Abstract
We evaluated three nonreplicating dengue virus type 2 (DENV-2) vaccines: (i) a DNA vaccine containing the prM-E gene region (D), (ii) a recombinant subunit protein vaccine containing the B domain (i.e., domain III) of the E protein as a fusion with the Escherichia coli maltose-binding protein (R), and (iii) a purified inactivated virus vaccine (P). Groups of four rhesus macaques each were primed once and boosted twice using seven different vaccination regimens. After primary vaccination, enzyme-linked immunosorbent assay (ELISA) antibody levels increased most rapidly for groups inoculated with the P and DP combination, and by 1 month after the second boost, ELISA titers were similar for all groups. The highest plaque reduction neutralization test (PRNT) titers were seen in those groups that received the DR/DR/DR combination (geometric mean titer [GMT], 510), the P/P/P vaccine (GMT, 345), the DP/DP/DP combination (GMT, 287), and the R/R/R vaccine (GMT, 200). The next highest titers were seen in animals that received the D/R/R vaccine (GMT, 186) and the D/P/P vaccine (GMT, 163). Animals that received the D/D/D vaccine had the lowest neutralizing antibody titer (GMT, 49). Both ELISA and PRNT titers declined at variable rates. The only significant protection from viremia was observed in the P-vaccinated animals (mean of 0.5 days), which also showed the highest antibody concentration, including antibodies to NS1, and highest antibody avidity at the time of challenge.
Dengue is a mosquito-transmitted viral disease of global importance. It is caused by four antigenically related but distinct dengue virus (DENV) serotypes (family Flaviviridae, genus Flavivirus, species Dengue virus) estimated to cause up to 100 million infections annually. Infection with any serotype can produce a spectrum of disease that ranges from asymptomatic or mild febrile illness to the more severe dengue hemorrhagic fever/dengue shock syndrome (17). There are no licensed vaccines available for dengue. The leading candidates are live-attenuated virus (LAV) vaccines made by cell culture passage of natural virus isolates or by genetic manipulation of infectious DENV cDNA clones. However, it has proven difficult to identify LAV vaccine candidates that are highly immunogenic and at the same time sufficiently attenuated (2, 11, 23, 30). Some alternatives being explored are purified inactivated whole virus (PIV), recombinant subunit protein, and nucleic acid-based vaccines (19, 20, 26).
Nonreplicating vaccines such as PIV and recombinant subunit protein vaccines mainly elicit humoral immune responses but they may not be as effective at stimulating cell-mediated immune responses, which require intracellular antigen processing and presentation. Nucleic acid (DNA) vaccines, which express antigen-coding sequences intracellularly, may be more effective at eliciting cell-mediated immune responses. However, most dengue virus DNA vaccines tested thus far elicit only moderate neutralizing antibody titers in mice and rhesus macaques. Several reports have shown that the use of a combination DNA vaccine together with a recombinant subunit protein vaccine in a prime-boost strategy can induce higher antibody titers than either vaccine alone (1, 25, 28). Our previous studies demonstrated that priming and boosting with a combination DENV-2 DNA and DENV-2 recombinant subunit protein vaccine can generate higher titers of long-lasting neutralizing antibodies in mice (27), with the highest neutralizing antibody titers seen in animals that received both vaccines simultaneously. Combining different types of vaccines should increase the complexity and perhaps the effectiveness of the immune response. The order in which the vaccines are administered might also be important, and a prime-boost strategy might be used to moderate the reactogenicity of LAV vaccines.
In the present study we used a rhesus macaque animal model to evaluate the immunogenicity and protective efficacy of various vaccine combinations in a prime-boost vaccination approach, using a DENV-2 DNA vaccine expressing the prM and E genes (D), a recombinant fusion protein containing 103 amino acids of the B domain of the DENV-2 envelope protein fused to the maltose-binding protein of Escherichia coli (R), and a DENV-2 PIV vaccine (P) (19, 20, 26).
MATERIALS AND METHODS
Virus.
Cell culture supernatant harvested from Vero cells infected with DENV-2 (S16803) was used as virus stock for the plaque reduction neutralization test (PRNT) and to prepare antigen for the enzyme-linked immunosorbent assay (ELISA).
Plaque reduction neutralization assay.
PRNTs were performed as previously described (22). Vero cell monolayers were seeded in six-well plates (Falcon; Becton Dickinson, Lincoln Park, NJ) and incubated at 37°C in a CO2 incubator. Sera from immunized rhesus macaques were tested using twofold dilutions starting at 1:10. Plaques were visualized on day 6 by staining with 0.02% neutral red in Hank's balanced salt solution. Each rhesus macaque serum was tested in duplicate, and the number of plaques reported was the average of the two determinations. The percent reduction of plaques was calculated by comparison of the results obtained with sera from unimmunized rhesus macaques, and the dilution at which a 50% plaque reduction occurred (PRNT50 titer) was determined by probit analysis.
Dengue virus challenge and assay for viremia.
Vaccinated and control rhesus macaques were challenged by subcutaneous injection with 10,000 PFU of DENV-2 strain S16803. Sera were collected daily after virus challenge for 10 consecutive days. Virus was detected by incubating the sera on Vero cell monolayers to amplify any virus present (amplified assay). Briefly, Vero cells were grown as monolayers in 25-cm2 flasks (T25) with Eagle's minimal essential medium, nonessential amino acids (BioWhittaker), 10% heat-inactivated fetal bovine serum, and penicillin-streptomycin. Duplicate flasks were inoculated with 0.3 ml of 1:2-diluted postchallenge serum samples and incubated at 37°C for 14 days, with a medium change on day 7. Cells from each flask were harvested, washed with phosphate-buffered saline (PBS), and spotted in duplicate onto immunofluorescence slides. The presence of DENV-2 was assessed by using DENV-2-specific monoclonal antibody 3H5 as well as hyperimmune ascitic fluid and fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin (Ig). The fluorescence was rated as positive or negative compared to uninfected control cells. Virus infectivity titers were also determined by plaque assay of sera directly on Vero cell monolayers.
Preparation of plasmid DNA.
The DNA vaccine (D) was prepared at Powderject, Inc., using a DENV-2 prM-E gene insert (New Guinea C) provided by WRAIR (18). The DENV-2 genes were cloned into a proprietary plasmid vector under the control of the cytomegalovirus immediate-early promoter. The insides of “shot” tubes were coated with the purified plasmid for delivery by gene gun. A single shot tube was formulated to contain 250 ng of DNA.
Preparation of purified inactivated vaccine.
The purified inactivated vaccine (P) was prepared from DENV-2 (S16803) grown in Vero cells. The virus was concentrated by ultrafiltration, purified on a sucrose gradient, and inactivated with formalin as previously described (19).
