Abstract
Objective
To determine the relation between substance use and cognition in individuals experiencing their first episode of psychosis.
Design
Prospective cross-sectional and longitudinal study.
Setting
An Early Psychosis Treatment and Prevention Program, an outpatient clinic in a psychiatry department at a university-affiliated hospital.
Participants
Individuals with a psychotic illness who were admitted to an Early Psychosis Program; 266 patients were assessed at initial presentation, 159 at 1 year and 90 at 2 years. Most were outpatients.
Measures
The effects of substance use (alcohol, cannabis, hallucinogens, cocaine, stimulants) on cognition were assessed. Substance use was determined by DSM-IV criteria, and the Case Manager Rating Scale was used to determine the level of substance use. A comprehensive cognitive battery of tests was used, and the Positive and Negative Syndrome Scale for schizophrenia was administered to all subjects to determine levels of positive and negative symptoms.
Results
Overall, both cross-sectionally and longitudinally, there were no significant associations between cognitive functioning and the use of various substances. Substance use was associated with higher positive symptoms.
Conclusions
Individuals with psychotic disorders who show mild-to-moderate abuse of substances, in particular alcohol and cannabis, do not exhibit more cognitive impairment than those who do not do use the substances. However, substance use may have other detrimental effects on the process of the psychotic illness.
Medical subject headings: age of onset, alcohol drinking, cannabis, cognition disorders, psychotic disorders, schizophrenia, substance-related disorders
Abstract
Objectif
Établir le lien entre la consommation d'alcool et de drogues et la cognition chez des personnes vivant leur premier épisode de psychose.
Conception
Étude transversale et longitudinale prospective.
Contexte
Un programme de traitement précoce et de prévention de la psychose en clinique externe d'un service de psychiatrie dans un hôpital universitaire.
Participants
Personnes atteintes de psychose admises dans un programme de traitement précoce de la psychose; 266 patients ont été évalués lors de la première consultation, 159 l'ont été après un an et 90, après deux ans. La plupart d'entre eux étaient suivis en clinique externe.
Mesures
On a évalué les effets de la consommation de diverses substances (alcool, cannabis, hallucinogènes, cocaïne, stimulants) sur la fonction cognitive. La consommation d'alcool et de drogues a été établie selon le diagnostic du DSM-IV, et l'échelle d'évaluation du responsable de cas (Case Manager Rating Scale) a servi à établir le degré de consommation de drogues ou d'alcool. Une batterie exhaustive de tests cognitifs ont été utilisés et on a soumis tous les sujets à l'échelle d'appréciation des symptômes positifs et négatifs pour la schizophrénie afin de déterminer le niveau des symptômes positifs et négatifs.
Résultats
Dans l'ensemble, on n'a pas constaté de lien significatif entre le fonctionnement cognitif et la consommation des diverses substances tant sous l'angle transversal que longitudinal. La consommation d'alcool et de drogues était associée avec des symptômes positifs accrus.
Conclusions
Les personnes aux prises avec des troubles psychotiques qui ont des problèmes légers à moyens d'abus de substances, en particulier l'alcool et le cannabis, ne présentent pas plus de troubles cognitifs que celles qui n'en consomment pas. La consommation d'alcool et de drogues pourrait néanmoins avoir d'autres effets nocifs au chapitre du processus morbide de la psychose.
Introduction
Individuals with a psychotic disorder are at high risk for substance use disorders.1,2 As many as 60% of patients with schizophrenia use illicit drugs.3 Substance use and misuse is associated with many detrimental effects on individuals with psychotic disorders. Studies have shown that misuse of substances among those with psychotic disorders is associated with greater severity of symptoms and poorer prognosis, significantly more admissions to hospital and outpatient visits, higher medication dose and medication nonadherence.4,5,6,7,8,9 Additionally, some studies have also indicated that some individuals with schizophrenia who abuse substances (particularly alcohol and cannabis) have higher functioning than nonusers.10 Also, those who have the skills and motivation to obtain substances and use them in a social setting tend to have a better prognosis in terms of their psychotic disorder.11 However, if they were not using substances, it is likely that they would have improved outcomes. Given the high rates of comorbidity between substance abuse and psychotic disorders and the resulting detrimental clinical effects, further study of other possible consequences of substance use is warranted.
