Abstract
The primate TRIM5 proteins constitute a class of restriction factors that prevent host cell infection by retroviruses from different species. The TRIM5 proteins act early after virion entry and prevent viral reverse transcription products from accumulating. We recently found that proteasome inhibitors altered the rhesus monkey TRIM5α restriction of human immunodeficiency virus type 1 (HIV-1), allowing reverse transcription products to accumulate even though viral infection remained blocked. To assess whether sensitivity to proteasome inhibitors was a common feature of primate TRIM5 proteins, we conducted a similar analysis of restriction mediated by owl monkey TRIM-cyclophilin A (CypA) or human TRIM5α. Similar to rhesus monkey TRIM5α restriction, proteasome inhibition prevented owl monkey TRIM-CypA restriction of HIV-1 reverse transcription, even though HIV-1 infection and the output of 2-LTR circles remained impaired. Likewise, proteasome inhibition alleviated human TRIM5α restriction of N-tropic murine leukemia virus reverse transcription. Finally, HIV-1 reverse transcription products escaping rhesus TRIM5α restriction by proteasome inhibition were fully competent for integration in vitro, demonstrating that TRIM5α likely prevents the viral cDNA from accessing chromosomal target DNA. Collectively, these data indicate that the diverse TRIM5 proteins inhibit retroviral infection in multiple ways and that inhibition of reverse transcription products is not necessary for TRIM5-mediated restriction of retroviral infection.
Retroviruses invading host cells must overcome numerous cellular factors designed to inhibit infection and replication. One such class of cellular restriction factors that recognize retroviral capsids entering the cell includes murine Friend virus susceptibility factor 1 (Fv1) and the TRIM5 family of proteins in primates (reviewed in references 5 and 13). These restriction factors protect host cells from infection by different retroviruses and thereby determine the species specificities of these retroviruses. However, their mechanism of action remains poorly defined.
The Fv1 locus was first identified in the 1970s and mediates the ability of different murine leukemia virus (MLV) strains to infect various types of mice. Two predominant Fv1 alleles have been identified: Fv1n and Fv1b, found in NIH Swiss and BALB/c mice, respectively. Sensitivity to different Fv1 alleles classifies MLV strains as N tropic or B tropic. Fv1n allows infection by N-tropic strains of murine leukemia virus (N-MLV strains) but restricts the infection of B-tropic MLV strains (B-MLV strains). Conversely, Fv1b permits B-MLV infection but restricts N-MLV infection (27). Restriction resulted in an early block to MLV infection after reverse transcription (RT) but before integration into the host cell genome (16, 28) and could be overcome or saturated by excess amounts of incoming, affected virus (11, 27). The gene responsible for mediating this restriction encodes a protein with homology to the Gag protein of the ERV-L family of endogenous retroviruses (4), although its precise mechanism of action remains unclear.
Similar restrictions to human immunodeficiency virus type 1 (HIV-1) or N-MLV infection of primate cells have since been identified (35). Like Fv1, sensitivity to these restrictions was mediated by determinants in the retroviral capsid protein (35), restriction could be overcome with excess amounts of incoming affected virus (9, 14), and restriction manifested as an early block to viral replication in target cells. However, unlike Fv1, these primate cell restriction factors acted at an earlier step in replication, preventing the accumulation of reverse transcription products (9, 33, 35). Recently, the rhesus TRIM5α protein (rhTRIM5α) was shown to mediate the restriction of HIV-1 infection in rhesus monkey cells (33). Subsequent work isolated TRIM5α orthologues in other primate species, including humans, African green monkeys, and squirrel monkeys, all of which facilitated restriction of invading retroviruses originating from different species (reviewed in references 23 and 36). For instance, human TRIM5α (huTRIM5α) was found to protect cells from infection by N-MLV, even though B-MLV escaped this restriction (24, 40). Furthermore, a novel fusion protein between TRIM5 and cyclophilin A (TRIM-CypA) was identified in owl monkey cells (31). Formed by retrotransposition of the CypA pseudogene into the TRIM5 locus, producing a fusion protein where CypA replaces the SPRY domain of TRIM5α, TRIM-CypA potently inhibits HIV-1 infection. Therefore, the TRIM5 proteins are a family of restriction factors protecting primate cells from infection by a variety of different retroviruses.
The presence of an E3 ubiquitin ligase domain within TRIM5α suggested that this protein may mediate restriction by inducing the proteasomal degradation of incoming viral cores. However, mutating key cysteine residues required for E3 ubiquitin ligase activity does not abrogate rhTRIM5α-mediated restriction, although the degree to which this mutant can restrict HIV-1 infection is reduced (15, 33). Other work using proteasome-inhibiting drugs or temperature-sensitive cell lines that block ubiquitination at nonpermissive temperatures failed to prevent TRIM5 proteins from restricting retroviral infection (25, 34), again suggesting that ubiquitination and proteasome function is not required for TRIM5α-mediated restriction of infection. We have also found that proteasome inhibitors fail to overcome rhTRIM5α restriction of HIV-1 infection (38). However, we did find that proteasome inhibition rescued HIV-1 RT products from restriction, even though infection and the production 2-LTR circles remained impaired (38). The ability of proteasome inhibitors to uncouple the restriction of infection from the restriction of RT products therefore exposes an intermediate stage in the rhTRIM5α restriction of HIV-1 reminiscent of Fv1 restriction. In this case, reverse transcription proceeds but integrated provirus formation is impaired (35). This suggests that rhesus TRIM5α inhibits HIV-1 infection via a multistep mechanism and that the phase resulting in a loss of reverse transcription products likely involves ubiquitination and proteasome activity (38). Here, we demonstrate that proteasome inhibition relieves the restriction of retroviral RT by diverse TRIM5 family members, thus unveiling a common intermediate of TRIM5-mediated restriction. Moreover, we find that RT products rescued from rhTRIM5α restriction by MG132 treatment are functional for integration, demonstrating that the intermediate detected after proteasome inhibition is on the functional infectious pathway.
