Abstract
Herpes simplex virus (HSV) stifles cellular gene expression during productive infection of permissive cells, thereby diminishing host responses to infection. Host shutoff is achieved largely through the complementary actions of two viral proteins, ICP27 and virion host shutoff (vhs), that inhibit cellular mRNA biogenesis and trigger global mRNA decay, respectively. Although most cellular mRNAs are thus depleted, some instead increase in abundance after infection; perhaps surprisingly, some of these contain AU-rich instability elements (AREs) in their 3′-untranslated regions. ARE-containing mRNAs normally undergo rapid decay; however, their stability can increase in response to signals such as cytokines and virus infection that activate the p38/MK2 mitogen-activated protein kinase (MAPK) pathway. We and others have shown that HSV infection stabilizes the ARE mRNA encoding the stress-inducible IEX-1 mRNA, and a previous report from another laboratory has suggested vhs is responsible for this effect. However, we now report that ICP27 is essential for IEX-1 mRNA stabilization whereas vhs plays little if any role. A recent report has documented that ICP27 activates the p38 MAPK pathway, and we detected a strong correlation between this activity and stabilization of IEX-1 mRNA by using a panel of HSV type 1 (HSV-1) isolates bearing an array of previously characterized ICP27 mutations. Furthermore, IEX-1 mRNA stabilization was abrogated by the p38 inhibitor SB203580. Taken together, these data indicate that the HSV-1 immediate-early protein ICP27 alters turnover of the ARE-containing message IEX-1 by activating p38. As many ARE mRNAs encode proinflammatory cytokines or other immediate-early response proteins, some of which may limit viral replication, it will be of great interest to determine if ICP27 mediates stabilization of many or all ARE-containing mRNAs.
Host protein synthesis is rapidly shut off during productive infection with herpes simplex virus type 1 (HSV-1), thereby tempering host responses to infection and allowing viral mRNAs to dominate the host translational apparatus (69). Host shutoff is achieved largely through the complementary actions of ICP27 and the virion host shutoff (vhs) protein, which inhibit host mRNA biogenesis and trigger accelerated mRNA decay, respectively (26, 27, 41, 64, 75).
The immediate-early ICP27 protein is an essential multifunctional regulator of viral and cellular gene expression, possessing both activating and repressive functions (47, 76). It binds RNA (50) and shuttles between the nucleus and the cytoplasm (46, 49, 59, 70, 77), properties that likely contribute to its ability to activate expression of certain viral genes. In this context, it has been proposed to aid in the transport of intronless viral messages to the cytoplasm via interactions with the cellular mRNA transport factors Aly/Ref (7, 39) and/or TAP1 (8); however, the functional significance of the interaction with Aly/Ref remains uncertain, as a mutation that eliminates the interaction interface has a relatively minor effect on viral replication (46). ICP27 also binds components of the transcriptional apparatus (31, 94) and translation initiation machinery (25) and stimulates transcription and translation of viral and reporter mRNAs (17, 30, 44). In addition to activating viral gene expression, ICP27 inhibits the expression of most cellular genes, at least in part by preventing the removal of introns from primary RNA transcripts and down-regulating cellular transcription (26, 27, 71, 76, 78).
vhs is a tegument protein that destabilizes cellular and viral mRNAs (41, 42, 82), thus magnifying the repressive effects of ICP27 on cellular gene expression. In contrast to ICP27, vhs is dispensable for HSV-1 lytic replication in cell culture (24, 63, 64, 73); however, vhs-deficient viruses are highly attenuated in animal models (80, 81). vhs displays amino acid similarity to the FEN-1 family of cellular nucleases (14), and recent data demonstrate that it functions as an endoribonuclease in the absence of other cellular or viral proteins (86); in addition, mutations in the nuclease domain of vhs eliminate its shutoff activity (22). Although vhs accelerates the decay of both cellular and viral mRNAs, other types of cytoplasmic RNA are spared (42, 55, 93), implying that vhs employs an mRNA-specific targeting mechanism. vhs binds host translation initiation factors eIF4B and eIF4H, and these interactions have been suggested to target vhs to mRNA substrates (13, 23). In infected cells, vhs degrades the 5′ end of the thymidine kinase mRNA before the 3′ end (35), while studies employing an in vitro assay system using rabbit reticulocyte lysates provided evidence that the initial endonucleolytic cleavage event occurs near regions of translation initiation (15, 16) followed by 5′-to-3′ decay (57).
Although most cellular mRNAs are depleted by the combined actions of ICP27 and vhs, some instead increase in abundance following infection (61, 84). We and others have provided evidence that one such virus-induced mRNA, encoding the immediate-early stress-inducible IEX-1 protein, is actively stabilized during HSV infection (18, 29). IEX-1, encoded by the human IER3 gene, is a potent regulatory protein described to have both pro- and antiapoptotic activities depending on cell type and context (1, 72, 91, 92). IEX-1 mRNA is one of many labile cellular messages that contain an AU-rich instability element (ARE) in their 3′-untranslated regions (UTRs). AREs are found in many mRNAs that code for inflammatory cytokines, transcription factors, and growth factors and act as cis signals to promote rapid mRNA degradation and regulate translation (3, 4, 21). Over 950 human mRNAs contain ARE motifs, which are grouped into several classes based on sequence (4). Degradation of ARE RNAs is typically initiated by deadenylation, likely mediated by the sequential actions of the poly(A) nucleases PAN2/3 and CCR4, followed by decay of the mRNA body in a 3′-to-5′ direction by the exosome, a large RNase complex (reviewed in reference 52). Although AREs are functionally diverse and thus display substantial regulatory heterogeneity, many class II ARE mRNAs are stabilized in response to proinflammatory cytokines which activate the stress-inducible p38 mitogen-activated protein kinase (MAPK) pathway (12, 90). Examples of such p38-stabilized ARE mRNAs are those encoding cyclooxygenase-2 (10, 68), interleukin-6 (53), tumor necrosis factor alpha (10), granulocyte-macrophage colony-stimulating factor (20, 89), and many other proteins involved in inflammation (reviewed in reference 9). In vivo studies have shown that activated p38 blocks decay of ARE mRNAs by inhibiting the initial deadenylation reaction (11); however, the mechanisms involved have yet to be fully defined. Activated p38 phosphorylates the downstream kinase MK2, leading to phosphorylation of the ARE-binding protein tristetraprolin, decreasing its ability to promote RNA decay (79). However, tristetraprolin cannot be the only relevant target of the p38 pathway, as it is not expressed in HeLa cells where p38 exhibits stabilizing activity (11). The Kaposi's sarcoma herpesvirus (KSHV) kaposin B protein activates the p38/MK2 pathway and thus stabilizes certain ARE mRNAs, an effect that may account for the enhanced production of proinflammatory cytokines by cells harboring latent KSHV (48).
The observation that HSV infection stabilizes IEX-1 mRNA (18, 29) implies that one or more features of this transcript render it resistant to vhs-mediated degradation and also raises the possibility that HSV enhances the stability of many ARE transcripts in a fashion similar to KSHV. Thus, elucidating the mechanisms by which HSV stabilizes IEX-1 mRNA is of great interest. Esclatine et al. concluded that vhs is the viral stabilizing factor (18); in marked contrast, we found that vhs has little if any effect on the stability of IEX-1 mRNA during HSV infection (29). The hypothesis that vhs stabilizes IEX-1 mRNA (18) is difficult to reconcile with the known activity of vhs as an mRNA-destabilizing RNase; it also seems to conflict with conclusions drawn in other studies by the same group, where it was argued that IEX-1 mRNA is a preferred target for degradation by vhs (19, 85, 86), a conclusion that we have recently challenged (29). Inasmuch as vhs did not obviously stabilize (or destabilize) IEX-1 mRNA in our experiments, our data implied that one or more viral factors other than vhs are the IEX-1 mRNA-stabilizing factors (29).
Previous studies have shown that ICP27 binds to the ARE elements in the 3′-UTRs of beta interferon (IFN-β) and c-myc mRNAs to increase their stability (6). Furthermore, ICP27 activates the Jun N-terminal protein kinase and p38 stress-activated MAPK pathways (28), suggesting a possible mechanism for stabilization of certain ARE mRNAs. Therefore, we initiated the present studies to clarify the roles of vhs and ICP27 in the stabilization of IEX-1 RNA during HSV-1 infection. Our results indicate that intact polyadenylated IEX-1 mRNA is stabilized at middle to late times postinfection and that this effect requires ICP27, not vhs. Moreover, experiments using both a panel of ICP27 mutant viruses and a p38 kinase inhibitor suggest that ICP27 stabilizes this ARE mRNA by activating the p38 MAPK pathway.
