Abstract
Adeno-associated virus serotype 8 (AAV8) is currently emerging as a powerful gene transfer vector, owing to its capability to efficiently transduce many different tissues in vivo. While this is believed to be in part due to its ability to uncoat more readily than other AAV serotypes such as AAV2, understanding all the processes behind AAV8 transduction is important for its application and optimal use in human gene therapy. Here, we provide the first report of a cellular receptor for AAV8, the 37/67-kDa laminin receptor (LamR). We document binding of LamR to AAV8 capsid proteins and intact virions in vitro and demonstrate its contribution to AAV8 transduction of cultured cells and mouse liver in vivo. We also show that LamR plays a role in transduction by three other closely related serotypes (AAV2, -3, and -9). Sequence and deletion analysis allowed us to map LamR binding to two protein subdomains predicted to be exposed on the AAV capsid exterior. Use of LamR, which is constitutively expressed in many clinically relevant tissues and is overexpressed in numerous cancers, provides a molecular explanation for AAV8's broad tissue tropism. Along with its robust transduction efficiency, our findings support the continued development of AAV8-based vectors for clinical applications in humans, especially for tumor gene therapy.
Adeno-associated virus (AAV) is an increasingly popular gene transfer vector with a number of inherent advantages over other vectors, including a lack of pathogenicity and the ability to mediate long-term gene expression in a variety of tissues in vivo. Of particular benefit is the feasibility to pseudotype recombinant AAV genomes (typically derived from the AAV serotype 2 [AAV2] prototype) with capsids from any of the over 100 identified naturally occurring human or nonhuman viral isolates (4, 8, 9, 11, 25) or with synthetic “designer” shells engineered via capsid DNA shuffling or mutagenesis (18). This results in vector particles with distinct properties, including unique tissue biodistribution and transduction profiles, and thus significantly contributes to the versatility of the AAV vector system.
Thus far, AAV2 has been the primary serotype tested in clinical trials, but many alternative serotypes that offer specific advantages for certain diseases are currently in preclinical development. Among these, perhaps the most interesting candidate is AAV8. We and others recently documented unusually robust and sustained transgene expression from AAV8 vectors in numerous tissues, including the liver, heart, and skeletal muscle (9, 20, 36). In fact, AAV8 resulted in up to 20-fold-higher liver transduction in mice than AAV2 did, despite an 83% amino acid similarity of the two viruses (20). We also showed that the higher rate of AAV8 capsid uncoating compared to AAV2 may be responsible for the increased transduction efficiency (31), allowing AAV8 to be transduced in almost 100% of hepatocytes (20). A second advantage of AAV8 is that since it is a primate virus (isolated from rhesus monkeys), vectors derived therefrom are likely not recognized by prevailing antibodies in humans and thus less prone to causing adverse immunological side effects. In contrast, use of AAV2 vectors is limited in humans due to a prevalent preexisting humoral immunity in the population and due to the risk of induction of a cellular immune response against capsid-containing cells in vivo (24).
In view of the increasing interest in AAV8 as a human gene transfer vector, it is essential to understand the full mechanism behind AAV8 transduction, including the initial steps of AAV8 binding and infection. The tissue tropism of different AAV serotypes is diverse, partly due to their binding to alternate cellular receptors. However, the cellular receptor(s) for AAV8 and most other serotypes remains unknown to date. The few exceptions include AAV2, which was shown to bind various receptors (heparan sulfate proteoglycans [HSPGs], αVβ5 integrin, and the fibroblast or hepatocyte growth factor receptors) (16, 22, 28, 29). AAV3 appears to bind cells in a manner similar to that of AAV2, i.e., via attachment to HSPG and the fibroblast growth factor receptor (3, 23). In contrast, AAV5 preferentially attaches to cells through binding to sialic acid and the platelet-derived growth factor receptor (PDGFR) (7, 15, 34). Like AAV5, AAV4 was also shown to interact with sialic acid; however, the required specific carbohydrate linkage differs between the two serotypes (15).
To further our understanding of the mechanisms behind AAV8 transduction, we conducted a yeast two-hybrid screen for host cellular proteins able to physically interact with AAV8 capsid proteins (B. Akache et al., unpublished data). Interestingly, one of the most frequently recovered clones encoded the extracellular domain of the 37/67-kDa laminin receptor (LamR). Since LamR is highly expressed in tissues shown to be transduced efficiently by AAV8, we decided to study the functional significance (if any) of the physical interaction between these two proteins. Here, we report that LamR is important for cellular binding and transduction by AAV8 in vitro and in vivo. In addition, it appears to play a role in transduction by three closely related virus serotypes. Our study provides the first report of a cellular receptor for AAV8, explaining the unusually wide tropism of this particular virus, and should have implications for its future use as a gene therapy vector.
MATERIALS AND METHODS
Plasmids.