The use of alcohol and cocaine, the most commonly studied substances, has been associated with deleterious consequences on cognitive functions.12,13,14,15 Although some studies have demonstrated cognitive impairments with cannabis abuse, little research exists on the cognitive effects of long-term cannabis use and abuse.16 Cognitive impairments have been clearly demonstrated in individuals with chronic schizophrenia and in individuals experiencing their first episode of a psychotic disorder.17,18 Thus, it is important to examine the combined effects of a psychotic disorder and substance use to determine whether substance use has a detrimental effect on already compromised cognitive functioning.
The literature on the cognitive functioning of individuals with schizophrenia who abuse substances is inconclusive; results depend largely on the substance studied.11,19,20,21,22,23 For example, some researchers have found significantly greater cognitive deficits in patients with schizophrenia who use substances than in those who do not,21,22 whereas others report no differences between users and nonusers.11,19,20,23 Interestingly, studies suggesting an association between cognitive deficits and substance use typically involve cocaine abuse, whereas those reporting no association focus more on alcohol or cannabis. These results are consistent with the non-schizophrenia literature.
Limited research has been conducted to assess the effects of substance use on cognition in patients experiencing their first-episode of psychosis. Thus, the purpose of this exploratory study was to examine whether there was an association between substance misuse and cognitive functioning in this population. Additionally, because using substances has been reported to have an impact on positive symptoms in individuals with a psychotic disorder,24,25,26,27 we examined the effect of substance use on both positive and negative symptoms.
Methods
Subjects for this study were 266 individuals (175 men, 91 women) who were newly admitted to the Calgary Early Psychosis Program, a well-established comprehensive treatment program for individuals with a first episode of psychosis.28 On admission, patients were experiencing their first episode of psychosis and had not received more than 3 months of previous adequate treatment.29 In keeping with Larsen and colleagues' description of the first episode,29 “adequate” treatment of initial psychosis may or may not include admission to hospital but will include administration of antipsychotic medication “in sufficient amount (e.g., haloperidol 5 mg/d) given for a sufficient period of time (e.g., 3 wk) that would generally lead to a clinically significant response in nonchronic, non-treatment-resistant patients.” Subjects were excluded from the study if they met any of the following criteria: evidence of an organic central nervous system disorder (e.g., epilepsy, traumatic brain injury, infectious or toxic cerebrovascular disease), mental retardation, age less than 16 years or greater than 50 years, or inability to speak English.
Subjects at the initial assessment were single (87.6%), with a mean age of 24.2 years (standard deviation [SD] 7.9 yr). Subjects at 1-year follow-up (n = 158) were single (89.2%), with a mean age of 23.6 years (SD 7.5 yr). Subjects at 2-year follow-up (n = 90) were single (90%), with a mean age of 23.1 years (SD 7.2). Individuals with previous admissions to psychiatric hospitals (57%, 53% and 48% at each assessment, respectively) had been out of hospital for 1–3 months. To maximize the number of participants, all available subjects were used at each time period. Thus, the decrease in numbers at the 1- and 2-year follow-up assessments is because of a 20% drop-out of subjects and because not all subjects assessed at either the initial or 1-year assessment had been in the program long enough to complete subsequent assessments.
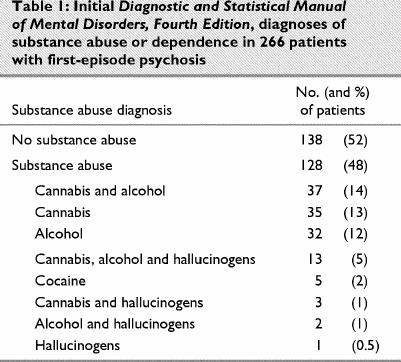
Subjects were diagnosed according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria,30 using the Structured Clinical Interview for DSM-IV (SCID-I).31 All subjects met criteria for a schizophrenia-spectrum disorder. Diagnoses were conducted at the initial assessment and confirmed at the 1-year assessment. Diagnoses were completed by J.A. and 2 experienced psychiatrists who have demonstrated reliability on this measure. Subjects' DSM-IV diagnoses at 1 year were: 72% schizophrenia, 14% schizophreniform, 4% psychotic disorder not otherwise specified and 10% other psychotic disorder. Initial DSM-IV diagnoses of substance abuse or dependence are presented in Table 1.