MATERIALS AND METHODS
Cells and viruses.
HeLa and Vero cells were maintained at 37°C in a 5% CO2 atmosphere in Dulbecco's modified Eagle's medium containing 100 U of penicillin and streptomycin per ml and 10% or 5% heat-inactivated fetal bovine serum, respectively. V27 cells are Vero derived and were engineered to express ICP27 upon HSV-1 infection (65). The HSV-1 wild-type strain used in this study was KOS. The following mutant viruses were derived from KOS: ΔSma, which contains a 588-nucleotide deletion in the UL41 gene (63); GFP vhs−, in which all but the last 7 codons of the UL41 open reading frame are deleted and replaced with enhanced green fluorescent protein (eGFP) coding sequences (see below); 5dl1.2, an ICP27 null mutant (47); and LJS1, an ICP27/vhs double knockout virus generated by recombination between 5dl1.2 and ΔSma. The ICP27 deletion virus d27-1 was derived from KOS1.1, as were the ICP27 in-frame deletions dLeu, d1-2, d2-3, d3-4, d4-5, d5-6, and d6-7 and the point mutants M11 and M50T. These viruses have been described previously (46, 50, 51, 54, 58, 65-67), and all but M50T were kindly provided by Stephen Rice (University of Minnesota). Virus absorption, infections, and mock infections were carried out at 37°C in order to minimize stress-mediated induction of IEX-1 mRNA. All viruses were propagated and titers were determined in Vero cells, except the ICP27 mutant viruses, for which V27 cells were used.
Construction of mutant viruses.
The HSV-1 strain KOS vhs null mutant GFP vhs− was constructed as follows. First, the vhs open reading frame present in the 2.3-kb PstI-HincII fragment of viral DNA carried by pUC8 vhs Xba2 (33) was altered by site-directed mutagenesis to create an NcoI cleavage site at the vhs initiation codon. Specifically, the A residue at the −2 position relative to the initiating ATG was converted to C by use of the QuikChange system (Stratagene), generating pUC8 vhs Xba2 NcoI. An NcoI-NotI fragment bearing eGFP coding sequences isolated from peGFP-N1 (Clontech) was then cloned between the NcoI and AatII sites of pUC8 vhs Xba2 NcoI, substituting the vhs open reading frame for eGFP coding sequences. The resulting construct bears eGFP coding sequences linked to the vhs promoter and lacks all but the last 7 codons of the vhs open reading frame. The remaining 7 vhs codons are located downstream of the eGFP termination codon and thus cannot be translated readily. The vhs deletion/substitution mutation was then transferred to the vhs locus of the intact HSV-1 KOS genome by in vivo homologous recombination using standard methods (74). Candidate recombinants were identified as green fluorescent protein-positive plaques and then screened by Southern blot hybridization to verify acquisition of the desired mutation at the vhs locus. One such isolate was designated GFP vhs−. The ICP27/vhs double mutant LJS1 was derived by in vivo recombination between 5dl1.2 and ΔSma, essentially as previously described (43), with the exception that the in vivo recombination and plaque purification of candidate recombinants were done in V27 cells. The KOS1.1 ICP27 mutant M50T was derived as follows. First, the ICP27 coding sequences present in pM27 (67) were altered by converting codon 50 from ATG to CCG, as described above. The resulting plasmid was then used to rescue the lethal phenotype of d27-1 by use of standard methods, and one plaque-purified rescue product was amplified and designated M50T. As previously described (54), the M50T mutation confers resistance to 10 ng/ml leptomycin B.
Antibodies and inhibitors.
H1113 is a mouse monoclonal antibody that recognizes residues 109 to 137 of HSV-1 ICP27 (Rumbaugh-Goodwin Institute for Cancer Research). Rabbit polyclonal anti-p38 and anti-phospho-p38 were from Cell Signaling Technologies. Actinomycin D was from Sigma. The SB203580 inhibitor of p38 kinase activation was obtained from Calbiochem.
RNA preparation and Northern blotting.
Total RNA was isolated from cells in 60-mm culture dishes by use of TRIzol reagent (Invitrogen) according to the manufacturer's instructions. RNA samples (10 μg) were subjected to 1.2% agarose-formaldehyde gel electrophoresis and transferred to a Genescreen membrane (NEN). Blots were hybridized to a radiolabeled probe specific for IEX-1 that was generated by random priming of the 710-bp EcoR1-XhoI fragment from the previously described IEX-1 cDNA IMAGE clone (29). Hybridization was performed at 68°C in ExpressHyb solution (Clontech) according to the user's manual. All quantification was performed with a STORM 860 phosphorimager (Molecular Dynamics). The signal of IEX-1 RNA band A was quantified independently from that of band B, and the averages ± standard errors of three independent experiments were calculated.
Protein preparation and Western blotting.
Six-well plates of mock-infected or infected cells were washed once with phosphate-buffered saline and lysed directly in 1× sodium dodecyl sulfate (SDS) sample buffer. Proteins were denatured by boiling. Equivalent amounts of protein were subjected to SDS-10% polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Amersham). Membranes were blocked in Tris-buffered saline-Tween containing 5% milk and probed overnight in Tris-buffered saline-Tween at 4°C with anti-ICP27 (1:2,000), anti-p38 (1:1,000), or anti-phospho-p38 (1:1,000). Horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse immunoglobulin secondary antibodies were purchased from Sigma and used at a 1:5,000 dilution. Secondary antibody was detected using ECL Plus detection reagents (Amersham Biosciences) according to the manufacturer's instructions.
RESULTS AND DISCUSSION
ICP27, not vhs, is required for HSV-mediated stabilization of intact IEX-1 mRNA.
As reviewed in the introduction, previous studies from another laboratory have reached varied and seemingly conflicting conclusions about the effects of HSV infection and vhs on the stability of the cellular stress-inducible IEX-1 transcript (18, 19, 85): two early reports argued that HSV infection causes selective vhs-dependent degradation of IEX-1 mRNA (19, 85), while a more recent report suggested instead that the mRNA is stabilized in a vhs-dependent fashion in HSV-infected cells (18). As reported previously, we also found that IEX-1 mRNA is stabilized following HSV-1 infection; however, our data indicated that vhs is not required for this effect (29). The present study was initiated to investigate the discrepancies between our results and those of Esclatine et al. (18) and, if warranted, to identify viral factors other than vhs that contribute to stabilization of IEX-1 mRNA.
One possibly relevant difference between our study (29) and that of Esclatine and coworkers (18) was the use of different vhs mutants. Esclatine et al. used R2621 (60), an HSV-1 strain F construct in which the first 343 residues of the 489-residue vhs (UL41) protein are fused in frame to Escherichia coli β-galactosidase; in contrast, we used the HSV-1 strain KOS mutant ΔSma (63), which bears an in-frame deletion of UL41 codons 148 to 343. Although both mutations inactivate the host shutoff function of vhs, it was conceivable that the C-terminal region of the internally deleted protein encoded by ΔSma retains an IEX-1 mRNA-stabilizing function that is absent from the truncated protein specified by R2621. We therefore examined the effects of deleting the majority of the UL41 open reading frame. To this end, we employed GFP vhs−, an HSV-1 KOS mutant in which vhs codons 1 to 483 are replaced with eGFP coding sequences (see Materials and Methods). Note that the 7 remaining vhs codons present in GFP vhs− are located downstream of the eGFP termination codon and thus cannot be translated readily.