The AAV2, AAV5, AAV6, and AAV8 bait plasmids were constructed by first PCR amplifying homologous regions (nucleic acid residues 439 to 1860 [AAV2], 436 to 1827 [AAV5], 442 to 1863 [AAV6], and 442 to 1869 [AAV8] of each AAV cap gene) from the AAV helper plasmids pDG (13), pDP5 or pDP6 (12), and pE5/V18 (9), respectively. The primers used were AAV2 (CGGAATTCGAGCACTCTCCTGTGGAGCCA and CGGGATCCGCTTTGCCCAGATGGGCCCCTG), AAV5 (CGGAATTCGACCACTTTCCAAAAAGAAAG and GAAGATCTACTTGGCCCAGATGGGTCCTTG), AAV6 (CGGAATTCCAGTCGCCACAAGAGCCAGAC and CGGGATCCGTTTGGCCCAAATAGGACCCTG), and AAV8 (CGGAATTCCCATCACCCCAGCGTTCTCCA and GAAGATCTGCTTGGCCCAGATGGGACCCTG). The AAV8B to -E portions were PCR amplified with the CGGAATTCCCATCACCCCAGCGTTCTCCA primer, in combination with either GAAGATCTGAGCCGTGTTTTGCTGCTGCAA (AAV8B), GAAGATCTGTTTGCCAAAAATCAGGATCCC (AAV8C), GAAGATCTGGACGCGTTGTTGGCGGTAACA (AAV8D), or GAAGATCTGGTAGTACAGGTACTGGTCAAT (AAV8E). The PCR products were then digested with BamHI and EcoRI for AAV2 and AAV6 or BglII and EcoRI for AAV5 and AAV8. They were then ligated to the BamHI- and EcoRI-linearized plasmid pGBK-T7 (BD Clontech, Palo Alto, CA). In the resulting constructs, the cap genes were fused in frame with a Gal4 DNA binding domain. The recovered laminin receptor clone contained the Gal4 activation domain fused to bp 411 to 968 (amino acids 111 to 295) of the 37/67-kDa laminin receptor (GenBank accession number AK011043) cDNA.
Two-hybrid analysis.
Plasmids were transformed into the Saccharomyces cerevisiae strain AH109 (MATa trp1-901 leu2-3 leu2-112 ura3-52 his3-200 gal4Δ gal80Δ LYS2::GAL1UAS-GAL1TATA-HIS3 GAL2UAS-GAL2TATA-ADE2 URA3::MEL1UAS-MEL1TATA-lacZ) (BD Clontech). Clones were tested for growth on SD medium (0.67% yeast nitrogen base without amino acids, 2% glucose, supplemented with the appropriate amino acids at a concentration of 0.004% but lacking adenine, histidine, leucine, and tryptophan). Alternatively, the activity of the lacZ reporter gene product was determined using β-galactosidase assays with chlorophenol red-d-galactopyranoside as a substrate according to BD Clontech. Briefly, the cells were grown overnight in selective SD medium lacking leucine and tryptophan and then diluted in YPD medium (1% Bacto yeast extract, 2% Bacto tryptone, 2% glucose). After 4 h, the optical density at 600 nm (OD600) of the yeast culture was measured (later used to calculate the Miller units; see below) before the cells were resuspended in buffer 1 (2.38% HEPES, 0.9% NaCl, 0.065% l-aspartate, 1% bovine serum albumin, and 0.05% Tween 20) and subsequently lysed via three freeze-thaw cycles in liquid nitrogen. The chlorophenol red-d-galactopyranoside substrate was dissolved to a concentration of 0.1335% in buffer 1 and added to the lysed cells. The reaction was stopped using 3 mM zinc chloride, and the OD578 of the sample was taken to calculate the Miller units [(1,000 × OD578)/(time [min] × OD600)].
Binding assay.
The binding assay was performed according to the method of Kaludov et al. (15). HeLa cells (7.5 × 103 per well) were plated on a 24-well plate and grown overnight. Five micrograms of a monoclonal antibody against the 37/67-kDa laminin receptor or platelet-derived growth factor receptor β (Abcam, Cambridge, MA) or 5 μg of soluble laminin or PDGF-AA (Sigma, St. Louis, MO) was incubated with the cells at 37°C for 1 hour. The cells were then incubated at 4°C for 30 min, before 6,500 particles/cell of AAV(hf.IX) were added and allowed to bind for 30 min at 4°C. Next, the cells were washed twice with cold medium, before the plates were allowed to warm to room temperature and 100 μl of Lyse-n-go PCR reagent (Pierce, Rockford, IL) was added to each well. Following a 10-minute incubation at room temperature, the cells were then frozen at −20°C. After the plates were thawed, 400 μl of water was added to each sample. One microliter of the cell lysate was used for a real-time PCR using SYBR green master mix (Applied Biosystems, Foster City, CA) and the primers CCTAAGCACCCCCAGAAAGC and CGTCGATTTCACAGCTGACATC. The construction and production of AAV(hf.IX) were described elsewhere (19, 31).
Stable laminin receptor expression.
