Abstract
Objective
To describe the effectiveness and tolerability of mirtazapine, a noradrenergic and specific serotonergic antidepressant, in the open-label treatment of patients with depression who were resistant to other antidepressant agents.
Methods
The charts of 24 patients who met the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, (DSM-IV) criteria for major depressive disorder and were treated with mirtazapine after partial or nonresponse to standard antidepressants were reviewed for clinical response. Outcome was determined by using the Clinical Global Impressions of Improvement (CGI-I) Scale.
Results
Symptomatic improvement was observed in 9 (38;) of 24 patients during an average of 14.1 months of mirtazapine treatment at a mean dose of 36.7 mg/day. Five (21%) patients discontinued mirtazapine because of side effects such as fatigue, weight gain and nausea. Five (21%) patients were receiving combination therapy with another antidepressant when mirtazapine treatment was initiated.
Conclusions
This open-label study suggests that a subgroup of patients with treatment-resistant depression may benefit from mirtazapine treatment. Further controlled studies are required to demonstrate the efficacy of mirtazapine in treatment-resistant depression.
Medical subject headings: antidepressive agents, chronic disease, depressive disorder, drug resistance, drug toxicity, treatment outcome
Abstract
Objectif
Décrire l'efficacité et la tolérabilité de la mirtazapine, antidépresseur noradrénergique et sérotoninergique spécifique, dans un traitement avec étiquetage en clair administré à des patients atteints de dépression qui ne répondaient pas à d'autres antidépresseurs.
Méthodes
Afin d'établir la réponse clinique, on a examiné les dossiers de 24 patients qui satisfaisaient aux critères de trouble dépressif majeur de la quatrième édition du Manuel diagnostique et statistique des troubles mentaux (DSM-IV) et qui ont reçu un traitement faisant appel à la mirtazapine après ne pas avoir répondu à des antidépresseurs courants ou n'avoir présenté qu'une réponse partielle. Le résultat a été établi au moyen de l'échelle des impressions globales cliniques de l'amélioration (CGI-I).
Résultats
Au cours d'un traitement d'une durée moyenne de 14,1 mois qui faisait appel à l'administration de 36,7 mg de mirtazapine par jour, en moyenne, on a observé l'amélioration des symptômes de neuf (38 %) des 24 patients. Cinq (21 %) des patients ont cessé de prendre le médicament à cause d'effets secondaires comme la fatigue, la prise de poids et les nausées. Cinq (21 %) des patients recevaient un traitement faisant appel à un autre antidépresseur au moment où le traitement par mirtazapine a été entrepris.
Conclusions
Cet essai ouvert indique qu'un sous-groupe de patients atteints de dépression réfractaire pourraient profiter de la mirtazapine. Il faudra effectuer d'autres études contrôlées pour démontrer l'efficacité de la mirtazapine dans le traitement de la dépression réfractaire.
Introduction
Despite the proven effectiveness of many antidepressants, some patients have depressive episodes that are resistant to antidepressant treatment. It is well recognized that up to 50% of depressed patients have either partial or no response to the first antidepressant they receive.1,2,3 Furthermore, as many as 20% of patients have chronic courses, remaining depressed long after the onset of illness despite multiple interventions.4,5 Currently, there is no generally accepted treatment algorithm for treatment-resistant depression (TRD).6 Optimizing antidepressant use by ensuring that patients receive an adequate dose for an adequate length of time is usually the first recommended strategy for managing poor response.7 Beyond optimization, however, there is limited evidence to guide clinical decisions in managing TRD. Medication strategies include augmenting the antidepressant with a medication that does not have an antidepressant effect itself (e.g., lithium or triiodothyronine), combining with another recognized antidepressant or switching to another antidepressant.7
Mirtazapine is a novel antidepressant in a new class referred to as the noradrenergic and specific serotonergic antidepressants. It enhances both central noradrenergic and serotonergic neurotransmission by directly inhibiting noradrenergic α2-autoreceptors and α2-heteroreceptors.8 It is highly specific, with no effect on monoamine reuptake and a relatively low affinity for dopaminergic receptors and some serotonergic receptor subtypes.9 Mirtazapine also selectively inhibits specific postsynaptic 5-HT2 and 5-HT3 receptors and histamine-H1 receptors,8 and this contributes to its favourable tolerability profile.
Treatment strategies involving novel mechanism antidepressants appear to be increasingly used for the management of TRD.10 Mirtazapine is a likely candidate because it has been shown to be as effective as amitriptyline in the treatment of severely depressed patients,11 provide a more rapid onset of action than citalopram12 and lead to less adverse events than venlafaxine.13 The objective of this naturalistic and retrospective review is to present additional data on the use of mirtazapine in patients who are resistant to antidepressant monotherapy.