Preparation of recombinant fusion protein.
The preparation of the DENV-2-maltose-binding protein fusion protein was previously described (26). Briefly, a gene fragment encoding amino acids 298 to 400 (B domain or domain III) of the DENV-2 (New Guinea C) envelope protein was expressed as a fusion protein with the maltose-binding protein of Escherichia coli. The fusion protein was purified by amylose affinity chromatography and polyacrylamide gel electrophoresis.
Enzyme-linked immunosorbent assay.
Antigen was prepared by centrifugation (27,000 rpm at 20°C) of DENV-2-infected and uninfected Vero cells for 2 h. Pellets were resuspended in PBS and pelleted again in 10% glycerol at 32,000 rpm for 2 h at 20°C. Purified virions and control antigen were resuspended in PBS and stored at −20°C until used. The analysis of sera from immunized rhesus macaques for DENV-2 antibodies was carried out as previously described (27). Briefly, microtiter plate wells were coated with purified DENV-2 virions in PBS at 4°C overnight, followed by blocking with 5% nonfat dry milk in PBS-0.01% Tween 20 for 1 h at 37°C. Plates were then incubated with the test sera diluted 1:100 in blocking buffer for 1 h at 37°C. The secondary antibody was peroxidase-conjugated goat anti-human IgG (Kirkegaard & Perry, Gaithersburg, MD) diluted in blocking solution and incubated for 1 h at 37°C. For the NS1 ELISA, purified recombinant NS1 protein was acquired from Hawaii Biotech Inc. (Aiea, Hawaii) and used as described above.
For the antibody avidity assay, twofold serial dilutions of the test sera starting at 1:100 were added in duplicate to antigen-coated microtiter plates. After 1 h at 37°C, plates were washed, 100 μl of a 1.5 M sodium thiocyanate (NaSCN) solution in PBS was added to one-half of the plates, and plain PBS was added to the other half. Plates were incubated for 15 min at 37°C and washed, and the assay was then continued as described. The 2.2′-azino-di[3-ethyl-benzthiazoline sulfonate (6)] (ABTS) peroxidase substrate system (Kirkegaard & Perry) was used to visualize DENV-2-specific antibody.
For the indirect ELISA, assays were performed in duplicate with a positive and negative control on every plate. The net optical density (OD) values were determined by subtracting the absorbance of test serum with negative control antigen from the absorbance of test serum with the DENV-2 antigen. The cutoff value for seropositivity was set at ≥0.10, since the mean adjusted OD plus 3 standard deviations for negative control sera was consistently below this value. For the avidity analysis, OD values of both curves were plotted against the dilution to determine the optical density of the treated assay mixture corresponding to an OD value at 1.5 (50% of the maximal optical density) of the untreated assay mixture. This allowed for the determination of avidity results at the same IgG concentration in each sample. The avidity index (AI) was the percentage of antibodies that remained bound at the antigen coat after treatment with sodium thiocyanate. Percent avidity was calculated by dividing the OD of the NaSCN-treated samples by the OD of the samples not treated with NaSCN [(OD of DENV-2 with NaSCN treatment − OD of control antigen with NaSCN treatment)/(OD of DENV-2 without NaSCN treatment − OD of control antigen without NaSCN treatment)]. A positive control sample was run in every avidity assay to monitor assay-to-assay variability. High avidity was interpreted as an avidity index of >50, and low avidity was an avidity index of <30. To eliminate the possibility of strain differences influencing ELISA titers, we prepared antigens from both virus strains using the same method. An assay was performed on the same plate in replicate wells coated with equal concentrations of both antigens to allow for a direct comparison. Sera of groups P/P/P and D/D/D from the day of challenge were diluted to achieve end points and assayed with both antigens. There was no difference in reactivity between the S16803 and NGC strains.
Monkey immunizations.
Groups of Indian origin rhesus macaques (Macaca mulatta) were inoculated with 2 μg (eight inoculations of 0.25 μg) of the DENV-2 DNA vaccine (D), 6 μg of DENV-2 purified inactivated vaccine (P), or 500 μg of DENV-2 recombinant subunit vaccine (R) on days 0, 30, and 60. The protein concentration, antigen content, and degree of purity for each vaccine preparation used in this experiment were previously determined. The doses selected for each vaccine were established empirically, based upon their immunogenicity in rhesus macaques. The DNA vaccine was administered intradermally by gene gun at eight inoculation sites in the abdominal and groin regions. The protein vaccines were adsorbed on 0.1% aluminum hydroxide (alum) and administered intramuscularly by needle and syringe in the upper arm (deltoid). Monkeys were primed with either D, P, or R, or DNA and recombinant protein given simultaneously (DP and DR) and then boosted twice with either the P (D/P/P or P/P/P), R (D/R/R or R/R/R), or D (D/D/D) vaccines, or with the D/P (DP/DP/DP) or DR (DR/DR/DR) combination vaccines. Monkeys were bled before each vaccination and at monthly intervals thereafter. Sera were assayed for DENV-2 antibodies by ELISA and PRNT. Five months after the final immunization the animals were challenged with live wild-type DENV-2 (S16803) and then bled daily for 10 days to measure viremia and then on days 14, 21, and 28 postchallenge to monitor anamnestic antibody responses and affinity maturation.
Statistical analysis.
Data were entered into Microsoft Excel (Microsoft Office 2003; Microsoft, Inc., Redmond, Wash.) and analyzed with Minitab (version 13; Minitab, Inc., State College, Penn.). Tables of summary descriptive statistics and graphical displays were constructed to summarize the magnitude of immunity and protective efficacy against experimental challenge. In order to summarize data collected on individuals over time, the area under the response curve was calculated for each subject. Analysis of variance (ANOVA) for a single-factor experiment (vaccine regimen) having serial measurements was employed on this summary measure for outcome variables describing antibody response among vaccine combinations. Logarithmic transformations of the reciprocal PRNT50 titers of the rhesus macaques in each immunization group were made to stabilize variances. Tukey's simultaneous test was used to perform pair-wise comparisons at a family error rate of 0.05. The mean log titers and avidity indices were converted to geometric mean titers (GMT) for presentation. Using the direct plaque assay to measure virus from serum (log10 PFU/ml), the area under the curve for these serial measurements was computed for each animal. For the purpose of calculation, animals with no viremia detectable by direct plaque assay titer but viremia positive by virus isolation assay were assigned a log PFU titer of 0.5.
The research protocol using animals in this study was reviewed and approved by the Naval Medical Research Center's Animal Care and Use Committee according to the principles set forth in the Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animals, National Research Council (17a).
RESULTS
Serum antibody responses in rhesus macaques.
