Abstract
Objective
To investigate the effect of loxapine on peripheral dopamine D2-like and serotonin receptor binding and on psychotic symptoms.
Patients
Patients (n = 24) meeting the diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision, for schizophrenia were selected from an outpatient clinic (age range 18–70 yr).
Methods
Patients were given loxapine (dose determined by a physician) for a period of 12 weeks. There were clinic visits at before treatment began and at 6 weeks and 12 weeks of treatment. Scores on a variety of efficacy and safety scales were recorded at each visit, and blood was drawn for receptor assays.
Results
Patients showed significant improvement on most psychiatric assessment scales after 6 and 12 weeks of treatment with loxapine, and both lymphocyte D2-like and 5-HT2A platelet receptor binding were down-regulated after 6 and 12 weeks. The degree of receptor down-regulation was not significantly correlated with improvements in psychotic symptoms, however.
Conclusion
Loxapine down-regulated both lymphocyte D2-like and platelet 5-HT2A receptors to the same extent, suggesting that both receptors are involved in the mechanism of action of loxapine in patients with schizophrenia.
Medical subject headings: antipsychotic agents; blood platelets; brief psychiatric rating scale; loxapine; lymphocytes; psychiatric status rating scales; receptors, dopamine, D2; receptors, serotonin; schizophrenia
Abstract
Objectif
Examiner l'effet de la loxapine sur la liaison des récepteurs de type D2 de la dopamine et des récepteurs de la sérotonine du sang périphérique et sur les symptômes de psychose.
Patients
Des patients (n = 24) satisfaisant aux critères de diagnostic de la schizophrénie de la quatrième édition révisée du Manuel diagnostique et statistique des troubles mentaux ont été choisis dans une clinique externe (plage d'âges de 18 à 70 ans).
Méthodes
Les patients ont reçu de la loxapine (dose établie par un médecin) pendant 12 semaines. Il y a eu des consultations en clinique avant le début du traitement et aux sixième et douzième semaines de traitement. À chaque consultation, des cotes ont été attribuées selon diverses échelles d'évaluation de l'efficacité et de l'innocuité et du sang a été prélevé pour le dosage des récepteurs.
Résultats
Après 6 et 12 semaines de traitement par loxapine, les patients ont montré une amélioration importante selon la plupart des échelles d'évaluation psychiatrique et on a observé une rétrorégulation de la liaison des récepteurs de type D2 des lymphocytes et des récepteurs de la sérotonine 5-HT2A des plaquettes. Il n'y avait toutefois pas de lien significatif entre le degré de rétrorégulation des récepteurs et l'amélioration des symptômes de psychose.
Conclusion
La loxapine a entraÎné une rétrorégulation semblable des récepteurs de type D2 des lymphocytes et des récepteurs de la sérotonine 5-HT2A des plaquettes, ce qui laisse croire que ces récepteurs jouent un rôle dans le mécanisme d'action de la loxapine chez les patients atteints de schizophrénie.
Introduction
Most antipsychotic drugs currently used for the treatment of schizophrenia can be classified as either typical (classical) or atypical neuroleptics. The therapeutic action of a typical antipsychotic drug is generally accompanied by adverse side effects such as neuroleptic-induced Parkinsonism or tardive dyskinesia. Atypical neuroleptics, such as fluperlapine and clozapine, show a striking dissociation between antipsychotic activity and the adverse side effects seen with classical neuroleptics.1,2,3,4,5,6,7
Loxapine is a mid-potency, typical neuroleptic belonging to the tricyclic dibenzoxazepine family. Several reports have shown that although loxapine treatment produces extrapyramidal side effects (EPS), the incidence is less frequent than it is with treatment with higher potency antipsychotics such as haloperidol or fluphenazine.8,9,10,11 A study comparing the use of loxapine and haloperidol in the treatment of aggressive patients with dementia showed that, although both drugs were equally efficacious, loxapine produced significantly fewer side effects than haloperidol, including extra-pyramidal effects.12 Although loxapine is considered a typical neuroleptic, it shares many structural and pharmacologic properties with the atypical neuroleptic clozapine. However, unlike clozapine, loxapine does not cause agranulocytosis often associated with long-term clozapine treatment.13 Clozapine is more efficacious in treating both positive and negative symptoms of schizophrenia, whereas loxapine has the advantage of producing a calming effect in agitated patients that has not been demonstrated with clozapine.12,14
Earlier studies of loxapine suggested the major pharmacological mode of action involves dopamine D2 receptor antagonism, and to a lesser extent, blocking activity at D1 receptors as well.15 Molecular cloning techniques have facilitated the identification of additional dopamine receptor subtypes and helped to further classify serotonin (5-HT2A) receptor isoforms.16,17,18 Receptor binding studies indicate that central serotonergic mechanisms may also be involved in the action of antipsychotic drugs. Interactions of dopamine and serotonin in the nigrostriatal system are well documented,6,19 and loxapine has been shown not only to antagonize dopamine receptors, but also to potentiate serotonergic transmission by blocking serotonin reuptake.20 PET studies in human patients taking loxapine also show the occupancy of 5-HT2A as well as D2 receptors.21
A number of studies using ligand binding and molecular biology techniques (reverse transcriptase polymerse chain reaction) report the presence of dopamine and serotonin receptors on peripheral blood lymphocytes and platelets.22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 Some of these studies have provided consistent evidence of dopamine receptor-like binding that may have clinical relevance as a marker in psychiatric and neurological disorders.