The mouse 37/67-kDa laminin receptor cDNA was amplified from a mouse liver cDNA library (BD Clontech) using the primers ACGCCAATTGACAATGTCCGGAGCCCTTGACGT and ACGCGTCGACTCAGGACCACTCAGTGGTGG, digested with MfeI and SalI, and cloned into the pcDNA3 (Invitrogen, Carlsbad, CA) vector digested with EcoRI and XhoI. The plasmid and the empty pcDNA3 vector were then transfected into NIH 3T3 cells using Polyfect (QIAGEN, Valencia, CA) according to the manufacturer's instructions. The cells were split after 2 days and plated at low density in medium containing 2 mg/ml of G418. G418-resistant cells were selected with medium containing G418 for 1 month, at which point polyclonal pools of cells were plated in a 96-well plate. AAV(gfp) pseudotypes 1 to 6, 8, or 9 was added to the cells for 1 hour, before the viruses were removed and new medium was added. The number of green fluorescent protein (GFP)-positive cells was counted after 3 days by fluorescent light microscopy. The AAV(gfp) vector construct, consisting of a double-stranded genome expressing the gfp gene from a respiratory syncytial virus promoter, will be described in detail elsewhere (D. Grimm et al., unpublished data). Briefly, it was engineered by assembling a full-length AAV2 inverted terminal repeat (ITR) together with a subfragment of an AAV4 ITR, in a pBlueScript II (Stratagene, La Jolla, CA) backbone. The AAV4 fragment contained the entire ITR sequence except for the terminal resolution site, to prevent DNA nicking during vector DNA replication. The double-stranded AAV(gfp) vector plasmid was pseudotyped with eight different capsids using a standard triple plasmid transfection approach (10). The virus was purified using cesium chloride density gradient centrifugation and quantitated by dot blot assay. The resulting virus particles were then added to the cells at the indicated ratios of particles to cells.
RNA interference (RNAi) with laminin receptor.
A lamR short interfering RNA (siRNA; sense strand, GACCUUCACUAACCAGAUCCAUU) or control siRNA (sense strand, AGGAGGAAUUCCAGGGUGAAUUU), each a double-stranded RNA 21-mer plus a UU overhang (Dharmacon, Lafayette, CO), was transfected into NIH 3T3 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. After 3 days, the cells were incubated with the AAV(gfp) vectors described above, and the number of GFP-positive cells was counted after another 3 days.
Western blotting.
Cells were scraped and lysed in a modified radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 7.4, 150 mM sodium chloride, 1% NP-40, 0.25% sodium deoxycholate, 1 mM EDTA, 1 mM sodium orthovanadate, and 1 mM sodium fluoride) for 30 min at 4°C. The supernatant was collected after the debris was pelleted by centrifugation for 10 min at 12,000 rpm and 4°C. Thirty micrograms of each sample was boiled for 5 min in Laemmli buffer, resolved on a 7.5% sodium dodecyl sulfate-polyacrylamide gel, and analyzed by immunoblotting with a polyclonal goat anti-laminin receptor or antiactin antibody (both from Santa Cruz Biotechnology, Santa Cruz, California).
In vivo infection inhibition assay.
Female C57BL/6 mice (8 to 10 weeks old; Jackson Laboratory, Bar Harbor, ME) were injected via the tail vein with either phosphate-buffered saline (PBS) or 40 μg of monoclonal antibody against PDGFR-β or against the 37/67-kDa laminin receptor (both from Abcam). After 6 hours, 1 × 1011 particles of AAV6 or AAV8(hf.IX) were injected via the tail vein. Blood samples were collected after 3 days from the retro-orbital plexus, and human factor IX (hF.IX) levels were determined by an enzyme-linked immunosorbent assay (21). All animal procedures were conducted according to the animal care guidelines at Stanford University.
RESULTS
Specific interaction of AAV8 and AAV2 capsid proteins with LamR in yeast.
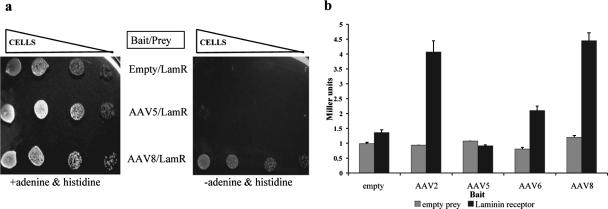
Clones containing the extracellular domain of LamR were frequently recovered when a mouse liver cDNA-Gal4p activation domain library was screened against an AAV8 bait (AAV8A, containing residues 147 to 623 of the AAV8 VP1 capsid protein fused to the Gal4 DNA binding domain) (Fig. 1a) (Akache et al., unpublished data). To confirm this AAV8-LamR interaction and quantify its extent, we measured the activation of a Gal4p-dependent lacZ reporter gene in yeast cotransformed with the recovered LamR prey and the AAV8A bait. To control for specificity of the interaction, baits containing the corresponding capsid sequences from serotype 2, 5, or 6 (the last two have approximately 61% and 84% amino acid sequence similarity to AAV8, respectively) or no capsid DNA at all were evaluated in parallel. Surprisingly, not only the AAV8 but also the AAV2 bait strongly interacted with the LamR prey, yielding a 3.5-fold-increased activity over the empty and AAV5 controls and twofold-increased activity over the AAV6 bait (Fig. 1b).
FIG. 1.
AAV8 and AAV2 capsid proteins bind the 37/67-kDa laminin receptor in yeast. (a) Yeast transformed with the LamR prey plasmid and the various bait plasmids was grown, serially diluted, and spotted on medium with or without adenine and histidine. The activation of both the ade2 and his3 reporter genes preceded by Gal4p binding sites is necessary for growth on medium lacking adenine and histidine. (b) Yeast transformed with the indicated combinations of bait and prey plasmids was assayed for activation of the reporter lacZ gene using β-galactosidase assays.
