Abstract
Newly assembled herpesvirus capsids travel from the nucleus to the plasma membrane by a mechanism that is poorly understood. Furthermore, the contribution of cellular proteins to this egress has yet to be clarified. To address these issues, an in vitro nuclear egress assay that reproduces the exit of herpes simplex virus type 1 (HSV-1) capsids from nuclei isolated from infected cells was established. As expected, the assay has all the hallmarks of intracellular transport assays, namely, a dependence on time, energy, and temperature. Surprisingly, it is also dependent on cytosol and was slightly enhanced by infected cytosol, suggesting an implication of both host and viral proteins in the process. The capsids escaped these nuclei by budding through the inner nuclear membrane, accumulated as enveloped capsids between the two nuclear membranes, and were released in cytosol exclusively as naked capsids, exactly as in intact cells. This is most consistent with the view that the virus escapes by crossing the two nuclear membranes rather than through nuclear pores. Unexpectedly, nuclei isolated at the nonpermissive temperature from cells infected with a UL26 thermosensitive protease mutant (V701) supported capsid egress. Although electron microscopy, biochemical, and PCR analyses hinted at a likely reconstitution of capsid maturation, DNA encapsidation could not be confirmed by a traditional SQ test. This assay should prove very useful for identification of the molecular players involved in HSV-1 nuclear egress.
Herpes simplex virus type 1 (HSV-1) replicates and assembles its capsids in the nucleus. It must then find its way to the plasma membrane for release in the extracellular environment. Given the restrictive nature of the much smaller nuclear pores, it has been assumed that the capsids could escape the nuclei only by budding through the inner nuclear membrane. Numerous electron microscopy (EM) observations of enveloped virions in the gap between the two nuclear envelopes, herein called the perinuclear space, indeed support this view (11, 23, 49, 72). Once in the perinuclear space, the virions must traverse the outer nuclear membrane. Given the continuity between the perinuclear space and the reticulum endoplasmic, a luminal model claims that the virions travel through the biosynthetic pathway (11, 30). They would thus reach the Golgi, trans-Golgi network, and plasma membrane in transport vesicles in much the same way that secreted proteins do. In this scenario, naked capsids would be released only accidentally in the cytoplasm and would not reach the extracellular medium. A second, more widely accepted model suggests that the perinuclear virions rather fuse with the outer nuclear membrane, thereby releasing naked capsids in the cytoplasm (70, 72). These capsids would then be reenveloped later on, likely at the trans-Golgi network (22-24, 26, 35, 74, 78). Interestingly, this deenvelopment/reenvelopment model may be valid for all members of the herpesvirus family (15, 43, 44). Finally, a third model has recently been proposed and implies the disassembly and dilatation of the nuclear pores, such that the capsids within the nucleus could reach the cytoplasm directly through the pores (39, 79). In this last model, naked cytoplasmic capsids would also be produced but would apparently quickly fuse with diverse membranes, including the outer nuclear membrane and Golgi apparatus. As for the previous model, naked cytoplasmic capsids would not be a dead-end product but an important intermediate. The route of HSV-1 nuclear egress is thus an issue not yet fully resolved that warrants further examination.
The molecular details of herpesvirus egress out of the nucleus are scarce. On the one hand, deletion of UL31 or UL34 results in the accumulation of naked capsids in the nucleus (5, 20, 32, 33, 57, 58, 61), while deletion of US3 causes the accumulation of enveloped virions in nuclear membrane invaginations (32, 58) or between the two nuclear envelopes (77). Interestingly, UL31 and UL34 form a complex targeted to the inner nuclear membrane (20, 40, 56, 57) and UL34 is a substrate for the US3-encoded viral kinase (53, 62). This complex is further regulated by UL13, a kinase that phosphorylates US3 (31). These interactions result in the depolymerization of the nuclear lamins via protein kinase C, presumably to allow the capsids to reach the nuclear periphery (3, 46, 51, 56, 66, 68, 69). Meanwhile, HSV-1 deletion mutants lacking VP16 or UL20 induce the accumulation of perinuclear virions (2, 45), though deletion of VP16 in equine herpesvirus blocked secondary reenvelopment (76). In the case of UL20, this phenotype has been attributed to UL20.5 or a combination of UL20 and UL20.5 (18). A recent report suggests that UL11 may also be involved in HSV-1 nuclear egress (1) but not in those of the two related viruses pseudorabies virus and equine herpesvirus (36, 64). Finally, Luxton and colleagues reported that UL36 and UL37 may modulate pseudorabies virus nuclear egress (41), a phenotype not yet seen by others (14, 19, 34). On the other hand, besides protein kinase C as mentioned above, no cellular protein has directly been implicated in HSV nuclear egress. Nonetheless, brefeldin A, a drug that typically inhibits transport from the endoplasmic reticulum to the Golgi (65), blocks HSV-1 nuclear egress under certain circumstances (6, 13, 29, 78). The mechanism by which the drug perturbs viral egress, however, remains unclear. Finally, actin has been shown to be required for the active transport of capsids within the nucleus, presumably from the site of assembly to the nuclear periphery (17). Thus, the molecular details of nuclear herpes egress remain unclear at this point.
In the past decade, much effort has been dedicated to the understanding of intracellular transport. In vitro reconstitution assays played an important role in identifying the molecules and mechanisms driving the transport of host and viral proteins (10). These assays have demonstrated their usefulness by virtue of their rapidity and flexibility compared with whole-cell experimentation. Most important, they accurately identified a variety of proteins and lipids involved in intracellular transport and addressed their molecular mechanisms (27, 37, 75, 81). For example, an in vitro assay reconstituting vesicular transport along the endocytic route could identify over 20 different effectors (7). Interestingly, a number of very informative in vitro assays reconstituting various steps of the herpesvirus life cycle are emerging (38, 47, 50, 71, 80). Given the limited knowledge about herpesvirus egress, for instance, from the nucleus to the cytoplasm, an in vitro assay could be very useful. Furthermore, an assay performed when other organelles are largely depleted would allow one to concentrate on that egress step.
To clarify the route of egress of herpesvirus capsids at the level of the nucleus and ultimately identify the molecular players involved, an in vitro nuclear egress assay for HSV-1 was set up. This assay is based on the isolation of nuclei from HSV-1-infected cells and their incubation in the test tube under various conditions. We now report that the assay reconstitutes HSV-1 egress from the nucleus to the cytoplasm. Interestingly, the assay has the usual hallmarks of intracellular transport assays, namely, temperature, time, and energy dependence. Surprisingly, HSV-1 egress is also supported by cytosol prepared from mock-treated cells, while cytosol prepared from infected cells enhances it, suggesting a potential role for both host and viral proteins. EM analysis of both isolated nuclei and intact cells revealed identical routes of egress, namely, the budding of capsids through the inner nuclear membrane, the accumulation of enveloped particles in the perinuclear space, and the release of exclusively naked capsids in the cytosol. These results are most consistent with the widely accepted deenvelopment/reenvelopment model of egress. Finally, nuclei isolated from cells infected with V701, a thermosensitive mutant for the viral UL26 protease (55, 74), were also competent to produce capsids in vitro and shared the same properties as those isolated from cells infected with the wild-type (WT) virus. The possible reconstitution of capsid maturation and nuclear egress is discussed. The assay should be useful for identification of molecules involved in herpesvirus egress.
MATERIALS AND METHODS
Cells and viruses.
HeLa cells adapted to culture in suspension were grown in Joklik's modified Eagle's medium (JMEM) (Sigma-Aldrich) supplemented with 5% fetal bovine serum (Medicorp), 0.1 mM MEM nonessential amino acid solution, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Wild-type HSV-1 17+ virus, provided by Beate Sodeik, and the V701 ts80-1C2 mutant (also of strain 17+), supplied by Bruce Register and Jules A. Shafer (55), were expanded on BHK cells and titrated on Vero cells as described previously(74).
Isolation of nuclei.
HeLa cells grown in suspension were infected with HSV-1 17+ at 37°C or with V701 at 39.5°C for 8 h at a multiplicity of infection of 3. For radiolabeled preparations, a protocol adapted from the work of Church and Wilson was used (9). Briefly, cells were starved for thymidine in JMEM and 3% dialyzed fetal bovine serum (Multicell), subsequently infected, and finally incubated in JMEM, 3% dialyzed fetal bovine serum, and 25 μCi/ml of [3H]thymidine (PerkinElmer). Eight hours postinfection (hpi), cold or 3H-labeled cells were pelleted, washed with phosphate-buffered saline (PBS)-5 mM MgCl2, and resuspended in reticulocyte standard buffer (10 mM NaCl, 10 mM Tris-Cl [pH 8.4], 5 mM MgCl2) before being broken mechanically by cavitation. The resulting cell lysate was centrifuged at 300 × g for 15 min on a 40% iodixanol cushion (Axis-shield), and the nuclei were collected and enriched on a 25 to 40% discontinuous iodixanol gradient at 10,000 × g for 30 min. The nuclear fraction was collected, adjusted to 50% glycerol and 1 mM dithiothreitol, and stored at −80°C. For wild-type infections, the isolation of the nuclei took place at 4°C, while V701-infected nuclei were isolated at 20°C.
Preparation of cytosol.