In this study, we examined the effect of treatment of patients with schizophrenia on lymphocyte dopamine D2-like and platelet serotonin (5-HT2A) receptor binding after 6 and 12 weeks of loxapine treatment.
Methods
This prospective, open-label, noncomparative study was conducted in one Canadian centre. Each patient was treated with haloperidol, as a washout, for at least 3 days before a 12-week treatment with loxapine commenced. The starting dose of loxapine was determined by the physician and was titrated appropriately to reach the optimal therapeutic dose. The daily dose did not exceed 250 mg. Clinic visits were scheduled at baseline, 6 weeks and 12 weeks of treatment. Assessments for efficacy and safety were performed at these times. As well, blood samples were drawn at these times for receptor assays on platelets and lymphocytes. Patients were withdrawn from the study in the event of lack of efficacy, a severe adverse event or withdrawal of consent. Trihexyphenidyl hydrochloride was given for the treatment of extrapyramidal side effects, if needed. No other neuroleptics were permitted during the study.
Selection criteria
Patients were eligible for the study if they were between 18 and 70 years of age, had been diagnosed with schizophrenia according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision, and if results of physical, neurological and laboratory tests and an electrocardiogram were normal. Patients were excluded if they had received depot neuroleptics or other investigational drugs within the 6-week period before the study. Also excluded were pregnant or lactating women, patients with a psychiatric diagnosis other than schizophrenia and patients with a clinically relevant history of alcohol or drug abuse within the last 6 months or a sensitivity or history of nonresponsiveness to loxapine.
Patient characteristics
Of the 25 patients initially enrolled in the study, only 24 were included in the analysis because 1 patient was removed from the study before the month 2 assessment due to inefficacy. Carbamazepine (Tegretol) was added to the loxapine treatment in 1 problematic patient. The mean age of the 5 women and 19 men was 39 years (range 26–68 yr).
Eight patients enrolled on low-dose loxapine (20 mg to 40 mg), 9 patients on medium dose (50 mg to 95 mg) and 7 patients on high dose (100 mg to 250 mg). By the end of 12 weeks, 4 patients were on low, 6 were on medium and 14 were on high-dose treatment. All patients were started initially with the dose of loxapine 50 mg twice daily, which was titrated individually to control the symptoms and stabilize the patients. At 6 weeks, the final titration was done, and in 7 patients the dose needed was 25 mg in the morning and 100 mg at bedtime, and in 15 patients the final titrated dose was 150 mg a day (25 mg in the morning and 125 mg at bedtime). The remaining 2 patients were taking 25 mg in the morning and 125 mg at bedtime.
Assessments
Efficacy was evaluated with the Brief Psychiatric Rating Scale (BPRS), Clinical Global Impression of Schizophrenia (CGI), Positive and Negative Symptoms Scale (PANSS), Quality of Life, Rajotte's Scale, Hamilton Depression Rating Scale and Hamilton Anxiety Scale. At study termination, a global evaluation was performed both by the patient and by the physician, and this was compared with baseline evaluations. Safety was assessed with the Treatment Emergent Symptom Scale (TESS), Udvalg for Kliniske Undersogelser (UKU) Side Effect Rating Scale and Abnormal Involuntary Movement Scale (AIMS).
Preparation of lymphocytes and dopamine D2-like receptor binding
The radioligands [3H]7-OH-DPAT (200 Ci/mmol; 1 Ci = 3.7 х 1010 Bq) and [3H]LSD (70 Ci/mmol) were obtained from Dupont NEN (Boston, Mass.). All other chemicals of the highest available grade were purchased from Sigma Chemical Co. (Oakville, Ont.). The ligand [3H]7-OH-DPAT was used to label D2-like receptors as [3H]spiroperidol results in very high nonspecific binding.25 Furthermore, [3H]7-OH-DPAT labels D2-like receptors in lymphocytes27,28,32 and, thus, it is a useful ligand to label D2-like receptors (D2 as well as D3). In addition, using reverse transcriptase polymerase chain reaction, Kwak et al30 have shown that dopamine D3 receptor is expressed on human lymphocytes and, in fact, D3 receptor transcript is increased in schizophrenia. Considering all of these factors, [3H]7-OH-DPAT was our ligand of choice.