Two separate AAV8 capsid subdomains mediate binding to LamR.
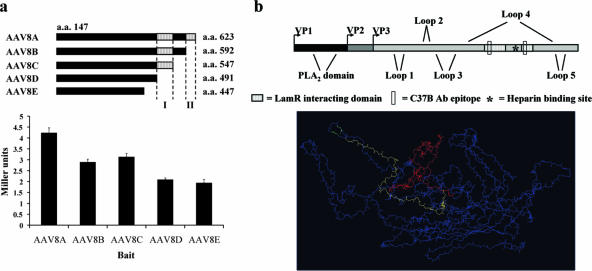
In an attempt to define the domain(s) mediating the LamR interaction, we constructed sequential C-terminal deletion mutants (AAV8B to -E, Fig. 2a) of the original AAV8 capsid bait. The C terminus was chosen based on comparison with the AAV2 prototype, where it contains most of the exterior domains. In fact, lacZ transactivation assays with all four mutants revealed two separate regions (VP1 residues 491 to 547 [I] and 593 to 623 [II]) whose deletion resulted in a decrease in activity (Fig. 2a). Notably, the AAV2 homologous regions form loops on the capsid exterior, and their binding by neutralizing antibodies or their point mutagenesis blocks AAV2 cellular attachment and transduction (17, 37) (Fig. 2b). Based on the sequence similarity between AAV serotypes 2 and 8, we speculate that these domains are also exposed on the assembled AAV8 capsid and thus able to mediate binding to LamR.
FIG. 2.
Two AAV8 capsid subdomains mediate binding to the 37/67-kDa laminin receptor. (a) Schematic of the AAV8 bait deletion constructs AAV8B to -E (top panel). Yeast transformed with the indicated combinations of bait and the LamR prey plasmid was assayed for activation of the reporter lacZ gene using β-galactosidase assays (bottom panel). Two C-terminal regions were identified (I and II in top panel) whose deletion led to a reduction in lacZ transactivation. (b) Schematic representation of the AAV capsid protein VP1 including the location of the previously identified AAV2 outer capsid loops, phospholipase A2 (PLA2) domain, the heparin binding motif, and the here-identified LamR-interacting domains (top panel). Also highlighted are two regions comprising the epitope for the monoclonal antibody (Ab) C37, whose binding blocks AAV2 cellular attachment. A three-dimensional representation of a single capsid protein subunit reveals that the two LamR-interacting domains, VP1 residues 491 to 547 (I in panel a) in red and 593 to 623 (II in panel a) in yellow, are on the outer surface of the capsid and in close proximity to the heparin binding motif (in green) (bottom panel).
AAV8 attaches to mammalian cells through LamR.
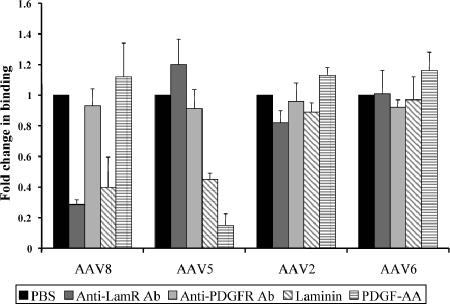
To assess whether AAV8 uses LamR for attachment to living mammalian cells, we performed a virus binding assay. To do this, we blocked LamR on HeLa cells by treatment either with a specific anti-LamR antibody or with laminin, the natural substrate for this receptor. The AAV5 receptor PDGFR served as a negative control and was blocked by treating the cells with an antibody specific to its β subunit or with its substrate PDGF-AA. Recombinant AAV serotype 2, 5, or 6 (as a control) or AAV8 was then added for 30 min, before cells and virus were lysed, and amounts of bound virus were determined via quantitative PCR. As shown in Fig. 3, none of the treatments significantly blocked AAV2 or AAV6 binding to the cells. This was initially unexpected for AAV2, as the two-hybrid assay data had suggested a strong interaction with LamR. However, we hypothesize that since AAV2 uses at least four other receptors (16, 22, 28, 29), blocking one (LamR) might have only marginal effects on AAV2 binding. As expected, AAV5 binding was inhibited sixfold by PDGF-AA, corroborating a previous report (7). The fact that its attachment was unaffected by the PDGFR antibody could be due to binding of the antibody and virus to different receptor domains. Soluble laminin caused a twofold decrease in AAV5 binding, which was expected as laminin binds sialic acid, another AAV5 receptor (5). Importantly, AAV8 was the only serotype whose binding was efficiently blocked both by soluble laminin and by the anti-LamR antibody, evidenced by the 2.5- or 3.5-fold decreases in detectable viral DNA, respectively. Similar to AAV2 and AAV6, soluble PDGF-AA and the anti-PDGFR antibody had no effect on AAV8 binding. Together, these findings suggested that LamR represents a main receptor for AAV8 binding to living cells and that its role for the virus is distinct from that for AAV2, despite a similar level of interaction in the yeast two-hybrid assay.
FIG. 3.