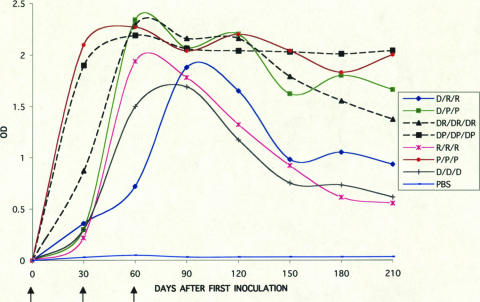
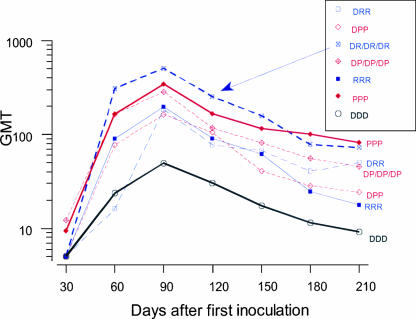
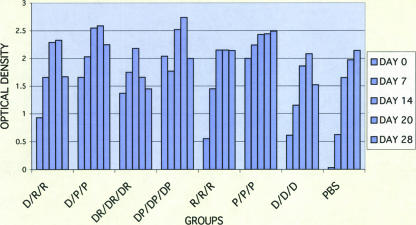
Animals inoculated with seven different combinations of D, P, and R vaccines exhibited IgG antibody responses against DENV-2 in an ELISA using purified virions as antigen (Fig. 1). Following primary vaccination, the ELISA titers increased most rapidly for the groups inoculated with preparations containing PIV (P and DP). One month after the third inoculation, the ELISA titers (reported as OD units) were similar for all vaccinated groups. This was followed by a variable decline in titers that was most marked for the R/R/R, D/D/D, and D/R/R groups. In the groups that received the PIV vaccine alone (P/P/P) or PIV in combination with DNA (DP/DP/DP), only two of four animals exhibited low-titer neutralizing antibodies after the first dose (Table 1), whereas no neutralizing antibody was detected with the other vaccine combinations at this early time point. One month after the third dose all vaccine recipients, regardless of regimen, demonstrated neutralizing antibodies, with the highest titers in groups that received combination DNA and protein vaccines (DR/DR/DR GMT, 510; DP/DP/DP GMT, 287), followed by groups that received protein vaccines alone (P/P/P GMT, 345; R/R/R GMT, 200), or DNA followed by protein vaccine (D/R/R GMT, 186; D/P/P GMT, 163). The group that received DNA vaccine alone (D/D/D) exhibited the lowest neutralizing antibody titer (GMT, 49). Following the course of vaccinations, all animals exhibited significant declines in PRNT titers. Figure 2 summarizes the antibody profiles over time for the seven regimens. The effect of vaccine type was significant by ANOVA (F [6, 21] = 3.26; P = 0.02). The serial measurements of neutralizing antibody responses among vaccine groups were summarized by calculating the area under the curve, and differences were assessed by analysis of variance. The only significant differences were seen between D/D/D and P/P/P (Tukey's test, P = 0.03) and D/D/D and DR/DR/DR (Tukey's test, P = 0.01). These significant pair-wise differences measured on day 90 were maintained until the day of challenge.
FIG. 1.
Serum IgG antibody response in rhesus macaques immunized with combinations of the DENV-2 DNA vaccine (D), DENV-2 PIV vaccine (P), and DENV-2 recombinant vaccine (R) in the ELISA using purified virions. Arrows indicate days of inoculation. The results are given as the means of the OD405 values for sera diluted 1:100.
TABLE 1.
Neutralizing antibody responses in rhesus monkeys following vaccination with combinations of the DENV-2 DNA, PIV, and recombinant vaccinesa
| Group | Monkey | PRNT50 titer (day after first inoculation)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 | 60 | 90 | 120 | 150 | 180 | 210c | 224 | ||
| D/R/R | 942 | <1:10 | 1:10 | 1:170 | 1:60 | 1:100 | 1:50 | 1:50 | 1:17,408 |
| 898 | <1:10 | 1:270 | 1:770 | 1:320 | 1:225 | 1:175 | 1:160 | 1:57,344 | |
| WTV | <1:10 | <1:10 | 1:150 | 1:45 | 1:30 | 1:15 | 1:30 | 1:8,705 | |
| XJX | <1:10 | <1:10 | 1:60 | 1:40 | 1:30 | 1:20 | 1:25 | 1:7,168 | |
| GMT | 1:5b | 1:16 | 1:186 | 1:77 | 1:67 | 1:40 | 1:50 | 1:15,849 | |
| D/P/P | 992 | <1:10 | 1:55 | 1:160 | 1:105 | 1:45 | 1:45 | 1:40 | 1:36,864 |
| VFE | <1:10 | 1:150 | 1:175 | 1:110 | 1:30 | 1:20 | 1:13 | 1:49,152 | |
| VAP | <1:10 | 1:40 | 1:320 | 1:105 | 1:50 | 1:35 | 1:25 | 1:30,720 | |
| XCW | <1:10 | 1:110 | 1:80 | 1:100 | 1:40 | 1:20 | 1:25 | 1:19,456 | |
| GMT | 1:5 | 1:78 | 1:163 | 1:105 | 1:41 | 1:28 | 1:24 | 1:32,359 | |
| DR/DR/DR | A25 | <1:10 | 1:480 | 1:960 | 1:290 | 1:210 | 1:135 | 1:95 | 1:15,456 |
| 939 | <1:10 | 1:830 | 1:480 | 1:320 | 1:225 | 1:130 | 1:160 | 1:30,720 | |
| 981 | <1:10 | 1:145 | 1:510 | 1:210 | 1:110 | 1:60 | 1:35 | 1:17,410 | |
| 973 | <1:10 | 1:160 | 1:290 | 1:210 | 1:120 | 1:35 | 1:50 | 1:16,384 | |
| GMT | 1:5 | 1:310 | 1:510 | 1:253 | 1:158 | 1:78 | 1:72 | 1:19,165 | |