Methods
We reviewed the medical charts of consecutive psychiatric patients at the University of British Columbia Hospital who were treated with mirtazapine under the Emergency Drug Release Program of the Therapeutic Products Programme Branch of Health Canada between May 1996 and June 2001. Institutional review board approval was obtained for the chart review. Diagnoses were made by attending physicians according to criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), and only patients with a primary diagnosis of major depressive disorder who previously failed to adequately respond to more than 1 class of antidepressants (i.e., tricyclic antidepressants and selective serotonin reuptake inhibitors) given at the highest tolerable dose for at least 8 weeks or longer, were eligible for mirtazapine use. Clinical and demographic information extracted from the charts included age, sex, diagnosis, details regarding patients' past and present depressive episodes, evidence of inadequate response to standard antidepressants before mirtazapine use, previous trials of electroconvulsive therapy (ECT) and augmentation, dose and duration of mirtazapine use, and any adverse events or reason for discontinuation of treatment. Concomitant medications were allowed. Therapeutic outcome was evaluated by reviewing the medical records of the patients' change from baseline in core depressive symptoms. On the basis of the results of the review, response to treatment was assessed retrospectively according to the Clinical Global Impression of Improvement (CGI-I) Scale as follows: 1 = very much improved, 2 = much improved, 3 = minimally improved, 4 = unchanged, 5 = minimally worse, 6 = much worse and 7 = very much worse. The statistical method included simple descriptive statistics and, where applicable, univariate analyses, chi-square test, Mann-Whitney U test, and Student's t-test. Unless otherwise stated, a 5% significance level was used with 2-tailed tests. All data are reported as means and standard deviations (SD).
Results
We identified 29 patients who met the initial inclusion criteria; 5 were excluded because of insufficient data regarding treatment and response, leaving 24 (17 inpatients, 7 outpatients) for further analysis. The sample consisted of 17 women and 7 men (mean age 45 yr, standard deviation [SD] 16 yr; range 20–81 yr). All patients had experienced previous episodes of depression. The duration of their current major depressive episode at the time of mirtazapine initiation ranged from 2 months to 11 years (mean 3 yr, > 6 mo in 18 cases); 10 patients had comorbid axis II diagnoses. The mean daily dose of mirtazapine was 38 mg/day (SD 17 mg/d), the range was 15–90 mg/day and the average duration of treatment was 7 months (SD 10 mo). Seventeen (71%) of the 24 patients remained on mirtazapine for 2 months or longer, and 5 patients had mirtazapine combined with another antidepressant (Table 1).
Table 1
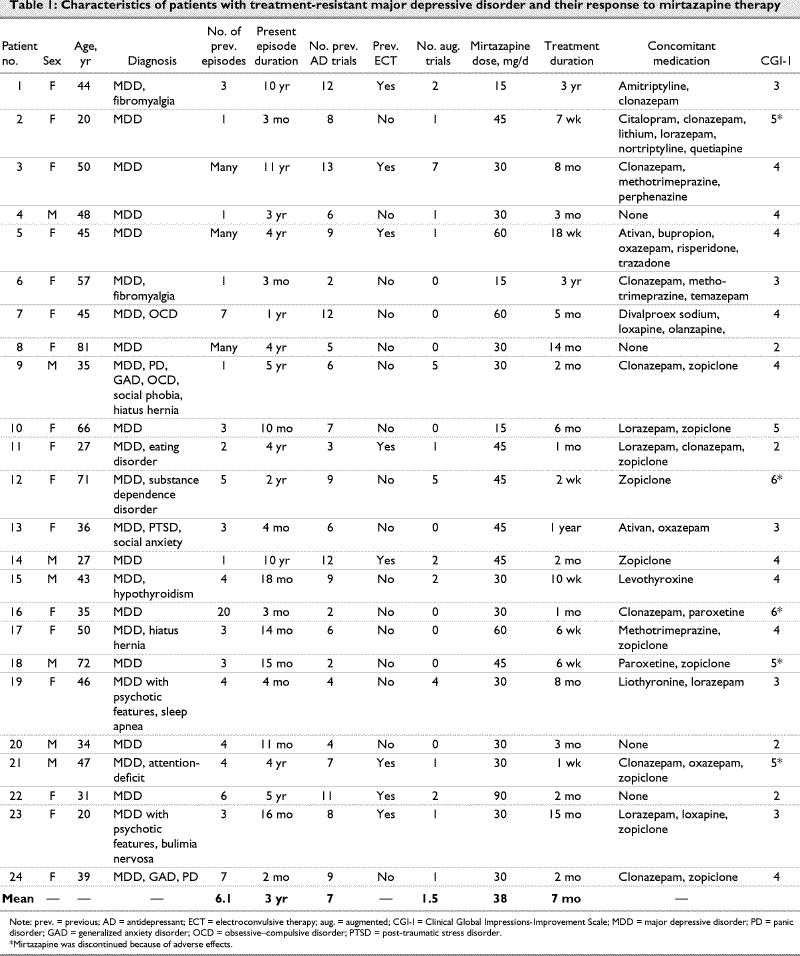
Positive response (i.e., ≤ 2 on the CGI-I at discharge or follow-up) or partial response (i.e., 3 on the CGI-I at discharge or follow-up) was observed in 9 (38%) patients. Only 1 of 9 was on combination mirtazapine; the rest were on mirtazapine as their only antidepressant. Ten (42%) patients did not respond to mirtazapine, despite an average trial duration of 3.7 months. As compared with responders, they were similar in age (t = –0.65, p = 0.53), duration of present episode (1-tailed test, U = 67, p = 1.0), number of previous antidepressant trials (t = –1.20, p = 0.68), previous ECT (χ2 = 2.67, p = 0.10) and augmentation trials (1-tailedtest, U = 59, p = 0.64) and dose (t = –0.32, p = 0.39). The only significant difference was in the duration of treatment (1-tailed test, U = 25, p < 0.05), which was expected because responders generally remain on a successful treatment. At the time of writing, 8 of the 9 original responders were still on maintenance treatment with mirtazapine (duration range 2–36 mo), at an average maintenance dose of 36.7 mg/day. For patients with axis II comorbidity, the response rate was 40% (4 of 10), which is similar to that for the entire sample. Five patients discontinued mirtazapine due to adverse effects, including fatigue (n = 3), sedation (n = 3), weight gain, nausea, dizziness, anergia and mild gastrointestinal discomfort.