Mock-treated or HSV-1 17+-infected HeLa cells were collected 8 hpi. They were pelleted, washed in PBS-5 mM MgCl2, and resuspended in KEHM (50 mM KCl, 10 mM EGTA, 50 mM HEPES [pH 7.4], 2 mM MgCl2) supplemented with 1 mM dithiothreitol and a cocktail of protease inhibitors (8.25 mM chymostatin, 1.05 μM leupeptin, 0.38 μM aprotinin, and 0.73 μM pepstatin A [Sigma-Aldrich]). They were then broken mechanically as described above, and the cell lysate was centrifuged at 4°C for 20 min at 800 × g and finally spun at 267,000 × g for 30 min. The resulting cytosol was stored at −80°C.
In vitro assay.
Nuclei were incubated in duplicates with nuclear buffer (20 mM Tris-Cl [pH 7.4], 5 mM MgCl2, 100 mM KCl, and 1 mM dithiothreitol) for various times and at different temperatures, as indicated in the figure legends. Cytosol, usually 4 mg/ml, and an energy-regenerating system (17.3 mM creatine phosphate, 87 μg/ml creatine kinase, 2.17 mM ATP; Roche) (28) were added unless otherwise indicated. At the end of the incubation period, the capsids released in vitro by the nuclei were recovered in the flowthrough of a spin column mounted with a 0.45-μm cellulose acetate filter (Costar) and centrifuged at 825 × g for 10 min at 4°C. After a wash with PBS, both the flowthrough and the wash were pooled and digested with 500 U/ml of DNase I (Roche) for 1 h at 37°C to ensure that only encapsidated DNA was quantified. The total encapsidated viral pool present in nuclei was evaluated by breakage of the nuclei with several cycles of freeze-thawing in distilled water and measurement of trichloroacetic acid (TCA)-precipitable, DNase I-resistant counts as described above. Efficiency of capsid release was determined by dividing the number of counts per minute (cpm) found in the virus released for 6 h in vitro by the number of cpm found in the nuclei at 0 h.
Detection by PCR.
Viral DNA from the capsids produced in the nuclear egress assay was extracted by phenol-chloroform and ethanol precipitation (4). The positive control was 100 ng of DNA extracted from extracellular viruses, while the negative control was devoid of DNA. To quantify the viral DNA, a 669-bp fragment of UL20 was amplified by conventional PCR with HSV-1-specific primers. The PCR products were then subjected to electrophoresis on a 2% agarose gel and stained with ethidium bromide before being photographed on a UV illuminator.
Detection by liquid scintillation.
Capsids produced in the in vitro assay were deposited on a paper filter (P5; Fisherband), dried, and subjected to trichloroacetic precipitation (63). Briefly, the samples were washed three times in ice-cold TP buffer (5% TCA, 20 mM sodium pyrophosphate), washed with 70% ethanol, and dried again. Levels of TCA-precipitated radioactivity were measured on an LKB Beta rack 1211 counter by liquid scintillation.
Exclusion of TRITC dextran.
Nuclei were incubated in vitro as described above for various times and at different temperatures, as indicated in Table 1. The capsids were then recovered with a spin column and incubated for 10 min on ice with 0.1 g/ml Hoechst 33342 (Sigma-Aldrich) to stain the nuclei and labeled with 0.2 mg/ml tetramethyl rhodamine isocyanate (TRITC) and 155-kDa dextran (Sigma-Aldrich). Untreated or 0.1% Triton X-100-permeabilized cells (10 min prior to addition of the TRITC-dextran) served as controls for the experiment. Cells were visualized with an Axiophot wide-field fluorescence microscope (Zeiss) equipped with filters and a Retiga 1300 camera (Q Imaging). The images were acquired and analyzed with Northern Eclipse imaging software (Empix Imaging) and manually counted. Intact nuclei stained for Hoechst but excluded the dextran.
TABLE 1.
Integrity of nuclei determined by their exclusion of dextran-TRITC
| Description of cells or nuclei | Value for incubation
|
% Impermeable material | |
|---|---|---|---|
| Time (h) | Temp (oC) | ||
| Control cells | 0 | 95 ± 0 | |
| Permeabilized cellsa | 0 | 0 ± 0 | |
| Wild-type nuclei | 0 | 86 ± 6 | |
| 6 | 4 | 88 ± 10 | |
| 6 | 37 | 82 ± 10 | |
| V701 nuclei | 0 | 62 ± 2 | |
| 6 | 4 | 63 ± 6 | |
| 6 | 31 | 72 ± 9 | |
| 6 | 39.5 | 66 ± 5 | |
Cells were treated for 10 min with 0.1% Triton X-100 on ice.
Western blot against PCNA.
Capsids produced in vitro and isolated as described above were boiled for 10 min in sample buffer (50 mM Tris-HCl [pH 6.8], 2% sodium dodecyl sulfate, 0.1% bromophenol blue, 10% glycerol, and 2% β-mercaptoethanol) and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Controls included total nuclei or the nuclear buffer used for the in vitro assay. Proteins were transferred to a polyvinylidene difluoride membrane and probed with a 1:500 dilution of E78 anti-PCNA (mouse anti-human proliferation cell nuclear antigen monoclonal antibody; Chemicon International) and a 1:5,000 dilution of horseradish peroxidase-coupled goat anti-mouse (Jackson Immunoresearch). The detection was done on Kodak BioMax MR film and Super Signal West Pico chemiluminescent substrate from Pierce.
Electron microscopy.
HeLa cells infected with HSV-1 17+ or V701 for 8 h at their respective permissive temperatures were fixed with 2.5% glutaraldehyde (Canemco and Marivac) in sodium cacodylate buffer (0.1 M, pH 7.2 to 7.4) for 1 h at room temperature and postfixed 1 h in 1% osmium tetroxide-0.1 M sodium cacodylate (Mecalab). They were then contrasted for 1 h at 4°C with 2% aqueous uranyl-acetate (Canemco and Marivac), gradually dehydrated in alcohol, embedded in Epon 812 (Mecalab), and ultrathin sectioned with a Reichert Ultracut S ultramicrotome. Seventy-five-nanometer sections were analyzed with a Philips 300 transmission electron microscope. For negative staining, the capsids released in vitro were deposited on hexagonal 200-mesh copper grids coated with Formvar and carbonated (Canemco and Marivac). Excess liquid was blotted away with filter paper, and the samples were contrasted with 2% of uranyl acetate (Canemco and Marivac). The grids were finally washed in distilled water and dried on filter paper. Samples were examined with the same transmission EM as that described above. When desired, these samples were quantified by counting mature and immature capsids in multiple fields from a minimum of three independent experiments.
SQ analysis.
DNA was extracted from capsids produced in vitro with phenol- chloroform and ethanol precipitation (4), digested with BamHI, and subjected to electrophoresis and Southern blot analysis. Membranes were probed with the BamHI SQ junction fragment of plasmid pNN9, provided by Sandra K. Weller (42), which was 32P labeled with a High Prime kit according to the manufacturer's instructions (Roche). As an encapsidated control, we used DNA extracted from extracellular HSV-1 17+ WT particles, while our unencapsidated control consisted of a bacmid containing the entire circular genome of HSV-1 provided by Beate Sodeik.
RESULTS
Isolation of infected nuclei.
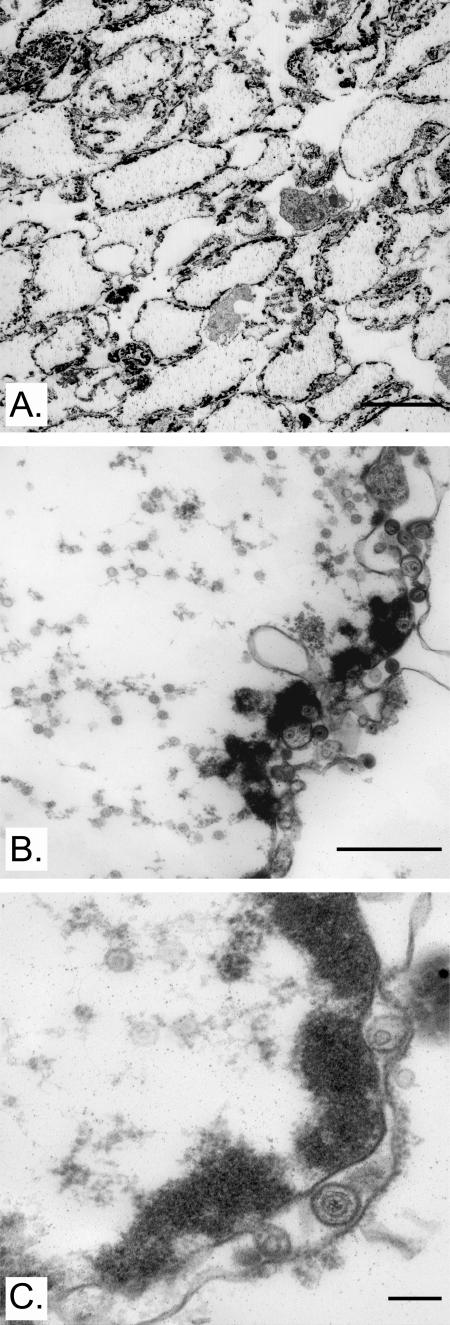
The aim of the work described here was to establish an in vitro assay that reconstitutes the exit of HSV-1 capsids from nuclei. Our approach was to isolate nuclei from infected cells and determine whether they could release capsids in the test tube. To this end, HeLa cells were chosen because they can be adapted to suspension culture and thus provide biochemical amounts of material, a must for any successful in vitro strategy. HeLa cells were therefore infected with WT HSV-1 and harvested at 8 hpi, a time when new capsid assembly is at an advanced stage but only at the onset of viral egress. After mechanical rupture of the cells, the nuclei were passed over an iodixanol gradient to enrich them and separate them from cytosolic components and other organelles. Since the integrity of the isolated nuclei was of primary importance, they were examined by EM. Figure 1 shows that the nuclei, despite their storage at −80°C, were relatively pure and intact, contained plenty of viral capsids, and retained both of their nuclear membranes. This made possible the storage of large preparations of nuclei, an important aspect, as it would technically be demanding to use freshly isolated nuclei for each assay. Finally and most importantly, the nuclei retained their functionality, as shown by their ability to release viral capsids in a controlled manner (see below). It was thus possible to isolate intact and functional nuclei.
