Abstract
Epstein-Barr virus (EBV) is a persistent, orally transmitted herpesvirus that replicates in B cells and epithelial cells and is associated with lymphoid and epithelial malignancies. The virus binds to CD21 on B cells via glycoprotein gp350/220 and infects efficiently. Infection of cultured epithelial cells has not typically been efficient but can occur in the absence of gp350/220 and CD21 and in vivo is thought to be important to the development of nasopharyngeal carcinoma. We report here that antibodies to gp350/220, which inhibit EBV infection of B cells, enhance infection of epithelial cells. The effect is not mediated by Fc receptor binding but is further enhanced by antibody cross-linking, which may patch gp350/220 in the virus envelope. Saliva from EBV-seropositive individuals has similar effects that can be reversed by depletion of antibody. The results are consistent with a model in which gp350/220 interferes with the access of other important players to the epithelial cell surface. The results may have implications for the development of nasopharyngeal carcinoma in high-risk populations in which elevated titers of antibody to EBV lytic cycle proteins are prognostic.
Epstein-Barr virus (EBV) is an orally transmitted human herpesvirus that infects more than 90% of the human population. Most infections are asymptomatic, but the virus can cause infectious mononucleosis and is associated with both lymphoid and epithelial malignancies (31). Of these, undifferentiated nasopharyngeal carcinomas (NPC), essentially all of which are latently infected with EBV, are probably the most common. NPC represents only 0.25% of the cancers in Western populations, but its incidence is elevated in Eskimo populations and in people of Mediterranean Africa, and it can represent as many as 20% of the cases of cancer in southern China and Southeast Asia (29).
In support of the relevance of EBV to the development of NPC is the finding that in virus-positive tumors the virus genome is typically “clonal.” This determination is based on the fact that the EBV genome is flanked by variable numbers of terminal repeats and each newly infected cell can be colonized by an episome in which a different, but distinct, number of repeats is retained (30). Thus, a population of infected cells will house a population of episomes with differing numbers of repeats, unless all of the cells within the population are derived from the same original infected cell.
There are two possible explanations for clonality. One is that infection and initiation of the tumor are concurrent and possibly causally related events. The other is that virus is not present at tumor initiation but that subsequent infection provides a growth advantage that promotes the tumor and leads to outgrowth of a single infected cell. The latter scenario is supported by two observations. First, although preinvasive lesions already carry clonal EBV (27), milder dysplasia may contain EBV in only a portion of the cells (3). Second, although simultaneous infection of epithelial cells in culture initially yields a polyclonal population carrying episomes with variable repeat sizes, a cell with a single repeat size rapidly emerges (22). Both scenarios envisage a role for EBV infection in driving tumor development. They are consistent with observations that high-titer antibodies to EBV lytic cycle proteins, which presumably represent an increase in virus replication, are not only characteristic of NPC (9-12) but are also prognostic in populations at risk (38, 39).
Despite the potential importance of epithelial cell infection, there remains uncertainty about how it actually occurs. The initial determinants of the tropism of EBV for lymphocytes are fairly well understood. The virus attaches to complement receptor type 2 (CR2) or CD21 via a large, heavily glycosylated envelope glycoprotein, gp350/220, so called because it is made in two alternatively spliced forms that both bind CD21 (5, 24, 25, 32). Fusion of the virus envelope and cell membrane is triggered by an interaction between HLA class II and virus glycoprotein gp42 which forms a complex with glycoproteins gHgL (18, 19). Fusion is completed by gHgL and glycoprotein gB (8). Fusion of virus with an epithelial cell is also mediated by gHgL and gB. The trigger is an as-yet-unidentified receptor, gHgLR, which interacts directly with gHgL (2, 21, 35). However, how the virus first attaches to epithelial cells is less clear. Many epithelial cell lines express at least low levels of CD21 (4, 13), and stable transfection of CD21 in an epithelial cell line facilitates a high level of infection (2, 20). Nevertheless, there remains considerable doubt as to whether CD21 is expressed on epithelial cells in vivo (37). The virus can use gHgLR for efficient attachment, but when it does so its ability to penetrate cells appears to be compromised (2). The multispan virus membrane protein encoded by the BMRF2 open reading frame carries an RGD motif and interacts with α5β1 integrins on polarized epithelial cells in vitro (34), but there is very little of this protein in the virus particle (16), and whether its major role is in attachment or cell signaling is unclear. Epithelial cells that carry the polymorphic immunoglobulin A (IgA) receptor can be infected in vitro with virus coated with IgA specific for gp350/220. This is of particular interest given the elevation of serum IgA, as well as IgG, antibody to EBV in patients with NPC (10, 12), although in polarized epithelial cells, virus coated with antibody to gp350/200 is preferentially transcytosed (6).
Infection of CD21-negative epithelial cells in culture with cell-free virus is generally inefficient (2, 13), but such cells occasionally express CD21 on prolonged culture and infection rates go up. One potential way to determine if CD21 is being expressed might be to examine whether infectivity is blocked by monoclonal antibodies (MAbs) to gp350/220 that block CD21-mediated B-cell infection. In the course of testing this possibility, we made the somewhat surprising discovery that antibodies to gp350/220 enhance rather than inhibit epithelial cell infection. We report here that this is not an Fc receptor-mediated event but may be mediated by clustering of gp350/220 in the virion envelope and facilitation of access of other essential virion proteins to the cell membrane. The effect can be at least partially recapitulated by antibodies in the saliva of seropositive individuals. If high-titer antibodies, including antibodies to gp350/220, predate the onset of NPC, these findings have potential implications for development of the disease.
