Abstract
Human immunodeficiency virus type 1 (HIV-1) assembly, budding, and release occur mostly at the plasma membrane in T lymphocytes as well as in established nonlymphoid cell lines, while in macrophages these processes occur primarily in intracellular compartments that harbor late endosomal/multivesicular body (LE/MVB) markers, including human leukocyte antigen DR (HLA-DR). Major histocompatibility complex class II molecules (MHC-II), which are expressed in macrophages and activated T cells, have been previously reported to induce the formation of multilaminar and multivesicular endocytic MHC-II-like structures analogous to MVB upon their expression in HEK 293 cells. Here, we have examined the role of MHC-II in HIV-1 Gag targeting as well as in virus assembly and release. Expression of HLA-DR in nonlymphoid cell lines induced a relocation of Gag to intracellular compartments that harbored LE/MVB markers and increased the accumulation of viral particles assembling intracellularly. Consequently, viral production and release from the cell surface was found to be substantially decreased in HLA-DR-expressing cells. This process was specific, since it was not observed with HLA-DR molecules lacking their cytoplasmic tails, nor with structurally related but functionally distinct MHC-II molecules such as HLA-DM or HLA-DO. Importantly, virus released intracellularly in HLA-DR-expressing cells retained infectivity. Overall, these results suggest a role of MHC-II molecules in promoting HIV-1 assembly and budding to LE/MVB and raise the possibility that this activity might be part of a normal pathway of virus production in cell types physiologically expressing MHC-II molecules, such as macrophages.
Production of retrovirus particles is a multistep process that requires the coordinated assembly of viral structural components at a membrane budding site. The human immunodeficiency virus type 1 (HIV-1) Gag polyprotein, Pr55gag, plays a central role in viral assembly and release, since Gag expression alone is sufficient for the production of noninfectious virus-like particles (16). Pr55gag is composed of four domains that are cleaved by the viral protease (PR) during the budding process to generate matrix (MA or p17), capsid (CA or p24), nucleocapsid (NC or p7), and p6, as well as two spacer peptides, SP1 and SP2 (12, 16). Functional domains that promote Gag binding to membrane and multimerization have been mapped in Pr55gag to the myristoylated N-terminal portion of MA and the region spanning from the C terminus of CA to the N terminus of NC, respectively (12, 16). p6, through its tetrapeptide (PTAP) late motif, plays a central role in the release of viral particles by recruiting Tsg101 and other components of the endosomal sorting complex required for transport involved in the biogenesis of multivesicular bodies (MVB) (14, 30, 49, 51).
HIV-1 has been recently reported to assemble and bud either at the plasma membrane or in late endosomes (LE)/MVB. In cells such as T lymphocytes and transformed human cell lines such as HeLa and HEK 293T, the majority of virus assembly takes place at the plasma membrane (12, 34, 36, 46). In contrast, in primary macrophages, assembly occurs primarily in intracellular compartments that express late endosomal or MVB markers, including major histocompatibility complex class II molecules (MHC-II), such as human leukocyte antigen DR (HLA-DR), CD63, and Lamp1 (33, 38, 40, 42). However, the mechanism governing whether virus release occurs via internal or plasma membranes remains poorly understood. Interestingly, several reports have established that in addition to directing Gag membrane binding, the HIV MA domain regulates the targeting of Gag to the site of virus assembly (7, 9, 13, 18, 37). On the other hand, the cell-type-dependent nature of HIV-1 assembly subcellular location strongly suggests that in addition to viral determinants, host cell factors must also play an active role in determining whether HIV-1 particle assembly and release occurs at the plasma membrane or in LE/MVB. However, the identity of cellular factors promoting HIV-1 targeting to LE/MVB remains to be defined.
Interestingly, MHC-II molecules, which are expressed in macrophages and activated T cells, have been previously reported to induce the formation of CD63/Lamp1-positive multilaminar and multivesicular endocytic structures, reminiscent of MHC-II-enriched compartments (MIIC), upon their ectopic expression in HEK 293 cells (4). Interestingly, the transmembrane and cytoplasmic tails of the class II α and β chains were found necessary for the induction of these prototypical MHC-II endocytic compartments in HEK 293 cells, indicating that MHC-II molecules contain information critical for the formation or maturation of MHC-II-like compartments. Since HIV-1 particles preferentially assemble at the plasma membrane in HEK 293T cells (18, 46), we investigated the impact of MHC-II expression on Gag localization as well as on assembly and release of HIV-1 particles. Our results suggest that expression of classical MHC-II molecules promotes assembly and budding of infectious HIV-1 to LE/MVB in a process that implicates the cytoplasmic domain of the α and β chains of MHC-II. These findings shed light on host cell factors governing the cell-type-dependent subcellular location of HIV-1 assembly and budding and reveal a novel effect of MHC-II molecules on HIV-1 replication and persistence.
MATERIALS AND METHODS
Cells and plasmids.
HEK 293T, HeLa-CD4-LTR-β-Gal (25), HeLa DR1 (DRα + DRβ0101) (43), and HeLa DRαTM/DRβTM (19) cells were maintained as described elsewhere (25). The HIV-1 molecular clone HxBc2 (24) and the MHC-II expression plasmids, including pBud-DO, pBud-DM (10), and pLNCX-DQ (19), were previously described. For the bicistronic pBud-DR construct, cDNAs encoding the DRα and DRβ chains were cloned into the pBudCE4-amp vector. A BamHI DRβ fragment originating from pBSDRβ was cloned into pBudCE4 (pBudCE4-amp DRβ), and a BamHI fragment encoding the DRα chain was cloned into the Bgl II site of pBudCE4-amp DRβ. For experiments where the TM/TM mutant was included, the following plasmids were used: RSV.5 DRα, RSV.3 DRβ, RSV.5 DRαTM, and RSV.3 DRβTM (19).
Transfections, immunoprecipitation, and viral release.
Transfections were performed as described previously (52). Immunoprecipitations were done using a mix of human anti-HIV serum together with a monoclonal anti-p24 antibody (Ab), as described elsewhere (52). For pulse-labeling experiments, transfectants were metabolically labeled with 1 mCi/ml [35S]methionine-cysteine ([35S] protein labeling mix; Perkin-Elmer) in Dulbecco's modified Eagle's medium lacking methionine and cysteine and supplemented with 5% dialyzed fetal bovine serum for 2 h. Viral release was calculated as described elsewhere (35). For pulse-chase experiments, cells were metabolically labeled for 30 min as described above and chased for different time intervals in Dulbecco's modified Eagle's medium containing an excess of unlabeled methionine and cysteine. Viral release was calculated as the amount of virion-associated Gag as a fraction of total (cell plus virion) Gag synthesized during 30 min of the metabolic labeling period (0-h chase).
Antibodies and immunostaining.