FIG. 1.
Purity and integrity of isolated nuclei. Nuclei isolated from HSV-1 17+-infected HeLa cells were mounted in Epon and analyzed by electron microscopy. Panel A indicates the relative purity of the preparation at low magnification (×960), while panels B and C show that the isolation procedure preserved the integrity of both nuclear envelopes at higher magnification (×12,400 and ×31,200, respectively). Note the presence of multiple capsids both in the nuclei matrix and in the perinuclear space. Bars represent (A) 10 μm, (B) 1 μm, and (C) 200 nm.
Separation of capsids and nuclei.
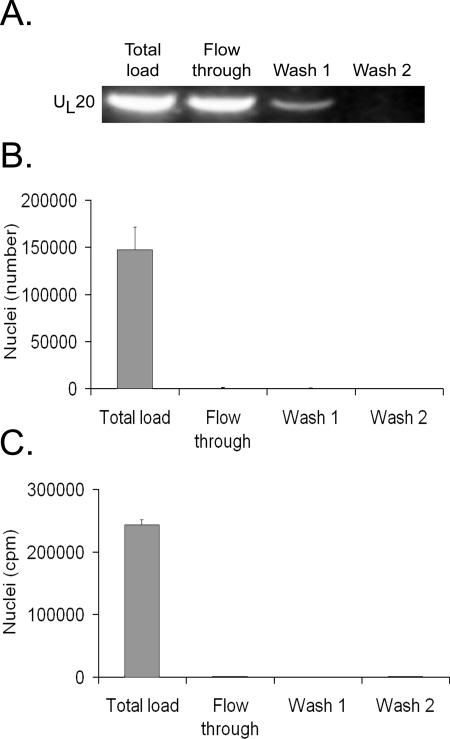
It was crucial to distinguish between the capsids present in the nuclei from the ones released in vitro. Given the propensity of the capsids to easily pellet, even at low speed (Taquet and Lippé, unpublished observations), we evaluated the ability of spin columns to quickly separate nuclei from the virus. To evaluate the efficiency of this procedure, extracellular virions were first passed over a column. Figure 2A shows that most virions were found in the flowthrough, with a few residual virions needing a washing step to fully recover them. In contrast, nuclei isolated from infected cells did not pass through the column (Fig. 2B). Moreover, the procedure did not rupture the nuclei, as their 3H-labeled content did not travel through the column (Fig. 2C). The data thus indicated that we could readily distinguish between capsids still present in the nuclei from the ones released in vitro.
FIG. 2.
Efficient separation of nuclei and virions. (A) Extracellular virions were passed over a spin column and washed twice, and the different fractions were collected. DNA from each fraction was extracted, PCR amplified using HSV-1 UL20-specific primers, and analyzed on an ethidium bromide agarose gel. (B) Nuclei isolated from [3H]thymidine-labeled, infected cells were passed over a spin column and the nuclei present in each fraction counted with a hemacytometer and phase contrast. (C) Same procedure as that shown in panel B, except that leakage of 3H-labeled virions from nuclei was measured by liquid scintillation. Average values for duplicate experiments are shown, and error bars represent the standard deviations of the means. Note that the nuclei remained intact during this procedure.
In vitro release of capsids.
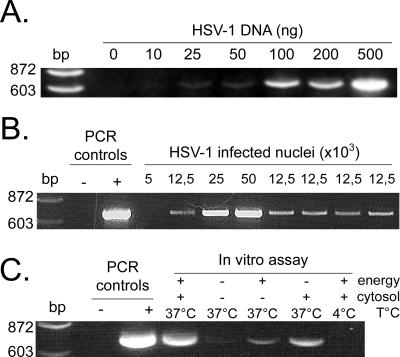
One of the main advantages of in vitro transport assays is their quantitative nature. Two complementary methods were used to quantify capsid egress. First, DNA extracted from the released capsids was quantified by PCR with HSV-1-specific primers. Although real-time PCR is a better option, semiquantitative PCR was initially performed to quickly establish whether the assay was working. As control, HSV-1 DNA purified from extracellular virions was used and amplified under various PCR cycling conditions to find appropriate parameters yielding a semilinear quantification of viral DNA (Fig. 3A). To confirm the results, viral DNA present in increasing numbers of nuclei isolated from infected cells was also quantified (Fig. 3B). The results clearly showed that it is possible to quantify viral egress by using this approach.
FIG. 3.
Quantitative analysis by PCR of capsids released. (A) Various amounts of purified HSV-1 DNA were amplified by PCR with UL20-specific primers under limited cycling conditions (see Materials and Methods). (B) The viral DNA from increasing numbers of nuclei isolated from infected cells was extracted and amplified by PCR as described above. (C) Nuclei isolated from HSV-1 17+-infected cells were incubated in vitro 6 h at 4°C or 37°C in the presence (4 mg/ml) or absence of cytosol from infected cells and with (+) or without (-) an energy regeneration system. Capsids released in the assay were separated from nuclei on spin columns, and DNA was extracted from the capsids and amplified by PCR as described in the text. PCR controls: -, no DNA; +, DNA from HSV-1 17+-infected cells.
Using our previously established PCR protocol, we proceeded to measure the amount of viral DNA released in vitro in the egress assay. Nuclei were incubated in the presence of energy, cytosol, and buffer. Since it was unclear whether host and/or viral cytosolic proteins were needed, high-speed cytosol derived from infected cells was included. It should be noted that this cytosol was completely devoid of virus, as determined by PCR and EM (data not shown). Figure 3C shows that the capsids indeed escaped the nuclei in vitro in a measurable way. As expected from intracellular transport assays, no egress occurred when the assay was performed at 4°C. In addition, little egress took place when cytosol or both cytosol and energy were omitted. In contrast, the sole omission of energy permitted some capsid release, which was attributed to the presence of energy in the cytosol preparation itself. Thus, as with most intracellular transport assays, our HSV nuclear egress assay was dependent on energy and temperature. Surprisingly, it was also dependent on cytosol, a finding that was not necessarily predictable since HSV-1 egress could conceivably have occurred without it. HSV-1 nuclear capsid egress was therefore measurable and dependent on the same factors as most intracellular transport steps.
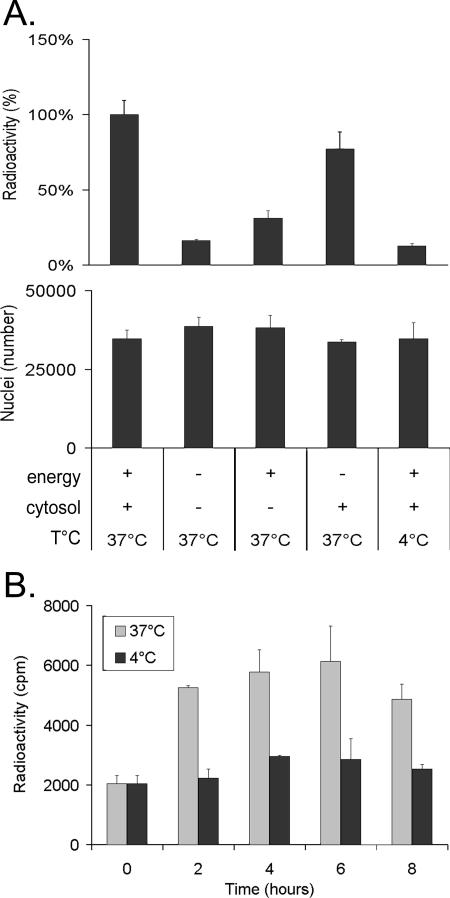
Having established that the assay was working and given the semiquantitative nature of normal PCR, we turned to [3H]thymidine and scintillation counting as a better tool by which to evaluate egress and confirm the results. Hence, after metabolic labeling of cells during the infection, nuclei were isolated as described above and used in the in vitro assay. Following the separation of nuclei and capsids with a spin column, the samples were treated with DNase I to ensure that only encapsidated DNA was measured (see Materials and Methods). Figure 4 shows that the results were identical to those obtained with PCR, namely, an indication of strong dependence on temperature, energy, and cytosol. Since intracellular transport is typically dependent on time, egress was also measured after different incubation times to determine the optimal moment to monitor capsid release (Fig. 4B). The data indicate that HSV-1 release increased steadily with time and peaked by 6 h, a time that was subsequently adopted. Given the more quantitative nature of liquid scintillation, this method of quantification was used for the remaining experiments. By comparison of the DNase-resistant nuclear pool and the capsids produced, it was estimated that up to 31% of the encapsidated DNA present in nuclei was released (data not shown).