Table 1
Measures
The SCID-I was used to make DSM-IV diagnoses of psychotic disorders and substance use disorders, and the Positive and Negative Syndrome Scale (PANSS) for schizophrenia was administered to all subjects to determine levels of positive and negative symptoms.32
The Case Manager Rating Scale (CMRS) for substance use disorder, a short checklist used to determine the level of substance use over the past year, was completed in all participants.33 Level of use was ranked as: none, mild, moderate, severe or extremely severe. This scale has been used in several studies.34,35
Cognitive functioning was assessed by a comprehensive battery of cognitive measures routinely used in studies with individuals with schizophrenia. The following cognitive functions were assessed (assessment tool in parentheses): verbal fluency (Controlled Oral Association Test and the Category Instance Generation), visual–spatial ability and motor speed (digit-symbol subtest from the WAIS-R and Trails A), visual memory (Rey-Osterrieth Complex Figure and the visual reproduction tasks from the Wechsler Memory Scale, Revised), verbal memory (immediate and delayed recall of the logical memory subtests from the Wechsler Memory Scale, Revised, and the Rey Serial Learning Test), executive functioning (Wisconsin Card-Sorting Test, computerized version, and Trails B), visual attention (Continuous Performance Test, a measure of visual sustained attention36,37) and early information processing (Forced-Choice Span of Apprehension Test, which measures the efficiency of early iconic memory and readout stages of visual information processing relatively independent of active short-term memory38,39).
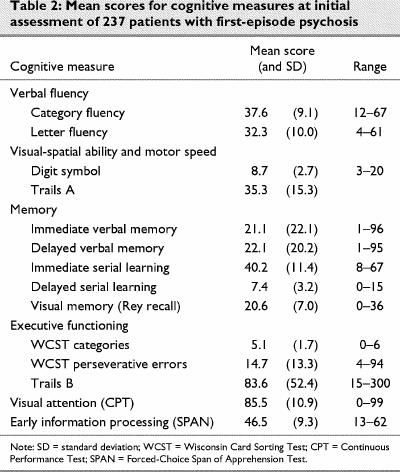
As a group, first-episode patients do not perform well relative to normal controls on these cognitive tests.40 The mean scores and ranges for all the cognitive tests are presented in Table 2.
Table 2
Procedures
These data were collected as part of an ongoing program evaluation. Patients sign an informed consent allowing the data to be used in research reports. All raters are experienced research clinicians who have used these measures in other research projects and have demonstrated adequate reliability. Diagnoses were obtained by the clinical research team using the SCID and included all available sources of information.
Results
Because not all subjects were willing to complete the cognitive testing, a series of t-tests were conducted to examine whether level of substance use (as measured by the CMRS) differed between participants for whom cognitive functioning data were available (i.e, 237 [89%] at initial assessment, 197 [74%] at 1-year follow-up and 194 [73%] at 2-year follow-up) and for participants who did not complete the cognitive testing. At the initial assessment, 1-year follow-up and 2-year follow-up, there was no difference in level of substance use between the 2 groups. Furthermore, these groups did not differ in positive symptoms, negative symptoms, age or male:female ratio.