| DP/DP/DP | 975 | 1:30 | 1:300 | 1:580 | 1:145 | 1:135 | 1:95 | 1:40 | 1:36,864 |
| 895 | <1:10 | 1:70 | 1:70 | 1:35 | 1:30 | 1:30 | 1:35 | 1:28,672 | |
| 921 | <1:10 | 1:100 | 1:290 | 1:80 | 1:40 | 1:25 | 1:25 | 1:17,410 | |
| JWK | 1:30 | 1:350 | 1:580 | 1:450 | 1:270 | 1:130 | 1:120 | 1:32,768 | |
| GMT | 1:12 | 1:165 | 1:287 | 1:115 | 1:81 | 1:55 | 1:45 | 1:28,022 | |
| R/R/R | 995 | <1:10 | 1:65 | 1:70 | 1:30 | 1:40 | 1:10 | <1:10 | 1:7,680 |
| C67 | <1:10 | 1:160 | 1:240 | 1:110 | 1:80 | 1:40 | 1:30 | 1:6,656 | |
| C51 | <1:10 | 1:160 | 1:510 | 1:145 | 1:70 | 1:35 | 1:35 | 1:53,250 | |
| B27 | <1:10 | 1:40 | 1:180 | 1:135 | 1:65 | 1:25 | 1:18 | 1:9,216 | |
| GMT | 1:5 | 1:90 | 1:200 | 1:90 | 1:62 | 1:24 | 1:18 | 1:12,589 | |
| P/P/P | A79 | <1:10 | 1:240 | 1:290 | 1:135 | 1:130 | 1:90 | 1:65 | 1:22,528 |
| A43 | 1:15 | 1:240 | 1:300 | 1:110 | 1:80 | 1:90 | 1:70 | 1:40,960 | |
| A65 | 1:20 | 1:100 | 1:770 | 1:290 | 1:175 | 1:160 | 1:135 | 1:45,055 | |
| C49 | <1:10 | 1:135 | 1:210 | 1:175 | 1:95 | 1:80 | 1:70 | 1:45,056 | |
| GMT | 1:9 | 1:167 | 1:345 | 1:165 | 1:114 | 1:100 | 1:81 | 1:36,728 | |
| D/D/D | 931 | <1:10 | 1:270 | 1:220 | 1:225 | 1:150 | 1:135 | 1:55 | 1:36,864 |
| GZ | <1:10 | <1:10 | 1:45 | 1:25 | 1:10 | <1:10 | <1:10 | 1:28,672 | |
| F483 | <1:10 | 1:15 | 1:15 | 1:10 | <1:10 | <1:10 | <1:10 | 1:5,632 | |
| 28R | <1:10 | 1:15 | 1:40 | 1:15 | 1:12 | <1:10 | <1:10 | 1:24,576 | |
| GMT | 1:5 | 1:24 | 1:49 | 1:30 | 1:17 | 1:11 | 1:9 | 1:19,611 | |
| PBS | 887 | <1:10 | <1:10 | <1:10 | <1:10 | <1:10 | <1:10 | <1:10 | 1:1,280 |
| C07 | <1:10 | <1:10 | <1:10 | <1:10 | <1:10 | <1:10 | <1:10 | 1:6,144 | |
| 923 | <1:10 | <1:10 | <1:10 | <1:10 | <1:10 | <1:10 | <1:10 | 1:770 | |
| B13 | <1:10 | <1:10 | <1:10 | <1:10 | <1:10 | <1:10 | <1:10 | 1:832 | |
| GMT | 1:1,505 | ||||||||
D, DNA vaccine; P, PIV; R, recombinant vaccine.
For the purpose of calculation, titers of <1:10 were assigned a titer of 1:5.
Day of challenge.
FIG. 2.
Neutralizing antibody responses in rhesus macaques vaccinated with combinations of the DENV-2 DNA vaccine (D), DENV-2 PIV vaccine (P), and DENV-2 recombinant vaccine (R). Reciprocal geometric mean neutralizing antibody titers for vaccine combinations are presented for all time points.
Viremia after challenge.
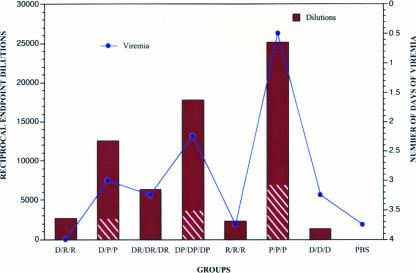
All vaccinated and control rhesus macaques were challenged 5 months after the last vaccination (month 7) with 104 PFU of near wild-type, infectious DENV-2. Sera obtained daily for 10 days after challenge were analyzed for the presence of infectious virus by amplified assay, and the viremia was quantified by direct plaque assay on Vero cell monolayers (Table 2). All of the PBS controls exhibited viremia (mean duration, 3.75 days; peak titer, 2.0 log10 PFU/ml). There was a significant reduction in the days of viremia only in the group vaccinated with the P vaccine alone (mean of 0.5 days), where two animals exhibited no detectable viremia and the remaining two animals exhibited 1 day of viremia each (P = 0.01). Comparison of viremia titers measured by plaque assay and virus isolation using Dunnett's simultaneous test revealed no significant difference between the vaccine groups and the PBS control group. Reduction in the mean number of days of viremia after challenge correlated directly with the total antibody titers on the day of challenge as measured by ELISA (correlation coefficient, −0.937) (Fig. 3) and with ELISA antibody titers to NS1 on the day of challenge in groups that received PIV. Thus, the highest ELISA titer and the shortest duration of viremia were seen in the P/P/P group (ELISA GMT, 25,119; 0.5 days of viremia), followed by groups that received DP/DP/DP (GMT, 17,783; 2.25 days), D/P/P (GMT, 12,589; 3.0 days) DR/DR/DR (GMT, 6,310; 3.25 days), D/R/R (GMT, 2,661; 4.0 days), R/R/R (GMT, 2,339; 3.75 days), and D/D/D (GMT, 1,334; 3.25 days).
TABLE 2.