MATERIALS AND METHODS
Cells and virus.
Akata-GFP, a CD21-positive Burkitt lymphoma-derived cell line carrying a recombinant EBV in which the thymidine kinase gene is interrupted with a double cassette expressing neomycin resistance and the green fluorescent protein (21), and EBV-negative Akata cells that have lost the EBV genome were grown in RPMI 1640 medium (Sigma) supplemented with 10% heat-inactivated fetal bovine serum (Gibco BRL). The EBV-positive Akata cells were also supplemented with 500 μg/ml active G418 (Mediatech). AGS, a CD21-negative gastric carcinoma-derived cell line (American Type Culture Collection) was grown in Ham's F12 nutrient mixture (Gibco BRL) supplemented with 10% heat-inactivated fetal bovine serum. 293 cells were grown in Dulbecco modified Eagle medium (Sigma) supplemented with 10% heat-inactivated fetal bovine serum. Akata-GFP virus was collected from clarified culture medium of cells that had been induced by treatment with anti-human immunoglobulin (21).
Antibodies.
The following MAbs were used: CL55 and L2 (17), reacting with gB; C1 (33) and CL3, reacting with gp350/220, and 1969, which recognizes the α5/β1 integrin complex (Chemicon International). C1, CL3, and CL55 were purified by chromatography on protein A (Sigma) coupled to Affigel-15 (Bio-Rad). L2, a gift from Gary Pearson, was ascites fluid, and the antibody concentration was determined by gel electrophoresis and comparison with known antibody concentrations. Fab′ and F(ab′)2 fragments of C1 and CL3 were made with an ImmunoPure IgG1 Fab and F(ab′)2 preparation kit (Pierce Biotechnology Inc., Rockford, IL) according to the manufacturer's instructions and separated by fast protein liquid chromatography with a column of Sephacryl 100 26/60 (GE Healthcare). Complete digestion into either Fab or F(ab′)2 was confirmed by polyacrylamide gel electrophoresis under nonreducing conditions.
Infection assays.
AGS or 293 cells were plated onto two-chamber Falcon slides (BD Biosciences Clontech) at a dilution that produced a 30% confluent monolayer 24 h later. Cells were incubated at 37°C with 500 μl of virus alone or virus that had been preincubated for 45 min at room temperature with 100 μg of antibody, preincubated with 100 μg of antibody and 100 μg of protein A/G (Pierce), or preincubated with saliva. In experiments where Fab fragments were used, 30 μg of goat anti-mouse heavy- and light-chain antibody (Jackson Immunoresearch Laboratories) was added at 45 min to some samples for an additional 45 min of incubation. After 2 h of incubation on cells, growth medium (1.5 ml) was added and the cells were reincubated and directly examined for GFP expression 48 h later by fluorescence microscopy. A total of 5 × 105 EBV-negative Akata cells were pelleted and resuspended in 100 μl of virus; virus and antibody; virus, antibody, and soluble protein A/G (Pierce); or virus and saliva for 2 h at 37°C, at which time growth medium (3 ml) was added. Cells were transferred to six-well plates and examined for GFP expression 48 h later. In some experiments, cells were preincubated with antibody 1969 to the α5/β1 integrin complex for 1 h at 37°C before addition of virus.
Virus binding assays.
The amount of virus that bound to AGS cells was determined by adding virus to adherent monolayers in six-well tissue culture plates on ice for 2 h, washing cells, and scraping them into ice-cold saline. DNA was isolated for real-time quantitative PCR with a QIAamp DNA Blood Mini Kit (QIAGEN Sciences).
Saliva.
Saliva was obtained by use of a Salivette (Sarstedt, Fisher Scientific International). Donors chewed a cotton swab for approximately 1 min, and the saliva-saturated swab was centrifuged at 840 × g in the Salivette to extract the saliva into the base and pellet out cells. The clarified saliva was then recentrifuged at 16,000 × g for 1 h and incubated overnight at 4°C with Sepharose CL4B. To deplete saliva of antibodies, it was incubated for 2 h with protein A-conjugated Sepharose (Sigma). All saliva was filtered through a 0.2-μm-pore-size filter before use.
Real-time quantitative PCR and reverse transcriptase real-time PCR.
