralysis and problems in neuromuscular transmission. Rivastigmine, a carbamate that reversibly inhibits both AChE and butyrylcholinesterase,21 also showed favourable effects on daily activities in patients with mild-to-moderate AD.22,23 Galantamine is a phenanthrene alkaloid that inhibits AChE reversibly and competitively and is also a nicotinic modulator.24,25,26 It was shown to significantly improve cognition and global function and had significant benefits on the daily activities in patients with mild-to-moderate AD.27
Not all patients with AD improve cognitively or behaviourally with ChEI therapy, however. For example, a review of donepezil therapy indicated that the proportion of patients with AD showing a significant improvement in cognition was less than 40% (21%–38%), but 11%–18% of those given placebo also improved.28 In an open-label study of donepezil treatment in 86 patients with AD, 41% showed a behavioural response to donepezil, whereas 28% worsened and 31% showed no behavioural change.29 Given the low response rate to ChEI therapy, it is important to be able to understand the correlates of response. Studying them may help explain the mechanisms of response development in certain patients.
This review presents the available data from studies that have focused on possible methods to identify which patients will respond to anticholinesterase therapy before treatment (i.e., predictors), as well as measurements made during treatment that correlate with response (i.e., correlates of response). The various predictors and correlates studied will be discussed, and the results of different studies will be compared to assess the validity of each method.
Methods
A search of the English literature from January 1966 to March 2002 was conducted to identify predictors of response to ChEI therapy. Potential papers were found by searching PubMed using the following keywords: AChase inhibitors, prediction, predictors, response, Alzheimer's disease. Searches were also conducted for individual AChE inhibitors (keywords: donepezil, E2020, Aricept, tacrine, tetrahydroaminoacridine or Cognex, galantamine, velnacrine, metrifonate, rivastigmine). Recent review articles and potential papers were manually cross-referenced for additional articles. Articles were organized into the following categories: predictors of cognitive improvement, predictors of behavioural response and correlates of response.
Results
Predictors of cognitive improvement
The predictors of cognitive improvement that have been evaluated include the apolipoprotein E genotype (plus or minus sex), pretreatment postural blood pressure drop, quantitative electroencephalography, disease stage and progression rate and neuropsychological profile.
Apolipoprotein E genotype
Apolipoprotein E (ApoE) is a cholesterol-transporting enzyme encoded by a gene on chromosome 19. The 3 major isoforms of ApoE are ApoE2, ApoE3 and ApoE4. Inheritance of the ApoE ε4 allele is a risk factor for late-onset AD and has been associated with a decreased mean age at onset, accumulation of senile plaques in the brain and reduction of choline acetyltransferase (ChAT) in the hippocampus.30,31 Because ApoE4 genotype is associated with lower ChAT levels and a more severe cholinergic deficit, the presence of the allele may predict a poorer response to ChEI therapy. Sex is thought to interact with ApoE genotype in producing cognitive decline32 and, thus, may also be a predictor of response.
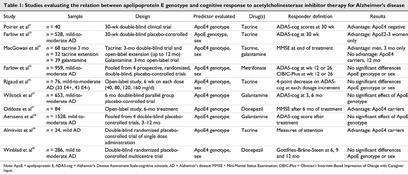
Ten studies27,31,33,34,35,36,37,38,39,40 have examined the relation between ApoE genotype and response to ChEI pharmacotherapy for cognitive outcomes (Table 1). Many of these studies concurrently evaluated the influence of sex on outcome. Piorier et al31 studied the effect of ApoE ε4 allele on responsiveness to tacrine in 40 patients with AD. The Alzheimer's Disease Assessment Scale cognitive subscale (ADAS-cog)41 score was used to assess outcome. The ADAS-cog measures memory, language and praxis on a 70-point scale, and a decrease in scores indicates improvement. Sixty percent of ApoE ε4 carriers achieved ADAS-cog scores worse than baseline, whereas more than 80% of ApoE ε4 noncarriers had improved ADAS-cog scores compared with baseline.31
Table 1
Farlow et al33,42 studied the effect of ApoE genotype and gender on clinical response to tacrine. In one study, data from 528 patients with mild-to-moderate AD who were assigned to placebo or daily tacrine dosages of 80, 120 or 160 mg/day for 30 weeks were analyzed. Intent-to-treat (ITT) analysis revealed no difference in treatment response on the basis of genotype, but sex was a factor in the association between treatment effect size and genotype such that the effect size was larger in ApoE2-3 women than in ApoE4 women, and no difference was detected in men. The authors suggest that ApoE genotype and sex might be used as predictors of response to tacrine treatment.33
A third study conducted by MacGowan and colleagues34 attempted to discover whether sex and ApoE genotype could predict response to anticholinesterase therapy. This study included 107 patients from double-blind or open-label trials of tacrine therapy for 3–12 months or an open-label trial of galantamine therapy for 3 months. After 3 months of treatment, men had a 73% greater chance of responding to therapy than women, indicating that sex could be a predictor of response to tacrine. This initial benefit did not last throughout the 12 months of treatment, however. ApoE genotype did not affect response to anticholinesterase therapy in the short term, which contradicted results of other studies. This may be attributed to the difference in therapy duration; ApoE genotype may affect response over a longer term (i.e., 12 months).34
Farlow et al35 retrospectively analyzed data pooled from 4 multicentre, randomized, double-blind, parallel-group, placebo-controlled metrifonate trials to investigate whether ApoE genotype affects treatment effect in patients with probable AD of mild-to-moderate severity. Compared with placebo, metrifonate significantly improved the cognitive performance of patients with AD regardless of ApoE genotype or sex. There was no significant interaction between ApoE ε4 allele copy number and treatment effect as assessed by the ADAS-cog score.35
Rigaud et al36 studied 76 patients with mild-to-moderate AD (33 ApoE ε4 allele carriers and 43 non-ε4 carriers) treated with incremental dosages of tacrine (40–160 mg/d, up to the highest dose tolerated, with 6 weeks allowed between each increment). ADAS-cog scores after treatment were compared with pretreatment scores. A 4-point decrease in ADAS-cog score was considered as responsive to tacrine treatment. There was no significant difference in treatment response between ε4 carriers and noncarriers, and response to tacrine therapy was independent of sex. The discrepancy between this study and the previous ones may be due to the longer duration of treatment,36 and goes against what was previously suggested by MacGowan et al34 that ApoE ε4 may have an effect in longer treatment trials.