The following antibodies were used: L243 (immunoglobulin G2a [IgG2a]), a murine monoclonal antibody that binds a specific HLA-DRα conformational determinant dependent on the correct conformation of the α/β heterodimer (39); mouse monoclonal antibody (IgG1), which recognizes p17 but not p55gag (catalog no. HB-8975) and mouse monoclonal anti-p24 (catalog no. HB-9725), isolated from supernatants of cultured hybridoma cells obtained from the American Type Culture Collection (Manassas, VA); rabbit anti-p24 polyclonal antibody (catalog no. 4250; NIH AIDS Research and Reference Reagent Program); the anti-HIV-1 serum (no. 162), obtained from an HIV-1-infected individual whose serum tested positive for the presence of HIV-1 antibodies by enzyme-linked immunosorbent assay (25); mouse anti-Lamp-1 (H5G11; IgG1; Santa Cruz Biotechnology, Santa Cruz, CA); anti-CD63 (H5C6; IgG1; Hybridoma Bank, NICHD, University of Iowa); mouse anti-lysobisphosphatidic acid (LBPA) monoclonal antibody (22), a kind gift from J. Gruenberg (University of Geneva, Geneva, Switzerland); and rabbit polyclonal anti-human class II alpha chain serum (31), a kind gift from J. Neefjes (Netherlands Cancer Institute). Alexa 488-conjugated anti-rabbit IgG, Alexa 594-conjugated anti-mouse IgG, Alexa 488-conjugated anti-mouse IgG1, and Alexa 594-conjugated anti-mouse IgG2 were obtained from Molecular Probes (Burlington, ON, Canada). Immunostaining was performed on 5 × 104 HEK 293T or 3 × 104 HeLa cells as follows: transfected cells were rinsed once with phosphate-buffered saline (PBS), cytospun for 4 min at 1,100 rpm in a Cytospin 2 (Shandon), and fixed with 4% paraformaldehyde for 30 min. HeLa cells were directly fixed in chambered coverglasses, where they were plated 24 h before. All procedures were carried out at room temperature unless otherwise indicated. Following a wash with PBS, fixed cells were permeabilized with PBS containing 0.2% Triton X-100 for 10 min, followed by an additional washing with PBS. Subsequently, cells were incubated in PBS containing 50 mM ammonium chloride for 10 min and exposed to primary antibodies diluted appropriately in 2% bovine serum albumin in PBS for 2 h at 37°C. Following three washes with PBS, cells were next incubated for 40 min with an appropriate secondary antibody diluted in PBS. Nuclei were then stained with 4′,6′-diamidino-2-phenylindole for 5 min. After extensive washing, cells were mounted with Permount (Fisher Scientific, Ottawa, ON, Canada) and examined by conventional epifluorescence micrographs on a Zeiss Cell Observer system (Zeiss, Toronto, ON, Canada) equipped with an Axiovert 200 M microscope using the 100× oil lens. Images were digitally deconvoluted with the AxioVision 3.1 software using the nearest-neighbor deconvolution method. Flow cytometry analysis was performed as described previously (3).
Cell-associated infectivity and Pr55gag processing.
HEK 293T cells (3 × 105) were cotransfected with 1.6 μg of HxBc2 provirus together with 0.8 μg of empty or HLA-DR vectors and washed 16 h later. Twenty-four hours posttransfection, indinavir sulfate (IVS; 10 μM; catalog no. 8145; NIH AIDS Research and Reference Reagent Program) was added to the culture medium to inhibit infectivity of newly produced virus. Forty-eight hours posttransfection, cells were extensively washed in PBS and either lysed in RIPA buffer (5% of cells) or homogenized (95% of cells) in homogenization buffer (0.25 M sucrose, 78 mM KCl, 4 mM MgCl2, 8.4 mM CaCl2, 10 mM EGTA, 50 mM HEPES-NaOH pH 7.0) during 60 s using a pellet pestle with a cordless motor (Kontes, Vineland, NJ). Homogenates were centrifuged at 1,000 × g for 5 min to pellet nuclei and any cell debris. Evaluation of cell lysis efficiency was accomplished by measuring β-hexosaminidase activity in pellets and supernatants using 4-methyl-umbelliferyl-N-acetyl-β-d-glucosamine (Sigma-Aldrich, Oakville, ON, Canada) as described elsewhere (50). Infectivity of virus present in postnuclear supernatants (PNS) was assessed by MAGI assay (20). Each sample was analyzed in duplicate. Of note, IVS treatment involved not only a 24-h exposure of transfected cells to the drug but also maintenance of IVS during PBS washes, the homogenization step, and infection of MAGI (HeLa-CD4-LTR-β-Gal) cells. Lysed cells were analyzed for Gag polyprotein precursor processing by Western blotting using the mouse monoclonal anti-p24 antibody as described previously (25).
Electron microscopy.
Cell pellets were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer. Postfixation of cell pellets was performed using 2% OsO4 in s-collidine buffer for 2 h at 21°C. Pellets were dehydrated in an acetone series before embedding and polymerization in SPURR resin. Thin sectioning was done with an ultramicrotome system (Ultrotome 2128, LKB, Sweden), and the sections were placed on copper-Formvar-carbon-coated grids. Cells were stained with 5% uranyl acetate in 50% ethanol and lead citrate (pH 12.0). For immunogold staining, cell pellets were fixed in 0.1% glutaraldehyde-4% paraformaldehyde in 0.1 M cacodylate buffer. Pellet dehydration, polymerization, and thin sectioning were performed as described above, and the sections were placed on nickel-Formvar-carbon-coated grids. Cells were labeled with a rabbit polyclonal anti-human class II serum (31) followed by incubation with a goat anti-rabbit antibody coupled to 12-nm gold beads before staining using 5% uranyl acetate in 50% ethanol and lead citrate (pH 12.0). The grids were examined on a transmission electron microscope (Hitachi 7100; Japan).
RESULTS
HLA-DR expression induces a relocation of HIV-1 Gag to LE/MVB.
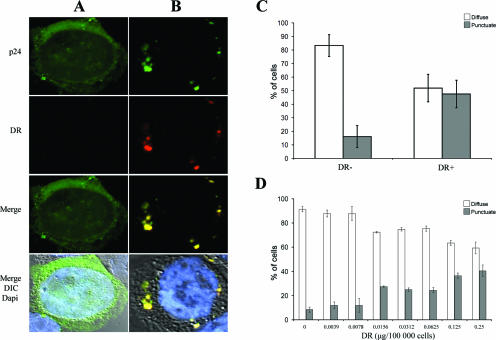
Expression of the α and β chains of MHC-II molecules in HEK 293 cells was found to be sufficient to induce the formation of multilamellar and multivesicular MIIC (4). To examine whether MHC-II molecules could affect Gag localization, HEK 293T cells were cotransfected with the HIV-1 molecular clone HxBc2 together with expression plasmids encoding the α and β chains of HLA-DR or an empty vector. In the absence of HLA-DR, the majority of Gag, as visualized with an anti-p24 antibody, was detected as diffuse cytoplasmic and cell membrane staining (Fig. 1A). In contrast, Gag-positive cells expressing HLA-DR often displayed a marked modification of Gag localization, with Gag staining accumulating in large intracellular vesicles (Fig. 1B). Interestingly, these intracellular Gag-containing vesicles were also HLA-DR positive (Fig. 1B, merge).
FIG. 1.