AAV8 attaches to mammalian cells through binding of LamR. Binding assays were performed by incubating HeLa cells with PBS, soluble laminin, PDGF-AA, an antibody (Ab) directed against the 37/67-kDa laminin receptor, or an antibody directed against PDGFR-β, followed by chilling and incubation with 6,500 recombinant particles/cell of each of the indicated serotypes. Quantitative PCR was performed to measure the amount of bound virus using primers specific to the viral genome encompassing the hf.IX gene.
Overexpression of LamR increases AAV8, AAV2, AAV3, and AAV9 transduction.
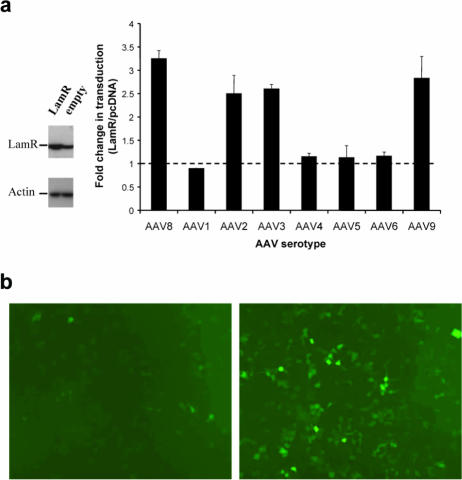
Since virus attachment to the cell surface is a prerequisite step in any viral transduction pathway, we speculated that beyond binding AAV8, LamR may also be crucial for functional virus transduction. To test this hypothesis, we stably transfected NIH 3T3 cells with a LamR expression plasmid, resulting in a pool of cells expressing threefold-higher LamR levels than control cells stably transfected with an empty plasmid (Fig. 4a). We then infected both populations with an AAV8 vector containing a gfp reporter gene and analyzed GFP expression 2 days later. As expected, we observed 3.3-fold-more positive cells in the LamR pool than in the control (Fig. 4a and b), which correlated well with a threefold increase in intracellular viral DNA (as determined by quantitative PCR [data not shown]). To assess whether this functional interaction was specific for AAV8, we subsequently infected the two cell pools with the gfp vector, pseudotyped with AAV1 through -6 or AAV9. We found virtually identical transduction efficiencies between the two cell pools with serotypes 1, 4, 5, and 6, but interestingly LamR overexpression led to a 2.5-fold increase in GFP-positive cells for serotypes 2, 3, and 9, similar to the result with AAV8 (Fig. 4a).
FIG. 4.
Overexpression of the 37/67-kDa laminin receptor increases transduction by AAV8, -2, -3, and -9 in vitro. (a) Total protein extracts from the stably transfected NIH 3T3 cells were analyzed by Western blot analysis using the anti-laminin receptor or antiactin antibody (left panel). The same two cell pools were incubated with 1 × 105 particles/cell of AAV1, -3, -4, -5, -6, -8, or -9(gfp) or 5 × 103 particles/cell of AAV2(gfp) for 1 hour, and amounts of transduced cells were determined after 3 days (right panel). (b) AAV8(gfp) (1 × 106 particles/cell) was incubated for 1 hour with a pool of NIH 3T3 cells stably transfected with the empty pcDNA plasmid (left panel) or with the laminin receptor expression plasmid (right panel). GFP expression, indicating successful AAV8 transduction, was visualized after 3 days.
RNAi against lamR inhibits AAV8, AAV2, AAV3, and AAV9 transduction.
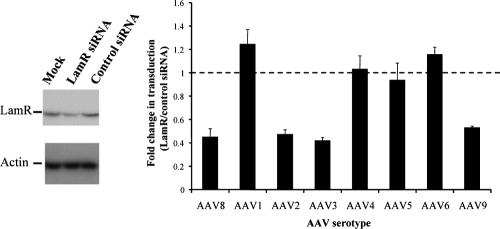
To further confirm the correlation of LamR expression levels and transduction with these four serotypes, we used RNAi to silence the lamR gene in wild-type NIH 3T3 cells. This was done by transfecting these cells with a previously reported anti-lamR siRNA (33), which in our hands resulted in a twofold reduction in LamR protein levels (Fig. 5). The cells as well as control siRNA-transfected cells were then incubated with the same eight AAV(gfp) vectors as above. As predicted, RNAi-mediated inhibition of LamR resulted in twofold-reduced transduction with AAV2, -3, -8 and -9, confirming a direct correlation of LamR expression levels and functional transduction by these serotypes. LamR knockdown had no effect on transduction with AAV1, -4, -5, or -6, showing that the receptor is not required for infection with these four isolates and thus also supporting our previous data.
FIG. 5.
Inhibition of 37/67-kDa laminin receptor expression by RNAi inhibits transduction by AAV8, -2, -3, and -9 in vitro. Total protein extracts from NIH 3T3 cells that were mock transfected or transfected with the indicated siRNA were analyzed by Western blotting using the anti-LamR or antiactin antibody (left panel). Cells transfected with the anti-lamR siRNA or control siRNA were incubated with 1 × 105 particles/cell of gfp-expressing AAV1, -3, -4, -5, -6, -8, or -9 or 5 × 103 particles/cell of gfp-expressing AAV2 for 1 hour, and amounts of transduced cells were determined after 3 days (right panel).