Wilcock et al27 analyzed data from a randomized, double-blind, parallel-group, placebo-controlled trial to evaluate the safety and efficacy of galantamine in the treatment of 658 patients with mild-to-moderate AD. Galantamine improved the ADAS-cog scores of participants regardless of the number of copies of the ApoE ε4 allele, and the authors conclude that ApoE genotype is not a predictor of response to ChEIs.27
Oddoze et al37 also studied the correlation between ApoE genotype and response to donepezil treatment in 84 patients with AD who were treated for 6 months. Response to treatment was measured by the change in Mini-Mental Status Examination (MMSE)43 scores from baseline. The ε4 carriers had a mean response of +1 from baseline on the MMSE, and non-ε4 patients had a change of –1.1 from baseline. This indicated that ApoE status might be a predictor of improved donepezil response in patients with mild AD pathology.37
Aerssens et al38 collected data from 4 similarly designed, international, placebo-controlled, multicentre clinical trials to study the association of ApoE genotype with age at onset, rate of decline and responsiveness to therapy. The study included 1528 subjects diagnosed with probable AD who had undergone 3–12 months of treatment with galantamine or sabeluzole. The mean change from baseline in total ADAS-cog score was a primary measure of efficacy, and mean change from baseline in total Disability Assessment for Dementia (DAD)44 score was a secondary outcome measure. In the population, 35% had no ε4 allele (1 ε4 allele, 49%; 2 ε4 alleles, 16%). There was no association between response to treatment and ApoE genotype.38
Almkvist et al39 also performed a study to find the characteristics of patients with AD who responded favourably to a single oral dose of tacrine. The study included 24 very mildly or mildly demented AD patients, and the authors found the frequency of ApoE ε4 alleles to be higher in responders to tacrine therapy than in nonresponders.39
Winblad et al40 collected samples for ApoE genotyping in a large (n = 286) multinational 1-year trial of the efficacy and safety of donepezil. The outcome variable was the score on the Gottfries-Bråne-Steen (GBS) scale,45,46 a global assessment tool for dementia symptoms used in northern European countries. At baseline, 98 (69%) of 142 patients taking donepezil and 98 (68%) of 144 patients randomized to placebo were ApoE4 positive (homozygous or heterozygous, 3/4 or 4/4 alleles) and (64%) 184 of 286 were women. Two-way analysis of covariance (ANCOVA) determined that donepezil response, as measured by the GBS or MMSE at week 52, was not predicted by either ApoE genotype or sex. Furthermore, 3-way ANCOVA demonstrated that there was no interaction between the donepezil treatment response, ApoE genotype or sex.
Of the 10 studies that assessed whether ApoE genotype was a predictor of response to ChEI therapy, 9 evaluated ApoE ε4 versus non-ε4 genotype.27,31,33,34,35,36,37,38,39,40 The discrepant results may be attributed to different study designs (sample size, single v. multiple doses, outcome definitions and length of trial) and pharmacokinetic and pharmacodynamic characteristics of the different inhibitors. Four randomized, placebo-controlled trials with large samples agreed that ApoE was not a good predictor of response to AChEIs.27,35,38,40 Given the large number of patients now characterized for ApoE genotype, it is doubtful that further study will reveal an important difference. Although male gender was found to be a correlate of response in 1 small study,34 another study found increased response in Apo2-3 females,33 and other studies, including 2 large double-blind trials, did not find any association between treatment response and sex.35,36,40
Pretreatment postural blood pressure drop
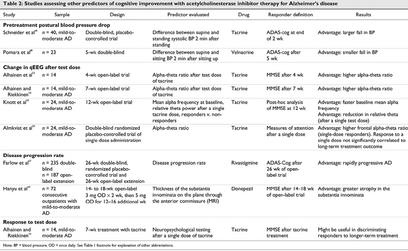
Both parasympathetic and sympathetic autonomic activities are important in the acute regulation of blood pressure. Animal data suggest that central cholinergic activity can modulate the baroreflex changes in heart rate after a change in blood pressure.47 Thus, blood pressure changes may reflect central cholinergic activity to some extent. Two groups of researchers have taken advantage of this information to study the relation between blood pressure and response to ChEIs (Table 2).
Table 2
Schneider et al48 studied whether pretreatment systolic orthostatic blood pressure could be used as a predictor of initial response to tacrine in patients with AD. The change in patients' blood pressure between supine and standing positions was determined before tacrine treatment, and then 40 patients were randomly assigned to 1 of 3 blinded 2-week trials of placebo, 40 mg of tacrine or 80 mg of tacrine per day. Those who improved 4 points on the ADAS were regarded as responders (n = 22) and were then randomized into a 6-week double-blind trial to receive either their best dose of tacrine or placebo. The 22 responders were significantly older than nonresponders and had a larger fall in pretreatment systolic orthostatic blood pressure 2 minutes after standing than nonresponders. It was concluded that both age and pretreatment orthostatic pressure drop contributed to the prediction of response to tacrine therapy.48
Pomara et al49 studied whether patients who responded to the ChEI velnacrine in the dose-finding phase of the clinical trial differed from nonresponders in terms of pretreatment postural blood pressure changes.49 Supine and sitting blood pressure were assessed before velnacrine treatment, and patients then entered a 5-week, double-blind dose-finding phase in which both drug and placebo were administered. Response was regarded as a 4-point or greater drug-to-placebo difference on the ADAS-cog. Contrary to the results of Schneider et al,48 nonresponders showed a larger decrease in pretreatment systolic blood pressure when going from a supine to a sitting position.
The divergent findings might be attributed to the different methodologies used in the 2 studies. Schneider and colleagues48 assessed change in blood pressure between supine and standing positions, whereas Pomara et al49 studied supine to sitting blood pressure. It has been speculated that different reflex mechanisms are responsible for maintaining blood pressure in the 2 situations.49 As well, different inhibitors were evaluated in the studies. Further studies are needed to clarify if there is any relation between blood pressure and treatment response in patients with AD.
Quantitative electroencephalography
Quantitative electroencephalography (qEEG) has played a role in the in vitro characterization of cerebral electrophysiologic features of neuropathologic ageing processes.50 qEEG recordings of AD patients show patterns of electric slowing evidenced by excessive theta (slow wave) activity in the early stages and reduced alpha and beta (fast wave) activity in the later stages.51 It is a potentially useful tool in demonstrating the effects of ChEI treatments. ChEIs shift activity patterns toward more normal profiles.52 Thus, it has been suggested that change in EEG after a test dose of tacrine might help to predict response to long-term treatment (Table 2).
Alhainen et al53 selected 14 probable AD patients and 7 age-matched healthy controls to study whether the change in qEEG after a single test dose of tacrine at the beginning of an open tacrine treatment trial could determine which patients would respond after 4 weeks of treatment. Baseline qEEGs were recorded for all participants, and the next day all were given peroral single doses of 50 mg tacrine, and a second qEEG recording was made. qEEG recordings were also carried out in the 14 AD patients after 4 weeks of tacrine treatment. A 3-point or more improvement in MMSE scores from baseline was considered a positive response to tacrine. Responders had increased alpha power, nonsignificantly decreased theta power and an increased alpha–theta ratio, the most sensitive indicator of response, compared with controls and nonresponders. The authors found that the relative change from baseline in the alpha–theta ratio 90 minutes after the test dose can discriminate responders and nonresponders to the 4-week tacrine treatment.53 The small number of patients studied and the lack of control group may limit the value of these results, however.