FIG. 4.
Quantitative analysis by scintillation counting of capsids released in vitro. (A) Isolated nuclei containing radiolabeled HSV-1 17+ capsids were incubated for 6 h at 4°C or 37°C with 0 or 4 mg/ml of infected cytosol and with (+) or without (-) an energy regeneration system. The capsids released in the test tubes were separated from nuclei on spin columns, their DNA was extracted, and TCA was precipitated on paper filters and finally analyzed in a liquid scintillation counter (top panel). The stabilities of the nuclei were determined by counting each sample with a hemacytometer after the incubation period of 6 h (middle panel). (B) To determine the optimal incubation time, nuclei were incubated for various times at 4°C or 37°C in the presence of energy and 4 mg/ml of infected cytosol and analyzed by scintillation counting as described above. The panels show average values for representative experiments, and error bars represent the standard deviations of the means, depicting the variation among the duplicates within each experiment.
Nuclear integrity.
The lack of capsid release at 4°C or in the absence of cytosol and energy suggested that HSV-1 egress was an active and regulated step. It also suggested that the capsids were not simply released owing to the rupture of the nuclei during the reaction. To confirm this, we performed several additional experiments. First, nuclei were examined by phase contrast and no noticeable difference in appearance before or after their incubation in vitro was found (data not shown). Second, no loss in the number of nuclei was detected upon incubation, as measured with a hemacytometer (Fig. 4A, lower panel). Third, we examined the ability of the nuclei to exclude large fluorescent dextrans, molecules too big to travel through the nuclear pores. In such an assay, only damaged or leaky nuclei can pick up the dextran (50). As shown in Table 1, control cells mixed with 155-kDa TRITC-coupled dextran were completely impermeable to this molecule, while none of the Triton X-100-permeabilized cells prevented the entry of the fluorescent probe. In comparison, nuclei mostly excluded the dextran both prior to and following incubation for 6 h in the test tube, suggesting that the much larger capsids could not leak out. Fourth, we probed by Western blotting the propensity of the nuclei to release PCNA, a 36-kDa nuclear protein, which would be indicative of instability. As anticipated, only marginal amounts of the nuclear marker leaked out (Fig. 5). Finally, if capsids were released by simple leakage or rupture of the nuclei, all capsids present in nuclei would indiscriminately be released. To test this directly, the capsid content of nuclei was compared by EM to the capsid produced in vitro. Table 2 shows that purified nuclei primarily contained immature capsids, as do intact cells (data not shown). This was even true after a 6-h incubation of the nuclei in vitro. In contrast, mature C capsids were preferentially released in the test tube. Altogether, these data indicate that the capsids were not simply produced by ruptured or leaky nuclei but that mature capsids were specifically released.
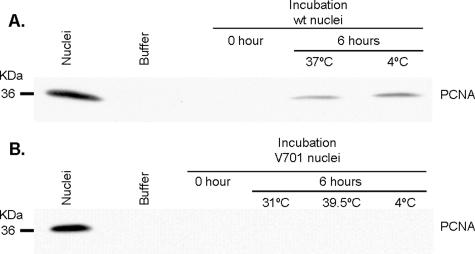
FIG. 5.
Lack of significant leakage of PCNA. To evaluate the leakiness of the nuclei during the in vitro assay, the presence in the flowthrough of spin columns of PCNA, a nuclear resident protein, was examined by Western blotting as described in Materials and Methods. The total PCNA nuclear pool is shown by loading the same number of nuclei as in the assay. The results indicate that both (A) WT and (B) V701 nuclei are relatively stable, with a minimal release of PCNA. Note that upon prolonged exposure, a signal can be seen for V701. The negative-control “Buffer” was the in vitro assay buffer.
TABLE 2.
Preferential release of mature wild-type capsids by nuclei in vitro
| Description of capsids | Incubationa time (h) | Value for capsids
|
||
|---|---|---|---|---|
| % Mature | % Immatureb | No. of capsids counted | ||
| Present in nuclei | 0 | 32 | 68 | 793 |
| 6 | 42 | 58 | 747 | |
| Released in vitro | 6 | 68 | 32 | 196 |
Incubation at 37°C in nuclear buffer, cytosol, and energy.
A, B, and atypical capsids.
Enhancement of nuclear egress by infected cytosol.
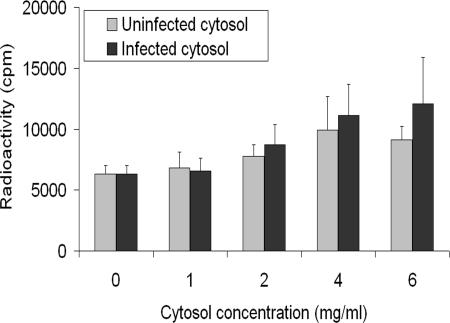
Given the ability of infected cytosol to support HSV-1 nuclear egress, it was interesting to evaluate whether cytosol from mock-treated cells could be equally effective. Cytosol from mock-treated cells was prepared and compared in the in vitro assay with cytosol obtained from infected cells. Interestingly, the presence of viral proteins in the cytosol was not obligatory, as cytosol from mock-treated cells also permitted egress (Fig. 6). However, a small and reproducible stimulation of egress was found when using cytosol from infected cells. Although not essential, cytosolic viral proteins enhanced HSV-1 nuclear egress.
FIG. 6.
Cytosol from infected cells is slightly more efficient than mock-treated cytosol in releasing capsids in vitro. Isolated nuclei containing radiolabeled HSV-1 17+ capsids were incubated for 6 h at 37°C with energy and increasing amounts of cytosol obtained from mock-treated or infected cells. The capsids released in vitro were separated from nuclei in spin columns and analyzed by liquid scintillation as in Fig. 4. The results combine average values for five independent experiments, each done in duplicate, and error bars represent the standard deviations of the means.
Release of mature naked capsids.
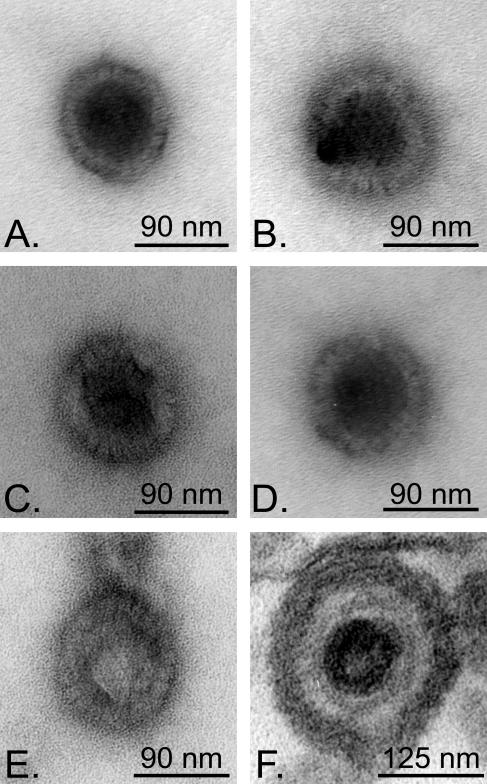
The deenvelopment/reenvelopment model of egress suggests that the capsids released should be naked, as newly assembled capsids in the nucleus would escape by budding through the inner nuclear membrane and subsequently fuse with the second nuclear envelope (15, 43, 44). Although much data support this view, one could always debate whether the naked capsids found in the cytoplasm of intact cells originated from the nucleus or from another compartment. We therefore examined this issue by using the assay, since it was relatively free of “contaminating” organelles (Fig. 1). An in vitro nuclear egress assay was performed, and the capsids produced were examined by negative staining. As can be seen in Fig. 7, all capsids were naked, without a single case of enveloped capsids being detected. For comparison, Fig. 7F shows a control extracellular enveloped capsid. Thus, capsids escaped the nuclei exclusively as naked particles.
FIG. 7.
Negative staining of capsids liberated during the in vitro assay. HSV-1 17+-infected nuclei were incubated for 6 h at 37°C with energy and (A) mock-treated or (B to E) infected cytosol. The capsids were then separated from the nuclei on spin columns, layered on copper grids, negatively stained, and examined by electron microscopy. Note that all capsids were unenveloped and most were mature. Some atypical capsids could also be seen (E). As a control for envelopment, panel F shows an extracellular virion mounted in Epon.