Substance use and cognitive functioning
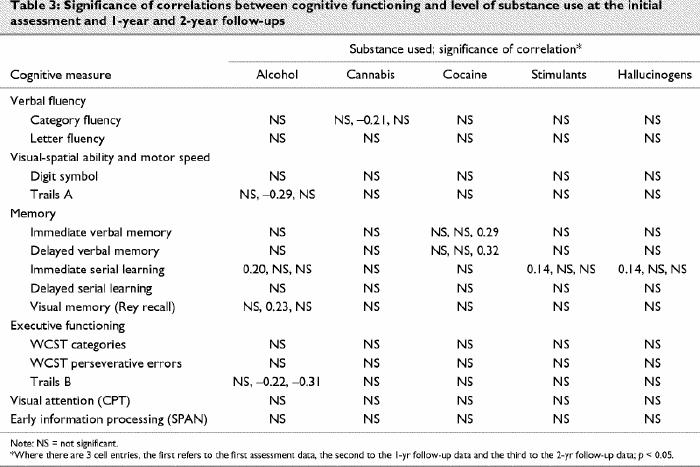
Pearson's product-moment correlations were conducted to examine the association between level of substance use (as measured by the CMRS) and cognitive performance. At the initial assessment, the 1-year follow-up and the 2-year follow-up, there were very few inconsistent significant correlations between level of use of a specific substance and cognitive performance tasks (Table 3).
Table 3
Participants were divided into the following 4 groups based on initial DSM-IV diagnoses: (1) no diagnosis of substance abuse (52%), (2) alcohol abuse or dependence (12%), (3) drug abuse or dependence (16%), (4) both alcohol and drug abuse or dependence (20%). Analyses of variance (ANOVA) were conducted with group membership as the independent variable and the mean scores on each of the cognitive tests as the dependent measures. At the initial assessment and at the 1-year follow-up, performance on any of the cognitive tests did not vary with group membership. Comparisons of diagnostic groups were not done for the 2-year assessment because the numbers were too small in the various groups.
Substance abuse and symptoms
Pearson's product-moment correlations were conducted to examine the association between level of substance use and negative symptoms and positive symptoms. At the initial assessment, 1-year follow-up and 2-year follow-up, there were no associations between negative symptoms and level of substance use on the CMRS. Furthermore, there were no associations between positive symptoms and level of substance use on the CMRS at the initial assessment and at the 2-year follow-up. However, there was a significant association between positive symptoms and level of cannabis use (r = 0.18, p < 0.05) at the 1-year follow-up.
To examine the relation between substance use diagnosis and negative symptoms, an ANOVA was conducted with group membership (as above) as the independent variable and the mean negative symptom scores as the dependent measure. At the initial assessment and 1-year follow-up, there were no differences in negative symptoms among the 4 groups. An ANOVA was also conducted with mean positive symptom scores as the dependent measure, and there were no differences in positive symptoms among the 4 groups with different substance use diagnoses at the initial assessment. However, there was a significant group difference for positive symptoms (p < 0.05) at 1-year follow-up. Individuals who had no substance use had significantly lower positive symptoms than those with a diagnosis of drug abuse.
Discussion
The main purpose of this study was to examine the impact of substance use on cognitive functioning in a sample of patients with first-episode psychosis. Overall, there were no significant associations between cognitive functioning and the use of various substances either cross-sectionally or longitudinally. In addition, there were no differences in cognitive functioning among individuals who did or did not meet criteria for a substance use or dependence diagnosis. These findings are consistent with other studies that have indicated no additional cognitive impairment in individuals with schizophrenia who use substances.11,19,20,23 The results are interesting given the extensive literature suggesting that the extended use of substances will lead to cognitive impairment and given that individuals with psychotic disorders often already have compromised cognitive functioning.41
There are some possible explanations for our results. First, this is a young sample, as the onset of psychotic disorders typically occurs between the late teens and the mid-30s. Yet, studies have indicated that the impairment in cognitive functioning associated with alcohol and drug use usually occurs after many years of prolonged and excessive use.42 Thus, given the young age of our sample, it is possible that the effects of substance use on cognitive impairment are not yet evident. Second, the level of substance use in the current sample was not very high. Although much of the sample used substances, the amounts used may not be enough to cause any additive cognitive impairment. Third, the present sample of first-episode patients may not be as impaired as individuals with more chronic schizophrenia. In other words, because these individuals exhibit higher functioning or have a better prognosis, it is possible that, as a group, they are not as vulnerable to the detrimental effects of substances on their cognitive functioning.
