Abstract
Host cell proteases that cleave the hemagglutinin (HA) of influenza viruses in the human respiratory tract are still not identified. Here we cloned two human type II transmembrane serine proteases with known airway localization, TMPRSS2 and HAT, into mammalian expression vector. Cotransfection of mammalian cells with plasmids encoding HA and either protease resulted in HA cleavage in situ. Transient expression of either protease in MDCK cells enabled multicycle replication of influenza viruses in these cells in the absence of exogenous trypsin. These data suggest that TMPRSS2 and HAT are candidates for proteolytic activation of influenza viruses in vivo.
The ability of the hemagglutinin protein (HA) of influenza viruses to mediate fusion between viral and endosomal membranes during virus entry into the cell depends on cleavage of fusion-incompetent precursor HA0 into disulfide-linked subunits HA1 and HA2 by a host endoprotease. Cleavage of HA is essential for infection and determines viral pathogenicity and tissue tropism (reviewed in references 8, 10, 11, and 22). Thus, the highly pathogenic avian influenza viruses of subtypes H5 and H7 are cleaved at the multibasic motif R-X-R/K-R by ubiquitous subtilisin-like cellular proteases (11, 23) and cause lethal systemic infection in birds. All other influenza A viruses, including human epidemic and pandemic strains, have a single arginine at the HA cleavage site; these viruses can only be cleaved in a limited number of tissues, such as the intestinal tract in birds and the respiratory tract in birds and mammals (11, 22).
Early studies demonstrated that influenza viruses with monobasic cleavage site can be proteolytically activated in cell culture by the addition of trypsin (12, 13). Less is known about proteases that cleave influenza viruses under conditions of natural infection. Several trypsin-like proteases isolated from rat and swine lung were shown to support replication of influenza viruses in vitro (3, 9, 18, 25). However, it remains unclear whether these proteases play a role in in vivo infection. In the case of human influenza, specific proteases responsible for HA cleavage in the human respiratory tract have not been identified thus far.
In search of such proteases, we use a new approach. Instead of isolating and characterizing influenza virus-activating enzymes from respiratory tissues, we clone and express genes of trypsin-like proteases known to be present in the human airway epithelium and test them for cleavage of HA. We have analyzed here two such proteases, TMPRSS2 (5, 14, 21) and HAT (human airway trypsin-like protease) (4, 24, 27, 28), given their previous detection in the human airways and the availability of their full-length coding sequences. As the source of human genetic material for cloning, we used differentiated cultures of human airway epithelial cells grown at the air-liquid interface in serum-free, hormone- and growth factor-supplemented medium as previously described (6, 15). These cultures support multicycle replication of human influenza viruses in the absence of exogenous proteases (15), indicating the presence of virus-activating proteases that cleave at the mono-basic cleavage site.
To begin, total RNA was extracted from 6-week-old cultures by using High-Pure RNA isolation kit (Roche). The full-length coding sequences of the proteases were amplified with specific primers from total RNA by using OneStep RT-PCR kit (QIAgen), and the PCR products were cloned into the beta-actin promoter-driven pCAGGS vector (20). Primers were designed on the basis of published sequences (GenBank accession numbers AB002134 and U75329 for HAT and TMPRSS2, respectively). We confirmed the identity of cloned protease genes by sequencing. A proteolytically inactive single-point mutant, TMPRSS2(S441A), was generated by substitution of the active-site serine to alanine at position 441 by using site-directed mutagenesis as described by Afar et al. (1). This mutation was shown to abolish enzymatic activity of TMPRSS2 without affecting the level of protein expression in transfected cells (1). The pCAGGS-HA plasmid encoding HA of A/Hong Kong/1/68 (H3N2) was generated by reverse transcription-PCR using viral RNA with HA-specific primers and subcloning of the product into the pCAGGS vector.
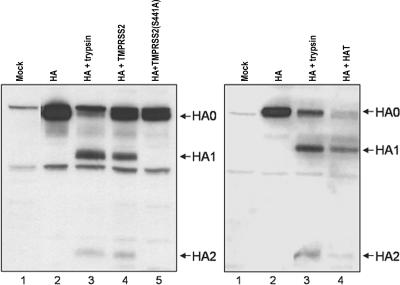
To test whether the proteases are able to cleave HA, we coexpressed HA and either protease in mammalian cells. A549 human lung carcinoma cells grown in six-well plates were cotransfected by using Lipofectamine 2000 (Invitrogen) with plasmids encoding HA (pCAGGS-HA) and the protease (either pCAGGS-TMPRSS2 or pCAGGS-HAT). Two days after transfection, we separated cell lysates by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12% gel) under reducing conditions. Proteins were transferred to a polyvinylidene difluoride membrane (Amersham Life Sciences) by semidry electroblotting and immunostained by sequential incubations with goat antibodies to A/Aichi/2/68 (H3N2) virus, peroxidase-labeled rabbit anti-goat immunoglobulin G antibodies (Dianova), and ECL peroxidase substrate (Pierce). Coexpression of the hemagglutinin together with either protease resulted in the cleavage of HA0 into two polypeptides with a mobility identical to that of HA1 and HA2 in the trypsin-treated control HA sample (Fig. 1). No cleavage was observed in cells that were cotransfected with either empty pCAGGS plasmid or plasmid encoding inactive protease mutant TMPRSS2(S441A) (Fig. 1, lanes 2 and 5, respectively). HA cleavage by either protease was not limited to A549 cells but was also observed in transfection experiments with MDCK and 293T cells (data not shown).