Lymphocytes were isolated as described by Boyum,38 and specific binding for dopamine receptors was determined using methods similar to those described by Rotstein et al.25 Lymphocytes were separated from 20 mL heparinized blood using a Ficoll Paque gradient within 1 hour and suspended in Hank's medium. For D2-like receptors, aliquots of 1 million cells were incubated in the dark at 37°C for 1 hour with 2.0 nM [3H]7-OH-DPAT in the presence or absence of 1 μM (+)butaclamol. The total assay volume was 1 mL, and samples were done in triplicate. After vacuum filtration through Whatman GF/B filters, the filters were washed 7 times with 2 mL of 50 mM Tris–HCl buffer (pH 7.5) and transferred to vials containing 5 mL Aquasol. After overnight equilibration at 4°C in the dark, the vials were subjected to liquid scintillation counting at an efficiency of about 50%. Less than 5% of the binding was accounted for by the platelets at the concentration that contaminated the lymphocyte preparation. Viability of lymphocytes as determined by exclusion of trypan blue dye was greater than 95% both after isolation and incubation.
Platelet membrane preparation and serotonin (5-HT2A) receptor binding
Platelets and membranes were prepared according to a method described by Steckler et al36 with minor alterations. Briefly, 12 mL of patients' blood was collected in vacutainer tubes coated with heparin. One part anticoagulant solution (585 mM trisodium citrate, 70 mM citric acid and 110 mM dextrose) was added to 6 parts blood, and samples were centrifuged at 300 х g for 10 min. The resulting supernatant (platelet-rich plasma) was aspirated and frozen at –70°C. To prepare membranes, 5 volumes of lysis buffer (5 mM Tris–HCL, 5 mM EDTA, pH 7.4) was added to the thawed plasma, the mixture was left on ice for 15 min, and was then homogenized (Brinkman Polytron, setting 6 for 15 s), with subsequent centrifugation at 40 000 х g for 20 min. The pellet was resuspended in assay buffer and sonicated (Sonic 300 Dismembrator, Artek, Farmingdale, NY) at a 30% setting for 15 seconds. Protein content was estimated using the Bradford method (Biorad protein dye reagent), and membranes were used in receptor binding assays.
[3H]LSD binding assay
Assay buffer (50 mM Tris–HCL, 120 mM NaCl, 5 mM KCl, 0.5% ascorbic acid, pH 7.3), 10 nM [3H]LSD and 1 μM (+)spiroperidol (to define nonspecific binding) were added to plastic disposable test tubes, in triplicates, in a total assay volume of 1 mL. The reaction was started with the addition of 200 μg of platelet membrane protein to each tube, and the incubation proceeded at 37°C for 3 hours. The reaction was terminated by filtering the tube contents through GF/B filters that had been presoaked in 0.3% polyethylenimine. The filters were washed 4 times with ice cold buffer (50 mM Tris–HCL, pH 7.4), placed in 5 mL of scintillation fluid (BCS, Amersham Bioscience, Oakville, Ont.), and the radioactivity was counted in a Beckman LS 5000TA scintillation counter.
The ligand [3H]LSD was chosen instead of [3H]ketanserin, because it has been shown by Steckler et al36 and Alda and Hrdina26 that, unlike [3H]ketanserin, [3H]LSD exhibits saturable specific binding only to the 5-HT2A receptors on platelet membranes and not to red blood cells or other sites. Furthermore, several other investigators have also used [3H]LSD as a ligand of choice to label 5-HT2A receptors in platelets.39,40,41
Statistical methods
Paired t-tests were used to compare the mean differences from baseline of D2-like and 5-HT2A receptor binding at 6 and 12 weeks. Repeated analysis of variance (ANOVA) was used to evaluate any time effect on each of the assessment scales: BPRS, PANSS modified, PANSS positive, PANSS negative, PANSS general, PANSS total, Rajotte, Hamilton anxiety, Hamilton depression, Quality of Life and CGI. Repeated ANOVA was used to assess the time effect on each of the UKU side effects (psychic, neurologic, other symptoms) and patient and physician assessments of side effects. Percent down-regulation of D2 and 5-HT2A receptors were calculated as the percent difference from baseline to 6 and 12 weeks divided by baseline.
Results
Efficacy
The mean efficacy parameters are listed in Table 1. Improvement at 6 weeks was significant, but a more significant improvement was noted in positive symptoms than in negative symptoms. At 12 weeks, both positive and negative symptoms showed similar significant improvements. BPRS scores were also significantly improved at 12 weeks (p < 0.001), and the Hamilton Anxiety and Hamilton Depression Rating Scale scores showed consistent improvements in anxiety and depression.