Viremia in rhesus monkeys immunized with combinations of the DENV-2 DNA, PIV, and recombinant vaccinesa
| Group | Monkey | Days of viremiab after virus challenge:
|
Mean viremia | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||
| D/R/R | 942 | − | − | +/1.4 | +/2.4 | −/2.4 | +/0.9 | − | − | − | − | |
| 898 | − | − | +/0.9 | +/2.6 | +/2.5 | −/1.2 | − | − | − | − | 4 | |
| WTV | − | −/0.9 | −/1.2 | +/2.4 | +/1.2 | − | − | − | − | − | ||
| XJX | − | − | +/1.5 | +/2.1 | +/1.5 | + | − | − | − | − | ||
| D/P/P | 992 | − | − | −/1.4 | +/1.8 | −/1.8 | − | − | − | − | − | |
| VFE | − | −/0.9 | +/1.7 | +/2.5 | +/2.0 | − | − | − | − | − | 3 | |
| VAP | − | − | −/1.7 | +/2.1 | −/0.9 | − | − | − | − | − | ||
| XCW | − | − | − | +/1.7 | +/2.7 | − | − | − | − | − | ||
| DR/DR/DR | A25 | − | − | −/0.9 | −/1.2 | +/2.2 | − | − | − | − | − | |
| 939 | − | − | − | + | −/0.9 | − | − | − | − | − | 3.25 | |
| 981 | − | − | −/0.9 | +/2.2 | +/2.7 | + | − | − | − | − | ||
| 973 | − | − | − | +/1.8 | +/2.2 | +/2.2 | −/1.8 | − | − | − | ||
| DP/DP/DP | 975 | − | − | − | −/1.2 | +/2.6 | − | − | − | − | − | |
| 895 | − | − | −/1.9 | −/1.4 | +/2.9 | +/2.7 | − | − | − | − | 2.25 | |
| 921 | − | − | + | +/2.4 | −/3.0 | − | − | − | − | − | ||
| JWK | − | − | − | − | − | − | − | − | − | − | ||
| R/R/R | 995 | − | − | − | +/2.2 | +/3.0 | +/3.8 | −/1.5 | − | − | − | |
| C67 | − | − | +/1.2 | +/2.1 | +/2.2 | − | − | − | − | − | 3.75 | |
| C51 | − | − | +/1.6 | +/2.9 | +/3.0 | +/2.8 | −/2.0 | − | − | − | ||
| B27 | − | − | − | +/1.2 | +/1.8 | +/1.5 | − | − | − | − | ||
| P/P/P | A79 | − | − | − | − | − | − | − | − | − | − | |
| A43 | − | − | − | − | − | −/1.2 | − | − | − | − | 0.50 | |
| A65 | − | − | − | − | − | − | − | − | − | − | ||
| C49 | − | − | − | −/0.9 | − | − | − | − | − | − | ||
| D/D/D | 931 | − | − | − | −/1.2 | −/0.9 | − | − | − | − | − | |
| GZ | − | − | − | +/1.8 | +/1.8 | − | − | − | − | − | 3.25 | |
| F483 | − | − | − | +/2.0 | +/2.2 | +/1.9 | +/2.2 | − | − | − | ||
| 28R | − | − | + | +/2.3 | +/2.6 | +/1.7 | −/1.8 | − | − | − | ||
| PBS | 887 | − | − | − | + | −/0.9 | +/1.7 | +/2.0 | +/0.9 | + | − | |
| C07 | − | − | + | +/1.9 | +/1.7 | −/0.9 | − | − | − | − | 3.75 | |
| 923 | − | − | − | +/1.5 | + | − | − | − | − | − | ||
| B13 | − | − | − | + | + | + | − | − | − | − | ||
D, DNA vaccine; P, PIV; R, recombinant vaccine.
Viremia results are presented as the result for isolation of virus by amplification in Vero cells (as + or −)/result on direct plaque assay of virus from serum (as log10 PFU/ml) (if present).
FIG. 3.
Serum IgG antibody response in rhesus macaques immunized with combinations of the DENV-2 DNA vaccine (D), DENV-2 PIV vaccine (P), and DENV-2 recombinant vaccine (R) measured by ELISA. Columns indicate geometric mean reciprocal serum dilution endpoint titers (≥0.1 OD) on the day of challenge, with the hatched area indicating antibody levels to NS1. Circles represent number of days of viremia after challenge.
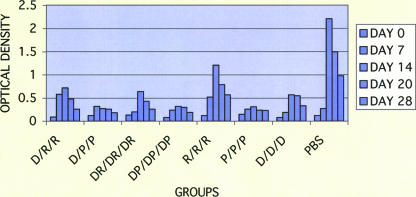
Immunological memory.
Sera obtained after DENV-2 challenge were assayed for IgM and IgG antibodies (Fig. 4 and 5). The PBS controls showed a vigorous primary IgM antibody response when compared with vaccine recipients, which had low titers of DENV-2-specific IgM antibody. Those animals immunized with the R vaccine alone had intermediate IgM antibody responses (probably due to epitopes not present on the vaccine), whereas low-level IgM antibody titers were seen in groups immunized with the DNA and R vaccine combinations (D/D/D, D/R/R, and DR/DR/DR). Those groups immunized with the P vaccine alone or in combination with DNA vaccine (P/P/P, D/P/P, and DP/DP/DP) exhibited the lowest levels of IgM antibody after challenge. These results suggest successful priming of all vaccinated rhesus macaques.
FIG. 4.
Serum IgM antibody response to dengue virus challenge in rhesus macaques immunized with combinations of the DENV-2 DNA vaccine (D), DENV-2 PIV vaccine (P), and DENV-2 recombinant vaccine (R) based on ELISA using purified virions. The results are given as the means of the OD405 values for sera diluted 1:100.
FIG. 5.
Serum IgG antibody response to dengue virus challenge in rhesus macaques immunized with combinations of the DENV-2 DNA vaccine (D), DENV-2 PIV vaccine (P), and DENV-2 recombinant vaccine (R) in an ELISA using purified virions. The results are given as the means of the OD405 values for sera diluted 1:100.
The PBS controls exhibited a rapid rise in IgG antibody beginning on day 7 and peaking on day 28 after challenge. All vaccinated animals exhibited existing levels of IgG antibody that increased after challenge. The lowest anamnestic antibody responses were observed in groups immunized with the P vaccine alone (P/P/P) and with the DP vaccine combination (DP/DP/DP).
Antibody avidity.
Measurement of antibody avidity by ELISA 1 month after the third inoculation demonstrated a low antibody avidity index (AI ≤ 30) in all animals except for those immunized with PIV alone (P/P/P) or PIV in combination with DNA (DP/DP/DP), which exhibited intermediate AI values of 34 and 33, respectively (Table 3). Animals inoculated with the recombinant subunit vaccine alone (R/R/R) had the lowest AI, 14. At the time of virus challenge, 5 months after the third inoculation, AI values increased to intermediate levels in five of the seven groups, and high AI values were seen in the P/P/P (AI = 64) and the D/P/P (AI = 50) groups. Significant differences in antibody avidity indices among vaccine combinations on the day of challenge were determined by ANOVA (F [6, 21], 6.03; P = 0.001). The AIs for groups that received D/R/R, DR/DR/DR, DP/DP/DP, R/R/R, and D/D/D vaccines were significantly different from the AI for the P/P/P group. There was no significant difference in AIs between the D/P/P and P/P/P groups. Four weeks after the virus challenge, an increase in AI was observed in all groups except those groups that received D/R/R and R/R/R vaccines.
TABLE 3.