Discussion
This study has a number of limitations. The sample is small, and the assessment of response was open, retrospective and uncontrolled, leaving the possibility of rater bias. The study was done in a naturalistic setting, complicated by the use of concomitant treatments (i.e., antidepressant combinations). Additionally, the clinical profiles of patients were diverse, with a high rate of axis II pathology.
Notwithstanding these limitations, mirtazapine was well tolerated in these 24 patients with TRD, with 4 (16%) displaying significant symptomatic improvement and another 5 (22%) showing enough improvement to continue taking the medication. This is clinically significant because this is such a refractory group of patients.
This response rate is somewhat less than a previously reported rate of 48% in a study of outpatients who did not respond to fluoxetine, paroxetine or sertraline for at least 4 weeks at standard doses and then were subsequently switched to open-label mirtazapine at a flexible dose (i.e, 15 mg/d to 45 mg/d).14 After 8 weeks, of the 94 patients in the intent-to-treat group, 48% (n = 43) responded to treatment (≥ 50% reduction in the 17-item Hamilton Depression Rating Scale scores). Twenty-six (25%) patients discontinued because of adverse events; the most commonly reported symptoms leading to discontinuation were somnolence (50%), increased appetite (30%), headache (29%), weight gain (23%), dizziness (21%) and nervousness (20%).14 In another study, mirtazapine was used in combination with other antidepressants in patients with TRD.15 Twenty patients failing to achieve adequate response to at least 4 weeks of treatment with high doses of standard antidepressants had open-label mirtazapine added. At 4 weeks, 11 (55%) patients were rated as responders, and only 3 (15%) discontinued due to side effects.15
The difference in response rates among studies may be explained by differences in the severity of TRD. In the present sample, there was a high proportion (71%) of inpatients with chronic symptoms. These patients had a long mean duration of current episode (i.e., 3 yr) and had failed many previous adequate antidepressant trials (mean = 7). Furthermore, fewer patients were on combination antidepressant treatment. Despite this lower response rate, a high proportion of responders (89%) sustained their improvement over follow-up, some for as long as 3 years, suggesting the possibility of mirtazapine use in maintenance-phase treatment of TRD.
Consistent with other reports,13,14,15 mirtazapine was generally well tolerated in our sample, even though many patients were taking concurrent medications. Irrespective of the clinical improvement, certain side effects were prominent enough to warrant treatment discontinuation in 5 (21%) cases. Fatigue (n = 3) was the most frequently reported side effect in these patients, followed by weight gain and nausea (n = 2).
In summary, mirtazapine appears to be beneficial in a subgroup of patients with TRD. Preliminary clinical data suggest that it is safe and well tolerated, and it may be effective in relapse prevention and maintenance of initial response. Further prospective, controlled trials are indicated to clarify the efficacy of mirtazapine in TRD.