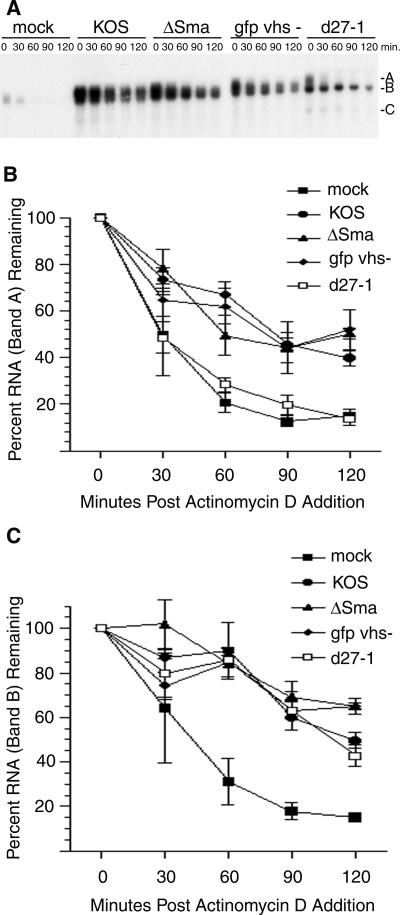
HeLa cells were infected with 5 PFU/cell of GFP vhs−, as well as ΔSma and the parental wild-type HSV-1 strain, KOS. Actinomycin D was added 6 h postinfection (hpi) to prevent de novo transcription, and total RNA harvested at various times thereafter was analyzed by Northern blotting using a radiolabeled probe corresponding to the 3′-UTR of the IEX-1 transcript in order to assess transcript stability (Fig. 1A). As described previously (19, 29, 85), three classes of IEX-1 transcripts are observed in cells infected with wild-type HSV-1: a diffuse band of 1.3 kb representing full-length poly(A)+ IEX-1 mRNA displaying various poly(A) tail lengths (band A), a discrete 1.1-kb band corresponding to deadenylated but otherwise intact mRNA (band B), and a 0.75-kb species corresponding to a truncated fragment lacking sequences 3′ of the ARE (band C) (Fig. 1A; see also Fig. 2 and 3). All of these transcript classes are also present in uninfected cells (29) but increase markedly in abundance following HSV infection. We quantified the intensities of bands A and B in three independent experiments and plotted the results as a function of time after addition of actinomycin D (Fig. 1B and C). Bands A and B both decayed rapidly in uninfected cells, with estimated half-lives of approximately 25 and 45 min, respectively (Fig. 1B and C). In contrast, bands A and B decayed much more slowly in cells infected with wild-type HSV-1 KOS, with estimated half-lives of 90 and 120 min. Previous studies of HSV-induced stabilization of IEX-1 mRNA did not distinguish between the stabilities of bands A and B (18, 29). The present data revealed that wild-type HSV-1 stabilizes both RNA species (see also Fig. 2, 3, and 5). Thus, HSV-1 infection appears to interfere with the initial deadenylation reaction as well as the subsequent decay of the deadenylated mRNA body, implying that two or more steps in the IEX-1 mRNA decay pathway are inhibited. Both bands A and B were also stabilized following infection with the vhs-deficient mutants ΔSma and GFP vhs−; however, in these cases the relative abundance of band C was somewhat reduced compared to levels after infection with wild-type KOS (Fig. 1A), as noted in earlier studies (29, 85). These data confirm that HSV infection stabilizes IEX-1 mRNA and support our previous conclusion that vhs plays little if any role in this process. In particular, the results obtained with GFP vhs− exclude the possibility that the discrepancy between our data and those obtained by Esclatine et al. (18) stems from residual activity of the internally deleted polypeptide specified by ΔSma.
FIG. 1.
ICP27, not vhs, is required for HSV-mediated stabilization of IEX-1 RNA. (A) HeLa cells were mock infected or infected with wild-type HSV-1 (strain KOS), either of two different vhs deletion mutants of KOS (ΔSma or GFP vhs−), or a deletion mutant of KOS1.1 (d27-1) at a multiplicity of infection of 5. Actinomycin D was added at 6 hpi, and total RNA was extracted at the indicated times. The IEX-1 transcript was visualized by Northern blotting. Letters at right indicate bands A, B, and C. (B and C) The relative intensities of band A and band B at each time at were quantified by phosphorimager analysis in three independent experiments. The averages ± standard errors are plotted.
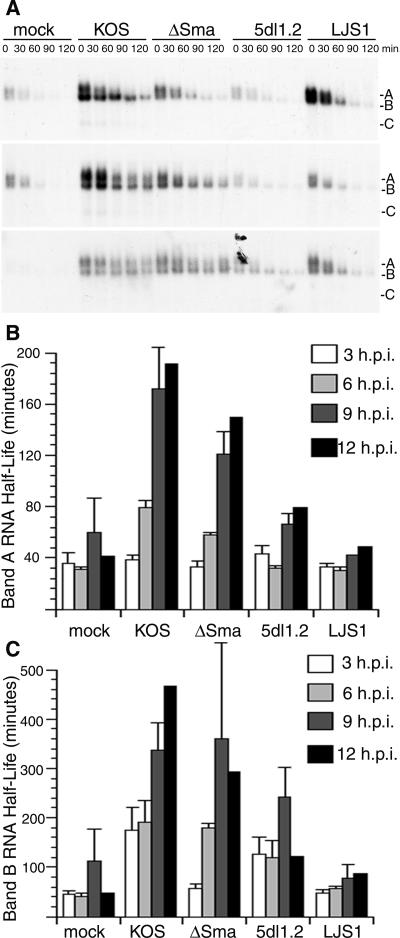
FIG. 2.
ICP27 stabilizes IEX-1 RNA at middle to late times after HSV-1 infection. HeLa cells were mock infected or infected with wild-type HSV-1 (strain KOS), a vhs deletion mutant of KOS (ΔSma), an ICP27 deletion mutant of KOS (5dl1.2), or a vhs and ICP27 double knockout of KOS (LJS1) at a multiplicity of infection of 5. Actinomycin D was added at 3, 6, 9, or 12 hpi. Total RNA was extracted at the indicated times and blotted for the IEX-1 transcript. (A) Representative blots are shown when actinomycin D was added at 3 (top panel), 6 (middle panel), or 9 (bottom panel) hpi. Letters at right indicate bands A, B, and C. (B and C) The half-lives of IEX-1 RNA bands A and B at various infection times were determined from phosphorimager analyses of three independent experiments, and the averages ± standard errors are plotted, except for the 12-h infection, where the averages of two independent experiments are plotted.
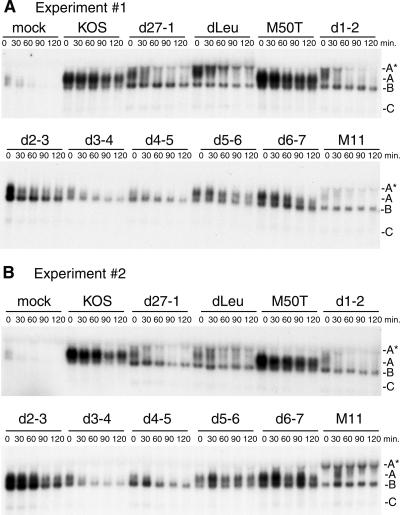
FIG. 3.
Regions of ICP27 required for stabilization of IEX-1 RNA band A. (A and B) HeLa cells were mock infected or infected with wild-type HSV-1 (strain KOS), the ICP27 null mutant of KOS (d27-1), one of the in-frame deletion mutants dLeu, d1-2, d2-3, d3-4, d4-5, d5-6, or d6-7, or one of the point mutants M50T or M11 at a multiplicity of infection of 10. Actinomycin D was added to the infected cultures at 7.5 hpi, and total RNA was extracted and blotted for the IEX-1 transcript as described in the legend for Fig. 1. Results from two representative experiments are displayed. Letters at right indicate bands A*, A, B, and C.
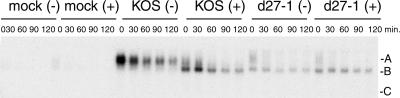
FIG. 5.
Effects of the p38 inhibitor SB203580 on HSV-induced stabilization of IEX-1 RNA band A. HeLa cells were pretreated for 30 min with either SB203580 (10 mM in dimethyl sulfoxide) (+) or an equivalent volume of dimethyl sulfoxide (−) and then mock infected or infected with wild-type HSV-1 KOS or d27-1 at a multiplicity of infection of 10. Inhibitor treatment was continued throughout the infection. Actinomycin D was added to the infected, treated cultures at 7.5 hpi, and total RNA was extracted and blotted for the IEX-1 transcript as described in the legend for Fig. 1. A representative blot is shown. Letters at right indicate bands A, B, and C.
As noted in the introduction, the immediate-early protein ICP27 has been implicated previously in stabilizing certain cellular ARE mRNAs (6) and also has been shown to activate the p38 stress kinase signaling pathway (28). We therefore included the ICP27 null mutant d27-1, derived from HSV-1 KOS1.1, in the experiments depicted in Fig. 1. Strikingly, d27-1-infected cells failed to stabilize IEX-1 band A relative to uninfected cells, while band B was as stable as in cells infected with wild-type KOS (compare Fig. 1B to C; see also Fig. 3 and 5) or KOS1.1 (data not shown). These data indicate that ICP27 is required for stabilization of the full-length polyadenylated form of IEX-1 RNA (band A), an effect that likely stems from inhibition of the initial deadenylation step in the IEX-1 mRNA decay pathway. The data further suggest that one or more viral factors other than ICP27 and vhs are responsible for the enhanced stability of the deadenylated decay product of the IEX-1 transcript (band B) in infected cells.