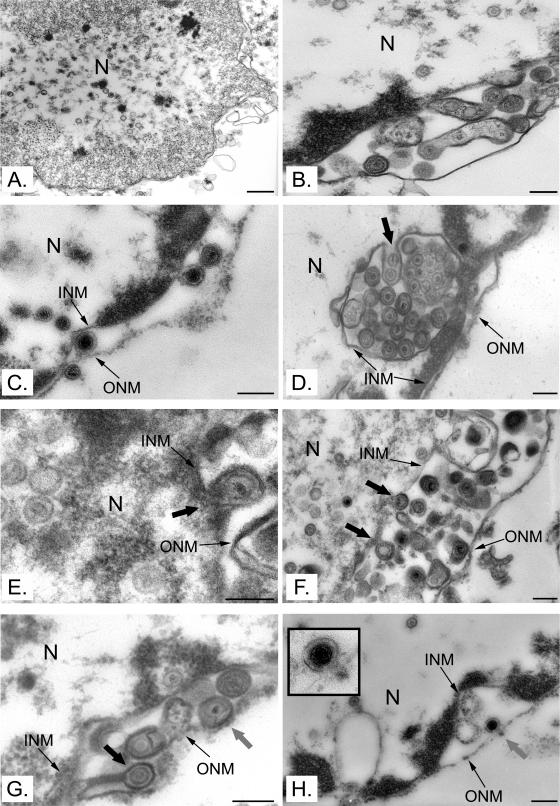
It has recently been suggested that naked cytosolic capsids could arise by the direct passage of newly assembled capsids from the nucleus through vastly enlarged nuclear pores (39, 79). To examine whether the virus used the same route of egress in isolated nuclei as in intact cells and to examine the possible role of enlarged pores in that egress, infected nuclei and cells were prepared for conventional Epon embedding and examined by EM. As expected from the literature, naked nuclear capsids, enveloped perinuclear virions, and capsids in the process of envelopment and deenvelopment at the nuclear membranes were observed in infected cells (data not shown). Similarly, numerous nuclear naked capsids were seen in isolated nuclei (Fig. 8A). Furthermore, enveloped perinuclear virions were often present in enriched nuclei (Fig. 8B and C). Finally, capsids seemingly in the process of envelopment at the inner nuclear membrane (Fig. 8D to G) and deenvelopment at the outer nuclear membrane (Fig. 8G and H) were detected. With the exception of rare cases of enlarged nuclear pores in damaged nuclei, only pores of normal size and appearance were seen in intact cells (data not shown). In isolated nuclei, it was unfortunately difficult to detect normal pores because of their propensity to have large perinuclear spaces when filled with virus. Nonetheless, the nuclear envelopes were usually intact all around the organelle (Fig. 1 and 8). Despite this limitation, the data reveal similar egress patterns in both isolated nuclei and cells and suggest that the capsids escaped nuclei in vitro by budding into the perinuclear space rather than exiting via enlarged nuclear pores.
FIG. 8.
Viral egress in vitro. Nuclei isolated from HeLa cells infected with HSV-1 17+ were analyzed by Epon embedding and electron microscopy. Capsids were present in the nuclei (A) and the perinuclear space (B and C). Capsids seemingly in the process of acquiring an envelope at the inner nuclear membrane (large black arrows in panels D to G) or interacting with the outer nuclear membrane (large gray arrows in panels G to H) were observed. Bars represent 500 nm in panel A and 200 nm in all other panels. N, nucleus; INM, inner nuclear membrane; ONM, outer nuclear membrane.
Likely reconstitution of capsid maturation.
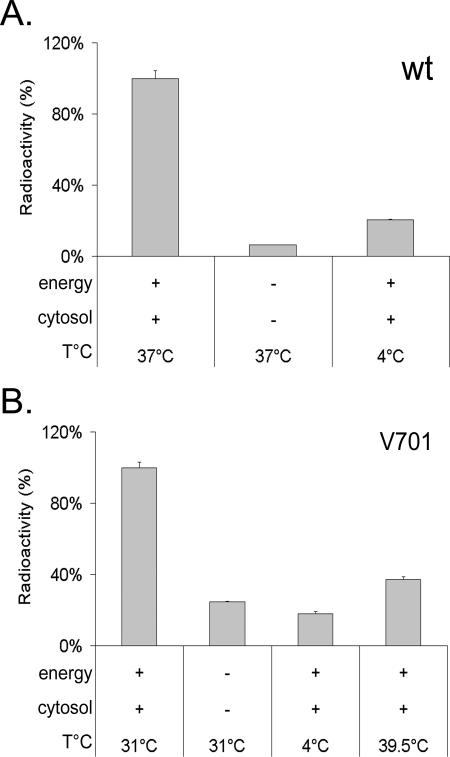
HSV-1 capsids are initially assembled in the nucleus into large B-core capsids (procapsids). These procapsids differ from mature C capsids by their lack of viral DNA, their shape, and their protein content. Their transformation into mature capsids involves the concomitant packaging of monomeric viral DNA, the cleavage of preVP22a and UL26, and the angularization of the capsids (25). The UL26 viral protease plays a central role in these processes. Thermosensitive mutants of the protease, such as ts1201 (52), tsProt.A (21), and V701 (55), are deficient for these steps at the nonpermissive temperature of 39.5°C but proceed normally at 31°C (9, 21, 52, 59, 74). Since the in vitro assay clearly reconstitutes nuclear egress of HSV-1 capsids, we were curious to determine whether it could also reconstitute DNA encapsidation and capsid maturation. To this end, HeLa cells were infected with V701 at 39.5°C and the nuclei isolated at 20°C instead of the usual 4°C, since these thermosensitive protease mutants readily disassemble their immature capsids at 4°C (60). These nuclei were then assayed for their ability to release capsids in vitro. To our surprise, capsid egress took place at the permissive temperature of 31°C (Fig. 9) and were equal in efficiency to wild-type virus (on average, 13,603 ± 4,948 cpm for wild-type virus and 14,202 ± 1,924 cpm for V701). As for wild-type virus, capsid egress was abolished at 4°C or in the absence of energy and cytosol. The protease mutant V701 thus seemingly shared with wild-type virus its dependence on energy, cytosol, and temperature (Fig. 9) as well as time (data not shown).
FIG. 9.
The thermosensitive protease A V701 mutant behaves in the same way as the wild-type virus in the in vitro assay. Isolated nuclei containing radiolabeled (A) HSV-1 17+ or (B) V701 capsids were incubated for 6 h at the specified temperatures with 0 or 4 mg/ml of infected cytosol and with (+) or without (-) energy. Released capsids were separated from nuclei, and DNA was TCA precipitated and measured by liquid scintillation as described in the text. The permissive temperature for V701 is 31°C and the nonpermissive temperature 39.5°C.
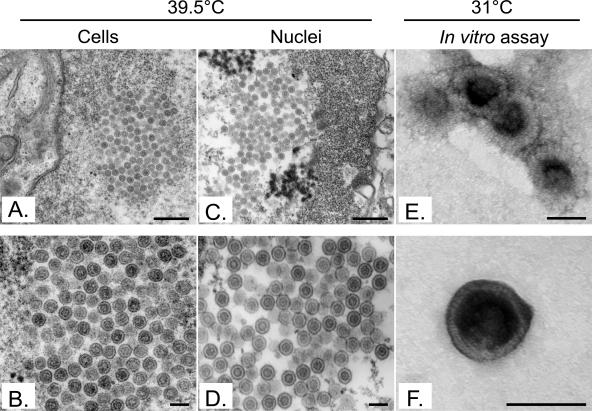
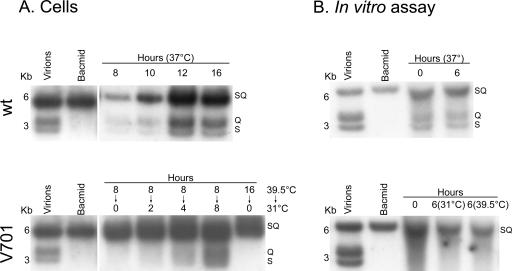
Given the leaky nature of the UL26 protease mutants (9; J. Duron and R. Lippé, unpublished observations), it was possible that only mature capsids already formed at the nonpermissive temperature escaped in vitro. To examine this issue, capsid egress at 39.5°C was evaluated. Figure 9 reveals a minimal level of egress barely above those seen for the 4°C control, suggesting that few preexisting mature capsids were released in vitro. As an additional control, cells infected with V701 at 39.5°C were examined by conventional Epon embedding and EM. As indicated above, they should contain immature capsids at the nonpermissive temperature but should release mature C-type capsids upon incubation at the permissive temperature. Figure 10A and B show that, as previously reported (55), large B-core capsids were indeed the predominant form of capsids in their nuclei. As anticipated, this was also the case in nuclei isolated from V701-infected cells incubated at the nonpermissive temperature (Fig. 10C and D). In comparison, capsids produced at 31°C in vitro and visualized by negative staining and EM revealed the exclusive presence of mature C-type capsids (Fig. 10E and F). As shown by Church and Wilson for a similar UL26 mutant (9), one sees limited numbers of mature capsids in the nuclei of tissue culture cells upon incubation at the permissive temperature. This is because they tend to egress quickly. Consistently, our EM data suggest that the distribution of capsid types in the nuclei of intact cells at 39.5°C is 0.1% type A, 93.2% type B, and 6.8% type C (n = 1125), while at 31°C, the distribution shifts to 3.2% type A, 78.3% type B, and 18.5% type C (n = 341). Notably, capsids released in vitro are exclusively of the C type (100%; n = 137). Hence, maturation of capsids seemingly takes place in our in vitro assay. To examine whether DNA encapsidation also occurred, we isolated the DNA from both wild-type and V701 capsids produced in the test tube and performed an SQ test. This test determines whether cleavage of the concatemers of viral DNA occurs and is often used to evaluate whether the concomitant encapsidation step takes place (8, 42). Figure 11 shows that cleavage of the SQ restriction fragment into the BamHI S and Q terminal fragments could indeed be detected for control wild-type DNA. The same was true for V701 in intact cells. However, no detectable encapsidation was seen for V701 in vitro using this assay, either prior to or after its incubation in the test tube. This contrasted with the obvious presence of DNA in the capsids seen by EM (Fig. 10) or detected by PCR (data not shown) and [3H]thymidine (Fig. 9). Taken together, these findings indicate that the V701-infected nuclei isolated at the nonpermissive temperature contained immature capsids and that they released mature capsids at the permissive temperature in vitro. Unfortunately, encapsidation could not be detected under these conditions.