Table 1
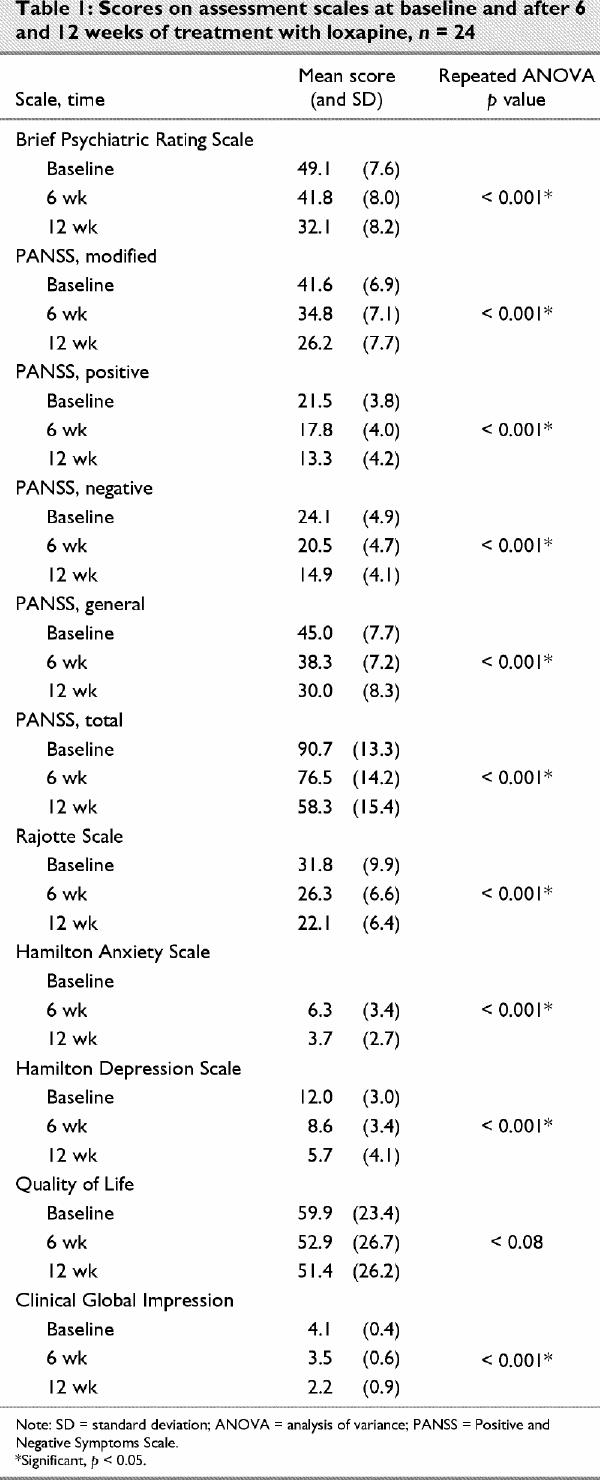
According to the physician's global evaluation, upon study completion, 2 of 24 patients were improved to a significant degree, 15 of 24 patients were much improved and 7 of 24 were mildly improved. According to the Rajotte Scale, a significant improvement in behaviour was seen at 12 weeks (p < 0.001). The Quality of Life Scale scores showed gradual but not significant improvement.
The correlation between the percentage of down-regulation and the percentage of improvement was calculated for each assessment scale. Improvement on the PANSS modified, PANSS general and PANSS total and 5-HT2A down-regulation was significantly correlated (p < 0.05). None of the other parameters, however, were significantly different.
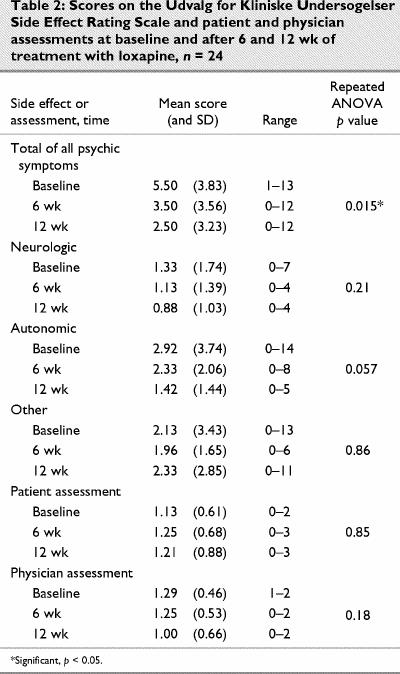
The UKU Side Effects Rating Scale indicated that the cluster of side effects (total of all psychic symptoms) was significantly reduced at 12 weeks compared with baseline and 6 weeks (Table 2). There were also nonsignificant improvements in clusters of side effects of neurologic and autonomic symptoms. Patient assessment scores were lower than physician scores at the beginning of the study and higher than physician scores after 12 weeks of treatment. Patients rated side effects as worse at 12 weeks, whereas physicians thought they had improved.