A 76-bp region of the EBV EBNA1 gene in the BamHI K fragment (primers 5′-GGATGCGATTAAGGACCTTGTT-3′ and 5′-CGTCAAAGCTGCACACAGTCA-3′, base coordinates 109677 and 109753, respectively; National Center for Biotechnology Information GenBank accession no. VO1555) was amplified together with a 101-bp DNA sequence of the human C-reactive protein (CRP) gene (primers 5′-CTTGACCAGCCTCTCTCATGC-3′ and 5′-TGCAGTCTTAGACCCCACCC-3′, base coordinates 132705 and 132605, respectively; accession no. AL445528) with the TaqMan Fluorogenic System (PE Applied Biosystems), in which real-time amplification is measured by cleavage of fluorescent-dye-labeled probes by the 5′-to-3′ exonuclease activity of Taq DNA polymerase. The EBNA1 probe (5′-CAAAGCCCGCTCCTACCTGCAATATCA-3′, base coordinate 109703) was labeled with 6-carboxyfluorescein, and the CPR probe (5′-TTTGGCCAGACAGGTAAGGGCCACC-3′, base coordinate 132682) was labeled with VIC (PE Applied Biosystems). Reactions were performed as described by others (7), in a 50-μl volume with TaqMan Universal Master Mix (PE Applied Biosystems), 300 nM primers, 200 nmol/liter probe, and 400 or 200 ng of DNA. Amplification consisted of 2 min at 50°C, 10 min at 95°C, and 50 two-step cycles of 15 s at 95°C and 60 s at 60°C. Each sample was run in duplicate, together with multiple template-negative controls. Serial dilutions of IB4 DNA, a Burkitt's lymphoma cell line containing five copies of EBV per cell, served as the standard. The EBV copy number per sample was normalized to the amount of CRP DNA representing the actual amount of amplifiable cellular DNA in each sample. Confirmation that the AGS epithelial cells were CD21 negative and that 293 cells were CD21 positive was obtained by nested reverse transcriptase real-time PCR. RNA was isolated with RNA STAT-60 (Tel-Test, Inc.) and reverse transcribed before amplification with outer primers (5′-GGG TTT TCT TGG CTC TCG TC-3′ and 5′-CCT TAT CAC GGT ACC AAC AGC-3′) located at bases 117 to 136 of the first exon of CD21 (bases 1 to 152; accession number NM_001877) and bases 218 to 238 in the second exon. A second round of PCR was carried out with the first-round PCR product mixed with the inner primers 5′-GGG TTT TCT TGG CTC TCG TC-3′ and 5′-AAT AAT AAC TAA TCC GGC CAT TTA GG-3′, located at bases 117 to 136 of the first exon and bases 181 to 206 of the second exon. The probe 5′-TCC TCG GGA TTT CTT GTG GCT CTC CT-3′ spanned the last six bases of exon 1 and the first 20 bases of exon 2 and was labeled with 6-carboxyfluorescein.
RESULTS
Antibodies to gp350/220 enhance infection of CD21-negative epithelial cells.
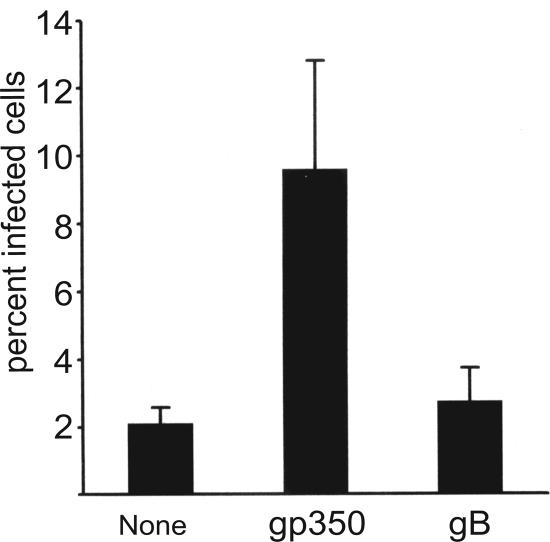
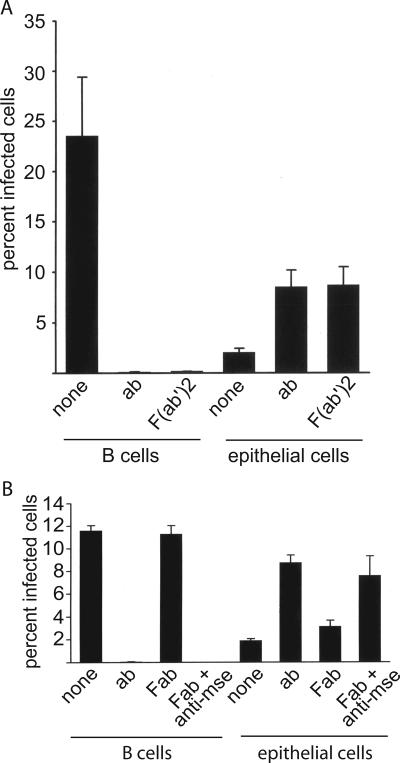
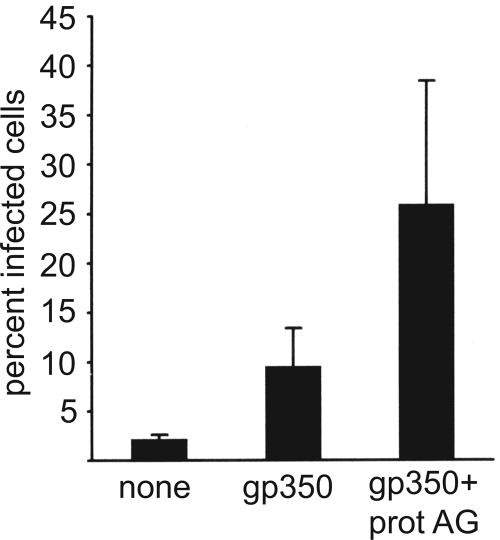
Virus was harvested from Akata cells carrying a recombinant EBV which expresses GFP and allows infection to be scored by counting the percentage of green cells at 48 h postinfection. AGS cells were confirmed to be CD21 negative by reverse transcriptase PCR and infected with this virus. As previously noted, infection was inefficient, with fewer than 5% of the cells typically expressing GFP. However, preincubation of the virus with two MAbs to gp350/220, one of which neutralizes B-cell infection (33), increased infection significantly; two nonneutralizing MAbs to gB had no such effect (Fig. 1). The failure of the antibodies to gB, which is also a relatively abundant envelope glycoprotein (16), to enhance infection suggested that enhancement could not simply be attributed to increased binding of virus via antibodies to Fc receptors on the epithelial cell surface. To confirm this, we made F(ab′)2 and Fab′ fragments of the two anti-gp350 antibodies. The F(ab′)2 fragments retained their ability to inhibit B-cell infection and enhance epithelial cell infection (Fig. 2A). Fab fragments failed either to inhibit B-cell infection or to enhance