Abstract
A number of flaviviruses are important human pathogens, including yellow fever, dengue, West Nile, Japanese encephalitis, and tick-borne encephalitis (TBE) viruses. Infection with or immunization against any of these viruses induces a subset of antibodies that are broadly flavivirus cross-reactive but do not exhibit significant cross-neutralization. Nevertheless, these antibodies can efficiently bind to the major envelope protein (E), which is the main target of neutralizing and protective antibodies because of its receptor-binding and membrane fusion functions. The structural basis for this phenomenon is still unclear. In our studies with TBE virus, we have provided evidence that such cross-reactive antibodies are specific for a cluster of epitopes that are partially occluded in the cage-like assembly of E proteins at the surfaces of infectious virions and involve—but are not restricted to—amino acids of the highly conserved internal fusion peptide loop. Virus disintegration leads to increased accessibility of these epitopes, allowing the cross-reactive antibodies to bind with strongly increased avidity. The cryptic properties of these sites in the context of infectious virions can thus provide an explanation for the observed lack of efficient neutralizing activity of broadly cross-reactive antibodies, despite their specificity for a functionally important structural element in the E protein.
The production of specific antibodies is a key component of the adaptive immune response to virus infections and, together with cellular immune reactions, contributes to virus clearance and the maintenance of long-lasting immunity (29, 73). While some of the antibody-mediated antiviral activities target infected cells (e.g., antibody-dependent cellular cytotoxicity) (68), the most important function of antibodies seems to be the neutralization of infectivity by binding to viral surface components and thereby interfering with functions that are essential for virus entry into cells (9, 29).
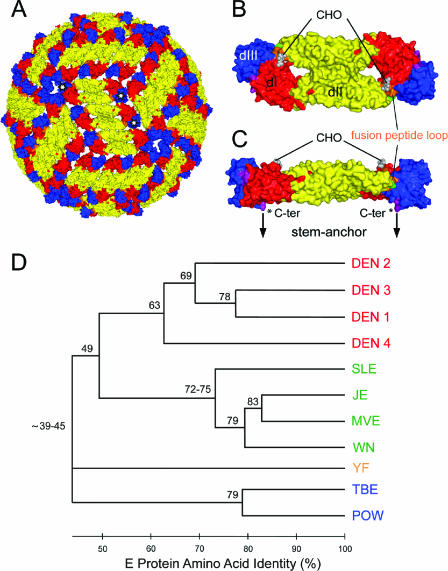
In the case of enveloped viruses, neutralizing antibodies are targeted to envelope glycoproteins that mediate receptor-binding and membrane fusion activities and usually form spiky projections at the virion surface. This spiky class of envelope proteins is found in a number of important human-pathogenic viruses, including members of the orthomyxovirus, paramyxovirus, retrovirus, filovirus, and coronavirus families (18, 66). Flaviviruses, on the other hand, display a completely different structure, the details of which have been revealed by X-ray crystallography and cryoelectron microscopy (Fig. 1) (50). They are small enveloped viruses with a smooth surface composed of tightly interacting envelope proteins (designated E) that, instead of being perpendicular, are oriented parallel to the viral membrane and cover the membrane completely (Fig. 1A) (42, 49). The basic building block of this cage-like protein shell is an antiparallel E protein dimer (Fig. 1B and C) (47, 48, 59, 75) that is inserted into the viral membrane through anchoring elements at its distal ends (74). Ninety copies of the E dimer form an unusual “herringbone-like” icosahedral lattice at the virion surface in which each of the three E monomers per asymmetric unit has a different chemical environment (42, 49) (Fig. 1A). The E protein mediates both receptor-binding and fusion activities after virus uptake by receptor-mediated endocytosis and, because of these functions, is the major target for virus-neutralizing antibodies. Fusion is triggered by the acidic pH in the endosome, which leads to E dimer dissociation and the exposure of the highly conserved fusion peptide (FP) loop at the tip of domain II (Fig. 1B and C), thus allowing its interaction with the target membrane (1, 71). Further structural rearrangements in E drive the fusion process to completion (30, 32, 41).
FIG. 1.
Structural organization of flavivirus particles (A, B, and C) and dendrogram displaying relationships among flaviviruses (D). (A) Model of the structure of the mature virus particle. The virion surface is covered by 90 E protein dimers in a herringbone-like arrangement as determined for dengue and West Nile viruses (42, 49). Domains III of three monomers belonging to one icosahedral asymmetric unit are labeled by white stars. The corresponding monomers thus have different chemical environments. (B and C) Surface representations of the X-ray crystal structure of an sE dimer in a top view (B) and a side view (C). Compared to the full-length E protein, sE lacks the double membrane anchor and an additional 50 amino acids (stem) that connect the anchor to the C terminus (C-ter) of sE. In panel C, the C terminus of sE is labeled by a star, and the arrows indicate the extension to the stem-anchor region. The three domains of sE are color coded in both monomeric subunits as follows: red, domain I (dI); yellow, domain II; blue, domain III. The regions linking domains I and III, and domain III and the stem, are shown in purple, the fusion peptide loop in orange, and amino acid 107 (which was mutated) in green. CHO indicates the attachment site of the single carbohydrate side chain (Asn 154 of domain I) in each E monomer. The images shown in panels A to C were prepared using the PyMol program (14). (D) Distance relationships between different flaviviruses based on amino acid sequence identity in the E protein. The dendrogram was constructed using ClustalX (72) and MEGA 3.1 (43). The different serocomplexes are shown in four colors: red, dengue virus serocomplex; green, Japanese encephalitis virus serocomplex; orange, yellow fever virus serocomplex; blue, tick-borne encephalitis virus serocomplex. SLE, Saint Louis encephalitis; MVE, Murray Valley encephalitis; POW, Powassan.