Alhainen and Riekkinen54 also studied the possibility of predicting response to tacrine treatment with pharmaco-qEEG. qEEG recordings were made in 14 AD patients before treatment, 90 minutes after a single dose of tacrine (50 mg) and after 7 weeks of tacrine treatment. There was a significant increase in mean absolute alpha power and alpha–theta ratio in responders compared with nonresponders and controls.54 Again, the small number of patients studied and the lack of control group may limit the value of the results.
Knott et al55 performed an open-label study to further examine the relation between change in qEEG and tacrine treatment. In this study of 24 probable AD patients, qEEG recordings were made before treatment (baseline), 2 hours after an oral dose of 30 mg tacrine and after 12 weeks of tacrine treatment. Patients, in general, exhibited EEG slowing compared with EEG norms. After 12 weeks of treatment, patients were divided into groups on the basis of change from baseline on MMSE scores, and analyses of single and long-term treatment effects on qEEG were performed. With a single dose of tacrine, relative alpha and delta power waves tended to increase in the higher scoring patients. There were also significant reductions in theta in highest scoring patients, but not in lower scoring patients. Discriminant function analysis was performed to test how much the acute response could predict the clinical response to tacrine. Test-dose response correctly classified 75%–79% of responders and nonresponders, indicating that test-dose EEG profiles efficiently predicted response to tacrine therapy.55
Almkvist et al39 attempted to identify the characteristics of individuals with a positive tacrine response after a double-blind randomized administration of a single oral dose of 40 mg tacrine or placebo. They also examined whether the response to a single tacrine dose correlated with response to long-term treatment. The 24 mildly to very mildly demented AD patients were given 40 mg tacrine or placebo in random order on 2 consecutive days, and the effects were evaluated within 2 hours using neuropsychological tests that assessed visuospatial ability, episodic memory and attention. qEEG activity was also recorded. Single-dose responders, defined as those with improved attentional performance, showed a higher right frontal alpha–theta ratio on qEEG at baseline than nonresponders. The response to a single dose of tacrine correlated, but not significantly, with long-term tacrine treatment. This study suggests the validity of baseline EEG profiles as predictors of response to ChEI therapy. Improvement in attentional skills after a single dose of tacrine may help to predict which patients are more likely to respond to long-term tacrine treatment.39
Thus, the results of all 4 studies suggest that qEEG profiles after a single test dose of an ChEI may be a good predictor of tacrine response.39,54,55,56 It should be noted, however, that 3 of the 4 studies were open label. Prospective trials with second-generation inhibitors are recommended.
Disease stage and progression rate
Disease stage and progression rate have also been implicated as possible predictors of response (Table 2). Although not directly linked to ChEI activity, disease stage and progression rate may differentiate between subgroups of patients with AD and may be indirectly related to drug response. Farlow et al57 studied the data from a multicentre, double-blind, randomized, placebo-controlled trial of rivastigmine (and open-label extension) to assess the relation between AD progression rate and treatment response. Patients who had received placebo for 26 weeks were divided into rapidly progressive (ADAS-cog score > 4 and a > 10% decline in Progressive Deterioration Scale [PDS] score) and slowly progressive groups. All patients were then given rivastigmine and monitored after 12, 18 and 26 weeks. The rapidly progressive patients showed a 4.97-point improvement in ADAS-cog score, whereas the slowly progressive group showed little improvement (i.e., 1.03-point). This effect persisted irrespective of initial disease severity, so patients with rapidly progressing symptoms were more likely to respond to rivastigmine treatment. Disease progression rate was a better predictor of response to rivastigmine than disease severity. Whether patients with more rapidly progressive AD are more responsive to treatment with other ChEIs remains to be determined.57
The substantia innominata, which contains the nucleus basalis of Meynert, can be readily visualized using superconductive magnetic resonance imaging (MRI).58 The pronounced atrophy of the substantia innominata in patients with AD (compared with age-matched controls) correlates significantly with cognitive impairment.59 Hanyu et al60 measured atrophy of the substantia innominata as an in vivo marker of cholinergic damage in the nucleus basalis of Meynert. Baseline MRI features of the substantia innominata were studied in 72 patients with mild-to-moderate probable AD, and then patients were treated with donepezil (5 mg/d х 3–4 mo) and divided into responders (n = 19, 26%) and nonresponders (n = 53, 74%) based on changes in MMSE scores (responder = increase of 4 or more points). Atrophy was more severe in the responder group, and MMSE change from baseline was significantly inversely correlated with thickness of the substantia innominata (r = –0.468, p < 0.001).
Thus, preliminary studies suggest that rapid progression of cognitive impairment and atrophy of the substantia innominata may help to predict degree of response to ChEI therapy. This remains to be confirmed in double-blind prospective trials, however.
Baseline neuropsychological profile
Although not directly reflective of central cholinergic activity, neuropsychological tests may help define subgroups of patients with AD. Alhainen and Riekkinen studied the possibility of predicting response to tacrine treatment with results on short neuropsychological tests.54 Tests were administered before and 2 hours after a single tacrine dose. A patient was considered a responder if there was a 3-or-more point increase in MMSE score after tacrine; 8 of 25 patients “responded” to a 50-mg dose of tacrine. After 7 weeks of tacrine treatment, 11 patients had responded, showing improvement in total MMSE score, orientation, attention and language abilities.54 The authors suggest that testing attention and working memory after tacrine administration might be useful in discriminating responders to long-term treatment.
Predictors of behavioural response
Predictors of improvement in behavioural (noncognitive) symptoms have also been studied. These include the baseline behavioural profile and single-photon emission computed tomographic (SPECT) profile (Table 3).
Table 3
Baseline behavioural profile
Mega et al29 performed an open-label retrospective study to assess whether baseline behavioural assessments might identify responders. These tests may also help define subgroups of AD patients who might respond and define target behaviours. Eighty-six patients underwent evaluation at baseline, week 4 (after 4 wk at 5 mg/d donepezil hydrochloride) and week 8 (after 4 wk at 10 mg/d of donepezil hydrochloride) of treatment. Patients were then divided into responder (≥ 4-point total Neuropsychiatric Inventory [NPI]61 score decrease), unchanged (± 3-point total NPI score change) or nonresponder (≥ 4-point total NPI score increase) groups. The NPI is a caregiver-based instrument that evaluates 10 neuropsychiatric behavioural symptoms that are common in dementia patients (e.g., delusions, hallucinations, agitation, depression, anxiety, etc.).61 The caregiver rates behaviour in terms of severity and frequency. Of the 86 patients, 41% responded to donepezil, 28% worsened and 31% showed no behavioural change from baseline. There was no significant improvement in cognition (measured by the MMSE) in any of the groups.29 When baseline behavioural profiles were compared, responders had a higher percentage of behavioural abnormalities across all behavioural domains evaluated except hallucinations. Nonresponders showed a lower percentage of all behaviours except hallucinations.