epithelial cell infection, but addition of sheep anti-mouse antibody, which would restore the ability of the antibodies to cross-link antigen, restored both the inhibition of B-cell infection and the enhancement of epithelial cell infection (Fig. 2B). To explore further if cross-linking of antigen was important to enhanced epithelial cell infection, we preincubated the virus with intact antibody and with soluble recombinant protein A/G, which combines four protein A and two protein G Fc binding domains and can thus increase antibody cross-linking. Consistent with a role for protein cross-linking, the soluble protein A/G increased the enhancement of infection beyond that mediated by antibody alone (Fig. 3).
FIG. 1.
Antibodies to glycoprotein gp350/220 enhance infection of CD21-negative epithelial cells. AGS epithelial cells infected with recombinant EBV expressing GFP that had been preincubated with medium alone (none), with two MAbs to gp350/220 (gp350), or with two nonneutralizing antibodies to glycoprotein gB (gB). The percentage of cells infected was determined by counting those expressing GFP at 48 h postinfection. Error bars indicate the standard deviation of seven separate experiments.
FIG. 2.
MAbs to gp350/220 lacking an Fc domain can inhibit B-cell infection and enhance epithelial cell infection, but only if they are bivalent. AGS epithelial cells or EBV-negative Akata B cells were infected with recombinant virus expressing GFP after preincubation with medium alone (none), intact antibodies (ab), or F(ab′)2 fragments (A) or with medium alone, intact antibodies, Fab fragments (Fab), or Fab fragments cross-linked by addition of anti-mouse antibody (Fab + anti-mse) (B). Error bars indicate standard deviation of three (A) or two (B) separate experiments. Different virus pools were used in panels A and B.
FIG. 3.
Addition of soluble protein A/G to MAbs to gp350/220 increases their ability to enhance infection of epithelial cells. AGS epithelial cells were infected with recombinant EBV expressing GFP that had been preincubated with medium alone (none), with two MAbs to gp350/220 (gp350), or with two MAbs to gp350/220 and 100 μg of soluble protein A/G (gp350 + prot AG). Error bars indicate the standard deviation of five separate experiments.
Antibodies to gp350/220 also enhance infection of epithelial cells expressing low levels of CD21.
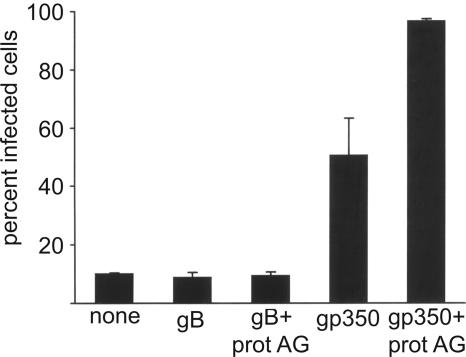
Some epithelial cell lines express at least low levels of CD21, and the question of whether epithelial cells express CD21 in vivo is still unresolved. In order to determine if antibodies to gp350/220 could also enhance infection of epithelial cell lines expressing CD21, 293 cells were infected with virus expressing GFP that had been preincubated with antibodies to gB, with antibodies to gp350/220, or with the same antibodies and with soluble recombinant protein A/G as well. Infection levels in the absence of any antibody were higher than for CD21-negative AGS cells, but again antibodies to gp350/220 and not antibodies to gB enhanced infection (Fig. 4). Addition of protein A/G increased infection even further. Real-time quantitative PCR analysis of cDNA reverse transcribed from 293 cells indicated that CD21 RNA levels were approximately one-sixth of the levels found in the Raji B cells that we currently have in culture.
FIG. 4.
MAbs to gp350/220, but not antibodies to gB, enhance infection of CD21-positive 293 cells. 293 cells were infected with recombinant EBV expressing GFP that had been preincubated with medium alone (none), with two MAbs to glycoprotein gB (gB), with two MAbs to gB and 100 μg of soluble protein A/G (gB + prot AG), with two MAbs to gp350/220 (gp350), or with two MAbs to gp350/220 and 100 μg of soluble protein A/G (gp350 + prot AG). Error bars indicate the standard deviation of four infections in two separate experiments.