In addition to their peculiar structural organization, flaviviruses have attracted great interest because of their worldwide roles as human disease agents. There are about 70 different flaviviruses (most of them transmitted by mosquitoes or ticks) that form a genus in the family Flaviviridae (34) and include a number of important human pathogens, such as yellow fever (YFV), dengue (DENV), Japanese encephalitis (JEV), West Nile (WNV), and tick-borne encephalitis (TBEV) viruses (7). The dramatic expansion of dengue virus endemic and epidemic areas, as well as the sudden appearance and spread of West Nile virus in the United States and neighboring regions since 1999, is an example that underscores the impact of flaviviruses as emerging and reemerging pathogens (45, 70).
Originally, flaviviruses (then designated group B arboviruses) were grouped together on the basis of their antigenic relationships as revealed by hemagglutination inhibition (HI) assays using polyclonal immune sera obtained after infection or immunization (58). Since the E protein is the viral hemagglutinin, the cross-reactivities measured in HI assays are E-protein specific, and cross-reactivity is also apparent in enzyme immunoassays using purified virions or isolated forms of E as an antigen (27, 60). Neutralization assays, on the other hand, are significantly more specific, and cross-reactions in these assays are largely confined to groups of more closely related flaviviruses, the so-called serocomplexes (11, 15, 34), as displayed in Fig. 1D. It is therefore apparent that infection or immunization with a given flavivirus induces two functionally distinct populations of E-protein-specific antibodies in the host: one with the potential to neutralize the homologous and closely related viruses (with a threshold of about 60 to 70% amino acid sequence identity in E [Fig. 1D]), and the other with the ability to inhibit hemagglutination of even the most distantly related flaviviruses (less than 40% amino acid sequence identity in E) but without having significant neutralizing activity. The induction of such nonneutralizing glycoprotein-specific antibodies has been described for other enveloped viruses as well (29, 56) and is an important facet of the models that have been discussed for the mechanism of virus neutralization (9). This phenomenon has also attracted considerable interest in the field of human immunodeficiency virus research (8, 55) because of the important implications it could have for the design of effective vaccines (8, 51).
The objective of this study was to gain insights into the structural basis of the extensive cross-reactivity that is observed between all flaviviruses in certain assays, despite the lack of significant cross-neutralization, by (i) identifying sites in the E protein where broadly flavivirus cross-reactive antibodies bind and (ii) finding a physical explanation for their relative lack of neutralizing activity. In our studies with TBE virus and TBE virus antigens with mutated forms of E, we show that a cluster of antigenic determinants involving the highly conserved fusion peptide loop at the tip of domain II in the E protein forms a dominant target for broadly flavivirus cross-reactive antibodies. We also provide evidence that these determinants are cryptic and mostly inaccessible at the surfaces of infectious virions. As a consequence, antibodies that are specific for such sites can bind only with low affinity (or not at all) to infectious virions, consistent with their lack of efficient neutralizing activity. The disintegration of the closed shell-like envelope structure, however, which is induced by the conditions of the HI assay and most enzyme immunoassays, leads to the exposure of conserved cryptic sites, allowing the cross-reactive antibodies to be efficiently recognized. Conversely, the presence of such antibodies in postinfection and postimmunization sera suggests that the corresponding epitopes also become exposed and presented to the immune system in the course of virus replication and/or immunization.
MATERIALS AND METHODS
Sequence alignments and analysis of amino acid sequence relationships.
Multiple sequence alignments of 41 representative flavivirus E protein amino acid sequences were performed with ClustalX software (72) and by manual adjustment. Distance matrices were determined by the analysis of p-distances (proportion of amino acid sites at which the two sequences to be compared are different) using MEGA 3.1 software (43) and were used to construct the dendrogram shown in Fig. 1 by the neighbor-joining method.
Production of virus, soluble E (sE) dimer, and subviral particles.
TBE virus strain Neudoerfl (46), JE virus strain Nakayama 3 Moskau (16), and WN virus strain NY99 (44) were grown in primary chicken embryo cells, harvested 48 h after infection, and purified by two cycles of sucrose density gradient centrifugation as described elsewhere (35). Viruses were inactivated with formalin (final dilution, 1:2,000) for 24 h at 37°C either before (WN virus) or after (TBE and JE viruses) purification.
Truncated sE dimers were produced as described previously (36). Briefly, purified TBE virus was treated with trypsin at 0°C, the residual particles were removed by ultracentrifugation, and the sE dimers were purified by anion-exchange chromatography. For the preparation of recombinant subviral particles (RSPs) in COS-1 (ATCC CRL 1650) cells, we used the wild-type plasmid SV-PEwt, a cDNA clone with the prM and E genes of TBE virus strain Neudoerfl under the control of the simian virus 40 early promoter, and plasmids derived from it containing amino acid substitutions at position 107 of the E protein (1). COS-1 cells were transfected with recombinant plasmids by electroporation as described previously (65). RSPs were harvested by pelleting them from cell culture supernatants 48 h after transfection and were either used directly after resuspension or further purified by rate-zonal centrifugation in a Beckman SW40 rotor (90 min at 38,000 rpm) on 5% to 20% sucrose gradients in TAN buffer (50 mM triethanolamine, 100 mM NaCl), pH 8.0. The peak fractions were pooled and centrifuged at 50,000 rpm at 4°C for 2 h in a Beckman Ti 90 rotor. The pellet was then resuspended in TAN buffer, pH 8.0, and quantitated by four-layer enzyme-linked immunosorbent assay (ELISA) after a 30-min denaturation with 0.4% sodium dodecyl sulfate at 65°C (37).
Monoclonal and polyclonal antibodies.