MATERIALS AND METHODS
Cells and pharmaceuticals.
293T, HeLa, and BHK21 cells (American Type Culture Collection), HeLa cells stably expressing huTRIM5α or rhTRIM5α (Joseph Sodroski, Harvard Medical School) (33), and owl monkey cells (Paul Bieniasz, Aaron Diamond AIDS Research Center) were cultured at 37°C with 7% CO2 in Dulbecco's modified Eagle's medium (HyClone). Media contained 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 292 μg/ml l-glutamine (Gibco), and 10 μg/ml ciprofloxacin (Cellgro). Puromycin (1 μg/ml; Sigma) was also added to HeLa cultures expressing huTRIM5α or rhTRIM5α to maintain selection. Stocks of MG132 (Sigma), cyclosporine (CsA; Sigma), and nevirapine (AIDS Research and Reference Program, Division of AIDS, NIAID, NIH) were prepared in ethanol or dimethyl sulfoxide and stored at −20°C. Drugs were used at final concentrations of 1 μg/ml MG132, 5 μM CsA, and 10 μM nevirapine.
Infections.
To generate virus, 10-cm plates of 293T cells were transfected with appropriate plasmids using polyethylenimine (molecular weight, 25,000; Polysciences). Vesicular stomatitis virus g protein (VSV-g)-pseudotyped R7ΔenvGFPHIV-1 was produced by transfecting 12 μg of R7Δenv and 8 μg of VSV-g plasmids. The VSV-g-pseudotyped N-MLVGFP or B-MLVGFP vector was generated by transfecting 8 μg of VSV-g, 8 μg of the MLVGFP vector, and 8 μg of either N-MLV Gag or B-MLV Gag packaging plasmids (provided by Greg Towers, Wohl Virion Institute, University College London, London, United Kingdom) (35). Virus was harvested as previously described (8). Virus yield in R7ΔenvGFPHIV-1 preparations was measured using an HIV-1 p24Gag enzyme-linked immunosorbent assay kit (Perkin Elmer). The N- and B-tropic MLV vectors were titrated on BHK cells to normalize the titer.
To measure R7ΔenvGFPHIV-1, N-MLVGFP, or B-MLVGFP virus infectivity, equivalent cell numbers in a 96- or 24-well plate were pretreated for 2 h with drug where appropriate and virus was added with or without drug. After 14 h of infection, the virus inoculum was replaced with fresh media without drug and green fluorescent protein (GFP) expression was measured 48 to 72 h later using a FACSCalibur flow cytometer (Becton Dickinson). The percentage of GFP-positive cells in each sample was graphed. The results of representative experiments are shown.
Quantitative real-time PCR.
For real-time PCR studies, 12-well plates were seeded with cells at either 1.5 × 105 cells/well (see Fig. 1 and 3) or 0.6 × 105 cells/well (see Fig. 2). The next day, cells were pretreated with drug 1 to 2 h before infection where appropriate. Virus was also pretreated with DNase I (20 U/ml; Sigma) in 10 mM MgCl2 for 1 h at room temperature before it was added to cells with drug as appropriate. Two hours into the infection, equal volumes of media with appropriate drug were added to infections and cultures were typically returned to 37°C for a further 12 h before being harvested. Cellular DNA was harvested using the QIAGEN DNeasy tissue kit (with optional RNase A treatment as per the instructions) and digested with DpnI (New England Biolabs) for 4 h at 37°C. Fifty nanograms of digested DNA was analyzed for DNA products via real-time PCR using the iCycler iQ real-time PCR detection system (Bio-Rad), iQ SYBR green Supermix (Bio-Rad), and published primers for HIV-1 late RT products, 2-LTR circles, or the GFP reporter in MLV RT products (2, 7). Human or rhesus β-actin primers were used to measure β-actin in HeLa-based cell types or in owl monkey and BHK21 samples, respectively (8, 38). Finally, 10-fold dilutions of the R7Δenv proviral plasmid, the R7Δenv 2-LTR junction plasmid, the MLVGFP vector plasmid, or genomic DNA, diluted in 30 ng/μl tRNA, were used to produce standard curves for HIV-1 late RT product, 2-LTR circle, MLVGFP, and β-actin quantification, respectively.
FIG. 1.
The MG132 proteasome inhibitor rescues HIV-1 reverse transcription from owl monkey TRIM-CypA and rhTRIM5α restriction. HeLa (white bars), stable rhTRIM5α HeLa (black bars), or OMK (gray bars) cells were infected with 84 ng p24Gag/well of VSV-g-pseudotyped R7-GFPHIV-1 for 14 h overnight in the absence of drug, with MG132, with CsA, or with both MG132 and CsA. Drug was removed and cells were analyzed for infection using flow cytometry for GFP 48 h later (A), or cellular DNA was harvested and analyzed for the numbers of late RT products (B) and 2-LTR circles (C) formed per cell (β-actin) using real-time PCR. (A) The percentages of GFP-positive cells from a representative experiment are shown. (B and C) Error bars indicate the standard deviations of triplicate values. Nev, nevirapine.