HLA-DR induces Gag accumulation in intracellular compartments in HEK 293T cells. HEK 293T cells were cotransfected with the HxBc2 provirus together with empty or HLA-DR vectors and analyzed 48 h later by immunofluorescence microscopy using a polyclonal anti-p24 and the monoclonal L243 HLA-DR Abs. (A) In the absence of HLA-DR, Gag displays a diffuse staining. (B) HLA-DR redirects Gag to large intracytoplasmic vesicles (punctuate staining), where it colocalizes with HLA-DR. (C) Quantification of Gag-associated staining. The number of cells displaying a diffuse versus punctuate Gag staining was evaluated in 200 cells per sample. Data shown represent the average of at least 25 independent experiments ± the standard deviation. (D) HLA-DR redirects Gag from a diffuse to a punctuate staining in a dose-dependent fashion. Data are representative of four independent experiments.
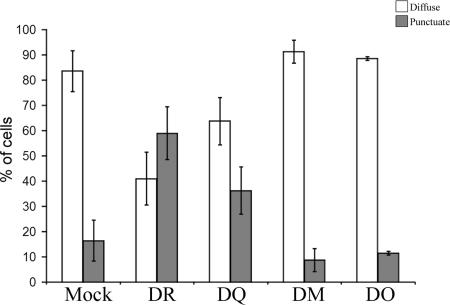
Since we were able to differentiate between diffuse and large punctuate Gag staining (Fig. 1A and B, respectively), we quantified the extent of Gag relocalization induced by HLA-DR in cell transfectants (Fig. 1C). In the absence of HLA-DR, 80 to 90% of cells showed a diffuse Gag staining, while less than 20% displayed a punctuate Gag staining. Conversely, upon HLA-DR expression, a punctuate Gag staining was detected in approximately 50% of the cells, most probably those expressing higher levels of HLA-DR. Indeed, the effect of HLA-DR expression on Gag localization in intracellular vesicles was dose dependent (Fig. 1D). Importantly, however, levels of HLA-DR expression required to induce Gag relocalization to intracellular vesicles (Fig. 1) were comparable to those detected in activated primary monocyte-derived macrophages (data not shown). Finally, to examine whether the effect of HLA-DR on HIV-1 Gag localization was restricted to the classical MHC-II molecules, such as HLA-DR and -DQ, or was also shared with other structurally related MHC-II proteins, such as HLA-DM and -DO (nonclassical MHC-II molecules), we tested the impact of their expression on Gag localization. HLA-DM is expressed in late endosomal/lysosomal compartments, including MVB and multilamellar compartments (28, 44), while HLA-DO resides in the endoplasmic reticulum when expressed by itself in transfected cells (26); neither HLA-DM nor -DO displayed any effect on Gag localization, while HLA-DQ partially recapitulated the effect of HLA-DR (Fig. 2).
FIG. 2.
Classical MHC-II molecules redirect Gag to intracellular compartments. HEK 293T cells were transfected with the proviral construct HxBc2 and plasmids encoding MHC-II-related molecules, including HLA-DR, -DQ, -DM, or -DO, and analyzed for Gag localization by immunostaining and fluorescence microscopy using a rabbit polyclonal anti-p24 antibody. Diffuse or punctuate Gag-associated staining patterns were quantified in 200 cells per sample. Data shown are means ± standard deviations of two independent experiments.
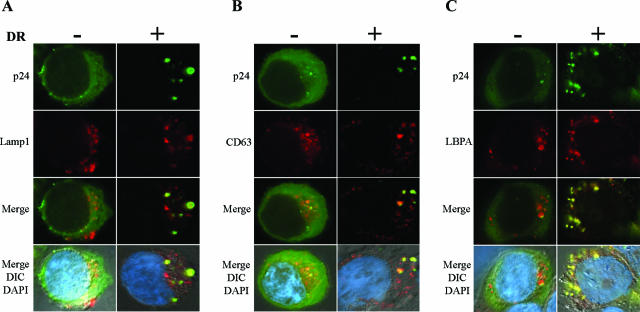
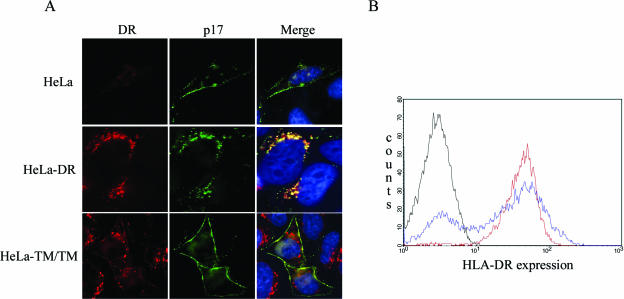
To further characterize the nature of the intracellular compartments where Gag accumulates in the presence of HLA-DR, we performed costaining experiments with antibodies directed against late endocytic markers. These experiments revealed that Gag-containing intracellular vesicles were positive for LE or MVB markers HLA-DR, Lamp1, CD63, and LBPA, a lipid found in the internal membranes of MVB (22) (Fig. 1B and 3A to C, respectively). Because results so far were obtained in a transient expression system where proteins are overexpressed, we also examined the effect of HLA-DR expression on Gag relocalization in HeLa cells stably expressing HLA-DR (Fig. 4A). Given that previous evidence suggested that the cytosolic domain of MHC-II molecules might be implicated in the induction of MHC-II-like compartments (4), we also analyzed Gag localization in HeLa cells stably expressing a truncated form of HLA-DR that lacks the cytoplasmic tails of the α and β chains (TM/TM). HLA-DR-expressing cell lines were transfected with the HxBc2 provirus and analyzed 48 h later by immunofluorescence microscopy, using an antibody that recognizes mature p17 (but not the MA domain in the context of Pr55gag) and an anti-HLA-DR antibody. Given that most processed MA is found associated with mature viral particles (16), the MA signal obtained with the anti-p17 antibody, in all likelihood, represents sites at which viral assembly occurs. In parental HeLa cells as well as in TM/TM cells, the majority of the MA signal was observed at the cell periphery on the plasma membrane (Fig. 4A), even though TM/TM molecules have been detected in intracellular vesicles that stained positive for MVB markers (A. Finzi and E. A. Cohen, unpublished data). In contrast, in HLA-DR-expressing cells, we observed a clear redistribution of MA staining to intracellular vesicles, where it colocalized with HLA-DR (Fig. 4A). Importantly, both HeLa-DR and HeLa-TM/TM expressed similar amounts of class II molecules as measured by flow cytometry (Fig. 4B).
FIG. 3.
HLA-DR redirects Gag to LE/MVB in HEK 293T cells. HEK 293T cells were transfected with the proviral construct HxBc2, together with HLA-DR or empty vector. Gag and MVB markers were detected by immunofluorescence microscopy 48 h later using a rabbit polyclonal anti-p24 antibody together with monoclonal antibodies against MVB markers. In the absence of HLA-DR, Gag shows primarily a diffuse staining (left panels of A, B, and C). Upon HLA-DR expression, Gag accumulates into Lamp1-positive (A), CD63-positive (B), and LBPA-positive (C) compartments (right panels).
FIG. 4.