The hybridoma cell line HB 112:D1-4G2-4-15 was obtained from the ATCC, and monoclonal antibody (MAb) 4G2 was purified from hybridoma cell supernatants using protein A Sepharose High Performance (GE Healthcare Life Sciences) according to the manufacturer's recommendations. The other cross-reactive MAbs were used in the form of ascitic fluids. Mabs 843, 813, 612, 528, and F7/3 were kindly provided by Ernest A. Gould (Centre for Ecology and Hydrology, Oxford, United Kingdom), and MAb 6B6C-1 was provided by John T. Roehrig (Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention, Fort Collins, CO). The human polyclonal DEN postinfection sera (low or no immunoglobulin M [IgM]) were kindly provided by Herbert Schmitz (Bernhard-Nocht Institute, Hamburg, Germany) and Suwanna Sinsawaiwong-Sethawacharawanit (Prince of Songkla University, Hat Yai, Songkla, Thailand). The DEN sera 1 and 2 were from German travelers to Brazil and Thailand, respectively, who returned from their journeys with dengue virus infections. DEN sera 3 to 6 were from patients hospitalized in Thailand with clinical diagnoses of dengue fever (DEN sera 4 and 6) and dengue hemorrhagic fever (DEN serum 5). No specific clinical information was available for DEN serum 3.
ELISA. (i) Solid-phase (three-layer) ELISA.
Microtiter plates were coated with purified virions (infectious or formalin inactivated) or RSPs at a concentration of 0.5 μg/ml in carbonate buffer, pH 9.6. Preliminary assays revealed the same titers with infectious and formalin-inactivated TBE and JE virions in this ELISA format. The ELISA reactivities of antibodies with DEN virus were determined using commercially available plates coated with formalin-inactivated DENV types 1 to 4 (Focus Technologies' Dengue Fever Virus IgG ELISA). Serial dilutions of MAbs (starting at a protein concentration of 100 or 50 μg/ml) or polyclonal human sera (starting at a dilution of 1:100) were added to the plates, and the plates were incubated for 1 h at 37°C. The bound antibody was then detected using peroxidase-labeled rabbit anti-mouse immunoglobulin G or peroxidase-labeled goat anti-human immunoglobulin G as described in reference 33.
(ii) Blocking ELISA.
In the blocking ELISA format, different forms of TBE virus antigens were preincubated with MAbs or polyclonal sera before addition to TBE virus-coated microtiter plates for determination (by conventional three-layer ELISA) of the fraction of antibodies that were not bound to the antigens during the preincubation step. The following blocking antigen preparations were used: untreated TBE virus and untreated TBE virus RSPs, TBE virus and TBE virus RSPs treated with Triton X-100 for 30 min at room temperature, and sE dimers. Serial dilutions of the blocking antigens containing equimolar amounts of E (starting at a virus protein concentration of 20 μg/ml, corresponding to 14 μg/ml E) were mixed with an equal volume of a predetermined fixed dilution of MAb or polyclonal human serum that would yield an absorbance value of around 1.0 at 490 nm in the standard three-layer ELISA. At the highest concentration of the blocking antigen, the molar excess of E protein over antibodies was at least 20-fold. After incubation for 1 h at 37°C, these mixtures were transferred to microtiter plates coated with purified TBE virus at a concentration of 1 μg/ml, and a conventional three-layer ELSA was carried out as described above. The results were expressed as percent blocking, defined as the percent reduction in absorbance in the presence of the blocking antigen compared to the absorbance value that was obtained with the corresponding antibody sample in the absence of a blocking antigen: 100 − (absorbance with blocking antigen/absorbance without blocking antigen) × 100.
(iii) Measurement of relative MAb avidities.
Two different formats were used to measure the ELISA avidities of MAbs to different TBE virus antigens.
(a) Four-layer ELISA.
Microtiter plates were coated with a guinea pig anti-TBE virus IgG and blocked with phosphate-buffered saline, pH 7.4, containing 2% lamb serum. Native TBE virions and TX-100-solubilized TBE virions (as described above for the blocking ELISA) at an E protein concentration of 0.4 μg/ml in phosphate-buffered saline, pH 7.4, containing 2% lamb serum were added and incubated for 1 h at 37°C. In the case of native virions, no detergent was used at any stage to avoid destabilization of the antigen. For the other antigens, 2% Tween 20 was added to the incubation buffers. Serial twofold dilutions of the MAbs (starting at a concentration of 200 nM) were added to the antigens, which were then incubated for 1 h at 37°C. The detection system was the same as that described above for the solid-phase three-layer ELISA. The MAb concentrations producing half-maximal binding were determined and defined as the ELISA avidity constants.
(b) Three-layer ELISA.
Microtiter plates were coated with TBE virus at an E protein concentration of 0.1 μg/ml. The further procedure of antibody titrations and determination of ELISA avidity constants was identical to that described above for the four-layer ELISA.
HI assay.
HI assays were performed at a final pH of 6.4 by the method of Clarke and Casals using goose erythrocytes (12).
NT assay.
TBE and JE virus neutralization (NT) assays were carried out in microtiter plates using baby hamster kidney cells (BHK-21), essentially as described previously (38). However, the assay was modified to contain a smaller virus inoculum and to use a shorter incubation period (3 instead of 4 days). Twofold serial dilutions of MAbs or polyclonal sera were mixed with 10 50% tissue culture infective doses of virus (starting MAb concentration in the mixture, 500 μg/ml; starting dilution of the serum in the mixture, 1:10) and incubated for 1 h at 37°C. BHK-21 cells were added, and incubation was continued for 3 days. The presence of virus in the supernatant was determined using a four-layer ELISA, and the virus neutralization titer was defined as the reciprocal of the serum/antibody dilution that gave a 90% or 50% reduction in the absorbance readout in the assay (NT90 or NT50) compared to the control without antibody (38).