Another possibly relevant difference between our previous study (29) and that of Esclatine et al. (18) was the time after infection when the actinomycin D chase was initiated: we added actinomycin D 6 h postinfection, compared to 3 h in the study by Esclatine et al. To determine if this difference contributed to the discrepancies between the two studies, we examined the time course of IEX-1 RNA stabilization following infection. HeLa cells were infected with wild-type HSV-1 KOS, ΔSma, 5dl1.2 (a KOS-derived ICP27 null mutant), and LJS1 (a vhs−/ICP27− virus that combines the ΔSma and 5dl1.2 mutations [see Materials and Methods]); IEX-1 bands A and B were then quantified following the addition of actinomycin D at 3, 6, 9, and 12 h postinfection (Fig. 2). None of the viruses, including wild-type KOS, stabilized intact IEX-1 mRNA early in infection (3 hpi) (Fig. 2A), as the half-life of band A did not differ from that observed for mock-infected cells (Fig. 2B). However, at middle to late infection times (6 to 12 hpi), the stability of IEX-1 band A increased in both KOS- and ΔSma-infected HeLa cells, albeit perhaps more slowly in the case of ΔSma; in contrast, band A was not stabilized significantly at any time postinfection with the ICP27-deficient viruses 5dl1.2 or LJS1 (Fig. 2B). A somewhat different pattern was observed for band B, which was significantly stabilized by 3 hpi with wild-type virus (Fig. 2C). However, stabilization of band B was delayed during infection with ΔSma. These data document that bands A and B are both progressively stabilized as infection proceeds, with band B being stabilized more rapidly. The data also indicate that stabilization of band B, and possibly that of band A, is somewhat delayed in the absence of vhs, perhaps accounting for the conclusion of Esclatine et al. (18) that vhs is required for stabilization of IEX-1 mRNA. In this context, we note that Escalatine et al. (18) monitored IEX-1 mRNA stability via reverse transcriptase PCR using probes for IEX-1 coding sequences and thus did not distinguish between the intact IEX-1 mRNA (band A) and its decay intermediates (bands B and C). 5dl1.2 was less effective at stabilizing band B than KOS or ΔSma, in contrast to d27-1. The reason for this difference has yet to be determined.
In summary, the data presented in this section document that HSV-1 infection stabilizes both intact IEX-1 mRNA (band A) and its deadenylated decay product (band B), implying that HSV-1 interferes with both the initial deadenylation reaction and the subsequent degradation of the deadenylated mRNA body. ICP27 is required for the stabilization of intact IEX-1 mRNA (band A) whereas vhs is dispensable, and neither ICP27 nor vhs is absolutely required for stabilization of band B. However, stabilization of band B appears to be delayed in the absence of vhs.
ICP27-dependent activation of p38 correlates with IEX-1 mRNA stabilization.
ICP27 displays several characteristics that could potentially contribute to its ability to stabilize IEX-1 mRNA. ICP27 is an RNA-binding protein that shuttles between the nucleus and cytoplasm (76), and it has previously been shown to enhance the stability of the IFN-β transcript by directly binding the 3′-UTR (6). In addition, ICP27 is the viral protein responsible for the activation of p38 MAPK and its downstream targets MK2 and MSK1 observed during HSV infection (28). As noted above, activation of the p38 MAPK pathway plays an important role in enhancing the stability of many ARE mRNAs (12, 79, 90) by blocking the initial deadenylation reaction (11). Moreover, KSHV has been shown to modulate the stability of a number of ARE-containing cytokine mRNAs via the activation of p38 and MK2 (48).
To investigate which regions of ICP27 are required for stabilization of IEX-1 mRNA, we examined cells infected with a panel of HSV-1 strain KOS1.1 ICP27 mutants, each of which encodes a different mutated version of ICP27. The properties of these mutants and their effects on viral replication are summarized in Table 1 and described below. The 512-residue ICP27 protein can be divided into several functional regions. The amino-terminal domain of the protein contains a leucine-rich nuclear export signal (NES) (amino acids 5 to 17), an acidic region (amino acids 12 to 63), a nuclear localization signal (NLS) (amino acids 110 to 137), and an RGG box (amino acids 138 to 152) responsible for RNA binding (17, 46, 76). The C-terminal domain (amino acids 262 to 512) is highly conserved and responsible for the transactivation and transrepression functions of ICP27 (67). The mutant panel included a number of in-frame deletion mutations that span the amino-terminal portion of the protein (dLeu, d1-2, d2-3, d3-4, d4-5, d5-6, and d6-7) and M50T and M11, which alter 1 and 2 amino acid residues, respectively (Table 1). Of these, dLeu is missing the leucine-rich NES, and d1-2, d3-4, and d4-5 lack the acidic region, NLS, and RGG box, respectively. M11 contains two mutations in the C-terminal domain (R340L and D341E) that prevent the expression of certain γ2 genes (67), and the single amino acid change in M50T renders the virus resistant to leptomycin B without altering the growth properties of the virus (54). Hargett et al. (28) have analyzed many of these mutants for their abilities to induce phosphorylation of the p38 MAPK in infected CV-1 cells, and their data are summarized in Table 1.
TABLE 1.
ICP27 mutants used in this study
| Mutant | Residue(s) altered | Region(s) affected | Competency or deficiencya
|
|||
|---|---|---|---|---|---|---|
| Replicationb | pp38 inductionc
|
mRNA stabilization | ||||
| Previous study | This study | |||||
| d27-1 | Δ1-512 | Entire ORFd | − | − | − | − |
| dLeu | Δ6-19 | NES | − | + | − | − |
| M50T | M50T | + | ND | + | + | |
| d1-2 | Δ12-63 | Acidic, NES | −/+ | − | − | − |
| d2-3 | Δ64-109 | + | + | −/+ | −/+ | |
| d3-4 | Δ109-138 | NLS | + | −/+ | − | − |
| d4-5 | Δ139-153 | RGG box | −/+ | + | − | − |
| d5-6 | Δ154-173 | + | + | + | + | |
| d6-7 | Δ174-200 | + | + | + | + | |
| M11 | R340L and D341E | − | + | − | − | |
HeLa cells were infected with each of the mutant viruses at 10 PFU/cell, actinomycin D was added at 7.5 hpi, and total RNA samples were extracted at various times thereafter. IEX-1 RNA stability was then determined by Northern blotting, and the results of two representative experiments are shown in Fig. 3A and B. As expected on the basis of previous results, all of the mutant viruses stabilized IEX-1 band B relative to uninfected cells. However, the levels of stability of band A varied among the isolates, and based on visual inspection of the Northern blots four phenotypes could be discerned. First, three of the mutants stabilized band A as well as wild-type KOS: M50T, d5-6, and d6-7. These three viruses are all replication competent in cell culture. Second, one recombinant, d2-3, displayed an intermediate ability to stabilize band A. Third, d27-1, dLeu, d1-2, and M11 failed to stabilize band A; in addition, in many but not all experiments these mutants induced the accumulation of significant quantities of a larger, more stable, ca.-1.7-kb IEX-1-related band that migrates just below 18S rRNA. We have termed this RNA band A*. Finally, two mutants, d3-4 and d4-5, failed to stabilize band A but did not induce the appearance of band A*.
We have not yet determined the structure of the transcript that gives rise to band A* and presently do not understand why it appears in cells infected with some ICP27 mutants and not others or why it varies in relative abundance between experiments. Oddly, when it is present it can be detected in preparations of both total cellular and cytoplasmic RNA, but it is not evident in poly(A)+ RNA prepared by oligo(dT) cellulose chromatography (data not shown). Thus, band A* does not appear to correspond to a discrete poly(A)+ transcript. Band A* complicated our analysis, because when it was present it interfered with accurate quantification of band A. In addition, band A was difficult to accurately quantify in cases where its intensity (steady-state level) prior to the addition of actinomycin D was reduced relative to that upon infection with wild-type virus (e.g., d1-2, M11, and d27-1). For these reasons, we were unable to accurately determine the half-life of band A for some of the mutants analyzed in this series of experiments and therefore relied on visual inspection to categorize the mutant phenotypes.
All of the ICP27 mutants that displayed severe defects in stabilizing IEX-1 band A (d27-1, dLeu, d1-2, d3-4, d4-5, and M11) also showed a noticeable increase in the ratio of band B to band A prior to the addition of actinomycin D (Fig. 3A and B). Inasmuch as band B is derived from band A by deadenylation, this finding provides additional evidence that these mutants display a reduced ability to stabilize band A relative to the ability of wild-type HSV-1. Taken in combination, these data indicate that multiple regions of ICP27, including sequences located at both the amino- and carboxy-terminal portions of the polypeptide, are required for stabilization of IEX-1 band A.