These results suggest that characterizing the pretreatment behavioural profile of patients with AD may improve the clinician's ability to target a subgroup most likely to benefit from medication.
Single-photon emission computed tomographic profile
High-resolution single-photon emission computed tomography (SPECT) may be used to obtain 3-dimensional representations of regional cerebral blood flow (rCBF) in the brain.62,63 SPECT scans produce both quantitative and qualitative measures of rCBF. The topography of rCBF deficits displays a marked heterogeneity among patients and correlates with cognitive profiles. SPECT can be used as a diagnostic adjunct in patients with mild cognitive or behavioural symptoms, suggesting a possible dementing disorder, or it can be used as an adjunct to diagnose probable AD in demented patients.62 Although not clearly linked to ChEI activity, SPECT profiles may be useful as a more generic marker of brain function and activity.
A study by Mega et al64 suggests that SPECT profiles might be used to predict improvement in behaviour after donepezil treatment. In a double-blind placebo-controlled trial, 33 patients with possible or probable AD received 5 mg donepezil for 4 weeks and 10 mg for another 4 weeks. Behavioural NPI profiles were assessed at baseline and after 8 weeks of treatment. Patients were divided into responders (≥ 4 point decrease in NPI score from baseline), nonresponders (≥ 4 point increase in NPI) and unchanged (± 3 points of baseline). The researchers then retrospectively studied the baseline SPECT profiles of each of the 3 groups; responders had a greater perfusion defect in the behaviourally relevant orbital frontal cortex and dorsolateral frontal cortex compared with the other groups. This indicated a dysfunction within the orbitofrontal cortex of patients who showed behavioural improvements with ChEI therapy. There were no significant changes in MMSE scores with treatment. Thus, baseline SPECT profiles of patients with AD might be used to predict a behavioural response to ChEI treatment, and may also be helpful in understanding the pathology of behavioural disturbances in AD and why some patients respond and others do not.64 Results of this study should be interpreted with caution because of the small number of patients and the lack of a control group. Future studies should evaluate post-treatment SPECT profiles to understand mechanism of response.
Correlates of response
As well as looking at factors that can be assessed a priori to predict response, researchers have also studied possible correlates that can be measured during treatment, including red blood cell (RBC) cholinesterase inhibition, cerebrospinal fluid (CSF) metabolites, pupillary response, amyloid precursor protein in platelets and central AChE activity as measured by positron-emission tomography (PET).
Red blood cell cholinesterase inhibition
AChE is present in large amounts in the human RBCs.65 Some researchers have attempted to compare peripheral AChE activity with AChE activity in the brain66,67 and tried to use RBC AChE inhibition as a surrogate marker for the therapeutic effectiveness of ChEIs.68 Thomsen et al68 showed that tacrine inhibited AChE activity in human postmortem brain tissue and RBCs with the same potency. Johansson et al69 reported 40%–60% RBC inhibition in 5 patients with AD who were on long-term tacrine treatment.69 RBC AChE inhibition has also been measured in several donepezil studies. Rogers et al19 reported that increased mean RBC AChE inhibition was associated with increased mean treatment effect. The relation between peripheral and central inhibition has not been confirmed in clinical trials, however. The extent to which RBC AChE inhibition reflects central pharmacodynamics and correlates with therapeutic outcome varies widely with the ChEI used.19,70 So far, no advantage over dose in predicting response has been demonstrated.70 Thus, a desired target level of RBC AChE inhibition associated with maximal short-term clinical response and minimal side effects has not yet been determined.
Cerebrospinal fluid metabolites
Alhainen et al54,56 studied the correlation between response to tacrine and possible changes in the CSF levels of 5-hydroxyindoleacetic acid (5-HIAA) and homovanillic acid (HVA) in 22 patients in an open-label trial. “Responding” was defined as a 3 or more point increase on the MMSE after 7 weeks. Those who did respond to tacrine treatment showed increased levels of these metabolites, indicating that clinical improvement may be partly mediated through the monoaminergic system. The increase in CSF 5-HIAA levels correlated significantly with improvement on cognitive tests and on the Instrumental Activities of Daily Living Scale.54,56
Pupillometry
Given that some evidence suggests that changes in the central cholinergic system may be accompanied by changes in peripheral cholinergic systems,71 pupillometry after the application of tropicamide (muscarinic antagonist) to the pupil was originally proposed as a method of diagnosing AD,71 but the results could not be reliably replicated.72 Naranjo et al73 used the pharmacological response to drugs that act on the cholinergic system of the iris to predict deficits in central cholinergic functioning. They performed a randomized double-blind controlled trial with pupillary tropicamide and pilocarpine (muscarinic agonist) to try to achieve a noninvasive peripheral probe to monitor or predict central cholinergic function. Pupil size of 10 healthy elderly and 9 young volunteers was measured at various time points after drug treatment, and there was an age-related pupillary response to pilocarpine that is not found with tropicamide. The authors suggested that this procedure might be used to monitor central cholinergic deficit and help predict response to ChEIs in patients with AD.73 This has yet to be tested.
Platelet amyloid precursor protein
The amyloid precursor protein (APP) undergoes protease cleavage to form extracellular deposits of filamentous β-amyloid or plaques. Diffuse plaques are associated with normal ageing, but AD neuropathology is characterized by the presence of senile plaques74 in a compact β-pleated conformation that leads to dystrophic neuritis.75,76,77 These later-stage plaques are thought to represent a more neurotoxic form.76 An interrelation between APP processing and AChE activity has been suggested on the basis of the role of AChE78,79 and butyrylcholinesterase75,76,77 in β-amyloid fibrillogenesis, the ability of AChE to modulate APP metabolism80 and the link between ACh receptors and enzymes involved in APP metabolism.81 Thus, evidence suggests a link between ACh and APP.
Borroni et al82 studied the correlation between APP forms in platelets of patients with AD. APP is present in 3 different forms (130 kd, 110 kd and 106 kd) in human platelets, and AD is associated with a decreased APP forms ratio (130 kd/(106 and 110) kd). The 20 patients treated with open-label donepezil for 30 days showed a significant improvement in MMSE scores, and this was accompanied by a significant increase in APP forms ratio compared with 10 untreated patients, but there was no significant correlation between change in MMSE and APP ratio changes.
Thus, the effect of ChEIs on APP processing can be demonstrated on platelets, and although this was not linked to cognitive changes in this study, a correlation may be found with a more sensitive outcome measure (e.g., ADAS-cog), larger samples or longer treatment periods.
PET neuroimaging
Brain AChE activity can be measured using a carbon-11-labelled ACh analogue such as N-[11C]methylpiperidin-4-yl acetate ([11C]MP4A) and PET.83 This can be used as an index of AChE activity. A preliminary study used [11C]MP4A to estimate brain AChE activity in 3 patients with AD before and after 1–1.5 months of donepezil (3–5 mg/d) treatment.84 Improvement in all 3 patients was accompanied by an average reduction in an index of AChE activity of 39%. Thus, PET can be used to measure inhibition of AChE in the brain, and this measure is sensitive enough to detect changes associated with ChEI therapy. Obviously, further study with larger samples is needed to evaluate this as a correlate or predictor of response.