Antibodies do not significantly increase virus binding.
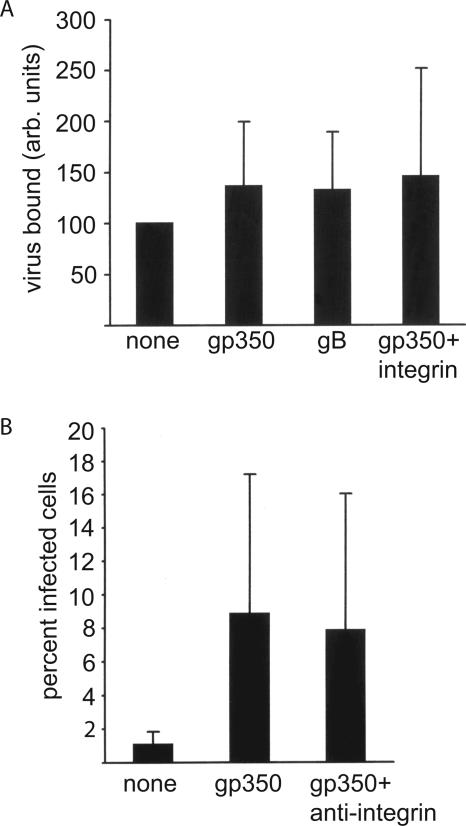
Although it did not appear that antibody-mediated enhanced infection of epithelial cells could be attributed to enhanced virus binding to Fc receptors, it remained possible that antibody was increasing the ability of additional virus proteins to attach virus to cells. To determine if this were the case, we used real-time quantitative PCR to measure the relative amounts of virus bound to AGS cells following preincubation of virus with MAbs. Antibodies to gp350/220 in six experiments, on average, did increase virus binding relative to virus alone (Fig. 5A). However, antibodies to gB that mediated no enhanced infection had the same effect and in neither case were the differences statistically significant. Since the virus BMRF2 protein interacts with α5β1 integrins, we also examined the binding of virus preincubated with MAbs to gp350/220 to cells which had been preincubated with a blocking antibody to α5β1 integrins. The slight increase in virus binding seen after preincubation of virus with antiviral antibodies was not affected. The same treatment (Fig. 5B) or preincubation of cells with fibronectin (not shown) also had no significant effect on the infectivity of virus pretreated with antibodies to gp350/220.
FIG. 5.
Enhanced infection is not a result of increased binding of virus and is not influenced by preincubation of AGS epithelial cells with antibody to α5β1 integrins. (A) Virus preincubated in medium (none), MAbs to gp350/220 (gp350), or MAbs to gB (gB) was incubated on ice for 2 h with cells or cells preincubated with antibody to α5β1 integrins (gp350 + integrin). Cells were then washed, and relative amounts of virus bound per cell were measured by real-time quantitative PCR for the BamHI K fragment of EBV and for cellular CRP. Virus bound, in arbitrary (arb.) units, represents the values obtained for EBV divided by the values obtained for CRP expressed relative to virus binding in the absence of antibody (set at 100). (B) AGS cells infected with recombinant EBV expressing GFP that had been preincubated with medium alone (none), with two MAbs to gp350/220 and AGS cells preincubated with antibody to α5β1 integrins before infection with virus bound to antibodies to gp350/220 (gp350 + integrin). Error bars indicate the standard deviation of six experiments. None of the values in panel A were statistically significantly different (P < 0.1), and in panel B the values for gp350 and gp350 + integrin were not significantly different (P < 0.1).
Antibody in saliva can enhance epithelial cell infection.
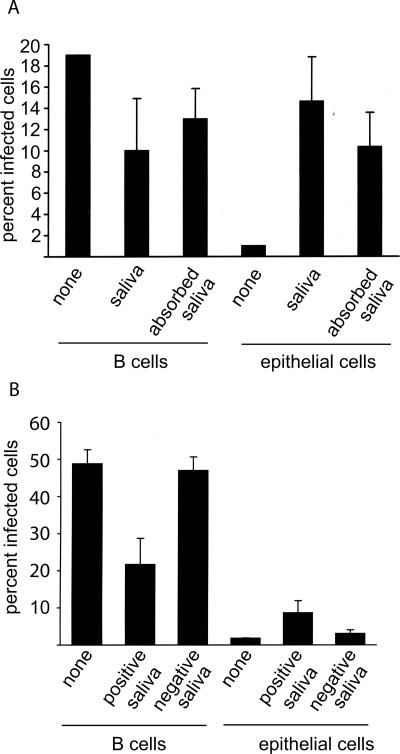
Antibodies to gp350/220, which is the most abundant glycoprotein in the virion (16), are found at low levels in the saliva of some normal healthy individuals (36). To determine if saliva might thus duplicate the effects of the MAbs to gp350/220, we collected cell-free saliva from EBV-seropositive individuals, absorbed it overnight on Sepharose CL4B, filtered it, and determined its ability to influence virus infection of B cells and epithelial cells. Saliva samples from four individuals that, preincubated with virus, could reduce infection of B cells also mediated, in parallel, an increase in infection of epithelial cells (Fig. 6A). Additional preabsorption of saliva on immobilized protein A modulated the decrease in B-cell infection and the increase in epithelial cell infection. Although in neither case were the infection rates restored to the same levels as those seen in the complete absence of saliva, the results were consistent with at least part of the activity being attributable to antibody. Consistent with this, saliva from four seronegative donors neither inhibited B-cell infection nor enhanced epithelial cell infection (Fig. 6B).