RESULTS
Mapping the binding sites of broadly flavivirus cross-reactive antibodies.
Consistent with the broad flavivirus cross-reactivity observed with polyclonal immune sera, a number of E-protein-specific mouse MAbs have been described (31, 61) (Table 1) that exhibit cross-reactivity even between the most distantly related flaviviruses. In order to get more detailed information about the epitopes recognized by such antibodies, we first determined the specific activities of a panel of 14 cross-reactive MAbs generated by immunization with different and distantly related flaviviruses (Table 1) in ELISA and HI and NT assays (Table 2). Consistent with their original description as “broadly flavivirus cross-reactive,” all of these MAbs reacted with each of the four representative flaviviruses tested (TBE, JE, WN, and DEN viruses, members of three different serocomplexes) (Fig. 1D) in solid-phase three-layer ELISA and HI assays. However, a significant degree of variation was observed with respect to the relative amounts of cross-reactivity with different flaviviruses (Table 2). MAb A2, for instance, had the highest ELISA titer with TBE virus, intermediate titers with JEV and WNV, and a very low titer with DENV, whereas MAb 6B6C1 displayed similarly high titers with JEV, WNV, and DENV but an at least 10-fold-lower titer with TBE virus. It is also apparent from Table 2 that the titers obtained in ELISA do not necessarily correlate with those in HI assays, as exemplified by MAbs IA3 and IS3, which had high titers in HI assays despite their comparatively low titers in ELISA. The MAbs A1 and IF3, on the other hand, displayed high titers in both assays.
TABLE 1.
Origin of broadly flavivirus cross-reactive MAbs used in this study
| MAb | Virus raised against | Serocomplex | Reference | Reference for mapping of binding site |
|---|---|---|---|---|
| A1 | TBEV | TBE | 27 | 1 |
| A2 | TBEV | TBE | 27 | 1 |
| IA3 | TBEV | TBE | 39 | |
| IF3 | TBEV | TBE | 39 | |
| IN3 | TBEV | TBE | 39 | |
| IO3 | TBEV | TBE | 39 | |
| IS3 | TBEV | TBE | 39 | |
| 4G2 | DENV | DEN | 22 | 13 |
| 843 | YFV | YF | 25 | |
| 813 | YFV | YF | 25 | |
| 528 | YFV | YF | 26 | |
| 612 | JEV | JE | 26 | |
| F7/3 | WNV | JE | 57 | |
| 6B6C1 | SLEVa | JE | 63 | 13 |
SLEV, Saint Louis encephalitis virus.
TABLE 2.
Specific titers of broadly flavivirus cross-reactive MAbs in ELISA and HI and NT assays
| MAb | ELISA titera
|
HI titerb
|
NT90 titerc
|
NT50 titerc
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TBEV | JEV | WNV | DENVd | TBEV | JEV | WNV | DENV-2 | TBEV | JEV | TBEV | JEV | |
| A1 | 208,900 | 513,300 | 733,300 | 154,800 | 512 | 2,048 | 4096 | 2,048 | <1 | <1 | 2-4e | 4-8e |
| A2 | 235,200 | 75,700 | 41,900 | 40 | 512 | 512 | 32 | <1 | <1 | <1 | <1 | <1 |
| IA3 | 1,200 | 2,700 | 3,700 | 300 | 512 | 2,048 | 4096 | 2048 | <1 | 1-2 | 1-2e | 4-8 |
| IF3 | 83,800 | 306,700 | 305,600 | 200,000 | 512 | 2,048 | 2,048 | 8192 | <1 | <1 | 2-4e | 4-8e |
| IN3 | 35,400 | 124,100 | 150,000 | 100,000 | 512 | 1,024 | 1,024 | 4096 | <1 | <1 | <1 | 4-8e |
| IO3 | 4,600 | 4,900 | 4,600 | 5,000 | 8 | 2 | 64 | 256 | <1 | <1 | <1 | <1 |
| IS3 | 6,900 | 12,300 | 11,400 | 5,000 | 1,024 | 2,048 | 2,048 | 2,048 | <1 | <1 | <1 | <1 |
| 4G2 | 26,600 | 118,600 | 144,400 | 83,300 | 256 | 512 | 512 | 512 | 1-2 | 1-2 | 4-8 | 4-8 |
| 843 | 200 | 1,600 | 800 | 200 | 2 | 16 | 2 | 2 | <1 | <1 | <1 | <1 |
| 813 | 200 | 5,200 | 5,000 | 80 | 2 | 4 | 4 | 2 | <1 | <1 | <1 | <1 |
| 528 | 800 | 2,400 | 2,700 | 2,000 | 16 | 64 | 64 | 16 | <1 | <1 | <1 | <1 |
| 612 | 11,200 | 119,600 | 105,500 | 100,000 | 256 | 2048 | 1,024 | 512 | <1 | <1 | <1 | <1 |
| F7/3 | 2,600 | 28,800 | 26,700 | 2,000 | 256 | 1,024 | 512 | 1,024 | <1 | 1-2 | 1-2e | 4-8 |
| 6B6C1 | 32,600 | 410,900 | 444,400 | 333,330 | 512 | 2,048 | 1,024 | 2,048 | <1 | <1 | <1 | <1 |
Specific titer for a MAb protein concentration of 100 μg/ml (cutoff for determination of titration endpoint, optical density of 0.1 at 490 nm); data are expressed as the average titer obtained in at least two (DENV) or four (TBEV, JEV, and WNV) independent experiments.
Specific titer for a MAb protein concentration of 100 μg/ml; data from at least two independent experiments.
Specific titer for a MAb protein concentration of 500 μg/ml; data from at least two independent experiments.
The ELISA antigen was a mixture of dengue virus types 1, 2, 3, and 4 (see Materials and Methods).