Antibody avidity indices of sera from individual monkeys immunized with combinations of the DENV-2 DNA, PIV, and recombinant vaccinesa
| Vaccination group | Monkey | Antibody avidity indexb
|
||
|---|---|---|---|---|
| Day 90 | Day 210 challenge | Day 28 postchallenge | ||
| DRR | 942 | 19 | 25 | 26 |
| 898 | 16 | 39 | 63 | |
| WTV | 39 | 49 | 33 | |
| XJX | 33 | 31 | 40 | |
| GMT | 25 | 35 | 38 | |
| DPP | 992 | 27 | 53 | 57 |
| VFE | 33 | 43 | 80 | |
| VAP | 26 | 50 | 42 | |
| XCW | 33 | 57 | 73 | |
| GMT | 30 | 50 | 61 | |
| DR/DR/DR | A25 | 19 | 31 | 52 |
| 939 | 30 | 40 | 60 | |
| 981 | 26 | 28 | 38 | |
| 973 | 20 | 56 | 50 | |
| GMT | 23 | 37 | 50 | |
| DP/DP/DP | 975 | 19 | 33 | 55 |
| 895 | 41 | 53 | 52 | |
| 921 | 33 | 35 | 55 | |
| JWK | 45 | 37 | 47 | |
| GMT | 33 | 39 | 52 | |
| RRR | 999 | 17 | 43 | 28 |
| C67 | 12 | 36 | 27 | |
| C51 | 13 | 30 | 29 | |
| B27 | 15 | 17 | 32 | |
| GMT | 14 | 30 | 29 | |
| PPP | A79 | 22 | 59 | 62 |
| A43 | 40 | 63 | 85 | |
| A65 | 47 | 68 | 64 | |
| C49 | 33 | 67 | 91 | |
| GMT | 34 | 64 | 75 | |
| DDD | 931 | 30 | 33 | 55 |
| GZ | 25 | 47 | 53 | |
| F483 | 29 | 45 | 43 | |
| 28R | 40 | 40 | 39 | |
| GMT | 30 | 41 | 47 | |
| PBS | 887 | —c | — | 3 |
| CO7 | — | — | 6 | |
| 923 | — | — | 6 | |
| B13 | — | — | 5 | |
| GMT | 5 | |||
D, DNA vaccine; P, PIV; R, recombinant vaccine.
Avidity index = (OD405 after NASCN treatment)/(OD405 without NASCN treatment) × 100.
—, no DEN-specific antibody was detectable.
DISCUSSION
Although the leading candidates for a dengue vaccine are LAV, it has proven difficult to produce LAV vaccines that are satisfactorily attenuated and at the same time sufficiently immunogenic (2, 11, 23, 30). Clearly, there is reason to explore alternatives. We previously reported that a DENV-2 DNA vaccine and a recombinant subunit protein vaccine when given in combination were able to generate high-titer antibodies in mice which persisted for more than 6 months (26). In the present study we further evaluated combinations of DNA, recombinant subunit, and purified inactivated virus vaccines in rhesus macaques. This was done by assessing the animals' immune responses to vaccination by measuring dengue virus-neutralizing antibody, total antibody, and antibody avidity and then assessing protection by challenging the vaccinated animals with live DEN-2 virus and measuring serum viremia (used as a surrogate for disease) and anamnestic antibody responses.
Dengue virus-neutralizing antibodies directed against the virion E antigen are thought to play a key role in protection against disease, an idea supported directly by passive antibody transfer experiments in animal models (9) and indirectly by epidemiological data from prospective studies in areas where dengue is endemic (6). However, minimum protective neutralizing antibody titers have not been established for dengue, and these might differ depending upon variables of the host (29), vaccine type (e.g., live or inactivated), and the genotype of the infecting virus (13). In a previous study in rhesus macaques vaccinated with DEN-2 PIV or 80% E protein vaccine, a statistically significant correlation was observed between higher virus-neutralizing antibody titers on the day of virus challenge and protection against viremia (19).
In the present study, all vaccinated macaques had dengue virus-neutralizing antibodies 1 month after the third dose (day 90) as measured by PRNT50 assay; the GMTs for each vaccine group ranged from 1:49 (D/D/D vaccine) to 1:510 (DR/DR/DR vaccine). By the time of the virus challenge 5 months after the third dose, the neutralizing antibody GMTs had fallen significantly, although they remained above the assay cutoff (1:10) except for the D/D/D vaccine group (1:9). The highest GMTs on the day of challenge (day 210) were seen in the P/P/P (1:81) and DR/DR/DR (1:72) groups, and the lowest titer was seen in the R/R/R vaccine group (1:18).
After the virus challenge, only the group that received the P/P/P vaccine showed a significant reduction in the number of days of viremia compared with the mock (PBS)-vaccinated controls. Only one animal that received a different vaccine (JWK, DP/DP/DP vaccine) was completely protected against viremia, and that animal had pre- and postchallenge PRNT50 titers similar in magnitude to the P/P/P recipients. There was no statistically significant correlation between PRNT50 titers on the day of challenge and viremia for any of the vaccine groups. All animals, whether or not they developed viremia, showed anamnestic antidengue antibody responses after challenge suggestive of some challenge virus replication, with PRNT50 titers at day 224 that were more than 10-fold higher than those in the PBS controls. It is possible that a sufficiently rapid and vigorous anamnestic neutralizing antibody response can prevent the development of viremia, but our study was not designed to investigate this.
PRNT titers by themselves, while a convenient measure of virus-neutralizing antibodies in vitro, might not provide an accurate measure of virus neutralization in vivo. Nonneutralizing antibody may also be important for protection, by binding to viral nonstructural proteins expressed on the surface of DENV-infected cells. Other studies in animals have also reported protection mediated by nonneutralizing antibodies (3, 4, 12, 15, 24). Other factors, such as antibody avidity, epitope specificity, and subclass (effector function), might play important roles in mediating protection, e.g., through complement-mediated lysis or phagocytosis of free virus and the elimination of virus-infected cells by antibody-dependent, cell-mediated cytotoxicity and complement-dependent cytotoxicity (3). Antibodies at concentrations higher than those required for the neutralization of free virus have been shown to act on infected cells to inhibit cell-to-cell transmission of viruses (7, 8, 14, 16, 18). In addition to E antigen, antibodies against prM and NS1 antigens are protective in animal models (5, 9), in the case of NS1 possibly by the targeting of infected cells.
The number and quality of immunogenic epitopes present in a vaccine might play an important role in protection. The PIV vaccine presents more epitopes to the immune system than does the recombinant subunit protein vaccine, which contains only domain III of the E protein. Immunogenicity of the DNA vaccine, in addition to being dependent upon the dose employed, is also dependent upon the efficiency of uptake and expression in antigen-presenting cells. Therefore, we used the ELISA to measure total anti-DENV antibodies to purified virions and also NS1 antigen, since the P vaccine contains NS1 as a result of its copurification with virions and empty particles (19). Not surprisingly, we found the highest total ELISA antibody titers against both viral antigens and NS1 antigen on the day of challenge in the P/P/P group followed by the DP/DP/DP and D/P/P groups, which was coincident with fewer days of viremia for those groups (although statistically significant only for the P/P/P group). While a direct comparison of the antibody responses following vaccination with the PIV vaccine to antibody responses after LAV vaccine or natural infection was not possible in this study, total and NS1-specific antibodies in the PBS controls 28 days after live virus challenge were 10-fold higher than in the P/P/P group on day 210, but a similar ratio existed between the total and anti-NS1 antibody titers (data not shown).