FIG. 10.
Electron microscopy analysis of V701 capsids. Cells and nuclei infected with V701 at the nonpermissive temperature (39.5°C) were prepared for Epon embedding and electron microscopy. Immature capsids were abundant in the nuclei of whole cells (A and B) and in isolated nuclei (C and D). V701 capsids released in vitro at the permissive temperature (31°C) were analyzed by negative staining (E and F). They exclusively consisted of mature naked C capsids. Bars represent 500 nm (A and C), 150 nm (B and D), and 90 nm (E and F).
FIG. 11.
SQ analysis of viral DNA released. DNA extracted from either (A) whole infected cells or (B) capsids released in the in vitro assay was digested with BamHI and analyzed by Southern blot hybridization with a probe recognizing the S, Q, and SQ BamHI fragments of the HSV-1 genome.
DISCUSSION
We have established an in vitro assay that reconstitutes HSV-1 egress from the nucleus to the cytoplasm. This assay relies on the isolation of nuclei from infected cells and their incubation in the test tube under controlled conditions. These nuclei maintain the integrity of their two envelopes (Fig. 1) and their functionality (Fig. 3, 4, and 6) and actively release capsids (Fig. 3 and 4). While the basal signal observed at 4°C or in the absence of energy and cytosol may be interpreted as meaning that some nuclei break in the course of the assay, the impact of free [3H]thymidine, thymidine incorporated in host DNA, and thymidine found in nonencapsidated viral genomes is limited since we measure only TCA-precipitated, DNase I-resistant material (i.e., only encapsidated viral DNA). Moreover, it is clear that leaky or ruptured nuclei played a minimal role, as confirmed by five independent other criteria. First, the data could not simply be explained by more active proteases and lipases at higher temperatures, since the V701 protease mutant yielded lower capsid release at 39.5°C (Fig. 9). Second, EM and phase-contrast examination of the samples also failed to detect nuclear damage over the course of the assay (data not shown). Moreover, the number of nuclei remained stable for the full 6 h of the assay (Fig. 4). Third, a minimal amount of PCNA was found outside the nucleus following the in vitro assay (Fig. 5). Fourth, the nuclei excluded large dextrans, molecules much smaller than capsids (Table 1). Finally, the preferential release of mature C capsids by nuclei otherwise containing a majority of immature capsids further supports this conclusion (Table 2). We therefore estimate that the assay reconstitutes nuclear HSV-1 egress in the test tube.
As expected from an intracellular transport assay, the egress of HSV-1 capsids is a temperature-, time-, and energy-dependent process (Fig. 3 and 4). Although a characteristic of most intracellular transport assays, viral egress was surprisingly also dependent on the presence of cytosol. This was not necessarily anticipated, since the virus moves from the nucleus and through the perinuclear space, where cytosolic components should be absent a priori. However, a role for cytosol is still possible, as the virus eventually reaches the cytoplasmic compartment. Perhaps it plays a role at the cytosolic face of the outer nuclear membrane, where fusion of perinuclear virions with the outer nuclear membrane occurs. Some cytosolic components may also be imported through the nuclear pores, since the pores are normally active under in vitro conditions (16, 50). Interestingly, cytosol prepared from mock-treated cells efficiently supported egress, while cytosol prepared from infected cells slightly and reproducibly enhanced it (Fig. 6). This suggests that viral proteins present in the cytosol, including cytosolic teguments, are not absolutely essential for this step of egress. It does, however, indicate that both host and viral proteins play some role. Given the modulation by HSV-1 of host protein expression in infected cells (54, 73), it is not clear whether cytosolic viral proteins play a direct role in nuclear egress or whether our results simply reflect a different host protein composition in infected cytosol. Further work will be required to clarify this issue. It will be interesting to analyze which teguments are found on the capsids released in vitro in the presence of either mock-treated or infected cytosol. From these results, it is clear that the assay reproduces nuclear HSV-1 egress in a quantifiable manner and has all the hallmarks of intracellular transport assays, i.e., temperature, time, energy, and even cytosol dependence. Interestingly, the efficiency of this assay (up to 30%) compares favorably to those of other intracellular assays(67).
Analysis by EM of the capsids released in vitro revealed the exclusive presence of naked capsids (Fig. 7). This is significant, as the capsids can come only from the nuclei in the assay. The absence of capsids in either the mock-treated cytosol or the infected cytosol preparations further supports this assertion (data not shown). This finding is consistent with the deenvelopment/reenvelopment model of egress but also partly coherent with the enlarged-nuclear-pore model of Wild and colleagues (39, 79). They suggest that newly assembled naked capsids reach the cytoplasm through enlarged and dissociated nuclear pores. These capsids apparently retrogradely bud into the perinuclear space from the cytosolic face of the nucleus rather than the inner nuclear face. In this model, nuclear capsids never interact with the inner nuclear membrane, but rather travel through dilated pores. Finally, they suggest that these perinuclear virions could travel to the Golgi via tubular structures directly linking the reticulum endoplasmic to the Golgi. While such events may be difficult to detect without rapid freezing, we find this model unlikely. First, aside from obviously much-damaged nuclei, we did not see any evidence for enlarged pores in either isolated nuclei or infected cells (data not shown). Second, EM analysis revealed abundant virions in the perinuclear space in both isolated nuclei and infected cells. Third, though we found evidence of deenvelopment at the outer nuclear membrane (Fig. 8), this cannot distinguish between the large-pore model of Wild and colleagues (39, 79) and the envelopment/deenvelopment/reenvelopment model. This is because it would look the same as budding from the cytosol (Wild's model). However, budding at the inner nuclear membrane can only be reconciled with the envelopment/deenvelopment/reenvelopment model, which is clearly shown in Fig. 8 and for which we found several examples. Fourth, the enlarged-pore model assumes that the perinuclear virions would leave the nucleus via the biosynthetic pathway. This is a well-characterized pathway that normally involves the transport of molecules from the reticulum endoplasmic to the Golgi in COPII-coated vesicles (48). Consistently, one would then predict the release of doubly enveloped virions, which was never observed in our nuclear enriched assay (Fig. 8). Finally, if perinuclear capsids were to travel as proposed via a continuous luminal passage to the Golgi, capsids should not egress in our assay since they are deprived of such an option. Although not a definitive proof, the data suggest that perinuclear virions are more likely to arise by budding from the nuclear side and that they are released as naked capsids in the cytosol, where they are later reenveloped.
An unexpected finding was the discovery that nuclei isolated from V701-infected cells arrested at the nonpermissive temperature could support HSV-1 egress when incubated at the permissive temperature in vitro (Fig. 9). This egress is unlikely to have originated from already-mature V701 capsids present in the nuclei since large B-core capsids and very few C capsids were found at 39.5°C in either isolated nuclei or intact cells (Fig. 10). Second, little egress took place at 39.5°C (Fig. 9). Third, although the UL26 protease mutation is slightly leaky, very few mature capsids are synthesized at the nonpermissive temperature, as evidenced by the 2- to 3-log decrease in viral titers in tissue culture (9; Duron and Lippé, unpublished observations). Fourth, nuclei exclusively released mature C capsids upon incubation at the permissive temperature (Fig. 9 and 10). Finally, no encapsidation was detected in V701-containing nuclei following their isolation at 39.5°C (i.e., prior to their incubation at the permissive temperature in vitro). Consequently, capsid maturation seemingly occurs upon shifting of nuclei to the permissive temperature. Unfortunately, the concomitant encapsidation step could not be observed with the classical SQ test (Fig. 11), despite the obvious presence of DNA in the capsids as judged by EM (Fig. 10), PCR (data not shown), and the presence of [3H]thymidine (Fig. 9). The reasons for this apparent contradiction are unclear to us. Unless concatemeric DNA can be packaged in capsids, which is unlikely (12), there must be DNA cleavage occurring. Since the nuclei are isolated at 20°C (see Materials and Methods), some of the DNA is perhaps the target of DNases that prevent us from detecting the more minor S and Q fragments (as evidenced by the slight smearing seen in Fig. 11 and many other attempts, including larger-scale preparations). Further work will be needed to elucidate this question.