Even at the highest MAb protein concentration (500 μg/ml), only partial inhibition of virus replication was observed.
In contrast to their reactivities in ELISA and HI assays, the cross-reactive MAbs had no or low activities in neutralization assays against TBE and JE viruses. Only at the highest protein concentrations tested (250 to 500 μg/ml) was complete neutralization of TBE and/or JE virus detectable with three of the MAbs (IA3, 4G2, and F7/3) (NT90s in Table 2). In addition, some of the MAbs exhibited incomplete neutralization at high concentrations (A1, IF3, and IN3), and the corresponding NT50 values are also listed in Table 2. Taken together, these data indicate that (i) the MAbs tested did not recognize a “universal” cross-reactive epitope that is identical on all flaviviruses but instead recognized antigenic sites displaying significant virus-specific variations, in addition to their cross-reactive nature, and (ii) these sites—at least in TBE and JE viruses—appear to play a minor role, if any, in virus neutralization.
Previous evidence had indicated that the binding sites of some cross-reactive MAbs involved residues of the highly conserved FP loop located at the tip of domain II in the E protein (1, 13, 24) (Fig. 1B and C). To map the epitopes of all of the cross-reactive MAbs listed in Table 1, we made use of three mutant RSPs of TBE virus as antigens in a three-layer ELISA. In the mutant RSPs, residue leucine 107 (L107) of the FP loop (Fig. 1B and C) had been replaced by either aspartic acid (L107D), threonine (L107T), or phenylalanine (L107F). These mutations were neutral with respect to the overall structural organization of E and did not impair particle formation (1).
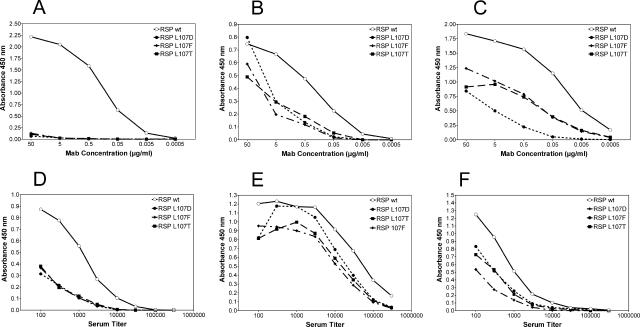
Because of their generally low reactivities with TBE antigens (Table 2), MAbs 843 and 813 did not yield conclusive results in these analyses. The other 12 cross-reactive MAbs (Table 2) exhibited lower reactivities with each of the mutant RSPs in comparison to the wild type. As illustrated by the examples displayed in Fig. 2A, B, and C, three different reactivity patterns could be discerned: (i) complete lack of reactivity with all three mutants (MAbs 4G2 and IN3) (Fig. 2A), (ii) equally reduced reactivities with all three mutants (MAbs A1, IA3, IO3, IS3, 528, 612, and F7/3) (Fig. 2B), and (iii) different degrees of reduced reactivity with individual mutants (MAbs A2, IF3, and 6B6C-1) (Fig. 2C). Consistent with the variation in reactivity patterns observed in ELISA and HI assays with different flaviviruses (Table 2), these results suggest differences in the fine specificities of the MAbs with respect to their binding sites in E and also indicate that all 12 of the cross-reactive MAbs tested recognized a cluster of epitopes at or around the FP loop.
FIG. 2.
Three-layer ELISA titration curves of broadly flavivirus cross-reactive MAbs (A to C) and polyclonal human postinfection dengue sera (D to F) using TBE wild-type (wt) RSP, as well as the mutant TBE RSPs L107D, L107F, and L017T, as coating antigens. (A, B, and C) Three representative examples of reactivity patterns obtained with the MAbs 4G2 (A), IS3 (B), and A2 (C). (D, E, and F) Three representative examples of reactivity patterns obtained with human polyclonal dengue sera 1 (D), 3 (E), and 5 (F) (Table 3). These data are representative of at least two independent experiments.
Mapping of cross-reactive antibodies in polyclonal immune sera.
In order to obtain information on the specificities of cross-reactive antibodies in a polyclonal immune response, we made use of a set of dengue virus postinfection sera. As expected, none of these sera was able to neutralize TBE virus completely, even at the lowest serum dilution of 1:10, and only a partial reduction of virus replication was observed at high antibody concentrations (NT50s in Table 3). In contrast, these sera were all strongly cross-reactive with TBE virus in HI assays and solid-phase three-layer ELISA (Table 3). To find out whether these polyclonal sera also contained antibodies directed to the principal site around the FP loop recognized by the panel of cross-reactive MAbs, we analyzed their reactivities with the same three TBE virus RSP FP loop mutants in a three-layer ELISA (Fig. 2D, E, and F). All of the DEN sera tested exhibited reduced binding to the three mutants compared to the wild-type RSP. The degrees of reduction, however, were variable, and different reactivity patterns were observed, similar to what was observed with the cross-reactive MAbs. Examples of such patterns are given in Fig. 2D, E, and F. The reactivities of four of the polyclonal sera (DEN sera 1, 2, 4, and 6) were reduced to the same extent with all three mutants (Fig. 2D), whereas two of them (DEN sera 3 and 5) exhibited variations in the degrees to which binding was reduced with the individual mutants (Fig. 2E and F). These data indicate that the polyclonal DEN sera contained antibodies specific for antigenic sites that included the FP loop and thus had specificities similar to those of the broadly flavivirus cross-reactive MAbs. As deduced by comparing the titers obtained with the mutants and the wild type, it can be estimated that on average about 30% of the cross-reactivity in the dengue sera was abolished by the mutations at amino acid position 107 in E.
TABLE 3.