LamR mediates AAV8 and AAV2 transduction of mouse liver in vivo.
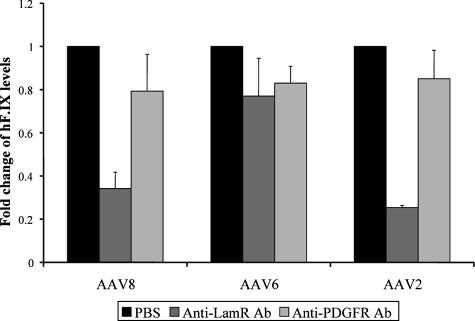
Finally, we studied the role of LamR for AAV8 binding and transduction in vivo by assaying the ability of recombinant virus to infect mouse liver in the presence of the anti-LamR antibody. To do this, mice were injected with 40 μg of this antibody or with 40 μg of an anti-PDGFR-β antibody or with PBS, followed 6 hours later by peripheral infusion of 1 × 1011 recombinant serotype 2, 6, or 8 particles expressing the human factor IX (hf.IX) gene from a liver-specific promoter. Blood was collected after 3 days, and plasma hF.IX was determined by enzyme-linked immunosorbent assay. Consistent with our previous in vitro findings, AAV2- and AAV8-mediated liver transduction was strongly inhibited in the presence of the anti-LamR antibody, as evidenced by a nearly threefold drop in plasma factor IX levels compared to the respective PDGFR/PBS controls (Fig. 6). In contrast and as predicted, AAV6 transduction was only marginally affected by the anti-LamR antibody.
FIG. 6.
AAV8- and AAV2-mediated transduction of mouse liver depends on the 37/67-kDa laminin receptor. An anti-LamR antibody (Ab), anti-PDGFR antibody, or PBS was intravenously injected into mice, followed 6 hours later by infusion of 1 × 1011 particles of AAV2, AAV6, or AAV8(hf.IX) per mouse via the same route. After 3 days, plasma levels of hF.IX were determined by enzyme-linked immunosorbent assay.
DISCUSSION
Collectively, the data in this paper allow us to conclude that the 37/67-kDa laminin receptor is functionally relevant for AAV8 cellular binding and transduction in vitro and in vivo and as such allow us to conclude that it is a receptor for AAV8. Interestingly, we identified a subset of three highly homologous (around 85% protein similarity) serotypes (AAV2, -8, and -9) which also utilize LamR for functional transduction. However, AAV8 clearly differs from the family prototype AAV2, which appears to rely on other receptors (e.g., HSPG) for cell attachment, while requiring LamR interaction for efficient internalization and transduction. Noteworthy in this respect are the findings that LamR and HSPG can form a complex on the cell surface (14), further substantiating the idea of a synergistic function of the two molecules for AAV2 transduction. AAV8 is likewise distinguished from four other serotypes (AAV1 and -4 to -6) that transduce cells independently of LamR. Among these, AAV4 and -5 are only 61 to 67% homologous to AAV8 and use sialic acids as receptors (15). AAV1 and -6, despite their 84% homology to AAV8, can also transduce cells through a sialic acid-based pathway (6, 26). Their preference for sialic acid may explain why we found neither evidence for in vitro interaction of these four serotypes with LamR nor an effect on their transduction from blocking/overexpressing this receptor.
The results of this study have three essential implications for the use of AAV8 or other viral serotypes as vectors for human gene therapy. Firstly, LamR is widely expressed in human tissues, where it is normally involved in interactions of extracellular laminin 1 with proteases and with the cell (1, 2). Interestingly, it also serves as a receptor for numerous viruses such as dengue virus and Sindbis virus, as well as for prions (14, 30, 35). The wide distribution of this receptor provides a molecular explanation for the unusually broad tropism of AAV8, and indeed, LamR is found on several tissues known to be efficiently transduced by the virus, such as the heart, liver, and skeletal muscle (27). Notably, it is specifically overexpressed in cancer cells, where it is critical for tumor invasion and metastasis. In fact, a Sindbis virus vector binding to LamR was shown to preferentially target tumor cells in vivo (32), implying that AAV8 might also prove to be an especially useful vector for tumor-directed gene therapy. This idea is particularly supported by our findings of a direct correlation of LamR expression levels and AAV8 transduction in our overexpression/inhibition studies. Secondly, our mapping of the LamR domains to the C terminus of the AAV8 VP proteins corroborates the evolving model, that this portion of AAV capsid proteins mediates receptor binding and antibody recognition, while the N-terminal domains are mostly involved in particle infectivity. Together with previously published data, our results suggest that AAV capsid proteins generally consist of individual functional domains, which can be swapped or molecularly altered to engineer chimeric viruses with distinct properties. Thirdly, our findings clearly highlight that the efficiency of AAV vector transduction is determined on multiple levels and that several steps in the infection pathway are essential, not only cell attachment but also cytoplasmic trafficking or nuclear uncoating. This is best exemplified by our result that three other AAV serotypes, including the widely used AAV2, can interact with LamR, while none of them achieves the transduction efficiencies of AAV8 in vivo. It thus remains an important goal of future work to identify further receptors or intracellular interaction partners involved in AAV8 transduction, as this may ultimately help unravel and overcome the obstacles seen with the less efficient serotypes. In conclusion, we believe that with its combination of high transduction efficiencies, lack of preexisting immunity in the population, and the here-identified widely expressed receptor, AAV8 may be an ideal vector for many applications in human gene therapy.