We have previously reported an improvement in substance use in this sample in the first year of treatment.43 However, by the second follow-up assessment, the more persistent and chronic users were still using substances, and there was still no association between use and cognitive functioning. It is possible that the use of substances leads to additive effects on cognitive impairment only in chronic users of many substances.44
There were no associations between negative symptoms and level of substance use and no differences in negative symptoms among individuals with and without substance abuse or dependence diagnoses. Although the use of most substances (mainly alcohol) was not associated with positive symptoms, increased use of cannabis was related to increased positive symptom scores at the 1-year follow-up. In addition, at the 1-year follow-up, individuals who had a drug use diagnosis (mainly cannabis) had significantly higher positive symptom scores than those with no substance use diagnosis. Thus, in the present sample, in which the main drugs used were alcohol and cannabis, there was an increase in positive symptoms for cannabis users only. Although small, this finding is consistent with previous research indicating that cannabis users have significantly more positive symptoms than nonusers.45
In summary, substance use does not appear to be related to cognitive functioning in this first-episode sample. There are, however, clinical implications of the present findings. First, the reduction or stopping of substance abuse in individuals with psychosis may lead to an improvement in positive symptoms. Second, if substance-abusing individuals with psychotic disorders do, in fact, comprise a higher functioning group, stopping substance use could lead to an improvement in cognitive functioning. Finally, although there is no evidence of additive cognitive impairment in this young sample, long-term use may lead to problems later on, particularly since it has been suggested that substance abuse might contribute to chronicity in general among individuals with schizophrenia.46 Thus, long-term substance use must be prevented. Intervention at the first episode may be the best time to help these young people and for them to understand the risk of continued substance use on an already compromised brain.43
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Jean Addington, Centre for Addiction and Mental Health, 250 College St., Toronto ON M5S 2S1; fax 416 979-4676; jean_addington@camh.net
Submitted Jul. 3, 2001 Revised Mar. 26, 2002; Aug. 7, 2002 Accepted Aug. 19, 2002
References
- 1.Cantwell R, Brewin J, Glazebrook C, Dalkin T, Fox R, Medley I, et al. Prevalence of substance misuse in first-episode psychosis. Br J Psychiatry 1999;174:150-3. [DOI] [PubMed]
- 2.Strakowski SM, Mauricio T, Stoll AL, Faedda G, Mayer PV, Kolbrener ML, et al. Comorbidity in psychosis at first hospitalization. Am J Psychiatry 1993;150:752-7. [DOI] [PubMed]
- 3.Dixon L, Haas G, Weiden PJ, Sweeney J, Frances AJ. Drug abuse in schizophrenic patients: clinical correlates and reasons for use. Am J Psychiatry 1991;48:224-30. [DOI] [PubMed]
- 4.Drake RE, Osher FC, Wallach MA. Alcohol use and abuse in schizophrenia. J Nerv Ment Dis 1989;177:408-14. [DOI] [PubMed]
- 5.Linszen DH, Dingemans PM, Lenior ME. Cannabis abuse and the course of recent-onset schizophrenic disorders. Arch Gen Psychiatry 1994;51:273-9. [DOI] [PubMed]
- 6.Bartels SJ, Teague GB, Drake RE, Clark RE, Bush PW, Noordsy DL. Substance abuse in schizophrenia: service utilization and costs. J Nerv Ment Dis 1993;181:227-32. [DOI] [PubMed]
- 7.Seibyl JP, Satel SL, Anthony D, Southwick SM, Krystal JH, Charney DS. Effects of cocaine on hospital course in schizophrenia. J Nerv Ment Dis 1993;181:31-7. [DOI] [PubMed]
- 8.Swofford CD, Scheller-Gilkey G, Miller AH, Woolwine B, Mance R. Double jeopardy: schizophrenia and substance use. Am J Drug Alcohol Abuse 2000;26:343-53. [DOI] [PubMed]
- 9.Pristach CA, Smith CM. Medication compliance and substance abuse among schizophrenic patients. Hosp Community Psychiatry 1990;41:1345-8. [DOI] [PubMed]
- 10.Arndt S, Tyrrel G, Flaum M, Andreasen NC. Comorbidity of substance abuse and schizophrenia: the role of premorbid adjustment. Psychol Med 1992;22:379-88. [DOI] [PubMed]
- 11.Addington J, Addington D. Substance abuse and cognitive functioning in schizophrenia. J Psychiatry Neurosci 1997;22:99-104. [PMC free article] [PubMed]
- 12.Beatty WW, Katzung VM, Moreland VJ, Nixon SJ. Neuropsychological performance of recently abstinent alcoholics and cocaine abusers. Drug Alcohol Depend 1995;37:247-53. [DOI] [PubMed]
- 13.Hoff AL, Riordan H, Morris L, Cestaro V, Wieneke M, Alpert R, et al. Effects of crack cocaine on neurocognitive function. Psychiatry Res 1996;60:167-76. [DOI] [PubMed]
- 14.Loberg T. Neuropsychological findings in early and middle phases of alcoholism. In: Grant I, Adams K, editors. Neuropsychological assessment of neuropsychiatric disorders. New York: Oxford University Press; 1986. p. 415-40.