FIG. 3.
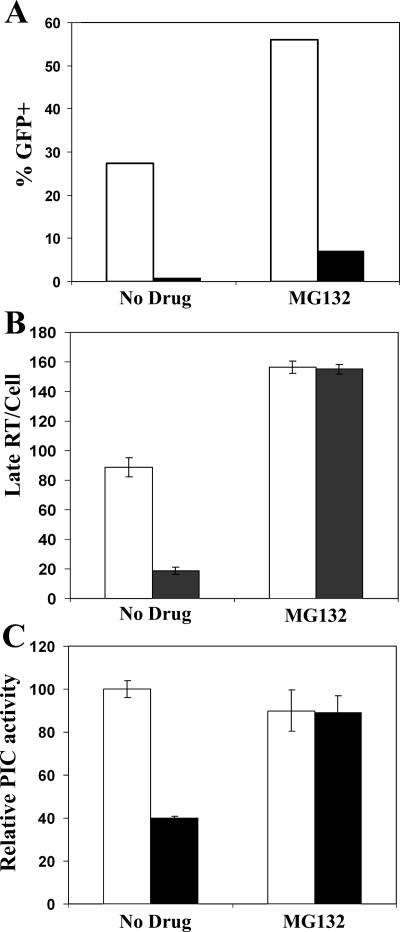
HIV-1 late RT products rescued from rhTRIM5α restriction by MG132 are integration competent. HeLa cells (white bars) and stable rhTRIM5α HeLa cells (black bars) were infected with VSV-g-pseudotyped R7-GFPHIV-1 in the presence or absence of MG132 for 7 h. (A) Cells were then incubated an additional 48 h and analyzed for infection via flow cytometry for GFP. (B) Cell DNA was analyzed for late RT products by real-time PCR. (C) Relative PIC activity was determined by assaying PIC-containing cytoplasmic extracts for in vitro integration.
FIG. 2.
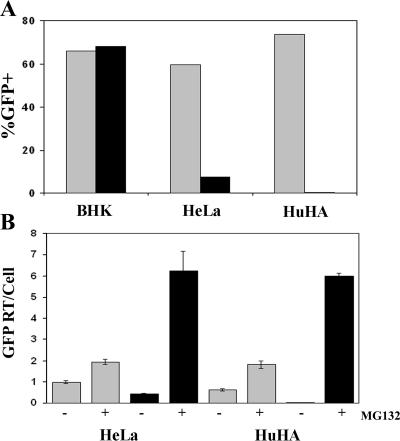
MG132 rescues N-MLV reverse transcription from human TRIM5α restriction. (A) BHK, HeLa, and stable huTRIM5α HeLa cells were infected for 14 h with 3.6 × 104 infectious units of VSV-g-pseudotyped B-tropic (gray bars) or N-tropic (black bars) MLV vectors expressing GFP. Cells were analyzed 48 h later for infection by flow cytometry for GFP; the percentages of GFP-positive cells from a representative experiment are shown. (B) HeLa and stable huTRIM5α HeLa cells were infected with B-tropic (gray bars) or N-tropic (black bars) MLV vectors in the presence or absence of MG132 as indicated, and cellular DNA was harvested and then analyzed for the number of GFP RT products per cell 14 h after infection using real-time PCR.
Integration assays.
For integration experiments, 10-cm plates of HeLa or rhTRIM5αHA.HeLa cells were pretreated for 1 h with MG132 where appropriate and infected with VSV-g-pseudotyped R7ΔenvGFPHIV-1 for 7 h with or without MG132. Cells were collected and divided into three samples. First, a small sample was reseeded into a 24-well plate and infection analyzed 48 h later by flow cytometry for GFP as described above. Second, cellular DNA was extracted from another small sample and HIV-1 late RT and β-actin were measured by real-time PCR as described above. Finally, the remaining cells were used to prepare preintegration complex (PIC)-containing cytoplasmic extracts as described previously (22) and assayed for in vitro integration.
For in vitro integration assays, 200 μl of PIC-containing cytoplasmic extract was incubated with 3 μg/ml pTZ18U/PL at 37°C for 45 min. Control reaction mixtures lacking target DNA were run in parallel. Deproteinized samples recovered after precipitation with ethanol were resuspended in 50 μl of 10 mM Tris-HCl (pH 7.5). HIV-1 late RT levels in 2 μl of plus-target samples were quantified by real-time PCR as described previously (21). PIC activities were quantified using real-time PCR as described elsewhere (22). Briefly, 5 μl of DNA subjected to 23 cycles of an initial PCR round was diluted 1:1,000 for subsequent real-time PCR. Integration activities were quantified by comparison to a 1:4 dilution series of PIC integration products derived from HeLa cells infected in the presence of MG132. PIC activities were normalized to the level of cDNA substrate in each sample.
RESULTS
MG132 rescues HIV-1 reverse transcription but not 2-LTR circle production or infection from TRIM-CypA restriction.