Stable HLA-DR expression in HeLa cells induces Gag accumulation into HLA-DR-positive intracellular vesicles. Parental, HLA-DR-, or TM/TM-expressing HeLa cells were transfected with the infectious molecular clone HxBc2. Gag and HLA-DR were detected by immunostaining and fluorescence microscopy using mouse monoclonal anti-p17 (MA) and anti-HLA-DR (L243) Abs. (A) In parental HeLa cells, Gag is localized at the plasma membrane, whereas in HLA-DR-expressing cells, Gag is predominantly detected in vesicles at the perinuclear region. Importantly, stable TM/TM expression did not modify Gag localization. (B) Flow cytometry analysis of total HLA-DR expression in cells depicted in panel A using the L243 anti-HLA-DR monoclonal Ab. Black line, HeLa cells; red line, HeLa-DR cells; blue line, HeLa-TM/TM cells.
Together, these results suggest that ectopic MHC-II expression in human nonlymphoid cell lines induces a redirection of HIV-1 Gag localization and assembly from the plasma membrane to MHC-II-containing LE/MVB. This effect is restricted to classical MHC-II molecules, such as HLA-DR and -DQ and, importantly, appears to involve the cytoplasmic domains of the α and β chains.
HIV-1 particles accumulate into intracellular compartments in MHC-II-expressing HEK 293T cells.
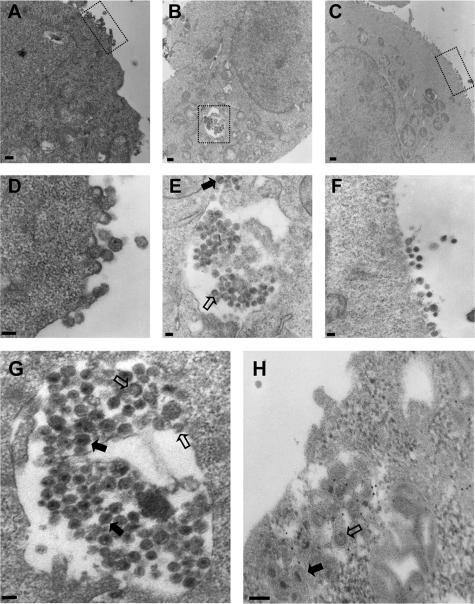
To obtain additional evidence that HLA-DR expression promotes HIV-1 particle assembly and budding in intracellular compartments, we performed electron microscopy analysis on HEK 293T cells transfected with HxBc2 alone or cotransfected with vectors encoding HLA-DR or the TM/TM mutant (Fig. 5). In HEK 293T cells transfected with HxBc2 alone or cotransfected with TM/TM, viral particle budding was observed predominantly at the plasma membrane (Fig. 5A and D and 5C and F, respectively). In HLA-DR-expressing cells, mature virions with typical condensed cores were observed in the lumen of large intracellular vesicles (Fig. 5B and E and 5G and H). Viral particles in the process of budding were also seen on the limiting membrane of these enlarged intracellular vesicles (Fig. 5G and H). Furthermore, immunogold staining experiments using an anti-HLA-DR antibody clearly revealed that the internal vesicles containing mature virions and budding viral particles stained positive for HLA-DR (Fig. 5H).
FIG. 5.
Mature and budding HIV-1 particles accumulate in intracellular compartments upon HLA-DR expression. HEK 293T cells were cotransfected with HxBc2 and empty, HLA-DR, or TM/TM vectors and observed by transmission electron microscopy (A to G) or processed for immunogold staining with a rabbit polyclonal anti-HLA-DRα Ab (H). In mock- or TM/TM-transfected cells, HIV-1 assembles at the plasma membrane (A and C, respectively). (B) HLA-DR expression induces accumulation of mature and budding HIV-1 particles into large intracellular compartments. (D to F) Magnified views from regions indicated in panels A to C, respectively. (G) Magnified view of intracellular HIV-1-containing compartments in HLA-DR-expressing cells. (H) HIV-1 particles accumulate into HLA-DR-positive compartments. Empty arrows indicate budding virus, whereas solid arrows indicate mature virus. Bar, 300 nm (A), 500 nm (B), 400 nm (C), or 100 nm (D to H).
Effect of HLA-DR expression on HIV-1 production.
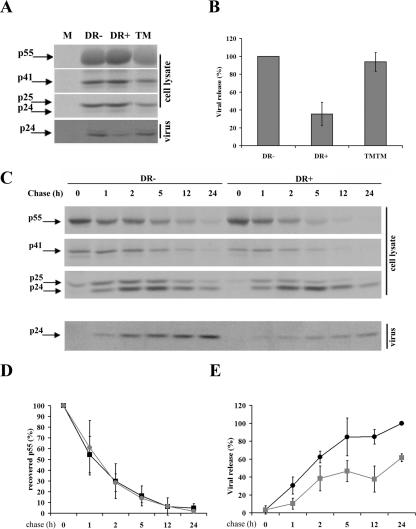
Having obtained evidence suggesting that HLA-DR expression promotes assembly and budding of HIV-1 particles to LE/MVB in HEK 293T cells, we next examined the impact of this relocation on HIV-1 particle production. HEK 293T cells were singly transfected with HxBc2 or cotransfected with expression vectors encoding HLA-DR or related molecules. Cells were pulse-labeled for 2 h, 48 h posttransfection, and cell and virus Gag-associated proteins were analyzed by immunoprecipitation (Fig. 6A). In transfected cell cultures expressing HLA-DR, virus release was reduced by two- to threefold compared to the mock-transfected control (Fig. 6A and B). In contrast, in TM/TM-, HLA-DM-, or HLA-DO-expressing cells, viral release efficiency was unaffected (Fig. 6A and B) (Finzi and Cohen, unpublished). Importantly, the observed impact of HLA-DR on HIV-1 release was not due to any marked defect at the level of Gag precursor processing, since measurements of Gag precursor cleavage in pulse-chase labeling/immunoprecipitation experiments revealed that the Pr55gag processing kinetics was identical in cells expressing HLA-DR and in the negative control (Fig. 6C and D). These results suggest that expression of MHC-II molecules, such as HLA-DR, can modulate viral release efficiency.
FIG. 6.
HLA-DR expression decreases HIV-1 release. (A) HEK 293T cells were mock transfected (M) or cotransfected with plasmids encoding HLA-DR (DR+), TM/TM (TM), or empty vector (DR-) together with the HxBc2 provirus. Two days after transfection, cells were metabolically labeled with [35S]Met-Cys for 2 h, and Gag-associated products in cell and virion lysates were immunoprecipitated using a mix of human anti-HIV serum together with a monoclonal anti-p24 Ab. (B) Quantitation of virus release efficiency. Data shown represent the average of at least four independent experiments ± the standard deviation. (C) Analysis of viral release kinetics by pulse-chase labeling analysis. Cell and virion lysates from HLA-DR+ and HLA-DR− cells were immunoprecipitated as for panel A after a 30-min metabolic labeling with [35S]Met-Cys or at different chase time intervals. (D) Gag precursor processing is represented as the percentage of p55gag-associated signal recovered from cell lysates after pulse-chase analysis as described for panel C. p55gag-associated signal after 30 min of labeling (0-h chase) was arbitrarily set to 100%. Data shown represent the average of five independent experiments ± the standard deviation. (E) Quantitation of viral release kinetics. Data from two independent experiments were quantified using a PhosphorImager equipped with ImageQuant software 5.0 and are shown as means ± standard deviations. Viral release efficiency was calculated as described in Materials and Methods. Gray lines, HLA-DR-expressing cells; black lines, HLA-DR-negative cells.