Discussion
The substantial heterogeneity in response to cholinergic therapy in patients with AD may be attributed to heterogeneity in the severity and type of underlying pathology. Although studying predictors and correlates of response to ChEI treatment may help explain the mechanisms associated with response, no consistently strong factors have emerged thus far.
One possibility for the varying results is that the studies involve different ChEIs. Although all of them inhibit AChE, other pharmacologic aspects of the compounds differ. Current ChEIs are divided into those that are competitive and reversible (galantamine),24 noncompetitive and reversible (donepezil),18 pseudo-irreversible (rivastigmine)21 and noncompetitive (tacrine)15,16 with respect to AChE inhibition. Other pharmacologic differences that may confer brain selectivity include dual inhibition of AChE and butyrylcholinesterase (rivastigmine and tacrine),21,85 allosteric modification of nicotinic receptors (galantamine)24,25,26 and preferential inhibition of the G1 form of AChE (rivastigmine).21 There are no comparative trials published, and all 3 second-generation ChEIs have been shown to improve cognition and function.86 In theory, these differences may translate to clinical differences, but the similarity of response rates among different AChEIs suggests that the mechanism of effect may be similar.
The designs of the evaluated studies also varied. In particular, choice of measure of efficacy, different lengths of treatment and time points of evaluation, and open-label versus randomized, placebo-controlled, double-blind designs could have influenced the ability to detect a difference. Not only were different outcomes measures for cognitive improvement used (ADAS-cog, MMSE and GBS), but also some studies focused on predictors of cognitive response, whereas others considered predictors of behavioural response. The MMSE was designed as a screening instrument for assessing cognitive function. As such, it is considered to be less sensitive to change than the ADAS-cog,87 yet all 6 studies that used the MMSE34,37,53,54,55,60 found positive predictive results. It may be that relatively large changes in cognition are needed before a link can be seen. A second possible variable contributing to discrepant results is that outcomes were evaluated at different time points. Times of evaluation in the studies reviewed here ranged from hours (single dose)39 to 12 months,34,38,40 with links being made to predictors throughout the times studied. It has been proposed that short-term (6 months or less) studies of ChEI measure symptomatic benefit but do not capture the disease-modifying activity of these medications that is seen after 12 or more months of therapy.88 Thus, studies of differing durations are likely measuring different drug effects. Third, designs included administration of medication under both double-blind27,31,33,35,38,39,40,48,49 and open-label34,36,37,53,54,55,57,60 conditions. Although there is no clear trend that only open-label studies provide positive results, 3 of the 4 positive qEEG studies53,54,55 and the 2 studies linking disease progression rate57,60 with outcome were of open-label design. Replication of these findings under double-blind conditions is necessary before conclusions can be reached. In addition, all of the large randomized controlled studies discussed are post hoc analyses of trials designed to demonstrate efficacy. More prospective studies are required to assess the validity, specificity and selectivity of each of these possible predictors. These study design issues limit the ability to interpret the results.
Researchers have assessed predictors of response with the possible goal of selecting appropriate patients for subsequent therapy. The decision to use a predictive test before ChEI therapy may depend on the availability, nature and acceptability of the test to patients. SPECT and EEG assessments are widely available, but patients or caregivers may not agree to SPECT evaluation because of the the ionic radiation exposure. Pretreatment postural blood pressure drop tests are fast, feasible, easily administered and do not require any additional intervention. The use of baseline behavioural profiles to predict response to therapy may be useful if the patients tested can tolerate the sometimes lengthy cognitive and behavioural testing. The feasibility and appropriateness of the predictive test will vary with the characteristics of the patient population being tested and the availability of the predictive tool. The practicality of the test may also depend on the setting in which it is used.
Future studies should consider evidence-based performance standards such as sensitivity and specificity. In the design of clinical trials, some researchers may prefer predictive tests with either a higher sensitivity or selectivity and decide to maximize one versus the other. Tests with lower false positives (i.e., higher specificity) may be chosen so that sample size can be kept to a minimum, or clinicians may want to decrease false negatives (i.e., higher selectivity) so that potential responders are not missed, given the safety and tolerability of the marketed drugs.
Although researchers have conducted these studies to be able to predict response before a clinical trial, the results may not be applicable in a clinical setting. Even if a certain test indicated that a patient was unlikely to respond to treatment with an ChEI, this information would not likely change the decision to treat. Given the benign side-effect profile of second-generation ChEIs and lack of other treatment options, it is likely that treatment would be initiated unless response could be definitively ruled out. The value of predictors may be more important in longer-term trials.
Summary
Disease progression rate, qEEG profiles after a test dose of drug, baseline SPECT profiles and baseline MRI measures of the substantia innominata all show association with response to ChEI therapy. Future studies should consider using a combination of these factors to study predictors and correlates of response. Most of the current studies have concentrated on predicting response after relatively short-term treatment. A valuable contribution would be to assess which biological or clinical characteristics might correlate with patterns of response studies that are expected to require 1 or more years of drug administration. Of the factors reviewed, analysis of platelet amyloid precursor protein may most closely reflect the disease-modifying effect of ChEIs. Determining predictors and correlates of response may help further our understanding of the mechanism of action of these medications, and this, in turn, may help guide the development of more effective drug therapies for patient with AD.
Acknowledgments
Dr. Lanctôt was partially supported by the Kunin-Lunenfeld Applied Research Unit of Baycrest Centre for Geriatric Care. Ms. LouLou was partially supported by a University of Toronto School of Graduate Studies open fellowship.
Footnotes
Competing interests: None declared for Ms. LouLou. Dr. Lanctôt has received consultantancy fees from AstraZeneca, Boehringer Ingelheim and Janssen-Ortho; has received research support from Janssen-Ortho, Novartis, Neotherapeutics and Bristol-Myers Squibb; and has received travel assistance from Pfizer. Dr. Herrmann has received speaker's fees from Janssen-Ortho, Pfizer, Eli Lilly and AstraZeneca; and has received research support from Janssen, Novartis, Lundbeck, Neotherapeutics, Eli Lilly and Bristol-Myers Squibb.