Specific titers of human DEN and TBE postinfection sera in ELISA and HI and NT assaysa
| Serum no. | ELISA titer
|
HI titer
|
NT90 titer (TBEV) | NT50 titer (TBEV) | ||
|---|---|---|---|---|---|---|
| DENVb | TBEV | DENVc | TBEV | |||
| DEN serum 1 | 100,000 | 21,000 | 2,560 | 160 | <10 | 40e |
| DEN serum 2 | 20,000 | 5,000 | 640 | 160 | <10 | 40e |
| DEN serum 3 | 3,500000 | 800,000 | 163,840 | 5,120 | <10 | 40e |
| DEN serum 4 | 2,000000 | 1,800000 | 163,840 | 10,240 | <10 | 40e |
| DEN serum 5 | 30,000 | 18,000 | 2,560 | 160 | <10 | 40e |
| DEN serum 6 | 80,000 | 31,000 | 1,280 | 160 | <10 | 80e |
| TBE serumd | 200,000 | 500,000 | 2,560 | 10,240 | 960 | >1,280 |
Data shown in this table are the results from at least two independent experiments.
The ELISA antigen was a mixture of dengue virus types 1, 2, 3, and 4 (see Materials and Methods).
Dengue virus type 2.
Postvaccination serum.
Even at the lowest serum dilution of 1:10, only partial inhibition of virus replication was observed.
Evidence for cryptic properties of the principal cross-reactive site.
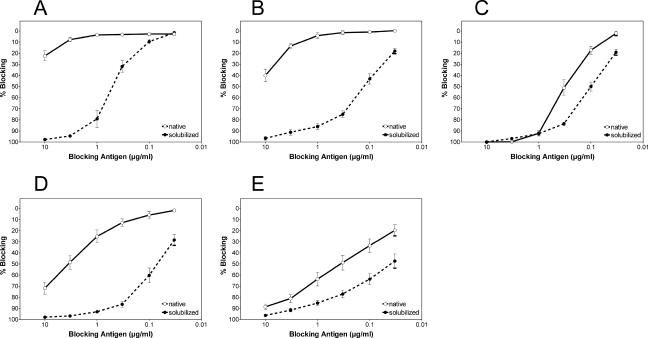
It is puzzling that antibodies that have high specific reactivities in three-layer ELISA and HI assays and are directed to the most highly conserved and functionally essential sequence element in E (the FP loop) are unable to neutralize the virus efficiently. One possible explanation for this phenomenon could be that the sites recognized are mostly buried or blocked at the surfaces of infectious virions but become accessible because of the way they are treated in the course of these assays. In the three-layer ELISA, it is well known that coating a solid phase with an antigen can lead to the antigen's partial denaturation (10, 20, 67) and also that the use of detergent (Tween 20 in our case) to reduce nonspecific binding leads to at least a partial disintegration of the viral envelope. In HI assays, the conditions are also quite harsh, including the exposure of the antigen to both strongly alkaline and slightly acidic pHs (12). In order to avoid these pitfalls, we established an assay format that allowed us to test the reactivities of antibodies with the surfaces of native infectious virions in solution (the blocking ELISA; see Materials and Methods). In this assay, the cross-reactive MAbs were preincubated with a dilution series of native infectious TBE virions before their addition to microtiter plates coated with purified TBE virus, enabling the detection by conventional three-layer ELISA of those antibodies that had not bound to the intact virions during the preincubation step. As in the previous experiments, the reactivities of MAbs 813 and 843 were too low to allow their inclusion in these analyses. Representative examples of the results obtained with the other 12 cross-reactive MAbs are shown in Fig. 3, and all of the data are summarized in Table 4. As indicated by the low degree of blocking even at the highest virus concentration tested (corresponding to at least a 20-fold molar excess of E proteins over antibodies), the cross-reactive MAbs were able to bind only weakly to native virions under the conditions used (Fig. 3A and B and Table 4). Solubilization of the virions with Triton X-100 before incubation with antibodies or the use of truncated sE dimers at the same molar amount of E protein, however, resulted in a significantly higher blocking activity (Fig. 3A and B and Table 4). In contrast, the non-cross-reactive TBE virus-specific control antibody (IM3) reacted strongly with native virions in solution, as indicated by the complete blocking of its reactivity with the solid-phase antigen (Fig. 3C). Also in this case, however, a slight shift in the blocking curve was observed at antigen concentrations of 0.33 μg/ml and below, i.e., in the range of antibody excess over the blocking antigen. This shift could indicate that only a fraction of the possible 180 binding sites on the native virion are occupied by MAb IM3, possibly as a result of steric hindrance and/or nonequivalent binding to different E molecules, which are present in three different chemical environments in the lattice structure (Fig. 1; see also Fig. 5) (42, 52). However, as observed with the cross-reactive MAbs (Fig. 3A and B), the high efficiency of blocking by soluble E protein compared to the low efficiency of blocking by native virions suggests that their epitopes are inaccessible or only partially accessible at the surfaces of infectious virions.
FIG. 3.
Blocking ELISA with TBE virus and cross-reactive monoclonal and polyclonal antibodies. A predetermined fixed dilution of the cross-reactive MAbs A2 (A) and 4G2 (B), the TBE virus-specific MAb IM3 (C), a pool of 11 polyclonal human dengue postinfection sera (D), or a pool of 10 polyclonal human TBE postinfection and postvaccination sera (E) was mixed with decreasing concentrations of TBE virus, either untreated (native) or solubilized with Triton X-100 (solubilized), as a blocking antigen. The molar ratios of E to MAb at the highest blocking antigen concentration were 250:1 (A2 and 4G2) and 500:1 (IM3). Then, the mixture was transferred to ELISA plates coated with purified virus to detect antibodies that had not bound to the blocking antigen in solution. The results are expressed as percent blocking, defined as the percent reduction in absorbance in the presence of the blocking antigen compared to the absorbance value that was obtained with the corresponding antibody sample in the absence of a blocking antigen: 100 − (absorbance with blocking antigen/absorbance without blocking antigen) × 100. The data are the averages of at least four independent experiments, and the error bars represent the standard errors of the mean.