In conclusion, we have set up an in vitro assay that reconstitutes the egress of HSV-1 capsids from the nucleus to the cytoplasm and may reconstitute capsid maturation and DNA encapsidation. To our knowledge, this is a significant breakthrough, as this is the first time that this has been achieved. This nuclear egress assay shares with other transport assays its dependence on cytosol, energy, time, and temperature. Host cytosolic proteins clearly play a role in this process, while viral proteins enhance it. This assay should now be useful for identification of molecules involved in nuclear egress and characterization of their functions.
Acknowledgments
We thank Joël Lanoix and Allégria Kessous for critical reading of the manuscript and excellent suggestions. We are indebted to Bruce Register and Jules A. Shafer for the V701 strain, Sandra Weller for the pNN9 plasmid, and Beate Sodeik for supplying wild-type viruses, the pHSV-1 bluelox bacmid, and continued support. We particularly wish to thank the excellent technical support of Johanne Duron.
This work was supported by the Canadian Institutes of Health Research (CIHR grant no. MOP 12679) and establishment grants from the Canadian Foundation for Innovation and the Fonds de la recherche en santé du Québec. R.L. is a recipient of a CIHR scholarship.
REFERENCES
- 1.Baines, J. D., and B. Roizman. 1992. The UL11 gene of herpes simplex virus 1 encodes a function that facilitates nucleocapsid envelopment and egress from cells. J. Virol. 66:5168-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baines, J. D., P. L. Ward, G. Campadelli-Fiume, and B. Roizman. 1991. The UL20 gene of herpes simplex virus 1 encodes a function necessary for viral egress. J. Virol. 65:6414-6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjerke, S. L., and R. J. Roller. 2006. Roles for herpes simplex virus type 1 U(L)34 and U(S)3 proteins in disrupting the nuclear lamina during herpes simplex virus type 1 egress. Virology 347:261-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, S. M., and A. R. MacLean. 1998. Herpes simplex virus protocols. Humana Press, Totowa, N.J.
- 5.Chang, Y. E., C. Van Sant, P. W. Krug, A. E. Sears, and B. Roizman. 1997. The null mutant of the UL31 gene of herpes simplex virus 1: construction and phenotype in infected cells. J. Virol. 71:8307-8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung, P., B. W. Banfield, and F. Tufaro. 1991. Brefeldin A arrests the maturation and egress of herpes simplex virus particles during infection. J. Virol. 65:1893-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christoforidis, S., H. M. McBride, R. D. Burgoyne, and M. Zerial. 1999. The Rab5 effector EEA1 is a core component of endosome docking. Nature 397:621-625. [DOI] [PubMed] [Google Scholar]
- 8.Church, G. A., A. Dasgupta, and D. W. Wilson. 1998. Herpes simplex virus DNA packaging without measurable DNA synthesis. J. Virol. 72:2745-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Church, G. A., and D. W. Wilson. 1997. Study of herpes simplex virus maturation during a synchronous wave of assembly. J. Virol. 71:3603-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook, N. R., and H. W. Davidson. 2001. In vitro assays of vesicular transport. Traffic 2:19-25. [DOI] [PubMed] [Google Scholar]
- 11.Darlington, R. W., and L. H. Moss III. 1968. Herpesvirus envelopment. J. Virol. 2:48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dasgupta, A., and D. W. Wilson. 1999. ATP depletion blocks herpes simplex virus DNA packaging and capsid maturation. J. Virol. 73:2006-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dasgupta, A., and D. W. Wilson. 2001. Evaluation of the primary effect of brefeldin A treatment upon herpes simplex virus assembly. J. Gen. Virol. 82:1561-1567. [DOI] [PubMed] [Google Scholar]
- 14.Desai, P. J. 2000. A null mutation in the UL36 gene of herpes simplex virus type 1 results in accumulation of unenveloped DNA-filled capsids in the cytoplasm of infected cells. J. Virol. 74:11608-11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enquist, L. W., P. J. Husak, B. W. Banfield, and G. A. Smith. 1998. Infection and spread of alphaherpesviruses in the nervous system. Adv. Virus Res. 51:237-347. [DOI] [PubMed] [Google Scholar]
- 16.Finlay, D. R., D. D. Newmeyer, P. M. Hartl, J. Horecka, and D. J. Forbes. 1989. Nuclear transport in vitro. J. Cell Sci. Suppl. 11:225-242. [DOI] [PubMed] [Google Scholar]
- 17.Forest, T., S. Barnard, and J. D. Baines. 2005. Active intranuclear movement of herpesvirus capsids. Nat. Cell Biol. 7:429-431. [DOI] [PubMed] [Google Scholar]
- 18.Foster, T. P., J. M. Melancon, J. D. Baines, and K. G. Kousoulas. 2004. The herpes simplex virus type 1 UL20 protein modulates membrane fusion events during cytoplasmic virion morphogenesis and virus-induced cell fusion. J. Virol. 78:5347-5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs, W., B. G. Klupp, H. Granzow, and T. C. Mettenleiter. 2004. Essential function of the pseudorabies virus UL36 gene product is independent of its interaction with the UL37 protein. J. Virol. 78:11879-11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuchs, W., B. G. Klupp, H. Granzow, N. Osterrieder, and T. C. Mettenleiter. 2002. The interacting UL31 and UL34 gene products of pseudorabies virus are involved in egress from the host-cell nucleus and represent components of primary enveloped but not mature virions. J. Virol. 76:364-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao, M., L. Matusick-Kumar, W. Hurlburt, S. F. DiTusa, W. W. Newcomb, J. C. Brown, P. J. McCann III, I. Deckman, and R. J. Colonno. 1994. The protease of herpes simplex virus type 1 is essential for functional capsid formation and viral growth. J. Virol. 68:3702-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gershon, A. A., D. L. Sherman, Z. Zhu, C. A. Gabel, R. T. Ambron, and M. D. Gershon. 1994. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J. Virol. 68:6372-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Granzow, H., B. G. Klupp, W. Fuchs, J. Veits, N. Osterrieder, and T. C. Mettenleiter. 2001. Egress of alphaherpesviruses: comparative ultrastructural study. J. Virol. 75:3675-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harley, C. A., A. Dasgupta, and D. W. Wilson. 2001. Characterization of herpes simplex virus-containing organelles by subcellular fractionation: role for organelle acidification in assembly of infectious particles. J. Virol. 75:1236-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Homa, F. L., and J. C. Brown. 1997. Capsid assembly and DNA packaging in herpes simplex virus. Rev. Med. Virol. 7:107-122. [DOI] [PubMed] [Google Scholar]
- 26.Homman-Loudiyi, M., K. Hultenby, W. Britt, and C. Soderberg-Naucler. 2003. Envelopment of human cytomegalovirus occurs by budding into Golgi-derived vacuole compartments positive for gB, Rab 3, trans-Golgi network 46, and mannosidase II. J. Virol. 77:3191-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong, W. 2005. SNAREs and traffic. Biochim. Biophys. Acta 1744:493-517. [PubMed] [Google Scholar]
- 28.Horiuchi, H., R. Lippe, H. M. McBride, M. Rubino, P. Woodman, H. Stenmark, V. Rybin, M. Wilm, K. Ashman, M. Mann, and M. Zerial. 1997. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell 90:1149-1159. [DOI] [PubMed] [Google Scholar]
- 29.Jensen, H. L., and B. Norrild. 2002. Temporal morphogenesis of herpes simplex virus type 1-infected and brefeldin A-treated human fibroblasts. Mol. Med. 8:210-224. [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, D. C., and P. G. Spear. 1982. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J. Virol. 43:1102-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato, A., M. Yamamoto, T. Ohno, M. Tanaka, T. Sata, Y. Nishiyama, and Y. Kawaguchi. 2006. Herpes simplex virus 1-encoded protein kinase UL13 phosphorylates viral Us3 protein kinase and regulates nuclear localization of viral envelopment factors UL34 and UL31. J. Virol. 80:1476-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klupp, B. G., H. Granzow, and T. C. Mettenleiter. 2001. Effect of the pseudorabies virus US3 protein on nuclear membrane localization of the UL34 protein and virus egress from the nucleus. J. Gen. Virol. 82:2363-2371. [DOI] [PubMed] [Google Scholar]
- 33.Klupp, B. G., H. Granzow, and T. C. Mettenleiter. 2000. Primary envelopment of pseudorabies virus at the nuclear membrane requires the UL34 gene product. J. Virol. 74:10063-10073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klupp, B. G., H. Granzow, E. Mundt, and T. C. Mettenleiter. 2001. Pseudorabies virus UL37 gene product is involved in secondary envelopment. J. Virol. 75:8927-8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Komuro, M., M. Tajima, and K. Kato. 1989. Transformation of Golgi membrane into the envelope of herpes simplex virus in rat anterior pituitary cells. Eur. J. Cell Biol. 50:398-406. [PubMed] [Google Scholar]
- 36.Kopp, M., H. Granzow, W. Fuchs, B. G. Klupp, E. Mundt, A. Karger, and T. C. Mettenleiter. 2003. The pseudorabies virus UL11 protein is a virion component involved in secondary envelopment in the cytoplasm. J. Virol. 77:5339-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreis, T. E., and R. Pepperkok. 1994. Coat proteins in intracellular membrane transport. Curr. Opin. Cell Biol. 6:533-537. [DOI] [PubMed] [Google Scholar]