FIG. 6.
Saliva from seropositive, but not seronegative, individuals inhibits B-cell infection and enhances epithelial cell infection, and antibody depletion partially reverses these effects. (A) EBV-negative Akata B cells or AGS epithelial cells infected with virus preincubated with medium (none) with saliva or with saliva from which antibody had been deleted by preabsorption with immobilized protein A (absorbed saliva). (B) EBV-negative Akata B cells or AGS epithelial cells infected with virus preincubated with medium (none) or with saliva from seropositive or seronegative donors as indicated. Error bars indicate the standard deviation of four donors. All saliva was clarified by centrifugation at 16,000 × g for 1 h and filtered through a 0.2-μm-pore-size filter before use. The experiments whose results are shown in panels A and B were done with different virus pools.
DISCUSSION
Saliva from EBV-seropositive individuals contains both cell-free and cell-associated virus, the latter of which may represent virus bound to a B-cell surface or virus that is being actively replicated in B cells. It has been shown in vitro that close contact with virus-producing B-cell lines results in significantly more efficient epithelial cell infection than does addition of cell-free virus (13, 34), which raises the question of the relevance of cell-free virus in saliva to virus transmission. The work reported here suggests that use of tissue culture-derived virus alone may underestimate the transmissibility of the cell-free virus in the oral cavity to uninfected epithelial cells in the same or in a new host.
It is clear that epithelial cells can be infected in the absence of CD21 and recombinant gp350/220-null virus has been shown to be able to infect epithelial cells (14). Nevertheless, the fortuitous finding that antibodies to gp350/220 can enhance epithelial cell infection was a surprise. The ability of F(ab′)2 fragments to mediate the same effect ruled out the possibility that it simply reflected an acquired ability to bind virus to Fc receptors on the epithelial cell surface. The inability of Fab fragments to block B-cell infection without the addition of anti-mouse antibody was slightly unexpected but presumably means that the epitope recognized by the neutralizing antibody C1 is not the same as the CD21 binding site of gp350/220 but one that when cross-linked on neighboring molecules results in masking of the CD21 binding site. The need for anti-mouse antibody to restore the ability of Fab fragments to enhance epithelial infection presumably cannot have a precisely similar explanation, but the ability of protein A/G to increase the effect of intact antibody does strongly suggest that cross-linking is relevant.
One obvious possibility is that cross-linking of gp350/220 causes it to patch in the virus membrane. This could then, in turn, facilitate the access of other glycoproteins, more relevant to epithelial cell infection, to the epithelial cell surface. One function of these proteins might be to increase attachment of virus to the cell surface. Antibodies to gp350/220, however, did not significantly impact virus binding or apparently increase the access of the BMRF2 protein to α5β1 integrins. In the absence of an antibody to the RGD domain on BMRF2, we cannot rule out the possibility that other integrins are relevant, although the failure of fibronectin to affect enhanced infection is not consistent with this. It seems more probable that it is access of proteins that are responsible for virus penetration that is being facilitated. Glycoprotein gp350/220, which has the greatest mass of all of the virus envelope proteins, as well as being the most abundant, has been modeled as a long, extended structure (23). This is consistent with a model in which gp350/220, not absolutely required for attachment to an epithelial cell, may get in the way of other important players. What these players might be is not clear. Virus derived from a B cell already binds efficiently to gHgLR on the epithelial cell surface, and gHgLR is probably the same receptor that initiates fusion with an epithelial cell (2). This, together with the fact that no significant increase in binding was observed, suggests that it is not access of gHgL to the cell surface that is enhanced. However, an increase in the amount of glycoprotein gB in a virion particle has been shown to increase the efficiency of epithelial cell infection (26), so it is possible that the antibodies to gp350/220 have increased the ability of gB to take part in virus-cell fusion.
Saliva from seropositive donors could substitute for antibodies to gp350/220 as an inhibitor of B-cell infection and an enhancer of epithelial cell infection. At least some of these effects can be attributed to antibody since absorbing saliva with immobilized protein A reversed them and the effect was not seen with saliva from seronegative individuals. We have no specific data to address the question of whether the antibody responsible is directed at gp350/220, although the reciprocal effects on B-cell and epithelial cell infection are at least consistent with this. We have been unable to reduce the effects of saliva any further by absorption with immobilized jacalin or protein A/G, which might do a better job of removing IgA. This perhaps suggests the interesting possibility that saliva contains other factors which affect the efficiency of infection.
Exactly where epithelial cells fit in the cycle of EBV persistence is not certain. Productive replication in epithelial cells at the initiation of primary infection presumably facilitates access to B cells in Waldeyer's ring, particularly since replication in an HLA class II-negative cell like an epithelial cell significantly increases the ability of virus to infect a B cell (1). Amplification in epithelial cells of virus reactivating from plasmablasts may also be important; certainly, epithelial cells are infected in healthy carriers (28) and most of the virus found in saliva appears to have been made in an HLA class II-negative cell (15). One event may be facilitated by antibody in saliva in which virus is shed or transmitted; the other may be facilitated by serum antibody. Facilitation of epithelial cell infection in individuals at risk for NPC is of particular concern. The findings reported here suggest that elevated titers of antibody to EBV gp350/220 may not only be prognostic for the tumor but also play a contributory role in its development.