Footnotes
Competing interests: None declared for Drs. Kundhur and Solomons. Dr. Yatham sits on advisory and speaker boards of and has received research grants from Eli Lilly Canada, Janssen Canada, AstraZeneca Canada and GlaxoSmithKline US. He has also received research grants from Bristol-Myers Squibb US. Dr. Lam sits on the advisory and speaker boards of Cyberonics, Inc., Eli Lilly Canada, GlaxoSmithKline, Litebook Company Ltd., Lundbeck Canada, Organon Canada, Pfizer Canada and Wyeth-Ayerst Canada and has received research grants from AstraZeneca Canada, Eli Lilly Canada, Merck-Frosst Canada, Roche Canada and Servier Canada.
Correspondence to: Dr. Raymond W. Lam, Department of Psychiatry, University of British Columbia, 2255 Wesbrook Mall, Vancouver BC V6T 2A1; fax 604 822-7922; r.lam@ubc.ca
Submitted Dec. 1, 2001 Revised May 29, 2002 Accepted Jul. 24, 2002
References
- 1.Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am 1996;19:179-200. [DOI] [PubMed]
- 2.Nierenberg AA, Amsterdam JD. Treatment-resistant depression: definition and treatment approaches. J Clin Psychiatry 1990;51(Suppl):39-47. [PubMed]
- 3.Burrows GD, Norman TR, Judd FK. Definition and differential diagnosis of treatment-resistant depression. Int Clin Psychopharmacol 1994;9(Suppl 2):5-10. [DOI] [PubMed]
- 4.Keller MB, Klerman GL, Lavori PW, Coryell W, Endicott J, Taylor J. Long-term outcome of episodes of major depression. Clinical and public health significance. JAMA 1984;252:788-92. [PubMed]
- 5.Paykel ES. Epidemiology of refractory depression. In: Nolen WA, Zohar J, Roose SP, Amsterdam JD, editors. Refractory depression: current strategies and future directions. New York: John Wiley and Sons; 1994. p. 3-18.
- 6.Nierenberg AA. Methodological problems in treatment resistant depression research. Psychopharmacol Bull 1990;26:461-4. [PubMed]
- 7.Kennedy SH, Lam RW, Cohen NL, Ravindran AV. Clinical guidelines for the treatment of depressive disorders. IV. Medications and other biological treatments. Can J Psychiatry 2001;46(Suppl 1):38S-58S. [PubMed]
- 8.de Boer TH, Maura G, Raiteri M, de Vos CJ, Wieringa J, Pinder RM. Neurochemical and autonomic pharmacological profiles of the 6-aza-analogue of mianserin, Org 3770 and its enantiomers. Neuropharmacology 1988;27:399-408. [DOI] [PubMed]
- 9.de Boer T. The effects of mirtazapine on central noradrenergic and serotonergic neurotransmission. Int Clin Psychopharmacol 1995;10(Suppl 4):19-23. [DOI] [PubMed]
- 10.Mitchell PB, Schweitzer I, Burrows G, Johnson G, Polonowita A. Efficacy of venlafaxine and predictors of response in a prospective open-label study of patients with treatment-resistant major depression. J Clin Psychopharmacol 2000;20:483-7. [DOI] [PubMed]
- 11.Kasper S, Zivkov M, Roes KC, Pols AG. Pharmacological treatment of severely depressed patients: a meta-analysis comparing efficacy of mirtazapine and amitriptyline. Eur Neuropsychopharmacol 1997;7:115-24. [DOI] [PubMed]
- 12.Leinonen E, Skarstein J, Behnke K, Agren H, Helsdingen JT. Efficacy and tolerability of mirtazapine versus citalopram: a double-blind, randomized study in patients with major depressive disorder. Nordic Antidepressant Study Group. Int Clin Psychopharmacol 1999;14:329-37. [DOI] [PubMed]
- 13.Guelfi JD, Ansseau M, Timmerman L, Korsgaard S. Mirtazapine versus venlafaxine in hospitalized severely depressed patients with melancholic features. J Clin Psychopharmacol 2001;21:425-31. [DOI] [PubMed]
- 14.Fava M, Dunner DL, Greist JH, Preskorn SH, Trivedi MH, Zajecka J, et al. Efficacy and safety of mirtazapine in major depressive disorder patients after SSRI treatment failure: an open-label trial. J Clin Psychiatry 2001;62:413-20. [DOI] [PubMed]
- 15.Carpenter LL, Jocic Z, Hall JM, Rasmussen SA, Price LH. Mirtazapine augmentation in the treatment of refractory depression. J Clin Psychiatry 1999;60:45-9. [DOI] [PubMed]