In previous work, we demonstrated that rhesus TRIM5α restriction of HIV-1 late RT products could be relieved by proteasome inhibitors, even though viral infection remained blocked (38). To assess whether restriction by other primate TRIM5α orthologues was similarly affected by proteasome inhibitors, we examined the impact of proteasome inhibition on restriction by the TRIM-CypA and human TRIM5α orthologues.
Restriction of HIV-1 infection in owl monkey kidney (OMK) cells is mediated by the TRIM-CypA orthologue of TRIM5α, and restriction can be abolished by adding the CsA immunosuppressive drug (31, 37). To determine the concentration of HIV-1 that was sensitive to TRIM-CypA restriction in OMK cells, twofold dilutions of VSV-g-pseudotyped HIV-1 containing a GFP reporter were added to equal amounts of OMK cells and infection was measured 48 h later via GFP expression (data not shown). HeLa cells or HeLa cells stably expressing rhesus TRIM5α fused to a hemagglutinin tag (rhTRIM5αHA) (33) were infected in parallel as unrestricted and restricted controls, respectively. HIV-1 infection was restricted in the rhTRIM5αHA-expressing cells at all virus dilutions compared to infection in the unrestricted HeLa cells. Infection of OMK cells was also restricted relative to that of HeLa cells at most concentrations but to a lesser extent than in the rhTRIM5α-expressing cells (data not shown). In subsequent experiments assessing the impact of proteasome inhibitors on TRIM-CypA restriction of HIV-1, cells were infected with a viral dilution that efficiently infected HeLa cells but that did not saturate restriction in OMK cells.
To assess whether proteasome inhibitors could relieve TRIM-CypA restriction of HIV-1 infection, OMK cells were infected in the presence or absence of MG132 and CsA (Fig. 1A). As previously observed, MG132 treatment did not relieve the ability of rhTRIM5α to inhibit HIV infection (34, 38). Similarly, TRIM-CypA restriction of HIV-1 in OMK cells was not affected by MG132 treatment (Fig. 1A), as previously reported (25). In contrast, CsA treatment did relieve restriction mediated by OMK cells irrespective of MG132, agreeing with published reports (31, 37). However, CsA treatment did not relieve rhTRIM5α restriction of HIV-1 infection (Fig. 1A), which is at odds with recent work (1, 18, 34), perhaps reflecting different cell types, rhTRIM5α expression levels, and/or HIV-1 infection levels used in the various studies.
As proteasome inhibitors rescue HIV-1 late RT product formation but not infection from rhTRIM5α restriction (38), we next assessed whether proteasome inhibitors similarly rescued HIV-1 late RT from TRIM-CypA restriction in OMK cells (Fig. 1B). Similar to rhTRIM5α-expressing cells, MG132 treatment induced a large increase in HIV-1 late RT products in OMK cells relative to the level in untreated cells (Fig. 1B). Furthermore, the late RT product level in MG132-treated OMK cells was comparable to late RT product levels when these cells were treated with CsA, which stops restriction to HIV-1 infection (Fig. 1A). Hence, MG132 treatment rescues HIV-1 late RT products from restriction in OMK cells.
When reverse-transcribed viral cDNA enters the nucleus, a proportion can form aberrant circles instead of integrating into the host chromosome to form a provirus. These circles may form through homologous recombination or end-to-end joining by the nonhomologous end-joining pathway, producing 1-LTR or 2-LTR circles, respectively (6, 20). As circles are speculated to form primarily in the nucleus, they serve as markers of nuclear localization of the viral cDNA (6). Given that proteasome inhibitors rescue HIV-1 late RT from TRIM-CypA restriction (Fig. 1B), we thus examined whether proteasome inhibitors also rescued HIV-1 2-LTR circle production from TRIM-CypA restriction (Fig. 1C). In correlation with the infection results (Fig. 1A), MG132 treatment had little impact on the restriction of HIV-1 2-LTR circles in OMK cells, even though CsA treatment, which blocks restriction, did increase 2-LTR circle output (Fig. 1C). The failure of MG132 to completely rescue HIV-1 2-LTR circles from TRIM-CypA restriction is similar to the results of previous work and the work shown here with rhTRIM5α, where MG132 did not rescue 2-LTR circle levels in rhTRIM5α-expressing HeLa cells to the level in unrestricted HeLa control cells (Fig. 1C). However, we note that MG132 did confer larger increases in 2-LTR circles in the rhTRIM5α-expressing cells than in OMK cells. Therefore, similar effects on rhTRIM5α restriction, the MG132 proteasome inhibitor can rescue HIV-1 late RT production from restriction in OMK cells (Fig. 1B), even though 2-LTR circle output (Fig. 1C) and viral infection (Fig. 1A) remain impaired.
MG132 treatment relieves human TRIM5α restriction of N-MLV reverse transcription.