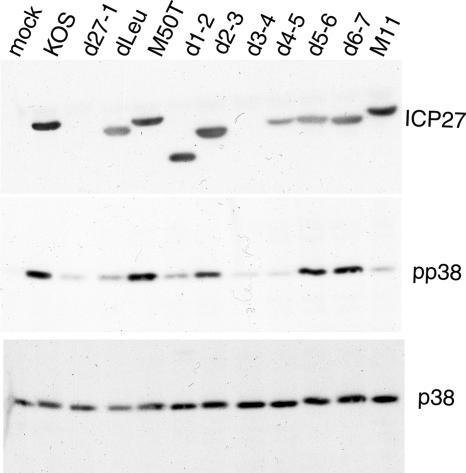
We next asked if the mRNA stabilization phenotypes of the various ICP27 mutants correlate with their abilities to induce phosphorylation of p38. At first glance this seemed unlikely, as the mRNA stabilization patterns of the mutants differed significantly from their previously published p38 activation profiles (28) (summarized in Table 1). For example, Hargett et al. reported that dLeu, d4-5, and M11 are all competent to activate p38 (28), while we found that these mutants fail to stabilize IEX-1 band A (Fig. 3; Table 1). However, the experiments of Hargett et al. were conducted with African green monkey CV-1 cells whereas our mRNA stabilization assays were done with human HeLa cells. We therefore determined the p38 activation profiles of the mutants in HeLa cells (Fig. 4). The various mutant viruses expressed approximately equivalent amounts of ICP27 as detected by Western blotting with H1113 monoclonal antibody, and the protein migrated at its expected molecular weight (Fig. 4, top panel), except, as expected, with d27-1 and d3-4, both of which lack the sequences encoding the epitope recognized by the H1113 antibody (28, 46). However, cells infected with d3-4 have been shown previously to express the ICP27 protein by use of the H1119 monoclonal antibody (28, 46). The infected-cell lysates were then immunoblotted for phospho-p38 (the active form) and total p38 (Fig. 4, middle and bottom panels, respectively). These experiments revealed a strong correlation between p38 phosphorylation and IEX-1 mRNA stabilization (summarized in Table 1). Specifically, all of the mutants that failed to stabilize IEX-1 band A (Fig. 3A and B) also failed to induce phosphorylation of p38 (Fig. 4, middle and bottom panels). These included d27-1, dLeu, d1-2, d3-4, d4-5, and M11. The mutant d2-3 displayed an intermediate ability to trigger phosphorylation of p38, consistent with the intermediate level of IEX-1 turnover in these cells. The p38 activation spectrum obtained in these experiments differs significantly from that reported by Hargett et al. (28) obtained using the same collection of ICP27 mutants (Table 1). We suspect that the different cell types used (HeLa versus CV-1) in the two studies may contribute to these discrepancies.
FIG. 4.
Effects of ICP27 mutations on activation of p38 MAPK. HeLa cells were mock infected or infected with wild-type HSV-1 (strain KOS), the ICP27 null mutant of KOS (d27-1), one of the in-frame deletion mutants dLeu, d1-2, d2-3, d3-4, d4-5, d5-6, or d6-7, or one of the point mutants M50T or M11 at a multiplicity of infection of 10. Infected cells were lysed at 7.5 to 8 hpi, and then total protein was separated by SDS-10% polyacrylamide gel electrophoresis and immunoblotted to verify the accumulation of ICP27 by use of the monoclonal antibody H1113 (top panel). A second blot was probed sequentially for the phosphorylated (pp38, middle panel) and nonphosphorylated (p38, bottom panel) forms of p38 kinase.
The foregoing data were consistent with the hypothesis that ICP27-induced activation of the stress-induced kinase p38 is responsible for the enhanced stability of IEX-1 mRNA in HSV-1-infected cells. To test this hypothesis more directly, HeLa cells were infected with KOS or d27-1 in the presence or absence of the p38 kinase inhibitor SB203580. The inhibitor blocked the ability of KOS to stabilize IEX-1 band A compared to untreated, KOS-infected cells and thus gave rise to an IEX-1 mRNA turnover profile similar to that of the ICP27 knockout d27-1 (Fig. 5). Indeed, the drug greatly reduced the level of band A and increased the level of band B in KOS-infected cells even in the absence of actinomycin D, suggesting that it specifically enhances IEX-1 mRNA deadenylation without affecting the rate of turnover of the deadenylated product. Consistent with this hypothesis, SB203580 had no effect on the stability of band B in cells infected with d27-1. Taken together, these data indicate that HSV-1 infection activates the p38 MAPK pathway, resulting in altered turnover of the ARE-containing RNA IEX-1, and that ICP27 is necessary for this process.
Although our experiments establish that ICP27 is required for stabilization of IEX-1 mRNA during HSV-1 infection, this finding does not necessarily indicate that ICP27 is the stabilizing factor. Indeed, ICP27 is required for the expression of certain early and late viral genes (17, 30, 47, 88), and it is therefore possible that ICP27 acts indirectly by inducing the synthesis of another viral protein that serves as the stabilizing factor. Unfortunately, our attempts to date to use transfection-based assays to determine if ICP27 is able to stabilize IEX-1 mRNA in the absence of other HSV gene products have been unsuccessful, as IEX-1 mRNA appears to be stabilized by the stresses associated with the transfection protocol (data not shown). Nonetheless, for the following reasons, we currently favor the hypothesis that ICP27 is directly responsible for the altered turnover of IEX-1. First, the ICP27 mutants d1-2, d2-3, d3-4, and d4-5 permit close-to-wild-type levels of early and late gene expression and yet these mutants fail to stabilize IEX-1 RNA (2, 46), thereby uncoupling the gene activation function mediated by ICP27 from that of stabilization. Along the same lines, SB203580 abrogates ICP27-dependent mRNA stabilization and yet has no detectable effect on the viral transcription program (34). Second, ICP27 has been shown previously to be both necessary and sufficient for the ability of HSV-1 infection to activate the MAPK p38 (28). These authors used a collection of recombinant viruses and inhibitors to eliminate other HSV-1 immediate-early, early, or late gene products as being responsible for p38 phosphorylation. Thus, if the sole mechanism utilized by ICP27 to stimulate ARE RNA stability is via p38 activation, it is likely the only viral protein product required for this effect. Third, although ICP27 is expressed at high levels at very early times, it is not observed in the cytoplasm until approximately 6 hpi (8, 46). Thus, the increase in IEX-1 RNA stability correlates with the time during infection when ICP27 accumulates in the cytoplasm. Further, the recombinant viruses dLeu and d1-2, which lack all or a portion of the NES, respectively, are restricted to the nucleus (8, 46) and cannot stabilize IEX-1 (Fig. 3). Fourth, the mutant form of ICP27 encoded by d4-5, which lacks the RNA-binding domain, is cytoplasmically located but cannot bind RNA; d4-5 also does not alter IEX-1 RNA turnover (Fig. 3). Brown et al. (6) demonstrated that ICP27 bound to the ARE-containing RNAs IFN-β and c-myc in vitro and suggested that this binding directly promotes RNA stability, though this binding specificity could not be replicated by others (50). These considerations raise the possibility that the RNA-binding activity of ICP27 may also contribute to the modulation of RNA stability in infected cells. However, it seems likely that cytoplasmic localization and RNA binding by ICP27 are insufficient for p38 activation and mRNA stabilization, as d3-4, which can be detected in the cytoplasm (46) and contains an intact RRG box, is devoid of these activities. Similarly, although to our knowledge the shuttling and RNA-binding activities of M11 have not been determined, the mutation is located outside the domains required for these activities and yet the mutant protein fails to activate p38 or stabilize IEX-1 mRNA.
The foregoing data indicate that the mRNA-stabilizing activity of ICP27 depends on its ability to activate p38, but they do not exclude the possibility that other mechanisms, including direct binding of ICP27 to ARE RNAs (6), also contribute to the effect. The ARE-binding and -destabilizing protein tristetraprolin modulates the stability of certain ARE-containing messages by altering the location of the target RNA in response to extracellular signals (79). As an RNA-binding protein, ICP27 might similarly modulate the association of ARE messages like IEX-1 with the cytoplasmic depots that form in response to stress (stress granules) or with sites of RNA decay (P or GW bodies) (36, 37). Recently, certain players of the RNA interference pathway (Dicer, Ago1, and miR16) have been implicated in the normal turnover of ARE-containing messages (32, 87). Indeed, the miR16 microRNA interacts with the ARE and tristetraprolin to trigger mRNA decay (32). Though these findings raise the possibility that ICP27 might enhance the stability of ARE RNAs by interfering with RNA silencing in a fashion similar to other recently described viral suppressors of RNA silencing that function in mammalian cells (5, 45, 83), we have not be able to demonstrate such activity by using a reporter assay system developed by Sullivan and Ganem (83) (data not shown).
Concluding remarks.