Acknowledgments
This work was supported by Public Health Services grant DE016669 from the National Institute of Dental and Craniofacial Research.
We thank Cliona Rooney for reagents.
REFERENCES
- 1.Borza, C. M., and L. M. Hutt-Fletcher. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8:594-599. [DOI] [PubMed] [Google Scholar]
- 2.Borza, C. M., A. J. Morgan, S. M. Turk, and L. M. Hutt-Fletcher. 2004. Use of gHgL for attachment of Epstein-Barr virus to epithelial cells compromises infection. J. Virol. 78:5007-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan, A. S. C., K. F. To, K. W. Lo, K. F. Mak, W. Pak, B. Chiu, G. M. K. Tse, M. Ding, X. Li, J. C. K. L. Lee, and D. P. Huang. 2000. High frequency of chromosome 3p deletion in histologically normal nasopharyngeal epithelia from southern Chinese. Cancer Res. 60:5365-5370. [PubMed] [Google Scholar]
- 4.Fingeroth, J. D., M. E. Diamond, D. R. Sage, J. Hayman, and J. L. Yates. 1999. CD21-dependent infection of an epithelial cell line, 293, by Epstein-Barr virus. J. Virol. 73:2115-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fingeroth, J. D., J. J. Weis, T. F. Tedder, J. L. Strominger, P. A. Biro, and D. T. Fearon. 1984. Epstein-Barr virus receptor of human B lymphocytes is the C3d complement CR2. Proc. Natl. Acad. Sci. USA 81:4510-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gan, Y., J. Chodosh, A. Morgan, and J. W. Sixbey. 1997. Epithelial cell polarization is a determinant in the infectious outcome of immunoglobulin A-mediated entry by Epstein-Barr virus. J. Virol. 71:519-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gan, Y.-J., B. I. Razzouk, T. Su, and J. W. Sixbey. 2002. A defective, rearranged Epstein-Barr virus genome in EBER-negative and EBER-positive Hodgkin's disease. Am. J. Pathol. 160:781-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haan, K. M., S. K. Lee, and R. Longnecker. 2001. Different functional domains in the cytoplasmic tail of glycoprotein gB are involved in Epstein-Barr virus induced membrane fusion. Virology 290:106-114. [DOI] [PubMed] [Google Scholar]
- 9.Henle, G., and W. Henle. 1976. Epstein-Barr virus-specific IgA serum antibodies is an outstanding feature of nasopharyngeal carcinoma. Int. J. Cancer 17:1-7. [DOI] [PubMed] [Google Scholar]
- 10.Henle, G., and W. Henle. 1975. Serum IgA antibodies of Epstein-Barr virus (EBV)-related antigens. A new feature of nasopharyngeal carcinoma. Bibl. Haematol. 43:322-325. [DOI] [PubMed] [Google Scholar]
- 11.Henle, W., G. Henle, H. G. Ho, P. Burtin, Y. Cachin, P. Clifford, A. de Schryver, G. de The, V. Diehl, and G. Klein. 1970. Antibodies to Epstein-Barr virus in nasopharyngeal carcinoma, other head and neck cancers and control groups. J. Natl. Cancer Inst. 44:225-231. [PubMed] [Google Scholar]
- 12.Henle, W., G. Henle, and H. C. Kwan. 1973. Antibodies to Epstein-Barr virus-related antigens in nasopharyngeal carcinoma. J. Natl. Cancer Inst. 51:361-369. [PubMed] [Google Scholar]
- 13.Imai, S., J. Nishikawa, and K. Takada. 1998. Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. J. Virol. 72:4371-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janz, A., M. Oezel, C. Kurzeder, J. Mautner, D. Pich, M. Kost, W. Hammerschmidt, and H. J. Delecluse. 2000. Infectious Epstein-Barr virus lacking major glycoprotein BLLF1 (gp350/220) demonstrates the existence of additional viral ligands. J. Virol. 74:10142-10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang, R., R. S. Scott, and L. M. Hutt-Fletcher. 2006. Epstein-Barr virus shed in saliva is high in B-cell-tropic gp42. J. Virol. 80:7281-7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johannsen, E., M. Luftig, M. R. Chase, S. Weicksel, E. Cahir-McFarland, D. Illanes, D. Sarracino, and E. Kieff. 2004. Proteins of purified Epstein-Barr virus. Proc. Natl. Acad. Sci. USA 101:16286-16291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kishishita, M., J. Luka, B. Vroman, P. J. F., and G. R. Pearson. 1984. Production of monoclonal antibodies to a late intracellular Epstein-Barr virus-induced antigen. Virology 133:363-375. [DOI] [PubMed] [Google Scholar]
- 18.Li, Q. X., M. K. Spriggs, S. Kovats, S. M. Turk, M. R. Comeau, B. Nepom, and L. M. Hutt-Fletcher. 1997. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J. Virol. 71:4657-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, Q. X., S. M. Turk, and L. M. Hutt-Fletcher. 1995. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J. Virol. 69:3987-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, Q. X., L. S. Young, G. Niedobitek, C. W. Dawson, M. Birkenbach, F. Wang, and A. B. Rickinson. 1992. Epstein-Barr virus infection and replication in a human epithelial system. Nature 356:347-350. [DOI] [PubMed] [Google Scholar]