- 15.O'Malley S, Adamse M, Heaton, RK, Gawin FH. Neuropsychological impairment in chronic cocaine abusers. Am J Drug Alcohol Abuse 1992;18:131-44. [DOI] [PubMed]
- 16.Pope HG, Gruber AJ, Yurgelun-Todd D. The residual neuropsychological effects of cannabis: the current status of research. Drug Alcohol Depend 1995;38:25-34. [DOI] [PubMed]
- 17.Hoff AL, Riordan H, O'Donnell DW, Morris L, DeLisi LE. Neuropsychological functioning of first-episode schizophreniform patients. Am J Psychiatry 1992;149:898-903. [DOI] [PubMed]
- 18.Saykin AJ, Gur BC, Gur RE, Mozley D, Mozley, LH, Resnick SM, et al. Neuropsychological function in schizophrenia: selective impairment in memory and learning. Arch Gen Psychiatry 1991; 48:618-24. [DOI] [PubMed]
- 19.Cleghorn JM, Kaplan RD, Szechtman B, Szechtman H, Brown GM, Franco S. Substance abuse and schizophrenia: effect on symptoms but not on neurocognitive function. J Clin Psychiatry 1991;52:26-30. [PubMed]
- 20.Nixon SJ, Hallford HG, Tivis RD. Neurocognitive function in alcoholic, schizophrenic, and dually diagnosed patients. Psychiatry Res 1996;64:35-45. [DOI] [PubMed]
- 21.Serper MR, Bergman A, Copersino ML, Chou J-CY, Richarme D, Canero R. Learning and memory impairment in cocaine dependant and comorbid schizophrenic patients. Psychiatry Res 2000; 93:387-94. [DOI] [PubMed]
- 22.Sevy S, Kay SR, Opler L, van Praag HM. Significance of cocaine history in schizophrenia. J Nerv Ment Dis 1990;178:642-8. [DOI] [PubMed]
- 23.Cooper L, Liberman D, Tucker D, Nuechterlein KH, Tsuang J, Barnett HL. Neurocognitive deficits in the dually diagnosed with schizophrenia and cocaine abuse. Psychiatr Rehab Skills 1999; 3:231-45.
- 24.Addington J, el-Guebaly N. Group treatment for substance abuse in schizophrenia. Can J Psychiatry 1998;43:843-5. [DOI] [PubMed]
- 25.Addington J, Duchak V. Reasons for substance use in schizophrenia. Acta Psych Scand 1997;96:329-33. [DOI] [PubMed]
- 26.Mueser KT, Bellack AS, Blanchard JJ. Comorbidity of schizophrenia and substance abuse: implications for treatment. J Consult Clin Psychology 1992; 60:845-56. [DOI] [PubMed]
- 27.Negrete JC, Knapp WP, Douglas DE, Smith WB. Cannabis affects the severity of schizophrenic symptoms: results of a clinical survey. Psychol Med 1986;16:515-20. [DOI] [PubMed]
- 28.Addington J, Addington D. Early intervention for psychosis: the Calgary Early Psychosis Treatment & Prevention Program. Can Psychiat Assoc Bull 2001;33:11-6.