ICP27 is a highly conserved and essential immediate-early protein and a prominent player in herpesvirus-induced shutoff of cellular gene expression. This report elucidates a new role for this multifunctional protein in mediating the stabilization of the normally labile IEX-1 mRNA. The ability of ICP27 to activate the p38 MAPK pathway is necessary for this effect. Activated p38 is able to stabilize a large number of ARE mRNAs encoding proinflammatory proteins (9), raising the possibility that HSV infection stabilizes many of these ordinarily labile mRNAs; however, this has yet to be determined directly. What is also not yet clear is why HSV-1 infection augments the accumulation of IEX-1 and perhaps other ARE mRNAs, some of which encode proteins that may limit viral replication.
The IEX-1 gene product is a potent regulator with the ability to alter cell survival in response to stress signals (1, 72, 91, 92). In some cell types, expression of IEX-1 promotes apoptosis (1), while in others, it is protective (92). The protein is found to be associated with ND10 domains (40) and shuttles between the nucleus and the cytoplasm in response to certain stimuli (38). Even though IEX-1 mRNA is up-regulated and stabilized in HSV-infected cells, Taddeo and colleagues report that the protein is not detectable later than 1 h after infection (85), implying that the protein is degraded and/or its mRNA is not translated. These authors suggested that the IEX-1 gene product might be detrimental to viral replication but demonstrated that overexpression of the protein has no effect on the replication or late gene expression of HSV (85). These data make it unclear whether the increased stability of IEX-1 RNA leads to enhanced protein production and, if so, whether the IEX-1 protein helps or hinders replication of HSV-1.
The latent KSHV gene product kaposin B increases the expression of certain cytokines by blocking the degradation of ARE-containing mRNAs via a mechanism that involves binding and activating the MK2 kinase, a target of activated p38 (48). This observation links the elevated levels of proinflammatory cytokines found in Kaposi's sarcoma tumor sites in vivo and the requirement for certain cytokines for the growth of Kaposi's sarcoma spindle cells in vitro with virus infection. It has also been shown that pseudorabies virus infection elevates the levels of the ARE-containing message for cyclooxygenase-2. This enzyme is required for virus replication, as cyclooxygenase-2 inhibitors abrogate virus production in cell culture (62). These recent observations suggest that diverse herpesviruses may have evolved the ability to modulate cellular gene expression by interfering with the decay of certain cellular mRNAs and, even more surprisingly, that the result of these efforts is the increased expression of proteins generally considered antiviral. Perhaps host inflammatory responses directly benefit herpesviruses by promoting host survival and establishing latency, features that are key to the maintenance of this group of viruses within host populations.
Finally, the work described in this study establishes that, contrary to a previous report (18), vhs is not the viral protein responsible for stabilizing IEX-1 mRNA. However, we also find that vhs does not destabilize IEX-1 mRNA detectably during middle to late times (6 to 12 hpi) of HSV-1 infection. Though we previously observed that ΔSma-infected cells accumulate the IEX-1 transcript to greater amounts at 12 and 24 hpi than cells infected with the wild-type virus (29), we suspect that this difference may result from the increased levels of ICP27 mRNA and protein found in cells infected with vhs mutants (56) rather than from direct action of vhs on IEX-1 mRNA. Thus, it is very intriguing that the IEX-1 RNA substrate may be resistant to the RNase activity of vhs in vivo, as vhs is generally believed to target all mRNAs indiscriminately and can cleave the IEX-1 RNA substrate in vitro (86). It is unclear at this point why the IEX-1 RNA is resistant to the activity of vhs in HSV-infected cells. Determining the molecular basis for this immunity will be of great interest.
Acknowledgments
We thank Holly Saffran and Rob Maranchuk for technical assistance, Henrietta Zanzotto and Leslie Shai for constructing viral mutants M50T and LJS1, respectively, and Stephen Rice for the panel of ICP27-deficient viruses.
This research was funded by an operating grant from the Canadian Institutes for Health Research.
J.R.S. is a Canada Research Chair in Molecular Virology.
REFERENCES
- 1.Arlt, A., O. Grobe, A. Sieke, M. L. Kruse, U. R. Folsch, W. E. Schmidt, and H. Schafer. 2001. Expression of the NF-kappa B target gene IEX-1 (p22/PRG1) does not prevent cell death but instead triggers apoptosis in Hela cells. Oncogene 20:69-76. [DOI] [PubMed] [Google Scholar]
- 2.Aubert, M., S. A. Rice, and J. A. Blaho. 2001. Accumulation of herpes simplex virus type 1 early and leaky-late proteins correlates with apoptosis prevention in infected human HEp-2 cells. J. Virol. 75:1013-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakheet, T., M. Frevel, B. R. G. Williams, W. Greer, and K. S. A. Khabar. 2001. ARED: human AU-rich element-containing mRNA database reveals an unexpectedly diverse functional repertoire of encoded proteins. Nucleic Acids Res. 29:246-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barreau, C., L. Paillard, and H. B. Osborne. 2005. AU-rich elements and associated factors: are there unifying principles? Nucleic Acids Res. 33:7138-7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennasser, Y., S. Y. Le, M. Benkirane, and K. T. Jeang. 2005. Evidence that HIV-1 encodes an siRNA and a suppressor of RNA silencing. Immunity 22:607-619. [DOI] [PubMed] [Google Scholar]
- 6.Brown, C. R., M. S. Nakamura, J. D. Mosca, G. S. Hayward, S. E. Straus, and L. P. Perera. 1995. Herpes simplex virus trans-regulatory protein ICP27 stabilizes and binds to 3′ ends of labile mRNA. J. Virol. 69:7187-7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, I.-H. B., K. S. Sciabica, and R. M. Sandri-Goldin. 2002. ICP27 interacts with the RNA export factor Aly/REF to direct herpes simplex virus type 1 intronless mRNAs to the TAP export pathway. J. Virol. 76:12877-12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, I.-H. B., L. Li, L. Silva, and R. M. Sandri-Goldin. 2005. ICP27 recruits Aly/REF but not TAP/NXF1 to herpes simplex virus type 1 transcription sites although TAP/NXF1 is required for ICP27 export. J. Virol. 79:3949-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark, A. R., J. L. Dean, and J. Saklatvala. 2003. Post-transcriptional regulation of gene expression by mitogen-activated protein kinase p38. FEBS Lett. 546:37-44. [DOI] [PubMed] [Google Scholar]
- 10.Dean, J. L., M. Brook, A. R. Clark, and J. Saklatvala. 1999. p38 mitogen-activated protein kinase regulates cyclooxygenase-2 mRNA stability and transcription in lipopolysaccharide-treated human monocytes. J. Biol. Chem. 274:264-269. [DOI] [PubMed] [Google Scholar]
- 11.Dean, J. L., S. J. Sarsfield, E. Tsounakou, and J. Saklatvala. 2003. p38 mitogen-activated protein kinase stabilizes mRNAs that contain cyclooxygenase-2 and tumor necrosis factor AU-rich elements by inhibiting deadenylation. J. Biol. Chem. 278:39470-39476. [DOI] [PubMed] [Google Scholar]
- 12.Dean, J. L. E., G. Sully, A. R. Clark, and J. Saklatvala. 2004. The involvement of AU-rich element-binding proteins in p38 mitogen-activated protein kinase pathway-mediated mRNA stabilization. Cell. Signal. 16:1113-1121. [DOI] [PubMed] [Google Scholar]
- 13.Doepker, R. C., W.-L. Hsu, H. A. Saffran, and J. R. Smiley. 2004. Herpes simplex virus virion host shutoff protein is stimulated by translation initiation factors eIF4B and eIF4H. J. Virol. 78:4684-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty, A. J., L. C. Serpell, and C. P. Ponting. 1996. The helix-hairpin-helix DNA-binding motif: a structural basis for non-sequence-specific recognition of DNA. Nucleic Acids Res. 24:2488-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elgadi, M. M., C. E. Hayes, and J. R. Smiley. 1999. The herpes simplex virus Vhs protein induces endoribonucleolytic cleavage of target RNAs in cell extracts. J. Virol. 73:7153-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elgadi, M. M., and J. R. Smiley. 1999. Picornavirus internal ribosome entry site elements target RNA cleavage events induced by the herpes simplex virus virion host shutoff protein. J. Virol. 73:9222-9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellison, K. S., R. A. Maranchuk, K. L. Mottet, and J. R. Smiley. 2005. Control of VP16 translation by the herpes simplex virus type 1 immediate-early protein ICP27. J. Virol. 79:4120-4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esclatine, A., B. Taddeo, and B. Roizman. 2004. The UL41 protein of herpes simplex virus mediates selective stabilization or degradation of cellular mRNAs. Proc. Natl. Acad. Sci. USA 101:18165-18170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esclatine, A., B. Taddeo, L. Evans, and B. Roizman. 2004. The herpes simplex virus 1 UL41 gene-dependent destabilization of cellular RNAs is selective and may be sequence-specific. Proc. Natl. Acad. Sci. USA 101:3603-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esnault, S., and J. S. Malter. 2002. Extracellular signal-regulated kinase mediates granulocyte-macrophage colony-stimulating factor messenger RNA stabilization in tumor necrosis factor-alpha plus fibronectin-activated peripheral blood eosinophils. Blood 99:4048-4052. [DOI] [PubMed] [Google Scholar]