FIG. 1.
Cleavage of influenza virus HA by coexpression with TMPRSS2 (left panel) and HAT (right panel). A549 cells were cotransfected with pCAGGS-HA (lanes 2 to 5) and empty pCAGGS vector (lanes 2 and 3), pCAGGS-TMPRSS2 or pCAGGS-HAT (lane 4), and pCAGGS-TMPRSS2(S441A) (lane 5, left panel). Mock transfections were done with empty pCAGGS plasmid (lane 1). We analyzed cell lysates prepared 48 h after transfection by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting as described in the text. For a positive control of HA cleavage, transfected cells were incubated with 10 μg of TPCK (tolylsulfonyl phenylalanyl chloromethyl ketone)-trypsin (Sigma)/ml for 20 min at 37°C prior to lysis (lane 3).
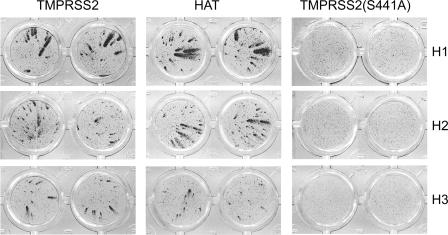
We then tested whether TMPRSS2 and HAT support influenza virus infection in MDCK cells, which lacked an endogenous HA-activating protease as judged by the inability of influenza viruses with monobasic cleavage site to undergo multicycle replication in these cells in the absence of trypsin. We transfected replicate MDCK cultures with plasmids encoding either active proteases or enzymatically inactive TMPRSS2(S441A) mutant and infected the cultures with influenza viruses 10 h after transfection. The cultures were fixed 24 h postinfection and immunostained for the expression of viral nucleoprotein as described previously (16). In cultures expressing TMPRSS2(S441A), only single infected cells could be detected (Fig. 2), confirming that cells transfected with the plasmid encoding inactive protease failed to support proteolytic activation and multicycle replication of virus progeny. In contrast, typical comet-like foci of virus spread were observed in cultures expressing TMPRSS2 and HAT. This pattern indicated that these proteases activated influenza virus infectivity, thus allowing multicycle viral replication. Importantly, either protease was able to support infection of influenza A viruses with all three hemagglutinin subtypes (H1, H2, and H3) associated with human epidemics and pandemics.
FIG. 2.
Multicycle viral replication in MDCK cells transfected with TMPRSS2 (left column), HAT (middle column), or proteolytically inactive protease mutant TMPRSS2(S441A) (right column). Ten hours after transfection with protease-encoding plasmids, the cultures were infected with influenza viruses A/Memphis/14/96 (H1N1) (top row), A/Mallard/Alberta/205/98 (H2N9) (middle row), and A/Texas/6/96 (H3N2) (bottom row) at a multiplicity of infection of 0.01. We incubated infected cultures for 24 h and immunostained them for viral nucleoprotein as described previously (16).
TMPRSS2 and HAT belong to the very few trypsin-like proteases with currently known expression in the human respiratory tract (5, 14, 24, 28). Both proteases are members of the family of type II transmembrane serine proteases, membrane-anchored multidomain proteases, which play complex regulatory roles at the plasma membrane and within the extracellular matrix (7, 19). In particular, TMPRSS2 and HAT were shown to regulate cellular functions via protease-activated receptor 2, with TMPRSS2 modulating epithelial sodium channels in human airways (5, 26) and HAT stimulating the proliferation of human bronchial fibroblasts (17). HAT was also demonstrated to increase the expression of mucin genes in airway epithelial cells (4).
Proteolytic activation of influenza viruses with monobasic HA cleavage site has long been believed to be a paracrine process mediated by secreted extracellular protease(s) (9-11, 22). However, a recent study in cultures derived from human adenoids demonstrated that the cleavage of influenza viruses in these airway-epithelial-like cultures is a cell-associated process taking place inside or on the surface of the cells in which the virus replicates (29). Another study revealed that activation of the WSN virus occurs in MDBK cells at the stage of virus entry, presumably by an endosomal protease (2). Interestingly, membrane-associated and soluble forms of TMPRSS2 and HAT have been detected (1, 27, 28). So, in principle either protease could account for all different modes of cleavage described above.