We also determined the antibody avidity index, which is an approximate measure of the strength or stability of an antibody-antigen interaction. High-avidity antibodies might be conducive for maintaining dengue viruses in a neutralized state. For most animals, the antibody avidity index increased from the time of vaccination to the time of virus challenge, possibly as a result of affinity maturation. The highest average antibody avidity index on the day of challenge was seen in the P/P/P vaccine group followed by the D/P/P group. Like higher neutralizing and total antibody titers, higher antibody avidity on the day of challenge correlated with a reduction in the total days of viremia, but only for the P/P/P group. Antibody subclass did not appear to play a role in the experimental outcome, since all rhesus macaques developed only DENV-specific IgG1 antibody after vaccination regardless of vaccine type. Our results suggest but do not prove that both neutralizing and nonneutralizing, high-avidity antibodies present at high titers are important for protection in this model.
In addition to the role of antibodies in vaccine-mediated protection, the antiviral activity of T cells might also play an important role. There is evidence that specific CD8+ T cells, as well as CD4+ T cells, have the ability to control viral replication by inducing apoptosis and release of cytokines. Nonreplicating vaccines, such as inactivated virus or recombinant protein vaccines, elicit neutralizing antibodies but possibly only weak CD8+ T-cell responses, whereas DNA vaccines induce CD8+ T-cell responses in mice and rhesus macaques (10, 21). The ability of our vaccines to elicit effective cell-mediated immune responses remains to be determined.
Some dengue virus vaccines might induce potentially harmful immune responses, such as those involved in antibody-dependent immune enhancement, which can lead to more severe disease most commonly seen in secondary DENV infections. It is believed that antibody-dependent immune enhancement and other immune mediators play a role in dengue hemorrhagic fever, e.g., by increasing the rate of infection of Fc receptor-bearing cells, thus increasing virus burden and disease severity. The development of vaccines with a low risk for potentiating severe disease after natural infection is a major concern for all dengue vaccine developers. Unfortunately, there are no reliable methods short of large field efficacy trials for assessing this risk. Although DENV-infected rhesus macaques do not exhibit signs of disease, some vaccinated animals in this study exhibited 10-fold higher titers of viremia than the PBS controls. However, the difference in viremia titer was not statistically significant. Whether these results were due to enhanced virus replication or animal-to-animal variation cannot be answered by this study.
Vaccine safety and efficacy are the paramount concerns. The Vero cells used to produce the vaccine are certified and now recently approved by the World Health Organization and U.S. Food and Drug Administration for inactivated vaccines intended for parenteral administration. A viable dengue virus vaccine candidate should also be economical enough to produce so that it can be marketed to developing nations, where it is needed most. For the DENV PIV vaccine, we were able to achieve virus titers of up to 5 × 107 PFU per ml, which after concentration and purification yielded about 30 doses from 1 liter of original culture fluid. Following appropriate commercial scale-up for current good manufacturing practices production, the vaccine should not be too expensive for widespread use, consistent with other vaccines, such as hepatitis A vaccine.
In summary, our results suggest that DENV-neutralizing antibodies and high-titer, high-avidity nonneutralizing antibodies, including antibodies against NS1, play a role in protection against a live virus challenge in the rhesus macaque model. There was significant correlation between the total antibody titers measured by ELISA and the reduction in the days of viremia after challenge in animals primed and boosted with the PIV vaccine. This vaccine may provide an alternate to LAV vaccines for immunizing against dengue virus, possibly with a lower risk for immediate reactogenicity. However, the long-term safety and efficacy of such a vaccine remain to be demonstrated.
Acknowledgments
We thank the personnel of the Division of Veterinary Medicine of Walter Reed Army Institute of Research for animal husbandry and technical assistance with the animal experiments. We gratefully acknowledge Heather Kelly for technical assistance, Robert Burge for statistical analysis, and Jim Fuller, Powderject, Inc., for providing the DNA vaccine.
Financial support for this research was provided by Naval Medical Research Center work unit 63002A 810S and the U.S. Army Medical Research and Materiel Command.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government.
REFERENCES
- 1.Barnett, S. W., S. Rajasekar, H. Legg, B. Doe, D. H. Fuller, J. R. Hayens, C. M. Walker, and K. S. Steimer. 1997. Vaccination with HIV-1 gp120 DNA induces immune responses that are boosted by a recombinant gp120 protein subunit. Vaccine 15:869-873. [DOI] [PubMed] [Google Scholar]
- 2.Bhamarapravati, N., and S. Yoksan. 1997. Live-attenuated tetravalent dengue vaccine, p. 367-377. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, Cambridge, United Kingdom.