TABLE 4.
Reactivities of broadly cross-reactive MAbs with native and solubilized TBE virus in a blocking ELISAa
| MAb | Reactivityb
|
||
|---|---|---|---|
| Native TBEV | TX-100-solubilized TBEV | sE dimer | |
| A1 | + | ++++ | ++++ |
| A2 | +/− | ++++ | ++++ |
| IA3 | − | ++++ | ++++ |
| IF3 | +/− | ++++ | ++++ |
| IN3 | − | ++++ | ++++ |
| IO3 | − | +++ | ++++ |
| IS3 | − | +++ | ++++ |
| 4G2 | + | ++++ | ++++ |
| 528 | + | +++ | ++++ |
| 612 | − | ++ | ++ |
| F7/3 | − | +/− | + |
| 6B6C1 | − | +/− | ++ |
| IM3 (control) | ++++ | ++++ | ++++ |
Data shown in this table are the results from at least two independent experiments.
Blocking of MAb reactivity at a virus protein concentration of 10 μg/ml; sE was used at the same molar E protein concentration: 0 to 15%, −; 16 to 30%, +/−; 31 to 45%, +; 46 to 70%, ++; 71 to 90%, +++; 91 to 100%, ++++.
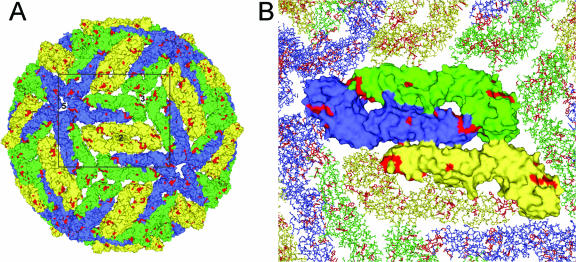
FIG. 5.
(A) Model of the structure of the mature virus particle using surface representations of the TBE virus E protein. The E monomers at the five-, three-, and twofold axes (corresponding to three different chemical environments) are colored blue, green, and yellow, respectively. Highlighted in red are amino acid residues that are surface exposed in all flaviviruses. (B) Enlargement of the section in panel A indicated by the rectangle. Shown is the exposed surface of one icosahderal asymmetric unit consisting of three E monomers, depicted as surface representations. The images were prepared using the PyMol program (14).
The general relevance of the data obtained with MAbs was also assessed using a pool of polyclonal dengue postinfection sera in the same type of blocking ELISA described above (Fig. 3D). The result was similar to that with the cross-reactive MAbs, i.e., the binding of the cross-reactive polyclonal antibodies in the dengue sera to the solid-phase TBE virus antigen was blocked much more efficiently by the solubilized virus than by the native virus preparation (Fig. 3D). As expected, much smaller differences were observed with a pool of control TBE sera (Fig. 3E), similar to those obtained with the neutralizing MAb IM3 (Fig. 3C).
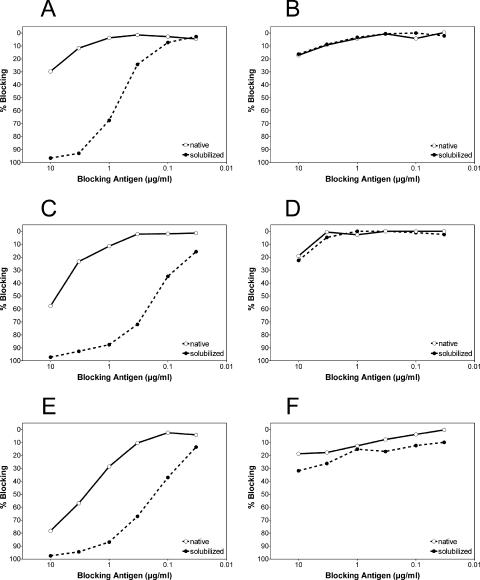
The data obtained so far indicate that the broadly flavivirus cross-reactive MAbs tested, as well as cross-reactive antibody populations present in polyclonal immune sera, recognize antigenic sites around the FP loop that are not fully accessible at the surfaces of native TBE virions but become significantly more exposed after disintegration of the envelope. If this conclusion is correct, one would expect that the observed enhancement of reactivity by solubilization (as displayed in Table 4 and Fig. 3) would be much less pronounced or absent with RSPs containing the FP mutations that were used for mapping the cross-reactive site. We therefore performed blocking experiments like the ones shown in Fig. 3 but, instead of native virions, used wild-type or mutant (L107D) RSPs in the preincubation step with antibodies. As shown in Fig. 4A, C, and E, RSPs with a wild-type FP sequence yielded results similar to those obtained with virions (compare Fig. 4 with Fig. 3A, B, and D), i.e., a dramatic solubilization-induced increase in the ability to block MAbs A2 and 4G2 (Fig. 4A and C), as well as cross-reactive antibodies in polyclonal dengue sera (Fig. 4E). In contrast, no such effect was observed when the same experiment was carried out with the mutant RSP L107D instead of wild-type RSP (Fig. 4B, D, and F). These results are consistent with the conclusions drawn from the previous experiments and confirm the dominant role of the FP loop in the solubilization-enhanced reactivities of broadly flavivirus cross-reactive antibodies.
FIG. 4.