Table 2
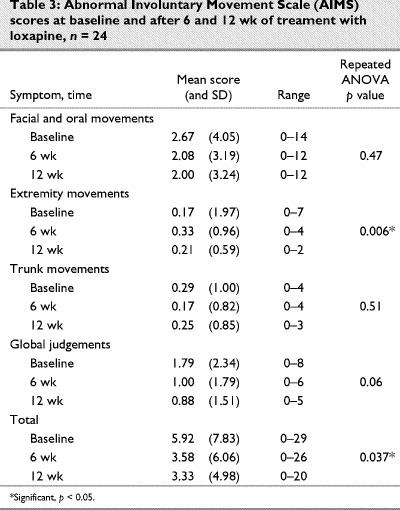
The results of the AIMS are shown in Table 3. Only extremity movements were significantly different than baseline.
Table 3
Laboratory parameters
Two patients experienced an unexpected rise in creatinine phosphokinase (CPK) levels. One rose to 320 U/L from 215 U/L at baseline, and the other rose to 819 U/L from 473 U/L at baseline. Both patients were asymptomatic, and all other tests were within normal levels. Interestingly, one patient's CPK level returned to normal while still in the loxapine post-study treatment period. The other patient's CPK level continued to rise while in the loxapine post-study treatment period. The prolactin levels were elevated to greater than 2 times normal for 11 of 24 patients at baseline. The mean prolactin level at baseline was 34.1 μg/L (standard deviation [SD] = 29.5 μg/L ). At the 12-week assessment, 3 of 21 patients had prolactin levels greater than 2 times normal. The mean prolactin level at 12 weeks was 19.3 μg/L (SD = 13.5 μg/L). No other clinically significant changes in hematology, biochemistry, urinalysis, electrocardiogram or thyroid function tests were seen.
Receptor binding assays
The results of the effect of loxapine treatment on receptor binding in patients with schizophrenia are shown in Fig. 1 and Fig. 2. Limited amounts of proteins and lymphocytes available from each patient (ethical regulations) did not allow for full saturation experiments. Thus, a single-point determination of receptor number was carried out at a saturating concentration. There was clear receptor down-regulation after 6 weeks and 12 weeks of treatment with loxapine. Both dopamine D2-like and serotonin 5-HT2A receptors were significantly reduced in number after loxapine treatment.
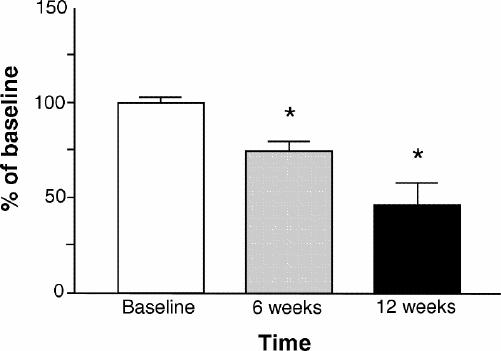
Fig. 1: Serotonergic 5-HT2A receptor binding to platelets after 6 and 12 weeks of loxapine treatment. The data are presented as a percent of baseline. (*p < 0.001).
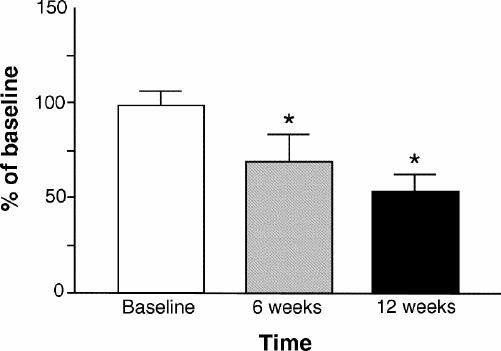
Fig. 2: Dopamine D2-like receptor binding to lymphocytes after loxapine treatment. The data are presented as a percent of baseline. (*p < 0.001).
To determine whether the down-regulation was due to residual presence of the drug after treatment, in a separate experiment, lymphocytes and platelets were incubated with 5 nM loxapine for 2 hours. At the end of incubation, the cells and the membranes were washed with incubation buffer 3 times, and receptor binding was carried out. The number of fmoles bound was similar to that of control baseline, suggesting the down-regulation of receptors was not due to the presence of any residual drug.
Discussion
For the past 2 decades in Canada, loxapine has been prescribed as an effective antipsychotic drug for the treatment of schizophrenia. Loxapine is known to act on negative as well as positive symptoms associated with schizophrenia, has sedative as well as neuroleptic qualities and has a relatively lower incidence of EPS compared with other typical high-potency neuroleptics. Our data clearly illustrate that loxapine causes significant down-regulation of lymphocyte dopamine D2-like and platelet 5-HT2A receptors in patients with schizophrenia, with a subsequent improvement of symptoms. However, the degree of down-regulation did not significantly correlate with the degree of improvement in symptoms.