Correspondence to: Dr. Krista L. Lanctôt, 2075 Bayview Ave., Rm. FG05, Sunnybrook and Women's College Health Sciences Centre, Toronto ON M4N 3M5; fax 416 480-6022; Krista.Lanctot@swchsc.on.ca
Submitted Oct. 26, 2001 Revised May 15, 2002 Accepted May 24, 2002
References
- 1.Perry EK, Perry RH, Blessed G, Tomlinson BE. A choline connection between normal aging and senile dementia in human hippocampus. Neurosci Lett 1977;6:85-9. [DOI] [PubMed]
- 2.Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol 1981;10(2):122-6. [DOI] [PubMed]
- 3.Perry EK, Perry RH, Blessed G, Tomlinson BE. Changes in brain cholinesterases in senile dementia of Alzheimer type. Neuropathol Appl Neurobiol 1978;4(4):273-7. [DOI] [PubMed]
- 4.Fishman EB, Siek GC, MacCallum RD, Bird ED, Volicer L, Marquis JK. Distribution of the molecular forms of acetylcholinesterase in human brain: alterations in dementia of the Alzheimer type. Ann Neurol 1986;19(3):246-52. [DOI] [PubMed]
- 5.Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, Perry RH. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. BMJ 1978; 2 (6150):1457-9. [DOI] [PMC free article] [PubMed]
- 6.Bartus RT, Dean RL 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982;217 (4558):408-14. [DOI] [PubMed]
- 7.Smith CM, Swash M, Exton-Smith AN, Phillips MJ, Overstall PW, Piper ME, et al. Choline therapy in Alzheimer's disease [letter]. Lancet 1978;2(8084):318. [DOI] [PubMed]
- 8.Christie JE, Blackburn IM, Glenn AIM, Zeiglel S, Shering A, Yates CM. Effects of choline and lecithin on CSF choline levels and on cognitive function in patients with presenile dementia of the Alzheimer type. In: Barbeau A, Growdon JH, Wartman RJ, editors. Nutrition and the brain. Vol 5. New York: Raven Press; 1979. p. 377-87.
- 9.Little A, Levy R, Chuaqui-Kidd P, Hand D. A double-blind, placebo-controlled trial of high-dose lecithin in Alzheimer's disease. J Neurol Neurosurg Psychiatry 1985;48(8):736-42. [DOI] [PMC free article] [PubMed]
- 10.Brinkman SD, Smith RC, Meyer JS, Vroulis G, Shaw T, Gordon JR, et al. Lecithin and memory training in suspected Alzheimer's disease. J Gerontol 1982;37(1):4-9. [DOI] [PubMed]
- 11.Christie JE, Shering A, Ferguson J, Glen AI. Physostigmine and arecoline: effects of intravenous infusions in Alzheimer presenile dementia. Br J Psychiatry 1981;138:46-50. [DOI] [PubMed]
- 12.Davis KL, Hollander E, Davidson M, Davis BM, Mohs RC, Horvath TB. Induction of depression with oxotremorine in patients with Alzheimer's disease. Am J Psychiatry 1987;144(4): 468-71. [DOI] [PubMed]
- 13.Felder CC, Porter AC, Skillman TL, Zhang L, Bymaster FP, Nathanson NM, et al. Elucidating the role of muscarinic receptors in psychosis. Life Sci 2001;68(22-23):2605-13. [DOI] [PubMed]
- 14.Mohs RC, Davis BM, Johns CA, Mathe AA, Greenwald BS, Horvath TB, et al. Oral physostigmine treatment of patients with Alzheimer's disease. Am J Psychiatry 1985;142(1):28-33. [DOI] [PubMed]
- 15.Kaye WH, Sitaram N, Weingartner H, Ebert MH, Smallberg S, Gillin JC. Modest facilitation on memory in dementia with combined lecithin and anticholinerestase treatment. Biol Psychiatry 1982; 17(2):275-80 [PubMed]
- 16.Summers WK, Majovski LV, Marsh GM, Tachiki K, Kling A. Oral tetrahydroaminoacridine in long-term treatment of senile dementia, Alzheimer type. N Engl J Med 1986;315(20):1241-5. [DOI] [PubMed]
- 17.Davis KL, Thal LJ, Gamzu ER, Davis CS, Woolson RF, Gracon SI, et al. A double-blind, placebo-controlled multicenter study of tacrine for Alzheimer's disease. The Tacrine Collaborative Study Group [see comments]. N Engl J Med 1992;327(18):1253-9. [DOI] [PubMed]
- 18.Rogers S, Yaminishi Y, Yamatsu K. E2020-the pharmacology of a piperidine cholinesterase inhibitor. In: Becker R, Giacobini E, editors. Cholinergic basis for Alzheimer therapy. Boston: Birkhauser; 1991. p. 314-20.
- 19.Rogers SL, Friedhoff LT. The efficacy and safety of donepezil in patients with Alzheimer's disease: results of a US Multicentre, Randomized, Double-Blind, Placebo-Controlled Trial. The Donepezil Study Group. Dementia 1996;7(6):293-303. [DOI] [PubMed]
- 20.Dubois B, McKeith I, Orgogozo JM, Collins O, Meulien D. A multicentre, randomized, double-blind, placebo-controlled study to evaluate the efficacy, tolerability and safety of two doses of metrifonate in patients with mild-to-moderate Alzheimer's disease: the MALT study. Int J Geriatr Psychiatry 1999; 14(11):973-82. [PubMed]
- 21.Enz A, Boddeke H, Gray J, Spiegel R. Pharmacologic and clinicopharmacologic properties of SDZ ENA 713, a centrally selective acetylcholinesterase inhibitor. Ann N Y Acad Sci U S A 1991;640:272-5. [DOI] [PubMed]
- 22.Corey-Bloom J, Anand R, Veach J. A randomized trial evaluating efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients wih mild to moderately severe Alzheimer's disease. Int J Geriatr Psychopharmacol 1998;1:55-65.