We have now shown that proteasome inhibitors can rescue HIV-1 reverse transcription, but not infection, from restriction by two different TRIM5α orthologues: rhTRIM5α and owl monkey TRIM-CypA. To investigate whether this phenomenon was specific to the restriction of HIV-1 or applied to the restriction of other retroviruses, the impact of proteasome inhibitors on human TRIM5α restriction of N-MLV was examined. For this work, we generated N-tropic and B-tropic MLV vectors expressing GFP (35) pseudotyped with the VSV-g envelope protein. As B-MLV is not restricted by human TRIM5α (24, 40), this virus serves as an unrestricted control. To normalize infection between the vectors for subsequent experiments, the vectors were first titrated on baby hamster kidney (BHK) cells (data not shown) that lack human TRIM5α and exhibit modest antiretroviral activity upon N-MLV infection versus B-MLV (3). To verify that huTRIM5α restricted N-MLV infection, BHK cell-normalized viral inputs were then added to BHK cells or HeLa cells expressing either endogenous huTRIM5α (HeLa) or endogenous plus exogenous hemagglutinin-tagged huTRIM5α (HuHA) (34). While N-MLV and B-MLV infected BHK cells to similar levels, N-MLV infection was restricted in both HeLa cell lines compared to B-MLV infection (Fig. 2A). Further, the extent of N-MLV restriction in the HeLa cell lines correlated with their huTRIM5α expression, with the restriction observed in the HuHA cells expressing endogenous and exogenous huTRIM5α being more severe than in parental HeLa cells expressing just endogenous huTRIM5α (Fig. 2A).
The restriction of MLV RT products was then measured in these cell lines via quantitative real-time PCR using primers directed to the GFP reporter in the MLV vectors. As this GFP reporter is reverse transcribed and thus detected by our primers following cDNA synthesis after the first-strand transfer jump in reverse transcription, this product therefore represents a middle phase in the reverse transcription process. In both HeLa cell types, the restriction to N-MLV infection was manifest as a reduction in RT products relative to the level in the B-MLV control (Fig. 2B). However, in the presence of MG132, RT product levels from N-MLV increased 14.6-fold in HeLa cells and about 300-fold in HuHA cells, rising to overall similar levels in both cell types despite the more severe restriction of N-MLV infection in HuHA cells without drug (Fig. 2A). In contrast, B-MLV RT product levels increased only two- to threefold in either cell line (Fig. 2B). Therefore, MG132 proteasome inhibitor rescued N-MLV RT products from restriction by huTRIM5α, demonstrating that this phenomenon was not unique to HIV-1 restriction by TRIM5α orthologues and also applied to N-MLV. Unfortunately, it was not possible to examine the effect of MG132 treatment on MLV infection in these experiments as MG132 treatment impaired cell division. Since MLV can infect only dividing cells (19, 30), MG132 treatment caused the infection levels of both N-tropic and B-tropic MLV vectors to decrease overall (data not shown), complicating data interpretation. Given this impact of MG132 on cell cycle and infection, combined with the point that viral 2-LTR circles form in the nucleus (6) and their production should also decrease if the nuclear access of MLV cDNA is perturbed by MG132, the impact of MG132 on MLV 2-LTR circle output was not assessed here.
HIV-1 cDNA rescued from rhTRIM5α restriction by MG132 is integration competent.
The results of these experiments and our previous work examining the restriction of HIV-1 by rhTRIM5α established that MG132 abrogated the ability of TRIM5 proteins to inhibit RT product formation. However, in no case was MG132 able to relieve the TRIM5-mediated restriction of infection. This suggested that the reverse-transcribed viral cDNA rescued from restriction by MG132 was most likely unable to perform functional integration. This may be due to one of two different possibilities. Either the nucleoprotein complexes housing the reverse-transcribed viral cDNA are inherently defective for integration or these complexes are integration competent but in some way fail to find their chromosomal targets. To distinguish between these two possibilities, HIV-1 cDNA complexes isolated from MG132-treated or untreated HeLa cells or HeLa cells stably expressing rhTRIM5α (33) were tested for in vitro integration activities (Fig. 3).
As expected (38), HIV-1 could not productively infect MG132-treated HeLa cells expressing rhTRIM5α (Fig. 3A), even though the block to reverse transcription was overcome under these conditions (Fig. 3B). Viral nucleoprotein complexes (preintegration complexes [PICs]) isolated from these cells were assessed for integration into a plasmid DNA target using a recently described real-time PCR assay (22). The resulting integration activities were normalized to the levels of viral late RT substrate in the different samples, thus revealing specific integration activities (Fig. 3C). In the control HeLa cells, PICs that formed in the presence or absence of MG132 displayed similar specific integration activities (Fig. 3C). Importantly, PICs recovered in the presence of MG132 from rhTRIM5α-expressing HeLa cells had activities similar to those recovered from control HeLa cells. In contrast, PICs recovered from rhTRIM5α-expressing cells in the absence of MG132 had at least twofold less integration activity when these samples were normalized for viral late RT content (Fig. 3C). However, MG132 treatment of rhTRIM5α cells also resulted in an approximately eightfold increase in late RT levels compared to cells infected in the absence of drug (Fig. 3B). When this difference in late RT levels is taken into account, MG132 treatment ultimately increased the overall PIC yield per cell by more than 15-fold in rhTRIM5α-expressing HeLa cells. From this, we conclude that HIV-1 nucleoprotein complexes housing reverse-transcribed cDNA that are rescued from rhTRIM5α restriction via MG132 treatment are fully integration competent.