- 21.Espel, E. 2005. The role of the AU-rich elements of mRNAs in controlling translation. Semin. Cell Dev. Biol. 16:59-67. [DOI] [PubMed] [Google Scholar]
- 22.Everly, D. N. J., P. Feng, I. S. Mian, and G. S. Read. 2002. mRNA degradation by the virion host shutoff (Vhs) protein of herpes simplex virus: genetic and biochemical evidence that Vhs is a nuclease. J. Virol. 76:8560-8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng, P., D. N. J. Everly, and G. S. Read. 2001. mRNA decay during herpesvirus infections: interaction between a putative viral nuclease and a cellular translation factor. J. Virol. 75:10272-10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fenwick, M. L., and R. D. Everett. 1990. Inactivation of the shutoff gene (UL41) of herpes simplex virus types 1 and 2. J. Gen. Virol. 71:2961-2967. [DOI] [PubMed] [Google Scholar]
- 25.Fontaine-Rodriguez, E. C., T. J. Taylor, M. Olesky, and D. M. Knipe. 2004. Proteomics of herpes simplex virus infected cell protein 27: association with translation initiation factors. Virology 330:487-492. [DOI] [PubMed] [Google Scholar]
- 26.Hardwicke, M. A., and R. M. Sandri-Goldin. 1994. The herpes simplex virus regulatory protein ICP27 contributes to the decrease in cellular mRNA levels during infection. J. Virol. 68:4797-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardy, W. R., and R. M. Sandri-Goldin. 1994. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J. Virol. 68:7790-7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hargett, D., T. McLean, and S. L. Bachenheimer. 2005. Herpes simplex virus ICP27 activation of stress kinases JNK and p38. J. Virol. 79:8348-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu, W. L., H. A. Saffran, and J. R. Smiley. 2005. Herpes simplex virus infection stabilizes cellular IEX-1 mRNA. J. Virol. 79:4090-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jean, S., K. M. LeVan, B. Song, M. Levine, and D. M. Knipe. 2001. Herpes simplex virus 1 ICP27 is required for transcription of two viral late (gamma 2) genes in infected cells. Virology 283:273-284. [DOI] [PubMed] [Google Scholar]
- 31.Jenkins, H. L., and C. A. Spencer. 2001. RNA polymerase II holoenzyme modifications accompany transcription reprogramming in herpes simplex virus type 1-infected cells. J. Virol. 75:9872-9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jing, Q., S. Huang, S. Guth, T. Zarubin, A. Motoyama, J. Chen, F. Di Padova, S. C. Lin, H. Gram, and J. Han. 2005. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell 120:623-634. [DOI] [PubMed] [Google Scholar]
- 33.Jones, F. E., C. A. Smibert, and J. R. Smiley. 1995. Mutational analysis of the herpes simplex virus virion host shutoff protein: evidence that vhs functions in the absence of other viral proteins. J. Virol. 69:4863-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karaca, G., D. Hargett, T. I. McLean, J. S. Aguilar, P. Ghazal, E. K. Wagner, and S. L. Bachenheimer. 2004. Inhibition of the stress-activated kinase, p38, does not affect the virus transcriptional program of herpes simplex virus type 1. Virology 329:142-156. [DOI] [PubMed] [Google Scholar]
- 35.Karr, B. M., and G. S. Read. 1999. The virion host shutoff function of herpes simplex virus degrades the 5′ end of a target mRNA before the 3′ end. Virology 264:195-204. [DOI] [PubMed] [Google Scholar]
- 36.Kedersha, N., G. Stoecklin, M. Ayodele, P. Yacono, J. Lykke-Andersen, M. J. Fritzler, D. Scheuner, R. J. Kaufman, D. E. Golan, and P. Anderson. 2005. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 169:871-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kedersha, N., and P. Anderson. 2002. Stress granules: sites of mRNA triage and regulate mRNA stability and translatability. Biochem. Soc. Trans. 30:963-969. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi, T., M. R. Pittelkow, G. M. Warner, K. A. Squillace, and R. Kumar. 1998. Regulation of a novel immediate early response gene, IEX-1, in keratinocytes by 1alpha,25-dihydroxyvitamin D3. Biochem. Biophys. Res. Commun. 251:868-873. [DOI] [PubMed] [Google Scholar]
- 39.Koffa, M. D., J. B. Clements, E. Izaurralde, S. Wadd, S. A. Wilson, I. W. Mattaj, and S. Kuersten. 2001. Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway. EMBO J. 20:5769-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kruse, M. L., A. Arlt, A. Sieke, F. Grohmann, M. Grossmann, J. Minkenberg, U. R. Folsch, and H. Schafer. 2005. Immediate early gene X1 (IEX-1) is organized in subnuclear structures and partially co-localizes with promyelocytic leukemia protein in HeLa cells. J. Biol. Chem. 280:24849-24856. [DOI] [PubMed] [Google Scholar]
- 41.Kwong, A. D., J. A. Kruper, and N. Frenkel. 1988. Herpes simplex virus virion host shutoff function. J. Virol. 62:912-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwong, A. D., and N. Frenkel. 1987. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc. Natl. Acad. Sci. USA 84:1926-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lam, Q., C. A. Smibert, K. E. Koop, C. Lavery, J. P. Capone, S. P. Weinheimer, and J. R. Smiley. 1996. Herpes simplex virus VP16 rescues viral mRNA from destruction by the virion host shutoff function. EMBO J. 15:2575-2581. [PMC free article] [PubMed] [Google Scholar]
- 44.Larralde, O., R. W. Smith, G. S. Wilkie, P. Malik, N. K. Gray, and J. B. Clements. 2006. Direct stimulation of translation by the multifunctional herpesvirus ICP27 protein. J. Virol. 80:1588-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lecellier, C. H., P. Dunoyer, K. Arar, J. Lehmann-Che, S. Eyquem, C. Himber, A. Saib, and O. Voinnet. 2005. A cellular microRNA mediates antiviral defense in human cells. Science 308:557-560. [DOI] [PubMed] [Google Scholar]
- 46.Lengyel, J., C. Guy, V. Leong, S. Borge, and S. A. Rice. 2002. Mapping of functional regions in the amino-terminal portion of the herpes simplex virus ICP27 regulatory protein: importance of the leucine-rich nuclear export signal and RGG box RNA-binding domain. J. Virol. 76:11866-11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCarthy, A. M., L. McMahan, and P. A. Schaffer. 1989. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J. Virol. 63:18-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCormick, C., and D. Ganem. 2005. The kaposin B protein of KSHV activates the p38/MK2 pathway and stabilizes cytokine mRNAs. Science 307:739-741. [DOI] [PubMed] [Google Scholar]
- 49.Mears, W. E., and S. A. Rice. 1998. The herpes simplex virus immediate-early protein ICP27 shuttles between nucleus and cytoplasm. Virology 242:128-137. [DOI] [PubMed] [Google Scholar]
- 50.Mears, W. E., and S. A. Rice. 1996. The RGG box motif of the herpes simplex virus ICP27 protein mediates an RNA-binding activity and determines in vivo methylation. J. Virol. 70:7445-7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mears, W. E., V. Lam, and S. A. Rice. 1995. Identification of nuclear and nucleolar localization signals in the herpes simplex virus regulatory protein ICP27. J. Virol. 69:935-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyer, S., C. Temme, and E. Wahle. 2004. Messenger RNA turnover in eukaryotes: pathways and enzymes. Crit. Rev. Biochem. Mol. Biol. 39:197-216. [DOI] [PubMed] [Google Scholar]
- 53.Miyazawa, K., A. Mori, H. Miyata, M. Akahane, Y. Ajisawa, and H. Okudaira. 1998. Regulation of interleukin-1beta-induced interleukin-6 gene expression in human fibroblast-like synoviocytes by p38 mitogen-activated protein kinase. J. Biol. Chem. 273:24832-24838. [DOI] [PubMed] [Google Scholar]