Given the ability of TMPRSS2 and HAT to activate different influenza viruses in vitro, the expression of these proteases in human airway epithelium, and their existence in both cell-surface-associated and secreted forms, we conclude that TMPRSS2 and HAT are plausible candidates for proteolytic activation of influenza viruses in humans. Since TMPRSS2 and HAT may not be the only virus-activating enzymes in the human respiratory tract, systematic screening for other proteases using the approach described here will have to be done in future studies.
Acknowledgments
We thank Robert Webster (St. Jude Children's Research Hospital, Memphis, TN) and Earl Brown (University of Ottawa, Ottawa, Ontario, Canada) for the influenza viruses, Alexander Klimov (Centers for Disease Control, Atlanta, GA) for the anti-nucleoprotein antibody, and Thomas Gray (National Institutes of Environmental Health Sciences, Research Triangle Park, NC) for advice on growing airway epithelial cultures.
This study was supported by grants from Deutsche Forschungsgemeinschaft (SFB 593) and VIRGIL European Network of Excellence on Antiviral Drug Resistance (EU grant LSHM-CT-2004-503359).
REFERENCES
- 1.Afar, D. E., I. Vivanco, R. S. Hubert, J. Kuo, E. Chen, D. C. Saffran, A. B. Raitano, and A. Jakobovits. 2001. Catalytic cleavage of the androgen-regulated TMPRSS2 protease results in its secretion by prostate and prostate cancer epithelia. Cancer Res. 61:1686-1692. [PubMed] [Google Scholar]
- 2.Boycott, R., H. D. Klenk, and M. Ohuchi. 1994. Cell tropism of influenza virus mediated by hemagglutinin activation at the stage of virus entry. Virology 203:313-319. [DOI] [PubMed] [Google Scholar]
- 3.Chen, Y., M. Shiota, M. Ohuchi, T. Towatari, J. Tashiro, M. Murakami, M. Yano, B. Yang, and H. Kido. 2000. Mast cell tryptase from pig lungs triggers infection by pneumotropic Sendai and influenza A viruses. Eur. J. Biochem. 267:3189-3197. [DOI] [PubMed] [Google Scholar]
- 4.Chokki, M., S. Yamamura, H. Eguchi, T. Masegi, H. Horiuchi, H. Tanabe, T. Kamimura, and S. Yasuoka. 2003. Human airway trypsin-like protease increases mucin gene expression in airway epithelial cells. Am. J. Respir. Cell. Mol. Biol. 30:470-478. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson, S. H., A. Hirsh, D. C. Li, G. Holloway, J. Chao, R. G. Boucher, and S. E. Gabriel. 2002. Regulation of the epithelial sodium channel by serine proteases in human airways. J. Biol. Chem. 277:8338-8345. [DOI] [PubMed] [Google Scholar]
- 6.Gray, T. E., K. Guzman, C. W. Davis, L. H. Abdullah, and P. Nettesheim. 1996. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am. J. Respir. Cell. Mol. Biol. 14:104-112. [DOI] [PubMed] [Google Scholar]
- 7.Hooper, J. D., J. A. Clements, J. P. Quigley, and T. M. Antalis. 2001. Type II transmembrane serine proteases. Insights into an emerging class of cell surface proteolytic enzymes. J. Biol. Chem. 276:857-860. [DOI] [PubMed] [Google Scholar]
- 8.Horimoto, T., and Y. Kawaoka. 2001. Pandemic threat posed by avian influenza A viruses. Clin. Microbiol. Rev. 14:129-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kido, H., Y. Yokogoshi, K. Sakai, M. Tashiro, Y. Kishino, A. Fukutomi, and N. Katunuma. 1992. Isolation and characterization of a novel trypsin-like protease found in rat bronchiolar epithelial Clara cells: possible activator of the viral fusion glycoprotein. J. Biol. Chem. 267:13573-13579. [PubMed] [Google Scholar]
- 10.Klenk, H. D., and R. Rott. 1988. The molecular biology of influenza virus pathogenicity. Adv. Virus. Res. 34:247-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klenk, H. D., and W. Garten. 1994. Host cell proteases controlling virus pathogenicity. Trends Microbiol. 2:39-43. [DOI] [PubMed] [Google Scholar]
- 12.Klenk, H. D., R. Rott, M. Orlich, and J. Blodorn. 1975. Activation of influenza A viruses by trypsin treatment. Virology 68:426-439. [DOI] [PubMed] [Google Scholar]
- 13.Lazarowitz, S. G., and P. W. Choppin. 1975. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology 68:440-454. [DOI] [PubMed] [Google Scholar]