- 21.Molesworth, S. J., C. M. Lake, C. M. Borza, S. M. Turk, and L. M. Hutt-Fletcher. 2000. Epstein-Barr virus gH is essential for penetration of B cell but also plays a role in attachment of virus to epithelial cells. J. Virol. 74:6324-6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moody, C. A., R. S. Scott, T. Su, and J. W. Sixbey. 2003. Length of Epstein-Barr virus termini as a determinant of epithelial cell clonal emergence. J. Virol. 77:8555-8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore, M. D., R. G. DiScipio, N. R. Cooper, and G. R. Nemerow. 1989. Hydrodynamic, electron microscopic and ligand binding analysis of the Epstein-Barr virus/C3dg receptor (CR2). J. Biol. Chem. 34:20576-20582. [PubMed] [Google Scholar]
- 24.Nemerow, G. R., C. Mold, V. K. Schwend, V. Tollefson, and N. R. Cooper. 1987. Identification of gp350 as the viral glycoprotein mediating attachment of Epstein-Barr virus (EBV) to the EBV/C3d receptor of B cells: sequence homology of gp350 and C3 complement fragment C3d. J. Virol. 61:1416-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nemerow, G. R., R. Wolfert, M. McNaughton, and N. R. Cooper. 1985. Identification and characterization of the Epstein-Barr virus receptor on human B lymphocytes and its relationship to the C3d complement receptor (CR2). J. Virol. 55:347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neuhierl, B., R. Feederle, W. Hammerschmidt, and H. J. Delecluse. 2002. Glycoprotein gp110 of Epstein-Barr virus determines viral tropism and efficiency of infection. Proc. Natl. Acad. Sci. USA 99:15036-15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pathmanathan, R., U. Prasad, R. Sadler, K. Flynn, and N. Raab-Traub. 1995. Clonal proliferation of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N. Engl. J. Med. 333:693-698. [DOI] [PubMed] [Google Scholar]
- 28.Pegtel, D. M., J. Middeldorp, and D. A. Thorley-Lawson. 2004. Epstein-Barr virus infection in ex-vivo tonsil epithelial cell cultures of asymptomatic carriers. J. Virol. 78:12613-12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raab-Traub, N. 2005. Epstein-Barr virus in the pathogenesis of NPC, p. 71-92. In E. S. Robertson (ed.), Epstein-Barr virus. Caister Academic Press, Norfolk, Great Britain.
- 30.Raab-Traub, N., and K. Flynn. 1986. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell 47:883-889. [DOI] [PubMed] [Google Scholar]
- 31.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2627. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 32.Tanner, J., J. Weis, D. Fearon, Y. Whang, and E. Kieff. 1987. Epstein-Barr virus gp350/220 binding to the B lymphocyte C3d receptor mediates adsorption, capping and endocytosis. Cell 50:203-213. [DOI] [PubMed] [Google Scholar]
- 33.Thorley-Lawson, D. A., and K. Geilinger. 1980. Monoclonal antibodies against the major glycoprotein (gp350/220) of Epstein-Barr virus neutralize infectivity. Proc. Natl. Acad. Sci. USA 77:5307-5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tugizov, S. M., J. W. Berline, and J. M. Palefsky. 2003. Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat. Med. 9:307-314. [DOI] [PubMed] [Google Scholar]
- 35.Wang, X., W. J. Kenyon, Q. X. Li, J. Mullberg, and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J. Virol. 72:5552-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao, Q.-Y., M. Rowe, A. J. Morgan, C. K. Sam, U. Prasad, H. Dang, Y. Zeng, and A. B. Rickinson. 1991. Salivary and serum IgA antibodies to the Epstein-Barr virus glycoprotein gp340: incidence and potential for virus neutralization. Int. J. Cancer 48:45-50. [DOI] [PubMed] [Google Scholar]
- 37.Young, L. S., C. W. Dawson, K. W. Brown, and A. B. Rickinson. 1989. Identification of a human epithelial cell surface protein sharing an epitope with the C3d/Epstein-Barr virus receptor molecule of B lymphocytes. Int. J. Cancer 43:786-794. [DOI] [PubMed] [Google Scholar]
- 38.Zeng, Y. 1985. Seroepidemiological studies on nasopharyngeal carcinoma in China. Adv. Cancer Res. 44:121-138. [DOI] [PubMed] [Google Scholar]
- 39.Zeng, Y., L. G. Zhang, Y. C. Wu, Y. S. Huang, N. Q. Huang, J. Y. Li, Y. B. Wang, M. K. Jiang, Z. Fang, and N. N. Meng. 1985. Prospective studies on nasopharyngeal carcinoma in Epstein-Barr virus IgA/VCA antibody-positive persons in Wuzhou City, China. Int. J. Cancer 36:545-547. [DOI] [PubMed] [Google Scholar]