Acknowledgments
We acknowledge Xuan Shen for his help with the computer modeling of the AAV capsid.
B.A. is supported by a fellowship from the Canadian Institute of Health Research. This work was supported by NIH grant HL66948 (M.A.K.).
REFERENCES
- 1.Ardini, E., B. Sporchia, L. Pollegioni, M. Modugno, C. Ghirelli, F. Castiglioni, E. Tagliabue, and S. Menard. 2002. Identification of a novel function for 67-kDa laminin receptor: increase in laminin degradation rate and release of motility fragments. Cancer Res. 62:1321-1325. [PubMed] [Google Scholar]
- 2.Ardini, E., E. Tagliabue, A. Magnifico, S. Buto, V. Castronovo, M. I. Colnaghi, and S. Menard. 1997. Co-regulation and physical association of the 67-kDa monomeric laminin receptor and the α6β4 integrin. J. Biol. Chem. 272:2342-2345. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn, S. D., R. A. Steadman, and F. B. Johnson. 2006. Attachment of adeno-associated virus type 3H to fibroblast growth factor receptor 1. Arch. Virol. 151:617-623. [DOI] [PubMed] [Google Scholar]
- 4.Bossis, I., and J. A. Chiorini. 2003. Cloning of an avian adeno-associated virus (AAAV) and generation of recombinant AAAV particles. J. Virol. 77:6799-6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouchara, J. P., M. Sanchez, A. Chevailler, A. Marot-Leblond, J. C. Lissitzky, G. Tronchin, and D. Chabasse. 1997. Sialic acid-dependent recognition of laminin and fibrinogen by Aspergillus fumigatus conidia. Infect. Immun. 65:2717-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, S., M. Kapturczak, S. A. Loiler, S. Zolotukhin, O. Y. Glushakova, K. M. Madsen, R. J. Samulski, W. W. Hauswirth, M. Campbell-Thompson, K. I. Berns, T. R. Flotte, M. A. Atkinson, C. C. Tisher, and A. Agarwal. 2005. Efficient transduction of vascular endothelial cells with recombinant adeno-associated virus serotype 1 and 5 vectors. Hum. Gene Ther. 16:235-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Pasquale, G., B. L. Davidson, C. S. Stein, I. Martins, D. Scudiero, A. Monks, and J. A. Chiorini. 2003. Identification of PDGFR as a receptor for AAV-5 transduction. Nat. Med. 9:1306-1312. [DOI] [PubMed] [Google Scholar]
- 8.Gao, G., L. H. Vandenberghe, M. R. Alvira, Y. Lu, R. Calcedo, X. Zhou, and J. M. Wilson. 2004. Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 78:6381-6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao, G. P., M. R. Alvira, L. Wang, R. Calcedo, J. Johnston, and J. M. Wilson. 2002. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA 99:11854-11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimm, D. 2002. Production methods for gene transfer vectors based on adeno-associated virus serotypes. Methods 28:146-157. [DOI] [PubMed] [Google Scholar]
- 11.Grimm, D., and M. A. Kay. 2003. From virus evolution to vector revolution: use of naturally occurring serotypes of adeno-associated virus (AAV) as novel vectors for human gene therapy. Curr. Gene Ther. 3:281-304. [DOI] [PubMed] [Google Scholar]
- 12.Grimm, D., M. A. Kay, and J. A. Kleinschmidt. 2003. Helper virus-free, optically controllable, and two-plasmid-based production of adeno-associated virus vectors of serotypes 1 to 6. Mol. Ther. 7:839-850. [DOI] [PubMed] [Google Scholar]
- 13.Grimm, D., A. Kern, K. Rittner, and J. A. Kleinschmidt. 1998. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum. Gene Ther. 9:2745-2760. [DOI] [PubMed] [Google Scholar]
- 14.Hundt, C., J. M. Peyrin, S. Haik, S. Gauczynski, C. Leucht, R. Rieger, M. L. Riley, J. P. Deslys, D. Dormont, C. I. Lasmezas, and S. Weiss. 2001. Identification of interaction domains of the prion protein with its 37-kDa/67-kDa laminin receptor. EMBO J. 20:5876-5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaludov, N., K. E. Brown, R. W. Walters, J. Zabner, and J. A. Chiorini. 2001. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J. Virol. 75:6884-6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashiwakura, Y., K. Tamayose, K. Iwabuchi, Y. Hirai, T. Shimada, K. Matsumoto, T. Nakamura, M. Watanabe, K. Oshimi, and H. Daida. 2005. Hepatocyte growth factor receptor is a coreceptor for adeno-associated virus type 2 infection. J. Virol. 79:609-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lochrie, M. A., G. P. Tatsuno, B. Christie, J. W. McDonnell, S. Zhou, R. Surosky, G. F. Pierce, and P. Colosi. 2006. Mutations on the external surfaces of adeno-associated virus type 2 capsids that affect transduction and neutralization. J. Virol. 80:821-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maheshri, N., J. T. Koerber, B. K. Kaspar, and D. V. Schaffer. 2006. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat. Biotechnol. 24:198-204. [DOI] [PubMed] [Google Scholar]