- 23.Rosler M, Anand R, Cicin-Sain A, Gauthier S, Agid Y, Dal-Bianco P, et al. Efficacy and safety of rivastigmine in patients with Alzheimer's disease: international randomised controlled trial. BMJ 1999;318(7184):633-8. [DOI] [PMC free article] [PubMed]
- 24.Thomsen T, Kewitz H. Selective inhibition of human acetylcholinesterase by galanthamine in vitro and in vivo. Life Sci 1990;46(21):1553-8. [DOI] [PubMed]
- 25.Bores GM, Huger FP, Petko W, Mutlib AE, Camacho F, Rush DK, et al. Pharmacological evaluation of novel Alzheimer's disease therapeutics: acetylcholinesterase inhibitors related to galanthamine. J Pharmacol Exp Ther 1996;277(2):728-38. [PubMed]
- 26.Vasilenko ET, Tonkopii VD. [Characteristics of galanthamine as a reversible inhibitor of cholinesterase]. Biokhimiia 1974;39 (4): 701-3. [PubMed]
- 27.Wilcock GK, Lilienfeld S, Gaens E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer's disease: multicentre randomised controlled trial. Galantamine International-1 Study Group. BMJ 2000;321(7274):1445-9. [DOI] [PMC free article] [PubMed]
- 28.Foster RH, Plosker GL. Donepezil. Pharmacoeconomic implications of therapy. Pharmacoeconomics 1999;16(1):99-114. [DOI] [PubMed]
- 29.Mega MS, Masterman DM, O'Connor SM, Barclay TR, Cummings JL. The spectrum of behavioral responses to cholinesterase inhibitor therapy in Alzheimer disease. Arch Neurol 1999;56(11):1388-93. [DOI] [PubMed]
- 30.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families [see comments]. Science 1993;261(5123):921-3. [DOI] [PubMed]
- 31.Poirier J, Delisle MC, Quirion R, Aubert I, Farlow M, Lahiri D, et al. Apolipoprotein E4 allele as a predictor of cholinergic deficits and treatment outcome in Alzheimer disease. Proc Natl Acad Sci U S A 1995;92(26):12260-4. [DOI] [PMC free article] [PubMed]
- 32.Mortensen EL, Hogh P. A gender difference in the association between APOE genotype and age-related cognitive decline. Neurology 2001;57(1):89-95. [DOI] [PubMed]
- 33.Farlow MR, Lahiri DK, Poirier J, Davignon J, Schneider L, Hui SL. Treatment outcome of tacrine therapy depends on apolipoprotein genotype and gender of the subjects with Alzheimer's disease. Neurology 1998;50(3):669-77. [DOI] [PubMed]
- 34.MacGowan SH, Wilcock GK, Scott M. Effect of gender and apolipoprotein E genotype on response to anticholinesterase therapy in Alzheimer's disease. Int J Geriatr Psychiatry 1998;13 (9): 625-30. [DOI] [PubMed]
- 35.Farlow MR, Cyrus PA, Nadel A, Lahiri DK, Brashear A, Gulanski B. Metrifonate treatment of AD: influence of APOE genotype. Neurology 1999;53(9):2010-6. [DOI] [PubMed]
- 36.Rigaud AS, Traykov L, Caputo L, Guelfi MC, Latour F, Couderc R, et al. The apolipoprotein E epsilon4 allele and the response to tacrine therapy in Alzheimer's disease. Eur J Neurol 2000;7 (3):255-8. [DOI] [PubMed]
- 37.Oddoze C, Michel BF, Lucotte G. Apolipoprotein E ε4 allele predicts a better response to donepezil therapy in Alzheimer's disease. Alzheimer's Reports 2000;3(4):213-6.
- 38.Aerssens J, Raeymaekers P, Lilienfeld S, Geerts H, Konings F, Parys W. ApoE genotype: no influence on galantamine treatment efficacy nor on rate of decline in Alzheimer's disease. Dement Geriatr Cogn Disord 2001;12(2):69-77. [DOI] [PubMed]
- 39.Almkvist O, Jelic V, Amberla K, Hellstrom-Lindahl E, Meurling L, Nordberg A. Responder characteristics to a single oral dose of cholinesterase inhibitor: a double-blind placebo-controlled study with tacrine in Alzheimer patients. Dement Geriatr Cogn Disord 2001;12(1):22-32. [DOI] [PubMed]
- 40.Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, Wimo A, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 2001; 57 (3):489-95. [DOI] [PubMed]
- 41.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry 1984;141(11):1356-64. [DOI] [PubMed]
- 42.Farlow MR, Lahiri DK, Poirier J, Davignon J, Hui S. Apolipoprotein E genotype and gender influence response to tacrine therapy. Ann N Y Acad Sc U S Ai 1996;802:101-10. [DOI] [PubMed]
- 43.Folstein MF, Folstein SE. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiat Res 1975;12:189-98. [DOI] [PubMed]
- 44.Gelinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer's disease: the disability assessment for dementia. Am J Occup Ther 1999; 53 (5):471-81. [DOI] [PubMed]
- 45.Gottfries CG, Brane G, Gullberg B, Steen G. A new rating scale for dementia syndromes. Arch Gerontol Geriatr 1982;1(4):311-30. [DOI] [PubMed]
- 46.Brane G, Gottfries CG, Winblad B. The Gottfries-Brane-Steen scale: validity, reliability and application in anti-dementia drug trials. Dement Geriatr Cogn Disord 2001;12(1):1-14. [DOI] [PubMed]
- 47.Brezenoff HE. Cardiovascular regulation by brain acetylcholine. Fed Proc 1984;43(1):17-20. [PubMed]
- 48.Schneider LS, Lyness SA, Pawluczyk S, Gleason RP, Sloane RB. Do blood pressure and age predict response to tacrine (THA) in Alzheimer's disease? A preliminary report. Psychopharmacol Bull 1991;27(3):309-14. [PubMed]
- 49.Pomara N, Deptula D, Singh R. Pretreatment postural blood pressure drop as a possible predictor of response to the cholinesterase inhibitor velnacrine (HP 029) in Alzheimer's disease. Psychopharmacol Bull 1991;27(3):301-7. [PubMed]
- 50.Saletu B. Neurophysiological aspects of aging and gerontopsychopharmacology. Mod Probl Pharmacopsychiatry 1989;23:43-55. [DOI] [PubMed]
- 51.Rosen I. Electroencephalography as a diagnostic tool in dementia. Dement Geriatr Cogn Disord 1997;8(2):110-6. [DOI] [PubMed]
- 52.Agnoli A, Martucci N, Manna V, Conti L, Fioravanti M. Effect of cholinergic and anticholinergic drugs on short-term memory in Alzheimer's dementia: a neuropsychological and computerized electroencephalographic study. Clin Neuropharmacol 1983;6 z(4):311-23. [DOI] [PubMed]
- 53.Alhainen K, Partanen J, Reinikainen K, Laulumaa V, Soininen H, Airaksinen M, et al. Discrimination of tetrahydroaminoacridine responders by a single dose pharmaco-EEG in patients with Alzheimer's disease. Neurosci Lett 1991;127(1):113-6. [DOI] [PubMed]
- 54.Alhainen K, Riekkinen PJ Sr. Discrimination of Alzheimer patients responding to cholinesterase inhibitor therapy. Acta Neurol Scand Suppl 1993;149:16-21. [DOI] [PubMed]
- 55.Knott V, Mohr E, Mahoney C, Ilivitsky V. Pharmaco-EEG test dose response predicts cholinesterase inhibitor treatment outcome in Alzheimer's disease. Methods Find Exp Clin Pharmacol 2000;22(2):115-22. [DOI] [PubMed]
- 56.Alhainen K, Helkala EL, Reinikainen K, Riekkinen P Sr. The relationship of cerebrospinal fluid monoamine metabolites with clinical response to tetrahydroaminoacridine in patients with Alzheimer's disease. J Neural Transm Park Dis Dement Sect 1993;5(3):185-92. [DOI] [PubMed]
- 57.Farlow MR, Hake A, Messina J, Hartman R, Veach J, Anand R. Response of patients with Alzheimer disease to rivastigmine treatment is predicted by the rate of disease progression. Arch Neurol 2001;58(3):417-22. [DOI] [PubMed]
- 58.Sasaki M, Ehara S, Tamakawa Y, Takahashi S, Tohgi H, Sakai A, et al. MR anatomy of the substantia innominata and findings in Alzheimer disease: a preliminary report. AJNR Am J Neuroradiol 1995;16(10):2001-7. [PMC free article] [PubMed]
- 59.Hanyu H, Asano T, Sakurai H, Tanaka Y, Takasaki M, Abe K. MR analysis of the substantia innominata in normal aging, Alzheimer disease, and other types of dementia. AJNR Am J Neuroradiol 2002;23(1):27-32. [PMC free article] [PubMed]
- 60.Hanyu H, Tanaka Y, Sakurai H, Takasaki M, Abe K. Atrophy of the substantia innominata on magnetic resonance imaging and response to donepezil treatment in Alzheimer's disease. Neurosci Lett 2002;319(1):33-6. [DOI] [PubMed]
- 61.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44(12):2308-14. [DOI] [PubMed]
- 62.Waldemar G, Hogh P, Paulson OB. Functional brain imaging with single-photon emission computed tomography in the diagnosis of Alzheimer's disease. Int Psychogeriatr 1997;9(Suppl 1): 223-7; discussion 247-52. [DOI] [PubMed]
- 63.Saha GB, MacIntyre WJ, Go RT. Radiopharmaceuticals for brain imaging. Semin Nucl Med 1994;24(4):324-49. [DOI] [PubMed]
- 64.Mega MS, Dinov ID, Lee L, O'Connor SM, Masterman DM, Wilen B, et al. Orbital and dorsolateral frontal perfusion defect associated with behavioral response to cholinesterase inhibitor therapy in Alzheimer's disease. J Neuropsychiatry Clin Neurosci 2000; 12(2):209-18. [DOI] [PubMed]
- 65.Galehr O, Plattner F. Uber das Schicksal des Acetylcholins im Blute. Pflugers Arch 1928;218:488-505.