- 54.Murata, T., F. Goshima, T. Koshizuka, H. Takakuwa, and Y. Nishiyama. 2001. A single amino acid substitution in the ICP27 protein of herpes simplex virus type 1 is responsible for its resistance to leptomycin B. J. Virol. 75:1039-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oroskar, A. A., and G. S. Read. 1989. Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J. Virol. 63:1897-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oroskar, A. A., and G. S. Read. 1987. A mutant of herpes simplex virus type 1 exhibits increased stability of immediate-early (alpha) mRNAs. J. Virol. 61:604-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perez-Parada, J., H. A. Saffran, and J. R. Smiley. 2004. RNA degradation induced by the herpes simplex virus vhs protein proceeds 5′ to 3′ in vitro. J. Virol. 78:13391-13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perng, G. C., C. Jones, J. Ciacci-Zanella, M. Stone, G. Henderson, A. Yukht, S. M. Slanina, F. M. Hofman, H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 2000. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science 287:1500-1503. [DOI] [PubMed] [Google Scholar]
- 59.Phelan, A., and J. B. Clements. 1997. Herpes simplex virus type 1 immediate early protein IE63 shuttles between nuclear compartments and the cytoplasm. J. Gen. Virol. 78:3327-3331. [DOI] [PubMed] [Google Scholar]
- 60.Poon, A. P., and B. Roizman. 1997. Differentiation of the shutoff of protein synthesis by virion host shutoff and mutant gamma (1)34.5 genes of herpes simplex virus 1. Virology 229:98-105. [DOI] [PubMed] [Google Scholar]
- 61.Ray, N., and L. W. Enquist. 2004. Transcriptional response of a common permissive cell type to infection by two diverse alphaherpesviruses. J. Virol. 78:3489-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ray, N., M. E. Bisher, and L. W. Enquist. 2004. Cyclooxygenase-1 and -2 are required for production of infectious pseudorabies virus. J. Virol. 78:12964-12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Read, G. S., B. M. Bradley, and K. Knight. 1993. Isolation of a herpes simplex virus type 1 mutant with a deletion in the virion host shutoff gene and identification of multiple forms of the vhs (UL41) polypeptides. J. Virol. 67:7149-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Read, G. S., and N. Frenkel. 1983. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of α (immediate early) viral polypeptides. J. Virol. 46:498-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rice, S. A., and D. M. Knipe. 1990. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J. Virol. 64:1704-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rice, S. A., V. Lam, and D. M. Knipe. 1993. The acidic amino-terminal region of herpes simplex virus type 1 alpha protein ICP27 is required for an essential lytic function. J. Virol. 67:1778-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rice, S. A., and V. Lam. 1994. Amino acid substitution mutations in the herpes simplex virus ICP27 protein define an essential gene regulation function. J. Virol. 68:823-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ridley, S. H., J. L. Dean, S. J. Sarsfield, M. Brook, A. R. Clark, and J. Saklatvala. 1998. A p38 MAP kinase inhibitor regulates stability of interleukin-1-induced cyclooxygenase-2 mRNA. FEBS Lett. 439:75-80. [DOI] [PubMed] [Google Scholar]
- 69.Roizman, B., and P. E. Pellett. 2001. The family Herpesviridae: a brief introduction, p. 2381-2397. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 70.Sandri-Goldin, R. M. 1998. ICP27 mediates HSV RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 12:868-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sandri-Goldin, R. M., and G. E. Mendoza. 1992. A herpesvirus regulatory protein appears to act post-transcriptionally by affecting mRNA processing. Genes Dev. 6:848-863. [DOI] [PubMed] [Google Scholar]
- 72.Shen, L., J. Guo, C. Santos-Berrios, and M. X. Wu. 2006. Distinct domains for anti- and pro-apoptotic activities of IEX-1. J. Biol. Chem. 281:15304-15311. [DOI] [PubMed] [Google Scholar]
- 73.Smibert, C. A., and J. R. Smiley. 1990. Differential regulation of endogenous and transduced β-globin genes during infection of erythroid cells in herpes simplex virus type 1 recombinant. J. Virol. 64:3882-3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smiley, J. R. 1980. Construction in vitro and rescue of a thymidine kinase-deficient deletion mutation of herpes simplex virus. Nature 285:333-335. [DOI] [PubMed] [Google Scholar]
- 75.Smiley, J. R. 2004. Herpes simplex virus virion host shutoff protein: immune evasion mediated by a viral RNase? J. Virol. 78:1063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith, R. W. P., P. Malik, and J. B. Clements. 2005. The herpes simplex virus ICP27 protein: a multifunctional post-transcriptional regulator of gene expression. Biochem. Soc. Trans. 33:499-501. [DOI] [PubMed] [Google Scholar]
- 77.Soliman, T. M., R. M. Sandri-Goldin, and S. J. Silverstein. 1997. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J. Virol. 71:9188-9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spencer, C. A., M. E. Dahmus, and S. A. Rice. 1997. Repression of host RNA polymerase II transcription by herpes simplex virus type 1. J. Virol. 71:2031-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stoecklin, G., T. Stubbs, N. Kedersha, S. Wax, W. F. C. Rigby, T. K. Blackwell, and P. Anderson. 2004. MK2-induced tristetraprolin:14-13-3 complexes prevent stress granule association and ARE-mRNA decay. EMBO J. 23:1313-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Strelow, L. I., and D. A. Leib. 1996. Analysis of conserved domains of UL41 of herpes simplex virus type 1 in virion host shutoff and pathogenesis. J. Virol. 70:5665-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Strelow, L. I., and D. A. Leib. 1995. Role of the virion host shutoff (vhs) of herpes simplex virus type 1 in latency and pathogenesis. J. Virol. 69:6779-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Strom, T., and N. Frenkel. 1987. Effects of herpes simplex virus on mRNA stability. J. Virol. 61:2198-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sullivan, C. S., and D. Ganem. 2005. A virus-encoded inhibitor that blocks RNA interference in mammalian cells. J. Virol. 79:7371-7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taddeo, B., A. Esclatine, and B. Roizman. 2002. The patterns of accumulation of cellular RNAs in cells infected with a wild-type and a mutant herpes simplex virus 1 lacking the virion host shutoff gene. Proc. Natl. Acad. Sci. USA 99:17031-17036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taddeo, B., A. Esclatine, W. Zhang, and B. Roizman. 2003. The stress-inducible immediate-early responsive gene IEX-1 is activated in cells infected with herpes simplex virus 1, but several viral mechanisms, including 3′ degradation of its RNA, preclude expression of the gene. J. Virol. 77:6178-6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Taddeo, B., W. Zhang, and B. Roizman. 2006. The UL41 protein of herpes simplex virus 1 degrades RNA by endonucleolytic cleavage in absence of other cellular or viral proteins. Proc. Natl. Acad. Sci. USA 103:2827-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takahashi, H., M. Maeda, H. Sawa, H. Hasegawa, M. Moriyama, T. Sata, W. W. Hall, and T. Kurata. 2006. Dicer and positive charge of proteins decrease the stability of RNA containing the AU-rich element of GM-CSF. Biochem. Biophys. Res. Commun. 340:807-814. [DOI] [PubMed] [Google Scholar]
- 88.Uprichard, S. L., and D. M. Knipe. 1996. Herpes simplex ICP27 mutant viruses exhibit reduced expression of specific DNA replication genes. J. Virol. 70:1969-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Winzen, R., G. Gowrishankar, F. Bollig, N. Redich, K. Resch, and H. Holtmann. 2004. Distinct domains of AU-rich elements exert different functions in mRNA destabilization and stabilization by p38 mitogen-activated protein kinase or HuR. Mol. Cell. Biol. 24:4835-4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Winzen, R., M. Kracht, B. Ritter, A. Wilhelm, C.-Y. A. Chen, A.-B. Shyu, M. Muller, M. Gaestel, K. Resch, and H. Holtmann. 1999. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeting mechanism. EMBO J. 18:4969-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu, M. X. 2003. Roles of the stress-induced gene IEX-1 in regulation of cell death and oncogenesis. Apoptosis 8:11-18. [DOI] [PubMed] [Google Scholar]
- 92.Wu, M. X., Z. Ao, K. V. Prasad, R. Wu, and S. F. Schlossman. 1998. IEX-1L, an apoptosis inhibitor involved in NF-kappaB-mediated cell survival. Science 281:998-1001. [DOI] [PubMed] [Google Scholar]
- 93.Zelus, B. D., R. S. Stewart, and J. Ross. 1996. The virion host shutoff protein of herpes simplex virus type 1: messenger ribonucleolytic activity in vitro. J. Virol. 70:2411-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou, C., and D. M. Knipe. 2002. Association of herpes simplex virus type 1 ICP8 and ICP27 proteins with cellular RNA polymerase II holoenzyme. J. Virol. 76:5893-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]