DISCUSSION
While numerous studies have recently found that proteasome inhibition does not affect the ability of TRIM5α proteins to restrict infection (25, 34, 38), we recently found that proteasome inhibition does relieve the rhTRIM5α-mediated inhibition of HIV-1 RT product accumulation (38). Here, we examined the ability of other TRIM5 proteins to restrict retroviral infection in the presence of proteasome inhibitor. We find that, similar to rhTRIM5α restriction of HIV-1, proteasome inhibition uncoupled TRIM-CypA restriction of HIV-1 RT from infection, allowing viral DNA products to accumulate to levels observed in CsA-treated OMK cells, where restriction is abrogated (Fig. 1B). Similarly, restriction of N-MLV RT products by huTRIM5α was substantially relieved by MG132 treatment (Fig. 2B), rising to a similar level in the HeLa cells irrespective of exogenous huTRIM5α expression and the degree of restriction (Fig. 2A). In contrast, MG132 caused only minor increases in RT products by unrestricted B-MLV in huTRIM5α-expressing cells (Fig. 2B). Therefore, the MG132-induced increase in RT products measured during TRIM-CypA restriction of HIV-1 and huTRIM5α restriction of N-MLV clearly arose from the specific relief of TRIM5 restriction of retroviral RT.
As MG132 allows completely reverse-transcribed, integration-competent viral DNA to be generated (Fig. 3C) but does not relieve TRIM5α restriction of infection, this suggests that the TRIM5 restriction mechanism involves at least two steps (Fig. 4). The first step, which involves the recognition and binding of the incoming viral core, is MG132 insensitive and sufficient to prevent infection, consistent with other reports demonstrating that proteasome inhibition does not stop TRIM5α restriction of infection (25, 34, 38). However, the data presented here and our previous work (38) argue for the existence of a second step in TRIM5α restriction that is MG132 sensitive and required for TRIM5α to inhibit RT product accumulation (Fig. 4). While inhibiting this second step with MG132 does not relieve the restriction of infection, it does allow fully formed, integration-competent viral DNA to be generated during rhTRIM5α restriction of HIV-1 (Fig. 3C). This suggests that the viral RT products rescued from restriction by MG132 are in fact on a productive pathway but fail to find their host cell chromosomal target for the ensuing infection (Fig. 4). Interestingly, TRIM5 restriction in this case becomes indistinguishable from restriction mediated by the unrelated murine restriction factor Fv1. That is, Fv1 restriction allows reverse transcription to proceed normally to form nucleoprotein complexes that are functional for integration in vitro but are prevented from integrating into chromosomal DNA in vivo (17, 28).
FIG. 4.
Model for retroviral restriction by TRIM5 proteins. Upon entry into the cytoplasm, determinants on the viral core are recognized and bound by TRIM5 proteins (1). In the absence of proteasome inhibition, the viral core is destroyed via a proteasome-dependent mechanism (2). However, in the presence of proteasome inhibition (e.g., MG132), the core stabilizes and viral cDNA reverse transcribes, unveiling an integration-competent PIC intermediate whose nuclear localization remains impaired analogous to Fv1 restriction (3).
In hindsight, hints that TRIM5α could restrict retroviral infection in multiple steps was evident in an earlier study demonstrating that huTRIM5α restriction of N-MLV RT products was below the magnitude of restriction to infection (26). This indicated that any N-MLV cDNA being reverse transcribed during restriction was blocked at a further step in replication to impede viral infection more than the generation of RT products. More recently, the ability of TRIM5α to restrict retroviral infection at multiple steps is consistent with work from the Stoye laboratory exploring HIV-1 restriction by novel fusion proteins between TRIM1, 18 or 19 domains, and CypA. While all fusion proteins restricted HIV-1 integration and infection, they clustered into two groups that restricted HIV-1 replication either at reverse transcription (analogous to most TRIM5α proteins) or after reverse transcription (39). Therefore, these structure/function-based studies reveal that TRIM proteins can restrict HIV-1 after reverse transcription, similarly to wild-type TRIM5 proteins after proteasome inhibition. Furthermore, the squirrel monkey TRIM5α protein was recently shown to restrict simian immunodeficiency virus from rhesus macaques after reverse transcription (41), as does murine Fv1. Therefore, the ability of TRIM-like proteins to restrict retroviral replication after reverse transcription can be observed in nature and recapitulated genetically (39) or pharmacologically with proteasome inhibitors (Fig. 1 to 3). The identification of this common block to retroviral infection after reverse transcription in all of these diverse cases supports the notion that TRIM5 inhibits retroviral infection through multiple mechanisms.
Recent work tracking the fate of incoming viral capsid in target cells with or without TRIM5α restriction proposes that TRIM5α orthologues recognize incoming retroviral capsid cores and accelerate their core disassembly (34). Here, we find that inhibiting proteasome function relieves TRIM5α restriction of RT products, indicating that proteasome activity is also involved in the restriction of viral reverse transcription. Therefore, it is possible that proteasome activity might play a role in the proposed loss of pelletable capsid during TRIM5α restriction (34) to perturb RT product formation (12).
Based on the available data, we propose the following model for the TRIM5α-mediated restriction of retroviral infection where TRIM5α proteins (i) interact with viral capsid cores (32, 34) and (ii) target these cores for degradation via proteasomes, thereby impairing RT product accumulation. Hence, if proteasome degradation is blocked either with proteasome-inhibiting drugs or by genetic manipulation, RT products can now persist. As TRIM5α proteins assemble into cytoplasmic bodies in vivo (29, 33), it is formally possible in the context of viral infection that TRIM5α proteins might restrict by initially binding and sequestering viral cores into cytoplasmic bodies before proteasome destruction. Thus, when proteasome destruction is blocked via drugs, such sequestering in the cytoplasm might allow subsequent blocks to viral infection, like preventing the reverse-transcribed viral cDNA from accessing the nucleus to limit both 2-LTR-circle formation and integration. Ultimately, using MG132 as a tool to generate the common intermediate of TRIM5α restriction now provides an avenue to more completely define the mechanism underlying the TRIM5α restriction of retroviral infection (10, 29, 33).