- 14.Lin, B., C. Ferguson, J. T. White, S. Wang, R. Vessella, L. D. True, L. Hood, and P. S. Nelson. 1999. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res. 59:4180-4184. [PubMed] [Google Scholar]
- 15.Matrosovich, M. N., T. Y. Matrosovich, T. Gray, N. A. Roberts, and H. D. Klenk. 2004. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc. Natl. Acad. Sci. USA 101:4620-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matrosovich, M., T. Matrosovich, J. Carr, N. A. Roberts, and H. D. Klenk. 2003. Overexpression of the alpha-2,6-sialyltransferase in MDCK cells increases influenza virus sensitivity to neuraminidase inhibitors. J. Virol. 77:8418-8425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushima, R., A. Takahashi, Y. Nakaya, H. Maezawa, M. Miki, Y. Nakamura, F. Ohgushi, and S. Yasuoka. 2006. Human airway trypsin-like protease stimulates human bronchial fibroblast proliferation in a protease-activated receptor-2-dependent pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 290:L385-395. [DOI] [PubMed] [Google Scholar]
- 18.Murakami, M., T. Towatari, M. Ohuchi, M. Shiota, M. Akao, Y. Okumura, M. A. A. Parry, and H. Kido. 2001. Mini-plasmin found in the epithelial cells of bronchioles triggers infection by broad-spectrum influenza A viruses and Sendai virus. Eur. J. Biochem. 268:1-10. [DOI] [PubMed] [Google Scholar]
- 19.Netzel-Arnett, S., J. D. Hooper, R. Szabo, E. L. Madison, J. P. Quigley, T. H. Bugge, and T. M. Antalis. 2003. Membrane anchored serine proteases: a rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer Metastasis Rev. 22:237-258. [DOI] [PubMed] [Google Scholar]
- 20.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 21.Paoloni-Giacobino, A., H. Chen, M. C. Peitsch, C. Rossier, and S. E. Antonarakis. 1997. Cloning of the TMPRSS2 gene, which encodes a novel serine protease with transmembrane, LDLRA, and SRCR domains and maps to 21q22.3. Genomics 44:309-320. [DOI] [PubMed] [Google Scholar]
- 22.Steinhauer, D. A. 1999. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology 258:1-20. [DOI] [PubMed] [Google Scholar]
- 23.Stieneke-Gröber, A., M. Vey, H. Angliker, E. Shaw, G. Thomas, C. Roberts, H. D. Klenk, and W. Garten. 1992. Influenza virus hemagglutinin with multibasic cleavage site is activated by furin, a subtilisin-like endoprotease. EMBO J. 11:2407-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi, M., T. Sano, K. Yamaoka, T. Kamimura, N. Umemoto, H. Nishitani, and S. Yasuoka. 2001. Localization of human airway trypsin-like protease in the airway: an immunohistochemical study. Histochem. Cell. Biol. 115:181-187. [DOI] [PubMed] [Google Scholar]
- 25.Towatari, T., M. Ide, K. Ohba, Y. Chiba, M. Murakami, M. Shiota, M. Kawachi, H. Yamada, and H. Kido. 2002. Identification of ectopic anionic trypsin I in rat lungs potentiating pneumotropic virus infectivity and increased enzyme level after virus infection. Eur. J. Biochem. 269:2613-2621. [DOI] [PubMed] [Google Scholar]
- 26.Wilson, S., B. Greer, J. Hooper, A. Zijlstra, B. Walker, J. Quigley, and S. Hawthorne. 2005. The membrane-anchored serine protease, TMPRSS2, activates PAR-2 in prostate cancer cells. Biochem. J. 388:967-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaoka, K., K. Masuda, H. Ogawa, K. Takagi, N. Umemoto, and S. Yasuoka. 1998. Cloning and characterization of the cDNA for human airway trypsin-like protease. J. Biol. Chem. 273:11895-11901. [DOI] [PubMed] [Google Scholar]
- 28.Yasuoka, S., T. Ohnishi, S. Kawano, S. Tsuchihashi, M. Ogawara, K. Masuda, K. Yamaoka, M. Takahashi, and T. Sano. 1997. Purification, characterization, and localization of a novel trypsin-like protease found in the human airway. Am. J. Respir. Cell. Mol. Biol. 16:300-308. [DOI] [PubMed] [Google Scholar]
- 29.Zhirnov, O. P., M. R. Ikizler, and P. F. Wright. 2002. Cleavage of influenza A virus hemagglutinin in human respiratory epithelium is cell associated and sensitive to exogenous antiproteases. J. Virol. 76:8682-8689. [DOI] [PMC free article] [PubMed] [Google Scholar]