Although the therapeutic effects of typical neuroleptics have been attributed to D2 receptor blockade, so have the extrapyramidal syndromes such as tardive and acute dyskinesia. Loxapine, however, has relatively fewer EPS associated with its use than haloperidol. This may be due to haloperidol's stronger affinity for D2 receptors. Clozapine shows a distinct dissociation between antipsychotic activity and extrapyramidal effects and rarely displays the tardive dyskinesia and cataleptogenesis associated with other neuroleptic treatments. A possible explanation for this dissociation may be that clozapine has a higher affinity for the D4 receptor than the D2 receptor.42 Therefore, the clinical benefits of clozapine may lie in its D4 receptor antagonism, and the lack of EPS with its relative low affinity for the D2 receptor. Neuroleptic-induced EPS is thought to involve D2 receptors in the basal ganglia. In contrast, D4 receptors have been shown to have predominantly cortical and mesolimbic distribution in the brain. Thus, the lack of EPS associated with clozapine may also be due to its low affinity for striatal D2 receptors or its faster dissociation rate from D2 receptors as compared with haloperidol.3,42,43 The fact that loxapine and clozapine are very similar in molecular structure, and both are dissimilar to haloperidol suggests that their site of action may be similar.
Loxapine is regarded as a typical neuroleptic because it induces catalepsy and tardive dyskinesia and is thought to accelerate dopamine turnover in the striatum. EPS may result from the relatively higher affinity of loxapine for dopamine D2 receptors.39 The results support the hypothesis that D2 receptor antagonism could be the cause of neuroleptic-induced EPS; loxapine has an affinity and acts as a antagonist for both D2-like receptors and 5-HT2A receptors in both the human and bovine species.39 Yet, like clozapine, loxapine shows a preferential and higher binding affinity for dopamine D4 receptors.39 Thus, the major pharmacologic difference between loxapine and clozapine appears to be the higher affinity of loxapine, as compared with clozapine, for the D2 receptor. However, loxapine may produce fewer EPS than other typical neuroleptics because of its relatively stronger affinity for the D4 and 5-HT2A receptor over the D2 receptor.3,8,9,10
Our results indicate a down-regulation of D2-like and 5-HT2A receptors. This is somewhat opposite to the expected results, as most receptor antagonists up-regulate and agonists down-regulate receptors. However, in lymphocytes and platelets, the mechanism involved in receptor regulation may be quite different than in other tissues. It is also possible that loxapine in this system may act as an inverse agonist, thus, down-regulation of both the lymphocyte D2-like and platelet 5-HT2A receptors is noted.44
The dopaminergic mechanism of neuroleptic activity is now generally accepted to involve an increase of D2 receptor density in the brain. Several groups have also investigated the role of serotonin in the mediation of antipsychotic drug actin. Matsubara and Meltzer45 found that a single injection of clozapine or loxapine down-regulated 5-HT2A receptors in the rat frontal cortex. Long-term treatment with loxapine and clozapine also significantly decreased 5-HT2A receptors in the rat brain by approximately 50% but had no effect on D2 receptor density.45
In a previous study, we found that loxapine displayed a very strong affinity for the 5-HT2A receptor in bovine and human tissues and almost equal affinity for the D4 receptor in transfected COS cells,39 which suggests that loxapine may down-regulate serotonin receptors. This may explain loxapine's calming effect on agitated patients. Kapur et al46 has also demonstrated equipotent blockade of 5-HT2A and D2 receptors in humans using positron emission tomography.
To our knowledge there are no other reports in the literature on the effects of other antipsychotic drugs on platelet 5-HT2A receptors and lymphocyte D2-like dopamine receptors in patients with schizophrenia. The reported down-regulation of peripheral dopamine D2-like and 5-HT2A receptors after loxapine treatment suggests that both dopaminergic and serotonergic mechanisms contribute to the therapeutic action and extrapyramidal side effects associated with loxapine in the treatment of schizophrenia.
Acknowledgments
We thank Angela Hrincu for her contributions to the study.
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Amarendra N. Singh, Professor and Head, Division of Psychopharmacology, Department of Psychiatry, Queens University, Kingston ON K7L 5G2; fax 613 544-4643; singha@post.queensu.ca
Submitted Dec. 6, 2001 Revised May 29, 2002, Jul. 17, 2002 Accepted Jul. 24, 2002
References
- 1.Seeman P. Brain dopamine receptors. Pharmacol Rev 1980; 32: 229-313. [PubMed]
- 2.Seeman P. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse 1987;1:133-52. [DOI] [PubMed]
- 3.Seeman P, Van Tol HH. Dopamine receptor pharmacology. Trends Pharmacol Sci 1994;15:264-70. [DOI] [PubMed]
- 4.Van Tol HH, Bunzow JR, Guan HC, Sunahara RK, Seeman P, Niznik HB, et al. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature 1991;350:610-4. [DOI] [PubMed]
- 5.Burki HR, Sayers AC, Ruch W, Asper H. Effects of clozapine and other dibenzo-epines on central dopaminergic and cholinergic systems. Structure-activity relationships. Arzneimittelforschung 1977;27:1561-5. [PubMed]
- 6.Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J Pharmacol Exp Ther 1989; 251: 238-46. [PubMed]
- 7.Seeman P. Brain dopamine receptors. In: Cresse I, Fraser CM, editors. Dopamine receptors. New York: A.R. Liss Publications; 1988. p. 230-45.