- 3.Burton, D. R. 2002. Antibodies, viruses and vaccines. Nat. Rev. 2:706-713. [DOI] [PubMed] [Google Scholar]
- 4.Chanock, R. M., J. E. Crowe, B. R. Murphy, and D. R. Burton. 1993. Human monoclonal antibody Fab fragments cloned from combinatorial libraries: potential usefulness in prevention and/or treatment of major human viral diseases. Infect. Agents Dis. 2:118-131. [PubMed] [Google Scholar]
- 5.Chung, K. M., G. E. Nybakken, B. S. Thompson, M. J. Engle, A. Marri, D. H. Fremont, and M. S. Diamond. 2006. Antibodies against West Nile nonstructural protein NS1 prevent lethal infection through Fc γ receptor-dependent and -independent mechanisms. J. Virol. 80:1340-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endy, T. P., A. Nisalak, S. Chunsuttitwat, D. W. Vaughn, S. Green, F. A. Ennis, A. L. Rothman, and D. H. Libraty. 2004. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J. Infect. Dis. 189:990-1000. [DOI] [PubMed] [Google Scholar]
- 7.Fujinami, R. S., and M. B. Oldstone. 1979. Antiviral antibody reacting on the plasma membrane alters measles virus expression inside the cell. Nature 279:529-530. [DOI] [PubMed] [Google Scholar]
- 8.Gerhard, W. 2001. The role of the antibody response in influenza virus infection. Curr. Top. Microbiol. Immunol. 260:171-190. [DOI] [PubMed] [Google Scholar]
- 9.Henchal, E. A., L. S. Henchal, and J. J. Schlesinger. 1988. Synergistic interactions of anti-NS1 monoclonal antibodies protect passively immunized mice from lethal challenge with dengue 2 virus. J. Gen. Virol. 69:2101-2107. [DOI] [PubMed] [Google Scholar]
- 10.Hooks, J. J., W. Burns, K. Hayashi, S. Geis, and A. L. Notkins. 1976. Viral spread in the presence of neutralization antibody: mechanisms of persistence in foamy virus infections. Infect. Immun. 14:1172-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanesa-Thasan, N., W. Sun, G. Kim-Ahn, S. Van Albert, R. Putnak, A. King, B. Raengsakulsrach, H. Christ-Schmidt, K. Gilson, J. Zahradnik, D. Vaughn, B. Innis, J. Saluzzo, and C. Hoke. 2001. Safety and immunogenicity of attenuated dengue virus vaccines (Aventis Pasteur) in human volunteers. Vaccine 19:3179-3188. [DOI] [PubMed] [Google Scholar]
- 12.Kato, H., R. Kato, K. Fujihashi, and J. R. McGhee. 2001. Role of mucosal antibodies in viral infections. Curr. Top. Microbiol. Immunol. 260:201-228. [DOI] [PubMed] [Google Scholar]
- 13.Kochel, T., D. M. Watts, A. S. Gozalo, D. F. Ewing, K. R. Porter, and K. L. Russell. 2005. Cross-serotype neutralization of dengue virus in Aotus nancymae monkeys. J. Infect. Dis. 191:1000-1004. [DOI] [PubMed] [Google Scholar]
- 14.Levine, B., J. M. Hardwick, B. D. Trapp, T. O. Crawford, R. C. Bollinger, and D. E. Griffin. 1991. Antibody-mediated clearance of alpha virus infection from neurons. Science 254:856-860. [DOI] [PubMed] [Google Scholar]
- 15.Manzanec, M. B., C. L. Coudret, and D. R. Fletcher. 1995. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J. Virol. 69:1339-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCullough, K. C., D. Parkinson, and J. R. Crowther. 1988. Opsonization-enhanced phagocytosis of foot-and-mouth disease virus. Immunology 65:187-191. [PMC free article] [PubMed] [Google Scholar]
- 17.Monath, T. P. 1986. Pathology of the flaviviruses, p. 375-440. In S. Schlesinger and M. J. Schlesinger (ed.), The Togaviridae and Flaviviridae. Plenum, New York, N.Y.
- 17a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 18.Pantaleo, G., J. F. Demarest, M. Vaccarezza, C. Graziosi, G. P. Bansal, S. Koenig, and A. S. Fauci. 1995. Effect of anti-V3 antibodies on cell-free and cell-to-cell human immunodeficiency virus transmission. Eur. J. Immunol. 25:226-231. [DOI] [PubMed] [Google Scholar]
- 19.Putnak, R., D. Barvir, J. Burrous, D. Dubois, V. Dandrea, C. Hoke, J. Sadoff, and K. Eckels. 1996. Development of a purified, inactivated dengue 2 virus vaccine prototype in Vero cells: immunogenicity and protection in mice and rhesus macaques. J. Infect. Dis. 174:1176-1184. [DOI] [PubMed] [Google Scholar]
- 20.Putnak, R., J. Fuller, L. Vanderzanden, B. Innis, and D. Vaughn. 2003. Vaccination of rhesus macaques against dengue-2 virus with a plasmid DNA vaccine encoding the viral pre-membrane and envelope genes. Am. J. Trop. Med. Hyg. 68:469-476. [PubMed] [Google Scholar]
- 21.Rodriguez-Carreno, M. P., M. S. Nelson, J. Botten, K. Smith-Nixon, M. J. Buchmeier, and J. L. Whitton. 2005. Evaluating the immunogenicity and protective efficacy of DNA vaccine encoding Lassa virus nucleoprotein. Virology 335:87-89. [DOI] [PubMed] [Google Scholar]
- 22.Russell, P. K., A. Nisalak, P. Sukhavachana, and S. Vivona. 1967. A plaque reduction test for dengue virus neutralizing antibodies. J. Immunol. 99:285-290. [PubMed] [Google Scholar]
- 23.Sabchareon, A., J. Lang, P. Chanthavanich, S. Yoksan, R. Forrat, P. Attanath, C. Sirivichayakul, K. Pengsaa, C. Pojjaroen-Anant, W. Chokejindachai, A. Jagsudee, J. F. Saluzzo, and N. Bhamarapravati. 2002. Safety and immunogenicity of tetravalent live-attenuated dengue vaccines in Thai adult volunteers: role of serotype, concentration, ratio, and multiple doses. Am. J. Trop. Med. Hyg. 66:264-272. [DOI] [PubMed] [Google Scholar]
- 24.Schlesinger, J. J., M. W. Brandriss, C. B. Cropp, and T. P. Monath. 1986. Protection against yellow fever in monkeys by immunization with yellow fever virus nonstructural protein NS1. J. Virol. 60:1153-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sedegah, M., T. R. Jones, M. Kaur, R. Hedstrom, P. Hobart, J. A. Tine, and S. L. Hoffman. 1998. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc. Natl. Acad. Sci. USA 95:7648-7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simmons, M., W. M. Nelson, S. J. Wu, and C. G. Hayes. 1998. Evaluation of the protective efficacy of a recombinant dengue envelope B domain fusion protein against dengue 2 virus infection in mice. Am. J. Trop. Med. Hyg. 58:655-662. [DOI] [PubMed] [Google Scholar]
- 27.Simmons, M., G. S. Murphy, T. Kochel, K. Raviprakash, and C. G. Hayes. 2001. Characterization of antibody responses to combinations of a dengue-2 DNA and dengue-2 recombinant subunit vaccine. Am. J. Trop. Med. Hyg. 65:420-426. [DOI] [PubMed] [Google Scholar]
- 28.Someya, K., K. Q. Xin, K. Matsuo, K. Okuda, N. Yamamoto, and M. Honda. 2004. A consecutive priming-boosting vaccination in mice with simian immunodeficiency virus (SIV) gag/pol DNA and recombinant vaccinia virus strain DIs elicits effective anti-SIV immunity. J. Virol. 78:9842-9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephens, H. A., R. Klaythong, M. Sirikong, D. W. Vaughn, S. Green, S. Kalayanarooj, T. P. Endy, D. H. Libraty, A. Nisalak, B. L. Innis, A. L. Rothman, F. A. Ennis, and D. Chandanayingyong. 2002. HLA-A and -B allele associations with secondary dengue virus infections correlate with disease severity and the infecting viral serotype in ethnic Thais. Tissue Antigens 60:309-318. [DOI] [PubMed] [Google Scholar]
- 30.Vaughn, D. W., C. H. Hoke, S. Yoksan, R. LaChance, B. L. Innis, R. M. Rice, and N. Bhamarapravati. 1996. Testing of a dengue 2 live-attenuated vaccine (strain 16681 PDK 53) in ten American volunteers. Vaccine 14:329-336. [DOI] [PubMed] [Google Scholar]