Interestingly, when we analyzed both quantitatively and qualitatively cell- and virus-associated Gag-related products by pulse-chase labeling and immunoprecipitation experiments, we started detecting a reduction of viral release in HLA-DR-expressing cells as early as 1 h postchase. This reduction in viral release efficiency was observed throughout the 24-h chase period, with a peak between 5 and 12 h; during that time interval, HLA-DR-expressing cells were found to release approximately twofold less virus than control cells (Fig. 6C and E). Interestingly, this reduction in viral release efficiency was accompanied by a change in the p24/p25 ratio accumulating in HLA-DR-expressing cells; quantitative analysis over several experiments revealed that there was 1.5 to 2.0 times more p24 relative to p25 in HLA-DR-expressing cells than control cells (Fig. 6C; compare p24 and p25 levels between HLA-DR+ and -DR− cells). These results indicate that even though CA is accumulating intracellularly in HLA-DR-expressing cells, it is being processed in a mature form usually found associated with mature viral particles. Overall, these results are consistent with our findings suggesting that expression of MHC-II molecules in HIV-producing cells leads to increased assembly and budding of mature viral particles in LE/MVB.
Infectivity of viral particles assembling intracellularly in the presence of HLA-DR.
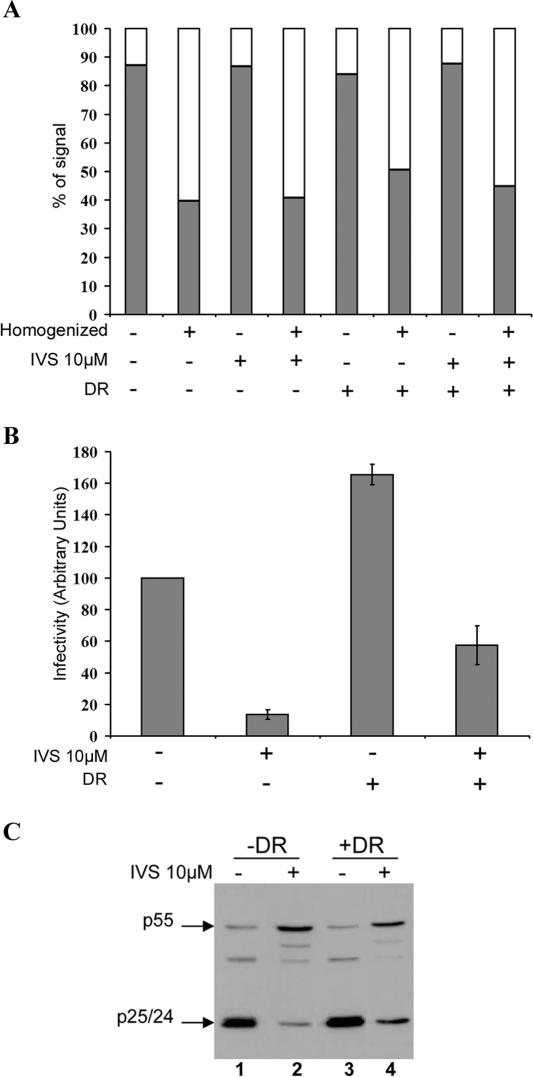
We next evaluated whether virions assembling intracellularly upon HLA-DR expression retained their infectivity. This is particularly important given that specific endosomal compartments are known to undergo acidification, a process that inactivates HIV infectivity (11), and participate in the degradation pathway that leads to lysosomes (17). To address this question, we adapted a recently described strategy that was used to evaluate the infectious stability of virions that assemble intracellularly in primary macrophages (45). This approach utilizes suprainhibitory concentrations (10 μM) of the protease inhibitor IVS to block de novo production of infectious particles. In the presence of 10 μM IVS, processing of Pr55gag is completely inhibited and virus particles that are produced are immature and hence noninfectious (45; Finzi and Cohen, unpublished). Consequently, infectious virus recovered from HIV-1-producing cells following IVS treatment should be formed prior to addition of the inhibitor. HEK 293T cells cotransfected with HxBc2 provirus and HLA-DR or empty vectors were allowed to produce infectious virus for 24 h prior to addition of IVS. Cells were homogenized and fractionated 24 h later to analyze cell-associated infectivity as described in Materials and Methods. Since cell disruption could affect recovery of the intracellular pool of virus, PNS and pellet fractions were analyzed for β-hexosaminidase activity as a marker for cell lysis and endocytic organelle disruption (23) (Fig. 7A). Consistent with the accumulation of intracellular viral particles (Fig. 5 and 6), untreated HLA-DR-expressing cells displayed more infectious activity (1.6-fold increase) in their PNS compared to control cells (Fig. 7B). IVS treatment drastically reduced intracellular infectious activity in control cells, thus suggesting that the bulk of cell-associated infectious activity produced during the first 24 h posttransfection (before adding IVS) was efficiently released in the extracellular medium in absence of HLA-DR. Nevertheless, some infectious activity (14%) was still detectable in PNS and most probably represents the background HIV-1 intracellular assembly detected in HEK 293T cells (Fig. 1 and 7C). Remarkably, IVS-treated HLA-DR-expressing cells retained four times more infectious activity in their PNS than control cells (57% versus 14%) (Fig. 7B). Interestingly, analysis of β-hexosaminidase activity released in PNS was found to be comparable between HLA-DR transfectants and control cells (Fig. 7A), thus indicating that differences observed in intracellular infectious activity cannot be attributed to variations in cell disruption efficiency. Furthermore, analysis of Gag processing in untreated or IVS-treated cells supported our observation that MHC-II molecules enhanced accumulation of mature virus particles into intracellular compartments (Fig. 7C). In the presence of IVS, as expected, there was a clear inhibition of Gag processing, as visualized by the decreased levels of p25/24 cleavage products and increased accumulation of p55gag (Fig. 7C; compare lanes 1 and 2 and lanes 3 and 4). Strikingly, the inhibitory effect of IVS on Gag processing was less efficient in HLA-DR transfectants than in control cells, as evidenced by the marked accumulation of p25/p24 (two- to threefold increase) in HLA-DR+ cells relative to the control (Fig. 7C, compare lanes 4 and 2). These completely processed intracellular Gag products, by definition, had to be produced during the 24-h time interval before addition of the drug.
FIG. 7.
Virions assembling intracellularly in the presence of HLA-DR remain infectious. HEK 293T cells were cotransfected with HxBc2 provirus and empty or HLA-DR vectors. IVS (10 μM) was added to the culture medium 24 h posttransfection to block production of new infectious virus. Cells were homogenized 24 h later, and cell disruption efficiency was determined by measuring β-hexosaminidase activity in pellets (filled bars) and PNS (empty bars) as described in Materials and Methods (A). Infectious activity was assessed in the PNS by MAGI assay (B). Data shown represent the average of two independent experiments ± the standard deviation. In parallel, Gag processing in each transfectant was analyzed by Western blotting using a monoclonal anti-p24 Ab (C). Data shown are representative of six independent experiments.