- 19.Miao, C. H., K. Ohashi, G. A. Patijn, L. Meuse, X. Ye, A. R. Thompson, and M. A. Kay. 2000. Inclusion of the hepatic locus control region, an intron, and untranslated region increases and stabilizes hepatic factor IX gene expression in vivo but not in vitro. Mol. Ther. 1:522-532. [DOI] [PubMed] [Google Scholar]
- 20.Nakai, H., S. Fuess, T. A. Storm, S. Muramatsu, Y. Nara, and M. A. Kay. 2005. Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J. Virol. 79:214-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakai, H., R. W. Herzog, J. N. Hagstrom, J. Walter, S. H. Kung, E. Y. Yang, S. J. Tai, Y. Iwaki, G. J. Kurtzman, K. J. Fisher, P. Colosi, L. B. Couto, and K. A. High. 1998. Adeno-associated viral vector-mediated gene transfer of human blood coagulation factor IX into mouse liver. Blood 91:4600-4607. [PubMed] [Google Scholar]
- 22.Qing, K., C. Mah, J. Hansen, S. Zhou, V. Dwarki, and A. Srivastava. 1999. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat. Med. 5:71-77. [DOI] [PubMed] [Google Scholar]
- 23.Rabinowitz, J. E., D. E. Bowles, S. M. Faust, J. G. Ledford, S. E. Cunningham, and R. J. Samulski. 2004. Cross-dressing the virion: the transcapsidation of adeno-associated virus serotypes functionally defines subgroups. J. Virol. 78:4421-4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabatino, D. E., F. Mingozzi, D. J. Hui, H. Chen, P. Colosi, H. C. Ertl, and K. A. High. 2005. Identification of mouse AAV capsid-specific CD8+ T cell epitopes. Mol. Ther. 12:1023-1033. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt, M., H. Katano, I. Bossis, and J. A. Chiorini. 2004. Cloning and characterization of a bovine adeno-associated virus. J. Virol. 78:6509-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seiler, M. P., A. D. Miller, J. Zabner, and C. L. Halbert. 2006. Adeno-associated virus types 5 and 6 use distinct receptors for cell entry. Hum. Gene Ther. 17:10-19. [DOI] [PubMed] [Google Scholar]
- 27.Sobel, M. E. 1993. Differential expression of the 67 kDa laminin receptor in cancer. Semin. Cancer Biol. 4:311-317. [PubMed] [Google Scholar]
- 28.Summerford, C., J. S. Bartlett, and R. J. Samulski. 1999. αVβ5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat. Med. 5:78-82. [DOI] [PubMed] [Google Scholar]
- 29.Summerford, C., and R. J. Samulski. 1998. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 72:1438-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thepparit, C., and D. R. Smith. 2004. Serotype-specific entry of dengue virus into liver cells: identification of the 37-kilodalton/67-kilodalton high-affinity laminin receptor as a dengue virus serotype 1 receptor. J. Virol. 78:12647-12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas, C. E., T. A. Storm, Z. Huang, and M. A. Kay. 2004. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J. Virol. 78:3110-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tseng, J. C., B. Levin, A. Hurtado, H. Yee, I. Perez de Castro, M. Jimenez, P. Shamamian, R. Jin, R. P. Novick, A. Pellicer, and D. Meruelo. 2004. Systemic tumor targeting and killing by Sindbis viral vectors. Nat. Biotechnol. 22:70-77. [DOI] [PubMed] [Google Scholar]
- 33.Umeda, D., H. Tachibana, and K. Yamada. 2005. Epigallocatechin-3-O-gallate disrupts stress fibers and the contractile ring by reducing myosin regulatory light chain phosphorylation mediated through the target molecule 67 kDa laminin receptor. Biochem. Biophys. Res. Commun. 333:628-635. [DOI] [PubMed] [Google Scholar]
- 34.Walters, R. W., S. M. Yi, S. Keshavjee, K. E. Brown, M. J. Welsh, J. A. Chiorini, and J. Zabner. 2001. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J. Biol. Chem. 276:20610-20616. [DOI] [PubMed] [Google Scholar]
- 35.Wang, K. S., R. J. Kuhn, E. G. Strauss, S. Ou, and J. H. Strauss. 1992. High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J. Virol. 66:4992-5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, Z., T. Zhu, C. Qiao, L. Zhou, B. Wang, J. Zhang, C. Chen, J. Li, and X. Xiao. 2005. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat. Biotechnol. 23:321-328. [DOI] [PubMed] [Google Scholar]
- 37.Wobus, C. E., B. Hugle-Dorr, A. Girod, G. Petersen, M. Hallek, and J. A. Kleinschmidt. 2000. Monoclonal antibodies against the adeno-associated virus type 2 (AAV-2) capsid: epitope mapping and identification of capsid domains involved in AAV-2-cell interaction and neutralization of AAV-2 infection. J. Virol. 74:9281-9293. [DOI] [PMC free article] [PubMed] [Google Scholar]