- 38.Lee, G. E., J. W. Murray, A. W. Wolkoff, and D. W. Wilson. 2006. Reconstitution of herpes simplex virus microtubule-dependent trafficking in vitro. J. Virol. 80:4264-4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leuzinger, H., U. Ziegler, E. M. Schraner, C. Fraefel, D. L. Glauser, I. Heid, M. Ackermann, M. Mueller, and P. Wild. 2005. Herpes simplex virus 1 envelopment follows two diverse pathways. J. Virol. 79:13047-13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang, L., and J. D. Baines. 2005. Identification of an essential domain in the herpes simplex virus 1 UL34 protein that is necessary and sufficient to interact with UL31 protein. J. Virol. 79:3797-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luxton, G. W., J. I. Lee, S. Haverlock-Moyns, J. M. Schober, and G. A. Smith. 2006. The pseudorabies virus VP1/2 tegument protein is required for intracellular capsid transport. J. Virol. 80:201-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez, R., R. T. Sarisky, P. C. Weber, and S. K. Weller. 1996. Herpes simplex virus type 1 alkaline nuclease is required for efficient processing of viral DNA replication intermediates. J. Virol. 70:2075-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mettenleiter, T. C. 2004. Budding events in herpesvirus morphogenesis. Virus Res. 106:167-180. [DOI] [PubMed] [Google Scholar]
- 44.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mossman, K. L., R. Sherburne, C. Lavery, J. Duncan, and J. R. Smiley. 2000. Evidence that herpes simplex virus VP16 is required for viral egress downstream of the initial envelopment event. J. Virol. 74:6287-6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muranyi, W., J. Haas, M. Wagner, G. Krohne, and U. H. Koszinowski. 2002. Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina. Science 297:854-857. [DOI] [PubMed] [Google Scholar]
- 47.Newcomb, W. W., F. L. Homa, D. R. Thomsen, Z. Ye, and J. C. Brown. 1994. Cell-free assembly of the herpes simplex virus capsid. J. Virol. 68:6059-6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nickel, W., and F. T. Wieland. 1998. Biosynthetic protein transport through the early secretory pathway. Histochem. Cell Biol. 109:477-486. [DOI] [PubMed] [Google Scholar]
- 49.Nii, S., C. Morgan, and H. M. Rose. 1968. Electron microscopy of herpes simplex virus. II. Sequence of development. J. Virol. 2:517-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ojala, P. M., B. Sodeik, M. W. Ebersold, U. Kutay, and A. Helenius. 2000. Herpes simplex virus type 1 entry into host cells: reconstitution of capsid binding and uncoating at the nuclear pore complex in vitro. Mol. Cell. Biol. 20:4922-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park, R., and J. D. Baines. 2006. Herpes simplex virus type 1 infection induces activation and recruitment of protein kinase C to the nuclear membrane and increased phosphorylation of lamin B. J. Virol. 80:494-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Preston, V. G., J. A. Coates, and F. J. Rixon. 1983. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J. Virol. 45:1056-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Purves, F. C., D. Spector, and B. Roizman. 1991. The herpes simplex virus 1 protein kinase encoded by the US3 gene mediates posttranslational modification of the phosphoprotein encoded by the UL34 gene. J. Virol. 65:5757-5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ray, N., and L. W. Enquist. 2004. Transcriptional response of a common permissive cell type to infection by two diverse alphaherpesviruses. J. Virol. 78:3489-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Register, R. B., and J. A. Shafer. 1996. A facile system for construction of HSV-1 variants: site directed mutation of the UL26 protease gene in HSV-1. J. Virol. Methods 57:181-193. [DOI] [PubMed] [Google Scholar]
- 56.Reynolds, A. E., L. Liang, and J. D. Baines. 2004. Conformational changes in the nuclear lamina induced by herpes simplex virus type 1 require genes UL31 and UL34. J. Virol. 78:5564-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reynolds, A. E., B. J. Ryckman, J. D. Baines, Y. Zhou, L. Liang, and R. J. Roller. 2001. UL31 and UL34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J. Virol. 75:8803-8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reynolds, A. E., E. G. Wills, R. J. Roller, B. J. Ryckman, and J. D. Baines. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 76:8939-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rixon, F. J., A. M. Cross, C. Addison, and V. G. Preston. 1988. The products of herpes simplex virus type 1 gene UL26 which are involved in DNA packaging are strongly associated with empty but not with full capsids. J. Gen. Virol. 69:2879-2891. [DOI] [PubMed] [Google Scholar]
- 60.Rixon, F. J., and D. McNab. 1999. Packaging-competent capsids of a herpes simplex virus temperature-sensitive mutant have properties similar to those of in vitro-assembled procapsids. J. Virol. 73:5714-5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roller, R. J., Y. Zhou, R. Schnetzer, J. Ferguson, and D. DeSalvo. 2000. Herpes simplex virus type 1 UL34 gene product is required for viral envelopment. J. Virol. 74:117-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ryckman, B. J., and R. J. Roller. 2004. Herpes simplex virus type 1 primary envelopment: UL34 protein modification and the US3-UL34 catalytic relationship. J. Virol. 78:399-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 64.Schimmer, C., and A. Neubauer. 2003. The equine herpesvirus 1 UL11 gene product localizes to the trans-Golgi network and is involved in cell-to-cell spread. Virology 308:23-36. [DOI] [PubMed] [Google Scholar]
- 65.Sciaky, N., J. Presley, C. Smith, K. J. Zaal, N. Cole, J. E. Moreira, M. Terasaki, E. Siggia, and J. Lippincott-Schwartz. 1997. Golgi tubule traffic and the effects of brefeldin A visualized in living cells. J. Cell Biol. 139:1137-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scott, E. S., and P. O'Hare. 2001. Fate of the inner nuclear membrane protein lamin B receptor and nuclear lamins in herpes simplex virus type 1 infection. J. Virol. 75:8818-8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simonsen, A., R. Lippe, S. Christoforidis, J. M. Gaullier, A. Brech, J. Callaghan, B. H. Toh, C. Murphy, M. Zerial, and H. Stenmark. 1998. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394:494-498. [DOI] [PubMed] [Google Scholar]
- 68.Simpson-Holley, M., J. Baines, R. Roller, and D. M. Knipe. 2004. Herpes simplex virus 1 UL31 and UL34 gene products promote the late maturation of viral replication compartments to the nuclear periphery. J. Virol. 78:5591-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simpson-Holley, M., R. C. Colgrove, G. Nalepa, J. W. Harper, and D. M. Knipe. 2005. Identification and functional evaluation of cellular and viral factors involved in the alteration of nuclear architecture during herpes simplex virus 1 infection. J. Virol. 79:12840-12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith, J. D. 1980. An additional role for the outer nuclear membrane in the morphogenesis of herpes simplex virus. Intervirology 13:312-316. [DOI] [PubMed] [Google Scholar]
- 71.Sodeik, B., M. W. Ebersold, and A. Helenius. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 136:1007-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stackpole, C. W. 1969. Herpes-type virus of the frog renal adenocarcinoma. I. Virus development in tumor transplants maintained at low temperature. J. Virol. 4:75-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stingley, S. W., J. J. Ramirez, S. A. Aguilar, K. Simmen, R. M. Sandri-Goldin, P. Ghazal, and E. K. Wagner. 2000. Global analysis of herpes simplex virus type 1 transcription using an oligonucleotide-based DNA microarray. J. Virol. 74:9916-9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Turcotte, S., J. Letellier, and R. Lippe. 2005. Herpes simplex virus type 1 capsids transit by the trans-Golgi network, where viral glycoproteins accumulate independently of capsid egress. J. Virol. 79:8847-8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Meer, G., and H. Sprong. 2004. Membrane lipids and vesicular traffic. Curr. Opin. Cell Biol. 16:373-378. [DOI] [PubMed] [Google Scholar]
- 76.von Einem, J., D. Schumacher, D. J. O'Callaghan, and N. Osterrieder. 2006. The α-TIF (VP16) homologue (ETIF) of equine herpesvirus 1 is essential for secondary envelopment and virus egress. J. Virol. 80:2609-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wagenaar, F., J. M. Pol, B. Peeters, A. L. Gielkens, N. de Wind, and T. G. Kimman. 1995. The US3-encoded protein kinase from pseudorabies virus affects egress of virions from the nucleus. J. Gen. Virol. 76:1851-1859. [DOI] [PubMed] [Google Scholar]
- 78.Whealy, M. E., J. P. Card, R. P. Meade, A. K. Robbins, and L. W. Enquist. 1991. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J. Virol. 65:1066-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wild, P., M. Engels, C. Senn, K. Tobler, U. Ziegler, E. M. Schraner, E. Loepfe, M. Ackermann, M. Mueller, and P. Walther. 2005. Impairment of nuclear pores in bovine herpesvirus 1-infected MDBK cells. J. Virol. 79:1071-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wolfstein, A., C. H. Nagel, K. Radtke, K. Dohner, V. J. Allan, and B. Sodeik. 2006. The inner tegument promotes herpes simplex virus capsid motility along microtubules in vitro. Traffic 7:227-237. [DOI] [PubMed] [Google Scholar]
- 81.Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2:107-117. [DOI] [PubMed] [Google Scholar]