Altogether, these results provide additional evidence indicating that mature virus particles accumulate more efficiently into intracellular compartments in the presence of HLA-DR and demonstrate that virions released intracellularly within HLA-DR-expressing cells retain their infectivity potential.
DISCUSSION
In this study, we examined the role of MHC-II molecules in HIV-1 Gag targeting as well as in virus assembly and release. Ectopic expression of classical MHC-II molecules, such as HLA-DR and -DQ, in nonlymphoid cell lines was found to promote Gag relocation to intracellular compartments that contained late endosomal and MVB markers (Fig. 1 to 3), in a process that strictly relied on the presence of the cytoplasmic tails of the α and β chains of MHC-II molecules (Fig. 4 and 5). This MHC-II-mediated relocalization of Gag correlated with an increased accumulation of mature viral particles in intracellular compartments (Fig. 5) and, as a consequence, resulted in decreased virus production and release from the cell surface (Fig. 6). Importantly, viral particles assembling intracellularly in HLA-DR-expressing cells retained their infectivity (Fig. 7). Together, these results provide evidence suggesting that MHC-II molecules promote assembly and budding of infectious HIV-1 virions to LE/MVB and raise the possibility that this process might be part of a normal pathway of virus production in cell types physiologically expressing MHC-II molecules, such as macrophages.
HIV-1 Gag contains motifs that are critical for its transport to the plasma membrane (36) and for interaction with LE/MVB (7, 27). Furthermore, recent evidence indicates that the cell-type-dependent targeting of HIV-1 assembly to the plasma membrane or LE/MVB can also be regulated by host cell factors. For instance, the human ubiquitin ligase POSH, a trans-Golgi network-associated protein (1), and more recently phosphatidylinositol (4,5)biphosphate [PI(4,5)P2], a member of the phosphoinositide family of lipids concentrated primarily on the cytoplasmic leaflet of the plasma membrane (35, 48), were found to regulate HIV-1 Gag targeting to the plasma membrane, such that depleting hPOSH or cellular PI(4,5)P2 redirected virus assembly from the plasma membrane to LE and inhibited virus release. Interestingly, in the case of MHC-II molecules, it is the expression of a host cell factor physiologically expressed in macrophages that relocalizes Gag and viral assembly to LE/MVB in HeLa or HEK 293T cells. Nevertheless, MHC-II-mediated relocation of viral assembly to LE/MVB in HEK 293T cells resulted in a reduction of virus release, as has been reported for hPOSH or cellular ([PI(4,5)P2] depletion.
How does expression of class II molecules result in a marked accumulation of HIV-1 into LE/MVB? One possibility is that MHC-II molecules interact with a structural component of HIV and retarget a fraction of viral assembly to intracellular compartments following their transit into the endocytic pathway. MHC-II molecules, such as HLA-DR, have been reported to be present at the surface of the HIV-1 virion (5, 6) and, based on a previous study, Env gp41 appears to be required for efficient insertion of HLA-DR molecules within HIV-1 (41). The mechanism underlying MHC-II-mediated relocalization of HIV-1 assembly and budding does not appear to involve an interaction between the viral envelope and HLA-DR, since the effect of HLA-DR on Gag relocalization and viral particle production was observed with proviral constructs lacking Env (Finzi and Cohen, unpublished). Although this redirection could also result from an interaction of Gag with HLA-DR, we were unable to detect any specific interaction between these molecules in coimmunoprecipitation experiments (Finzi and Cohen, unpublished). However, the lack of physical interaction between HLA-DR and Gag does not preclude the possibility that the two molecules can interact functionally. In fact, it was reported that Gag can affect HLA-DR trafficking (15). In this study, Gag expression was shown to be sufficient to specifically restore defective transport of HLA-DR from intracellular compartments to the cell surface in a subclone of the HUT78 human T-cell line, suggesting that at some point Gag and HLA-DR share the same trafficking pathway. Interestingly, it was reported that a significant pool of MHC-II molecules traffic to endosomal-lysosomal compartments by means of the cell surface (8, 29). Consequently, it is therefore possible that MHC-II-induced relocalization of Gag into LE/MVB and subsequent accumulation of mature viral particles in these intracellular compartments could result from an increased internalization of virions from the plasma membrane mediated by HLA-DR rather than an enhanced targeting of Gag to LE/MVB. Interestingly, a recent report indicates that expression of a dominant negative form of dynamin (K44A), known to inhibit clathrin-mediated endocytosis (47), prevented the accumulation of Gag at intracellular sites in the absence of Vpu in HeLa cells (32). Although our preliminary data indicate that the effect of HLA-DR on viral release was not affected by K44A expression, we did observe a partial reduction of Gag relocalization to intracellular compartments under these conditions (Finzi and Cohen, unpublished), thus suggesting that a pool of Gag retargeted by HLA-DR could be plasma membrane associated. Importantly, however, since Gag relocation to intracellular compartments could not be completely abolished upon K44A expression, this suggests that HLA-DR may also affect the cellular localization of Gag by enhancing its direct targeting to MVB.
Indeed, an alternative but not exclusive model to explain the effect of MHC-II molecules on HIV-1 assembly and budding to intracellular compartments postulates that expression of MHC-II may contribute to the formation or maturation of compartments to which Gag molecules would be targeted. Importantly, expression of MHC-II molecules, such as HLA-DR, in HEK 293 cells was found to be sufficient to induce a MIIC-like structure having a multilamellar and multivesicular morphology and expressing CD63 and Lamp1 (4). Both types of structures were proposed to reflect different maturation states of MIIC (21). One might envision that formation and/or maturation of MIIC-like compartments by MHC-II in HEK 293T cells may provide additional internal membrane platforms toward which Gag can be targeted for assembly and budding. Interestingly, treatment of U1 promonocytic cells with gamma interferon, a strong upregulator of MHC-II expression, was found to significantly increase the redirection of virus assembly from the plasma membrane to intracytoplasmic vesicles (2). More studies are required to fully understand the precise mechanism underlying the effect of MHC-II molecules on HIV-1 assembly and release. Furthermore, experiments aimed at depleting MHC-II molecules are currently in progress to elucidate whether MHC-II influences Gag targeting and assembly to MVB in primary macrophages and as such constitutes a cellular determinant governing HIV-1 production and egress in this cell type.
Acknowledgments
We thank G. Duisit for helpful discussions, as well as A. Robert and A. Vallée for expertise in electron microscopy analysis. We thank J. Gruenberg and J. Neefjes for providing Abs. The polyclonal anti-p24 Ab, IVS, and HeLa-CD4-LTR-β-Gal cells were obtained through the NIH AIDS Research and Reference Reagent Program.
A.F. is recipient of a studentship from a Canadian Institute of Health Research (CIHR) training program in cancer research. E.A.C. and J.T. are recipients of the Canada Research Chair in Human Retrovirology and a Fonds de la Recherche en Santé du Québec (FRSQ) scholarship award, respectively. This work was supported by grants from CIHR and FRSQ to E.A.C. and J.T. and from the FRSQ-AIDS network to E.A.C.