- 8.Fenton M, Murphy B, Wood J, Bagnall A, Chue P, Leitner M. Loxapine for schizophrenia. Cochrane Database Syst Rev 2000; (2):CD001943. [DOI] [PubMed]
- 9.Glazer WM. Does loxapine have “atypical” properties? Clinical evidence. J Clin Psychiatry 1999;60(Suppl 10):42-6. [PubMed]
- 10.Meltzer HY, Jayathilake K. Low-dose loxapine in the treatment of schizophrenia: is it more effective and more “atypical” than standard-dose loxapine? J Clin Psychiatry 1999;60(Suppl 10): 47-51. [PubMed]
- 11.Seeman MV. Pharmacologic features and effects of neuroleptics. CMAJ 1981;125:821-6. [PMC free article] [PubMed]
- 12.Carlyle W, Ancill RJ, Sheldon L. Aggression in the demented patient: a double-blind study of loxapine versus haloperidol. Int Clin Psychopharmacol 1993;8:103-8. [DOI] [PubMed]
- 13.DePaulo JR Jr., Ayd FJ Jr. Loxapine: fifteen years' clinical experience. Psychosomatics 1982;23:261-71. [DOI] [PubMed]
- 14.Sunderland T. Treatment of the elderly suffering from psychosis and dementia. J Clin Psychiatry 1996;57(Suppl 9):53-6. [PubMed]
- 15.Buckland PR, O'Donovan MC, McGuffin P. Changes in dopamine D1, D2 and D3 receptor mRNA levels in rat brain following antipsychotic treatment. Psychopharmacology (Berl) 1992; 106:479-83. [DOI] [PubMed]
- 16.Bradley PB, Engel G, Feniuk W, Fozard JR, Humphrey PP, Middlemiss DN, et al. Proposals for the classification and nomenclature of functional receptors for 5-hydroxytryptamine. Neuropharmacology 1986;25:563-76. [DOI] [PubMed]
- 17.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev 1994;46:157-203. [PubMed]
- 18.Saudou F, Hen R. 5-Hydroxytryptamine receptor subtypes in vertebrates and invertebrates. Neurochem Int 1994;25:503-32. [DOI] [PubMed]
- 19.Chesselet MF. Presynaptic regulation of neurotransmitter release in the brain: facts and hypothesis. Neuroscience 1984; 12:347-75. [DOI] [PubMed]
- 20.Delini-Stula A. Neuroanatomical, neuropharmacological and neurobiochemical target systems for antipsychotic activity of neuroleptics. Pharmacopsychiatry 1986;19:134-9. [DOI] [PubMed]
- 21.Kapur S, Remington G, Jones C, Wilson A, DaSilva J, Houle S, et al. High levels of dopamine D2 receptor occupancy with low-dose haloperidol treatment: a PET study. Am J Psychiatry 1996; 153:948-50. [DOI] [PubMed]
- 22.Bondy B, Ackenheil M, Birzle W, Elbers R, Frohler M. Catecholamines and their receptors in blood: evidence for alterations in schizophrenia. Biol Psychiatry 1984;19:1377-93. [PubMed]
- 23.Bondy B, Ackenheil M. 3H-spiperone binding sites in lymphocytes as possible vulnerability marker in schizophrenia. J Psychiatr Res 1987;21:521-9. [DOI] [PubMed]
- 24.Le Fur G, Meininger V, Phan T, Gerard A, Baulac M, Uzan A. Decrease in lymphocyte [3H]spiroperidol binding sites in Parkinsonism. Life Sci 1980;27:1587-91. [DOI] [PubMed]
- 25.Rotstein E, Mishra RK, Singal DP, Barone D. Lymphocyte 3H-spiroperidol binding in schizophrenia: preliminary findings. Prog Neuropsychopharmacol Biol Psychiatry 1983;7:729-32. [DOI] [PubMed]
- 26.Alda M, Hrdina PD. Distribution of platelet 5-HT(2A) receptor densities in suicidal and non-suicidal depressives and control subjects. Psychiatry Res 2000;94:273-7. [DOI] [PubMed]
- 27.Amenta F, Bronzetti E, Felici L, Ricci A, Tayebati SK. Dopamine D2-like receptors on human peripheral blood lymphocytes: a radioligand binding assay and immunocytochemical study. J Auton Pharmacol 1999;19:151-9. [DOI] [PubMed]
- 28.Barbanti P, Fabbrini G, Ricci A, Cerbo R, Bronzetti E, Caronti B, et al. Increased expression of dopamine receptors on lymphocytes in Parkinson's disease. Mov Disord 1999;14:764-71. [DOI] [PubMed]