- 29.Larsen TK, McGlashan TH, Moe LC. First-episode schizophrenia: I. Early course parameters. Schizophr Bull 1996;22:241-56. [DOI] [PubMed]
- 30.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington (DC): American Psychiatric Association; 1994.
- 31.Spitzer RL, Williams JB, Gibbon M, First MB. The structured clinical interview for DSM-IV SCID. I. History, rationale, and description. Arch Gen Psychiatry 1992;49:624-9. [DOI] [PubMed]
- 32.Kay SR, Fizbein A, Opler LA. The Positive and Negative Syndrome Scale PANSS for Schizophrenia. Schizophr Bull 1987; 13: 261-76. [DOI] [PubMed]
- 33.Drake RE, Osher FC, Noordsy DL, Hurlbut SC, Teague GB, Beaudett MS. Diagnosis of alcohol use disorders in schizophrenia. Schizophr Bull 1990;16:57-67. [DOI] [PubMed]
- 34.Drake RE, McHugo GJ, Noordsy DL. Treatment of alcoholism among schizophrenic outpatients: 4-year outcomes. Am J Psychiatry 1993;150:328-9. [DOI] [PubMed]
- 35.Drake RE, Osher FC, Noordsy DL, Hurlbut SC, Teague GB, Beaudett MS. Diagnosis of alcohol use disorders in schizophrenia. Schizophr Bull 1990;16:57-67. [DOI] [PubMed]
- 36.Nuechterlein KH. Vigilance in schizophrenia and related disorders. In: Steinhauer SR, Gruzelier JH, Zubin J, editors. Handbook of schizophrenia. Vol. 5. Neuropsychology, psychophysiology and information processing. Amsterdam: Elsevier Science; 1991. p. 397-433.
- 37.Nuechterlein KH, Asarnow RF. UCLA Continuous Performance Test [manual and computer program]. Version 4. Los Angeles: University of California, Los Angeles; 1992.
- 38.Asarnow RF, Granholm E, Sherman T. Span of apprehension in schizophrenia. In: Steinhauer SR, Gruzelier JH, Zubin J, editors. Handbook of schizophrenia. Vol. 5. Neuropsychology, psychophysiology and information processing. Amsterdam: Elsevier Science; 1991. p. 335-70.
- 39.Asarnow RF, Nuechterlein KH. UCLA Forced-Choice Span of Apprehension Test [manual and computer program]. Version 4. Los Angeles: University of California, Los Angeles; 1992.
- 40.Addington J, Addington D. Cognitive functioning in first-episode schizophrenia. J Psychiatry Neurosci 2002;27:188-92. [PMC free article] [PubMed]
- 41.Mueser KT, Bellack AS, Blanchard JJ. Comorbidity of schizophrenia and substance abuse: implications for treatment. J Consult Clin Psychol 1992;60:845-56. [DOI] [PubMed]
- 42.Eckhardt MJ, Stapleton JM, Rawlings RR, Davis EZ, Grondin DM. Neuropsychological functioning in detoxified alcoholics between 18 and 35 years of age. Am J Psychiatry 1995;153:53-9. [DOI] [PubMed]
- 43.Addington J, Addington D. Intervention strategies for substance use in early psychosis. J Psychiatr Rehab 2001;25:60-7. [DOI] [PubMed]
- 44.Tracy JI, Josiassen R, Bellack AS. Neuropsychology of dual diagnosis: understanding the combined effects of schizophrenia and substance use disorders. Clin Psychol Rev 1995;15:67-97.
- 45.Wolthaus JE, Dingemans PM, Schene AH, Linszen DH. Cannabis abuse, symptoms and personality traits in recent-onset schizophrenia [abstract]. Schizophr Res 2001;49:9.
- 46.Klegon DA, Fiedosewicz H, Chang HH, Bayog CR, Berman I. Clinical significance of substance abuse in patients with schizophrenia [abstract]. Schizophr Res 2001;49:36.