Acknowledgments
We thank Greg Towers (Wohl Virion Centre, University College London, London, United Kingdom) for providing the MLV-GFP reporter and MLV Gag packaging plasmids. Furthermore, we are grateful to Joseph Sodroski (Harvard Medical School, Boston, Mass.) and Paul Bieniasz (Aaron Diamond AIDS Research Center) for generously providing the HeLa cells stably expressing huTRIM5α or rhTRIM5α and owl monkey cells, respectively.
This work was supported by National Institutes of Health grants R01 AI47770 to T.J.H. and RO1 AI52014 to A.E. T.J.H. is an Elizabeth Glaser Scientist.
REFERENCES
- 1.Berthoux, L., S. Sebastian, E. Sokolskaja, and J. Luban. 2005. Cyclophilin A is required for TRIM5α-mediated resistance to HIV-1 in Old World monkey cells. Proc. Natl. Acad. Sci. USA 102:14849-14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Besnier, C., Y. Takeuchi, and G. Towers. 2002. Restriction of lentivirus in monkeys. Proc. Natl. Acad. Sci. USA 99:11920-11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besnier, C., L. Ylinen, B. Strange, A. Lister, Y. Takeuchi, S. P. Goff, and G. J. Towers. 2003. Characterization of murine leukemia virus restriction in mammals. J. Virol. 77:13403-13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Best, S., P. Le Tissier, G. Towers, and J. P. Stoye. 1996. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature 382:826-829. [DOI] [PubMed] [Google Scholar]
- 5.Bieniasz, P. D. 2004. Intrinsic immunity: a front-line defense against viral attack. Nat. Immunol. 5:1109-1115. [DOI] [PubMed] [Google Scholar]
- 6.Brown, P. O. 1997. Integration, p. 161-203. In J. M. Coffin, S. H. Hughes, and H. E. Varmus. (ed.), Retroviruses. Cold Spring Harbor Laboratory, Press, Cold Spring Harbor, N.Y.
- 7.Butler, S. L., M. S. Hansen, and F. D. Bushman. 2001. A quantitative assay for HIV DNA integration in vivo. Nat. Med. 7:631-634. [DOI] [PubMed] [Google Scholar]
- 8.Campbell, E. M., R. Nunez, and T. J. Hope. 2004. Disruption of the actin cytoskeleton can complement the ability of Nef to enhance human immunodeficiency virus type 1 infectivity. J. Virol. 78:5745-5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowan, S., T. Hatziioannou, T. Cunningham, M. A. Muesing, H. G. Gottlinger, and P. D. Bieniasz. 2002. Cellular inhibitors with Fv1-like activity restrict human and simian immunodeficiency virus tropism. Proc. Natl. Acad. Sci. USA 99:11914-11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dismuke, D. J., and C. Aiken. 2006. Evidence for a functional link between uncoating of the human immunodeficiency virus type 1 core and nuclear import of the viral preintegration complex. J. Virol. 80:3712-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duran-Troise, G., R. H. Bassin, A. Rein, and B. I. Gerwin. 1977. Loss of Fv-1 restriction in Balb/3T3 cells following infection with a single N tropic murine leukemia virus particle. Cell 10:479-488. [DOI] [PubMed] [Google Scholar]
- 12.Forshey, B. M., U. von Schwedler, W. I. Sundquist, and C. Aiken. 2002. Formation of a human immunodeficiency virus type 1 core of optimal stability is crucial for viral replication. J. Virol. 76:5667-5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goff, S. P. 2004. Retrovirus restriction factors. Mol. Cell 16:849-859. [DOI] [PubMed] [Google Scholar]
- 14.Hatziioannou, T., S. Cowan, S. P. Goff, P. D. Bieniasz, and G. J. Towers. 2003. Restriction of multiple divergent retroviruses by Lv1 and Ref1. EMBO J. 22:385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Javanbakht, H., F. Diaz-Griffero, M. Stremlau, Z. Si, and J. Sodroski. 2005. The contribution of RING and B-box 2 domains to retroviral restriction mediated by monkey TRIM5alpha. J. Biol. Chem. 280:26933-26940. [DOI] [PubMed] [Google Scholar]
- 16.Jolicoeur, P., and D. Baltimore. 1976. Effect of Fv-1 gene product on proviral DNA formation and integration in cells infected with murine leukemia viruses. Proc. Natl. Acad. Sci. USA 73:2236-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jolicoeur, P., and E. Rassart. 1980. Effect of Fv-1 gene product on synthesis of linear and supercoiled viral DNA in cells infected with murine leukemia virus. J. Virol. 33:183-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keckesova, Z., L. M. Ylinen, and G. J. Towers. 2006. Cyclophilin A renders human immunodeficiency virus type 1 sensitive to Old World monkey but not human TRIM5 alpha antiviral activity. J. Virol. 80:4683-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis, P. F., and M. Emerman. 1994. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J. Virol. 68:510-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, L., J. M. Olvera, K. E. Yoder, R. S. Mitchell, S. L. Butler, M. Lieber, S. L. Martin, and F. D. Bushman. 2001. Role of the non-homologous DNA end joining pathway in the early steps of retroviral infection. EMBO J. 20:3272-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Limon, A., E. Devroe, R. Lu, H. Z. Ghory, P. A. Silver, and A. Engelman. 