- 29.Bondy B, Ackenheil M, Ertl M, Minelli G, Mundt C, Peuker B, et al. 3H-spiperone binding capacity in mononuclear cells: a family study. Prog Neuropsychopharmacol Biol Psychiatry 1993; 17:373-81. [DOI] [PubMed]
- 30.Kwak YT, Koo MS, Choi CH, Sunwoo I. Change of dopamine receptor mRNA expression in lymphocyte of schizophrenic patients. BMC Med Genet 2001;2:3. [DOI] [PMC free article] [PubMed]
- 31.Levesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, et al. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci U S A 1992; 89: 8155-9. [DOI] [PMC free article] [PubMed]
- 32.Ricci A, Bronzetti E, Felici L, Tayebati SK, Amenta F. Dopamine D4 receptor in human peripheral blood lymphocytes: a radioligand binding assay study. Neurosci Lett 1997; 229: 130-4. [DOI] [PubMed]
- 33.Ricci A, Bronzetti E, Felici L, Greco S, Amenta F. Labeling of dopamine D3 and D4 receptor subtypes in human peripheral blood lymphocytes with [3H]7-OH-DPAT: a combined radioligand binding assay and immunochemical study. J Neuroimmunol 1998;92:191-5. [DOI] [PubMed]
- 34.Ricci A, Bronzetti E, Mignini F, Tayebati SK, Zaccheo D, Amenta F. Dopamine D1-like receptor subtypes in human peripheral blood lymphocytes. J Neuroimmunol 1999;96:234-40. [DOI] [PubMed]
- 35.Santambrogio L, Lipartiti M, Bruni A, Dal Toso R. Dopamine receptors on human T- and B-lymphocytes. J Neuroimmunol 1993; 45:113-9. [DOI] [PubMed]
- 36.Steckler T, Ruggeberg-Schmidt K, Muller-Oerlinghausen B. Human platelet 5-HT2 receptor binding sites re-evaluated: a criticism of current techniques [corrected]. J Neural Transm Gen Sect 1993;92:11-24. [DOI] [PubMed]
- 37.Takahashi N, Nagai Y, Ueno S, Saeki Y, Yanagihara T. Human peripheral blood lymphocytes express D5 dopamine receptor gene and transcribe the two pseudogenes. FEBS Lett 1992; 314: 23-5. [DOI] [PubMed]
- 38.Boyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol 1976;(Suppl 5):9-15. [PubMed]
- 39.Singh AN, Barlas C, Singh S, Franks P, Mishra RK. A neurochemical basis for the antipsychotic activity of loxapine: interactions with dopamine D1, D2, D4 and serotonin 5-HT2 receptor subtypes. J Psychiatry Neurosci 1996;21:29-35. [PMC free article] [PubMed]
- 40.Bixo M, Allard P, Backstrom T, Mjorndal T, Nyberg S, Spigset O, et al. Binding of [3H]paroxetine to serotonin uptake sites and of [3H]lysergic acid diethylamide to 5-HT2A receptors in platelets from women with premenstrumental dysphoric disorder during gonadotropin releasing hormone treatment. Psychoneuroendorinology 2001;26:551-64. [DOI] [PubMed]
- 41.Hrdina PD, Bakish D, Ravindran A, Chudzik J, Cavazzoni P, Lapierre YD. Platelet serotonergic indices in major depression: up-regulation of 5-HT2A receptors unchanges by antidepressant treatment. Psychiatry Res 1997;66:73-85. [DOI] [PubMed]
- 42.Seeman P. Dopamine receptors: clinical correlates. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The fourth general of progress. New York: Raven Press; 1995. p. 541-9.
- 43.Kapur S, Remington G. Atypical antipsychotics: New directions and new challenges in treatment of schizophrenia. Ann Rev Med 2001;52:503-17. [DOI] [PubMed]
- 44.Malmberg A, Mikaels A, Mohell N. Agonist and inverse agonist activity at the dopamine D3 receptor measured by guanosine 5'--gamma-thio-triphosphate--35S binding. J Pharmacol Exp Ther 1998;285:119-26. [PubMed]
- 45.Matsubara S, Meltzer HY. Effect of typical and atypical antipsychotic drugs on 5-HT2 receptor density in rat cerebral cortex. Life Sci 1989;45:1397-406. [DOI] [PubMed]
- 46.Kapur S, Zipursky R, Remington G, Jones C, McKay G, Houle S. PET evidence that loxapine is an equipotent blocker of 5-HT2 and D2 receptors: implications for the therapeutics of schizophrenia. Am J Psychiatry 1997;154:1525-9. [DOI] [PubMed]