- 66.Becker RE, Giacobini E. Mechanisms of cholinesterase inhibition in senile dementia of the Alzheimer type: clinical, pharmacological, and therapeutic aspects. Drug Development Res 1988; 12:163-95.
- 67.Becker RE, Giacobini E. Pharmacokinetics and pharmacodynamic of acetylcholinesterase inhibition: can acetylcholine levels in the brain be improved in Alzheimer's disease? Drug Development Res 1988;14:235-46.
- 68.Thomsen T, Kaden B, Fischer JP, Bickel U, Barz H, Gusztony G, et al. Inhibition of acetylcholinesterase activity in human brain tissue and erythrocytes by galanthamine, physostigmine and tacrine. Eur J Clin Chem Clin Biochem 1991;29(8):487-92. [DOI] [PubMed]
- 69.Johansson M, Hellstrom-Lindahl E, Nordberg A. Steady-state pharmacokinetics of tacrine in long-term treatment of Alzheimer patients. Dementia 1996;7(2):111-7. [DOI] [PubMed]
- 70.Sramek JJ, Cutler NR. RBC cholinesterase inhibition: a useful surrogate marker for cholinesterase inhibitor activity in Alzheimer disease therapy? Alzheimer Dis Assoc Disord 2000;14 (4): 216-27. [DOI] [PubMed]
- 71.Scinto LF, Daffner KR, Dressler D, Ransil BI, Rentz D, Weintraub S, et al. A potential noninvasive neurobiological test for Alzheimer's disease. Science 1994;266(5187):1051-4. [DOI] [PubMed]
- 72.Kardon RH. Drop the Alzheimer's drop test. Neurology 1998;50 (3): 588-91. [DOI] [PubMed]
- 73.Naranjo CA, Fourie J, Herrmann N, Lanctot KL, Birt C, Yau KK. Probing peripheral and central cholinergic system responses. J Psychiatry Neurosci 2000;25(4):325-36. [PMC free article] [PubMed]
- 74.Katzman R. Alzheimer's disease. N Engl J Med 1986;314(15): 964-73. [DOI] [PubMed]
- 75.Wright CI, Geula C, Mesulam MM. Neurological cholinesterases in the normal brain and in Alzheimer's disease: relationship to plaques, tangles, and patterns of selective vulnerability. Ann Neurol 1993;34(3):373-84. [DOI] [PubMed]
- 76.Guillozet AL, Smiley JF, Mash DC, Mesulam MM. Butyrylcholinesterase in the life cycle of amyloid plaques. Ann Neurol 1997; 42(6):909-18. [DOI] [PubMed]
- 77.Mesulam MM, Geula C. Butyrylcholinesterase reactivity differentiates the amyloid plaques of aging from those of dementia. Ann Neurol 1994;36(5):722-7. [DOI] [PubMed]
- 78.Inestrosa NC, Alvarez A, Perez CA, Moreno RD, Vicente M, Linker C, et al. Acetylcholinesterase accelerates assembly of amyloid-beta-peptides into Alzheimer's fibrils: possible role of the peripheral site of the enzyme. Neuron 1996;16(4):881-91. [DOI] [PubMed]
- 79.Inestrosa NC, Alvarez A, Calderon F. Acetylcholinesterase is a senile plaque component that promotes assembly of amyloid beta-peptide into Alzheimer's filaments. Mol Psychiatry 1996;1 (5):359-61. [PubMed]
- 80.Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science 1992;258 (5080): 304-7. [DOI] [PubMed]
- 81.Small DH, Moir RD, Fuller SJ, Michaelson S, Bush AI, Li QX, et al. A protease activity associated with acetylcholinesterase releases the membrane-bound form of the amyloid protein precursor of Alzheimer's disease. Biochemistry 1991;30(44):10795-9. [DOI] [PubMed]
- 82.Borroni B, Colciaghi F, Pastorino L, Pettenati C, Cottini E, Rozzini L, et al. Amyloid precursor protein in platelets of patients with Alzheimer disease: effect of acetylcholinesterase inhibitor treatment. Arch Neurol 2001;58(3):442-6. [DOI] [PubMed]
- 83.Irie T, Fukushi K, Namba H, Iyo M, Tamagami H, Nagatsuka S, et al. Brain acetylcholinesterase activity: validation of a PET tracer in a rat model of Alzheimer's disease. J Nucl Med 1996; 37 (4):649-55. [PubMed]
- 84.Iyo M, Namba H, Fukushi K, Shinotoh H, Nagatsuka S, Sudo Y, et al. Measurement of acetylcholinesterase by positron emission tomography in the brains of healthy controls and patients with Alzheimer's disease. Lancet 1997;349(9068):1805-9. [DOI] [PubMed]
- 85.Ballard CG. Advances in the treatment of Alzheimer's disease: benefits of dual cholinesterase inhibition. Eur Neurol 2002;47 (1):64-70. [DOI] [PubMed]
- 86.Gauthier S. Advances in the pharmacotherapy of Alzheimer's disease. CMAJ 2002;166(5):616-23. [PMC free article] [PubMed]
- 87.Burch EA Jr, Andrews SR. Comparison of two cognitive rating scales in medically ill patients. Int J Psychiatry Med 1987;17(2): 193-200. [DOI] [PubMed]
- 88.Giacobini E. Do cholinesterase inhibitors have disease-modifying effects in Alzheimer's disease? CNS Drugs 2001;15(2):85-91. [DOI] [PubMed]