2002. Nuclear localization of human immunodeficiency virus type 1 preintegration complexes (PICs): V165A and R166A are pleiotropic integrase mutants primarily defective for integration, not PIC nuclear import. J. Virol. 76:10598-10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu, R., N. Vandegraaff, P. Cherepanov, and A. Engelman. 2005. Lys-34, dispensable for integrase catalysis, is required for preintegration complex function and human immunodeficiency virus type 1 replication. J. Virol. 79:12584-12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nisole, S., J. P. Stoye, and A. Saib. 2005. TRIM family proteins: retroviral restriction and antiviral defence. Nat. Rev. Microbiol. 3:799-808. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Caballero, D., T. Hatziioannou, A. Yang, S. Cowan, and P. D. Bieniasz. 2005. Human tripartite motif 5alpha domains responsible for retrovirus restriction activity and specificity. J. Virol. 79:8969-8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez-Caballero, D., T. Hatziioannou, F. Zhang, S. Cowan, and P. D. Bieniasz. 2005. Restriction of human immunodeficiency virus type 1 by TRIM-CypA occurs with rapid kinetics and independently of cytoplasmic bodies, ubiquitin, and proteasome activity. J. Virol. 79:15567-15572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perron, M. J., M. Stremlau, B. Song, W. Ulm, R. C. Mulligan, and J. Sodroski. 2004. TRIM5alpha mediates the postentry block to N-tropic murine leukemia viruses in human cells. Proc. Natl. Acad. Sci. USA 101:11827-11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pincus, T., J. W. Hartley, and W. P. Rowe. 1975. A major genetic locus affecting resistance to infection with murine leukemia viruses. IV. Dose-response relationships in Fv-1-sensitive and resistant cell cultures. Virology 65:333-342. [DOI] [PubMed] [Google Scholar]
- 28.Pryciak, P. M., and H. E. Varmus. 1992. Fv-1 restriction and its effects on murine leukemia virus integration in vivo and in vitro. J. Virol. 66:5959-5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reymond, A., G. Meroni, A. Fantozzi, G. Merla, S. Cairo, L. Luzi, D. Riganelli, E. Zanaria, S. Messali, S. Cainarca, A. Guffanti, S. Minucci, P. G. Pelicci, and A. Ballabio. 2001. The tripartite motif family identifies cell compartments. EMBO J. 20:2140-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roe, T., T. C. Reynolds, G. Yu, and P. O. Brown. 1993. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 12:2099-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sayah, D. M., E. Sokolskaja, L. Berthoux, and J. Luban. 2004. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature 430:569-573. [DOI] [PubMed] [Google Scholar]
- 32.Sebastian, S., and J. Luban. 2005. TRIM5alpha selectively binds a restriction-sensitive retroviral capsid. Retrovirology 2:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stremlau, M., C. M. Owens, M. J. Perron, M. Kiessling, P. Autissier, and J. Sodroski. 2004. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427:848-853. [DOI] [PubMed] [Google Scholar]
- 34.Stremlau, M., M. Perron, M. Lee, Y. Li, B. Song, H. Javanbakht, F. Diaz-Griffero, D. J. Anderson, W. I. Sundquist, and J. Sodroski. 2006. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5α restriction factor. Proc. Natl. Acad. Sci. USA 103:5514-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Towers, G., M. Bock, S. Martin, Y. Takeuchi, J. P. Stoye, and O. Danos. 2000. A conserved mechanism of retrovirus restriction in mammals. Proc. Natl. Acad. Sci. USA 97:12295-12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Towers, G. J. 2005. Control of viral infectivity by tripartite motif proteins. Hum. Gene Ther. 16:1125-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Towers, G. J., T. Hatziioannou, S. Cowan, S. P. Goff, J. Luban, and P. D. Bieniasz. 2003. Cyclophilin A modulates the sensitivity of HIV-1 to host restriction factors. Nat. Med. 9:1138-1143. [DOI] [PubMed] [Google Scholar]
- 38.Wu, X., J. L. Anderson, E. M. Campbell, A. M. Joseph, and T. J. Hope. 2006. Proteasome inhibitors uncouple rhesus TRIM5alpha restriction of HIV-1 reverse transcription and infection. Proc. Natl. Acad. Sci. USA 103:7465-7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yap, M. W., M. P. Dodding, and J. P. Stoye. 2006. Trim-cyclophilin A fusion proteins can restrict human immunodeficiency virus type 1 infection at two distinct phases in the viral life cycle. J. Virol. 80:4061-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yap, M. W., S. Nisole, C. Lynch, and J. P. Stoye. 2004. Trim5alpha protein restricts both HIV-1 and murine leukemia virus. Proc. Natl. Acad. Sci. USA 101:10786-10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ylinen, L. M., Z. Keckesova, S. J. Wilson, S. Ranasinghe, and G. J. Towers. 2005. Differential restriction of human immunodeficiency virus type 2 and simian immunodeficiency virus SIVmac by TRIM5α alleles. J. Virol. 79:11580-11587. [DOI] [PMC free article] [PubMed] [Google Scholar]