REFERENCES
- 1.Alroy, I., S. Tuvia, T. Greener, D. Gordon, H. M. Barr, D. Taglicht, R. Mandil-Levin, D. Ben-Avraham, D. Konforty, A. Nir, O. Levius, V. Bicoviski, M. Dori, S. Cohen, L. Yaar, O. Erez, O. Propheta-Meiran, M. Koskas, E. Caspi-Bachar, I. Alchanati, A. Sela-Brown, H. Moskowitz, U. Tessmer, U. Schubert, and Y. Reiss. 2005. The trans-Golgi network-associated human ubiquitin-protein ligase POSH is essential for HIV type 1 production. Proc. Natl. Acad. Sci. USA 102:1478-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biswas, P., G. Poli, A. L. Kinter, J. S. Justement, S. K. Stanley, W. J. Maury, P. Bressler, J. M. Orenstein, and A. S. Fauci. 1992. Interferon gamma induces the expression of human immunodeficiency virus in persistently infected promonocytic cells (U1) and redirects the production of virions to intracytoplasmic vacuoles in phorbol myristate acetate-differentiated U1 cells. J. Exp. Med. 176:739-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunet, A., A. Samaan, F. Deshaies, T. J. Kindt, and J. Thibodeau. 2000. Functional characterization of a lysosomal sorting motif in the cytoplasmic tail of HLA-DOβ. J. Biol. Chem. 275:37062-37071. [DOI] [PubMed] [Google Scholar]
- 4.Calafat, J., M. Nijenhuis, H. Janssen, A. Tulp, S. Dusseljee, R. Wubbolts, and J. Neefjes. 1994. Major histocompatibility complex class II molecules induce the formation of endocytic MIIC-like structures. J. Cell Biol. 126:967-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantin, R., J. F. Fortin, G. Lamontagne, and M. Tremblay. 1997. The presence of host-derived HLA-DR1 on human immunodeficiency virus type 1 increases viral infectivity. J. Virol. 71:1922-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantin, R., J. F. Fortin, and M. Tremblay. 1996. The amount of host HLA-DR proteins acquired by HIV-1 is virus strain- and cell type-specific. Virology 218:372-381. [DOI] [PubMed] [Google Scholar]
- 7.Dong, X., H. Li, A. Derdowski, L. Ding, A. Burnett, X. Chen, T. R. Peters, T. S. Dermody, E. Woodruff, J. J. Wang, and P. Spearman. 2005. AP-3 directs the intracellular trafficking of HIV-1 Gag and plays a key role in particle assembly. Cell 120:663-674. [DOI] [PubMed] [Google Scholar]
- 8.Dugast, M., H. Toussaint, C. Dousset, and P. Benaroch. 2005. AP2 clathrin adaptor complex, but not AP1, controls the access of the major histocompatibility complex (MHC) class II to endosomes. J. Biol. Chem. 280:19656-19664. [DOI] [PubMed] [Google Scholar]
- 9.Facke, M., A. Janetzko, R. L. Shoeman, and H. G. Krausslich. 1993. A large deletion in the matrix domain of the human immunodeficiency virus gag gene redirects virus particle assembly from the plasma membrane to the endoplasmic reticulum. J. Virol. 67:4972-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faubert, A., A. Samaan, and J. Thibodeau. 2002. Functional analysis of tryptophans alpha 62 and beta 120 on HLA-DM. J. Biol. Chem. 277:2750-2755. [DOI] [PubMed] [Google Scholar]
- 11.Fredericksen, B. L., B. L. Wei, J. Yao, T. Luo, and J. V. Garcia. 2002. Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus. J. Virol. 76:11440-11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freed, E. O. 1998. HIV-1 gag proteins: diverse functions in the virus life cycle. Virology 251:1-15. [DOI] [PubMed] [Google Scholar]
- 13.Freed, E. O., J. M. Orenstein, A. J. Buckler-White, and M. A. Martin. 1994. Single amino acid changes in the human immunodeficiency virus type 1 matrix protein block virus particle production. J. Virol. 68:5311-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrus, J. E., U. K. von Schwedler, O. W. Pornillos, S. G. Morham, K. H. Zavitz, H. E. Wang, D. A. Wettstein, K. M. Stray, M. Cote, R. L. Rich, D. G. Myszka, and W. I. Sundquist. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107:55-65. [DOI] [PubMed] [Google Scholar]
- 15.Gluschankof, P., and M. Suzan. 2002. HIV-1 gag polyprotein rescues HLA-DR intracellular transport in a human CD4+ cell line. Virology 300:160-169. [DOI] [PubMed] [Google Scholar]
- 16.Gottlinger, H. G. 2001. The HIV-1 assembly machine. Aids 15(Suppl. 5):S13-S20. [DOI] [PubMed] [Google Scholar]
- 17.Gruenberg, J., and H. Stenmark. 2004. The biogenesis of multivesicular endosomes. Nat. Rev. Mol. Cell Biol. 5:317-323. [DOI] [PubMed] [Google Scholar]
- 18.Hermida-Matsumoto, L., and M. D. Resh. 2000. Localization of human immunodeficiency virus type 1 Gag and Env at the plasma membrane by confocal imaging. J. Virol. 74:8670-8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khalil, H., A. Brunet, I. Saba, R. Terra, R. P. Sekaly, and J. Thibodeau. 2003. The MHC class II beta chain cytoplasmic tail overcomes the invariant chain p35-encoded endoplasmic reticulum retention signal. Int. Immunol. 15:1249-1263. [DOI] [PubMed] [Google Scholar]
- 20.Kimpton, J., and M. Emerman. 1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J. Virol. 66:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleijmeer, M. J., S. Morkowski, J. M. Griffith, A. Y. Rudensky, and H. J. Geuze. 1997. Major histocompatibility complex class II compartments in human and mouse B lymphoblasts represent conventional endocytic compartments. J. Cell Biol. 139:639-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi, T., E. Stang, K. S. Fang, P. de Moerloose, R. G. Parton, and J. Gruenberg. 1998. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature 392:193-197. [DOI] [PubMed] [Google Scholar]
- 23.Kramer, B., A. Pelchen-Matthews, M. Deneka, E. Garcia, V. Piguet, and M. Marsh. 2005. HIV interaction with endosomes in macrophages and dendritic cells. Blood Cells Mol. Dis. 35:136-142. [DOI] [PubMed] [Google Scholar]
- 24.Lavallee, C., and E. A. Cohen. 1993. HIV-1 HxBc2 strain encodes a truncated vpr gene product of 78 amino acids. J. Acquir. Immune Defic. Syndr. 6:529-530. [DOI] [PubMed] [Google Scholar]
- 25.Levesque, K., Y. S. Zhao, and E. A. Cohen. 2003. Vpu exerts a positive effect on HIV-1 infectivity by down-modulating CD4 receptor molecules at the surface of HIV-1-producing cells. J. Biol. Chem. 278:28346-28353. [DOI] [PubMed] [Google Scholar]
- 26.Liljedahl, M., T. Kuwana, W. P. Fung-Leung, M. R. Jackson, P. A. Peterson, and L. Karlsson. 1996. HLA-DO is a lysosomal resident which requires association with HLA-DM for efficient intracellular transport. EMBO J. 15:4817-4824. [PMC free article] [PubMed] [Google Scholar]
- 27.Lindwasser, O. W., and M. D. Resh. 2004. Human immunodeficiency virus type 1 Gag contains a dileucine-like motif that regulates association with multivesicular bodies. J. Virol. 78:6013-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marks, M. S., P. A. Roche, E. van Donselaar, L. Woodruff, P. J. Peters, and J. S. Bonifacino. 1995. A lysosomal targeting signal in the cytoplasmic tail of the beta chain directs HLA-DM to MHC class II compartments. J. Cell Biol. 131:351-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCormick, P. J., J. A. Martina, and J. S. Bonifacino. 2005. Involvement of clathrin and AP-2 in the trafficking of MHC class II molecules to antigen-processing compartments. Proc. Natl. Acad. Sci. USA 102:7910-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morita, E., and W. I. Sundquist. 2004. Retrovirus budding. Annu. Rev. Cell Dev. Biol. 20:395-425. [DOI] [PubMed] [Google Scholar]
- 31.Neefjes, J. J., V. Stollorz, P. J. Peters, H. J. Geuze, and H. L. Ploegh. 1990. The biosynthetic pathway of MHC class II but not class I molecules intersects the endocytic route. Cell 61:171-183. [DOI] [PubMed] [Google Scholar]
- 32.Neil, S. J., S. W. Eastman, N. Jouvenet, and P. D. Bieniasz. 2006. HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane. PLoS Pathog. 2:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen, D. G., A. Booth, S. J. Gould, and J. E. Hildreth. 2003. Evidence that HIV budding in primary macrophages occurs through the exosome release pathway. J. Biol. Chem. 278:52347-52354. [DOI] [PubMed] [Google Scholar]
- 34.Nydegger, S., M. Foti, A. Derdowski, P. Spearman, and M. Thali. 2003. HIV-1 egress is gated through late endosomal membranes. Traffic 4:902-910. [DOI] [PubMed] [Google Scholar]
- 35.Ono, A., S. D. Ablan, S. J. Lockett, K. Nagashima, and E. O. Freed. 2004. Phosphatidylinositol(4,5)bisphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc. Natl. Acad. Sci. USA 101:14889-14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ono, A., and E. O. Freed. 2004. Cell-type-dependent targeting of human immunodeficiency virus type 1 assembly to the plasma membrane and the multivesicular body. J. Virol. 78:1552-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ono, A., J. M. Orenstein, and E. O. Freed. 2000. Role of the Gag matrix domain in targeting human immunodeficiency virus type 1 assembly. J. Virol. 74:2855-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orenstein, J. M., M. S. Meltzer, T. Phipps, and H. E. Gendelman. 1988. Cytoplasmic assembly and accumulation of human immunodeficiency virus types 1 and 2 in recombinant human colony-stimulating factor-1-treated human monocytes: an ultrastructural study. J. Virol. 62:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panina-Bordignon, P., X. T. Fu, A. Lanzavecchia, and R. W. Karr. 1992. Identification of HLA-DR alpha chain residues critical for binding of the toxic shock syndrome toxin superantigen. J. Exp. Med. 176:1779-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelchen-Matthews, A., B. Kramer, and M. Marsh. 2003. Infectious HIV-1 assembles in late endosomes in primary macrophages. J. Cell Biol. 162:443-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poon, D. T., L. V. Coren, and D. E. Ott. 2000. Efficient incorporation of HLA class II onto human immunodeficiency virus type 1 requires envelope glycoprotein packaging. J. Virol. 74:3918-3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raposo, G., M. Moore, D. Innes, R. Leijendekker, A. Leigh-Brown, P. Benaroch, and H. Geuze. 2002. Human macrophages accumulate HIV-1 particles in MHC II compartments. Traffic 3:718-729. [DOI] [PubMed] [Google Scholar]
- 43.Robadey, C., W. Ammerlaan, C. Muller, I. Cloutier, R. P. Sekaly, J. A. Haefliger, and S. Demotz. 1997. The processing routes determined by negatively charged residues in DR1-restricted T cell determinants. J. Immunol. 159:3238-3246. [PubMed] [Google Scholar]
- 44.Sanderson, F., M. J. Kleijmeer, A. Kelly, D. Verwoerd, A. Tulp, J. J. Neefjes, H. J. Geuze, and J. Trowsdale. 1994. Accumulation of HLA-DM, a regulator of antigen presentation, in MHC class II compartments. Science 266:1566-1569. [DOI] [PubMed] [Google Scholar]
- 45.Sharova, N., C. Swingler, M. Sharkey, and M. Stevenson. 2005. Macrophages archive HIV-1 virions for dissemination in trans. EMBO J. 24:2481-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sherer, N. M., M. J. Lehmann, L. F. Jimenez-Soto, A. Ingmundson, S. M. Horner, G. Cicchetti, P. G. Allen, M. Pypaert, J. M. Cunningham, and W. Mothes. 2003. Visualization of retroviral replication in living cells reveals budding into multivesicular bodies. Traffic 4:785-801. [DOI] [PubMed] [Google Scholar]
- 47.Sieczkarski, S. B., and G. R. Whittaker. 2002. Dissecting virus entry via endocytosis. J. Gen. Virol. 83:1535-1545. [DOI] [PubMed] [Google Scholar]
- 48.Simonsen, A., A. E. Wurmser, S. D. Emr, and H. Stenmark. 2001. The role of phosphoinositides in membrane transport. Curr. Opin. Cell Biol. 13:485-492. [DOI] [PubMed] [Google Scholar]
- 49.Strack, B., A. Calistri, S. Craig, E. Popova, and H. G. Gottlinger. 2003. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell 114:689-699. [DOI] [PubMed] [Google Scholar]
- 50.Stumptner-Cuvelette, P., M. Jouve, J. Helft, M. Dugast, A. S. Glouzman, K. Jooss, G. Raposo, and P. Benaroch. 2003. Human immunodeficiency virus-1 Nef expression induces intracellular accumulation of multivesicular bodies and major histocompatibility complex class II complexes: potential role of phosphatidylinositol 3-kinase. Mol. Biol. Cell 14:4857-4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von Schwedler, U. K., M. Stuchell, B. Muller, D. M. Ward, H. Y. Chung, E. Morita, H. E. Wang, T. Davis, G. P. He, D. M. Cimbora, A. Scott, H. G. Krausslich, J. Kaplan, S. G. Morham, and W. I. Sundquist. 2003. The protein network of HIV budding. Cell 114:701-713. [DOI] [PubMed] [Google Scholar]
- 52.Yao, X. J., R. A. Subbramanian, N. Rougeau, F. Boisvert, D. Bergeron, and E. A. Cohen. 1995. Mutagenic analysis of human immunodeficiency virus type 1 Vpr: role of a predicted N-terminal alpha-helical structure in Vpr nuclear localization and virion incorporation. J. Virol. 69:7032-7044. [DOI] [PMC free article] [PubMed] [Google Scholar]