(A, C, and E) Blocking ELISA using TBE virus wild-type RSP, either untreated (native) or solubilized with Triton X-100 (solubilized), as a blocking antigen. (A) Blocking of MAb A2. (C) Blocking of MAb 4G2. (E) Blocking of antibodies in a pool of 11 polyclonal human dengue postinfection sera. (B, D, and F) Blocking ELISA using mutant TBE virus RSP L107D, either untreated or solubilized with Triton X-100, as a blocking antigen. (B) Blocking of MAb A2. (D) Blocking of MAb 4G2. (F) Blocking of antibodies in a pool of 11 polyclonal human dengue postinfection sera. The results are expressed as percent blocking, defined as the percent reduction in absorbance in the presence of the blocking antigen compared to the absorbance value that was obtained with the corresponding antibody sample in the absence of a blocking antigen: 100 − (absorbance with blocking antigen/absorbance without blocking antigen) × 100. The data are average curves from at least two independent experiments.
When the blocking curves obtained with native virions (Fig. 3A, B, and D) are compared to those obtained with RSPs (Fig. 4A, C, and E), it is apparent that the cross-reactive epitopes are somewhat more accessible in untreated RSPs than in untreated virions. This could be due to a lower stability of the capsidless subviral particles or could reflect the different geometry and more open arrangement of E dimers on the RSP surface compared to the dense packing found on virions (19, 42, 49).
Relative avidities of cross-reactive antibodies in different assay formats.
To obtain quantitative figures on the observed differences in binding to whole virions, partially denatured or disintegrated virions, or soluble forms of E, we measured the relative avidities of two selected cross-reactive MAbs (A2 and 4G2) and the TBE virus type-specific control MAb IM3 in different ELISA formats that presented the E protein in different configurations and were expected to preserve native E protein structures to different degrees (see Materials and Methods). These included (i) a four-layer ELISA with native TBE virus in the absence of Tween 20, a format presumed to preserve the intact envelope structure of the virion; (ii) a four-layer ELISA with TX-100-solubilized virus in the presence of Tween 20; (iii) a three-layer ELISA with native TBE virus in the absence of Tween 20; and (iv) the same assay in the presence of Tween 20. As can be seen in Table 5, the avidities of the cross-reactive MAbs in the four-layer ELISA with native TBE virus were very low (saturation was still not reached at 200 nM, the highest MAb concentration tested). Significantly higher values (more than 400-fold in the case of A2 and more than 70-fold in the case of 4G2) were obtained when TX-100-solubilized virus was used as an antigen in the same assay format. With the control antibody IM3, almost no difference was observed under these conditions.
TABLE 5.
Relative ELISA avidities of the two flavivirus cross-reactive MAbs, A2 and 4G2, and the TBE-virus-specific MAb IM3a
| MAb | Four-layer ELISA avidity constant (nM)
|
Three-layer ELISA avidity constant (nM)
|
||
|---|---|---|---|---|
| Native TBEV | TX-100-solubilized TBEV | TBEV | TBEV + Tween | |
| A2 | >100b | 0.25 ± 0.09 | 1.81 ± 0.06 | 0.93 ± 0.11 |
| 4G2 | >100b | 1.38 ± 0.22 | 7.43 ± 0.97 | 5.79 ± 0.67 |
| IM3 (control) | 0.57 ± 0.19 | 0.41 ± 0.18 | 0.42 ± 0.20 | 0.23 ± 0.02 |
Results are expressed as the means of data from at least four independent experiments using duplicate antibody dilutions (mean ± SEM).
No saturation was observed up to a MAb concentration of 200 nM.
In the three-layer ELISA, the relative avidities between the cross-reactive MAbs and virions that coated the solid phase were significantly higher (and even more so in the presence of Tween 20) than in the four-layer ELISA with native virions (Table 5). The neutralizing MAb IM3, however, yielded similar results in all assay formats. These data, together with those shown in Fig. 3 and 4 and Table 4, support the conclusion that the lack of efficient neutralizing activity by the MAbs shown in Table 1 and broadly flavivirus cross-reactive antibodies in polyclonal sera is indeed due to inefficient binding to the surfaces of native infectious virions.
DISCUSSION
It is a characteristic feature of flavivirus postinfection or postimmunization sera that they contain a subset of strongly cross-reactive antibodies that lack significant cross-neutralizing activity. In our work with TBE virus, we have provided evidence that monoclonal and polyclonal antibodies of this type are directed to antigenic sites around the FP loop in the E protein that is largely occluded at the surfaces of infectious virions. This conclusion is primarily based on the finding that the reactivities of such antibodies were much higher with disintegrated virions and soluble forms of E than with native whole-virus preparations. Because of the three different chemical environments of E at the virion surface (Fig. 1 and 5), nonequivalent binding of antibodies to such monomers that could also be influenced by virus disintegration is theoretically possible. This structural peculiarity alone, however, cannot account for the extent of the phenomena observed. In any case, the cryptic properties of cross-reactive sites offer an explanation for the observed low specific neutralizing activities of the corresponding antibodies, which form the basis for the subdivision of flaviviruses into several serocomplexes (Fig. 1) (11, 15, 34). In the present study, neutralization of TBE and JE viruses was observed at high concentrations of some MAbs, which in most instances was incomplete (NT50s in Table 2). Only three of the MAbs were able to almost completely inhibit virus replication (NT90), albeit at concentrations of 250 to 500 μg/ml. In serum, the concentration of IgG antibodies to a specific virus has been calculated to be only about 100 μg/ml (21), and therefore, broadly cross-reactive antibodies with such low specific neutralizing activities would not be predicted to make a significant contribution to the overall neutralizing activities of polyclonal sera—consistent with the results of cross-neutralization data between flaviviruses from different serocomplexes (11, 15). In the flavivirus literature, the neutralizing activities of cross-reactive MAbs have also generally been classified as low compared to those of less broadly reactive MAbs, although specific activities are not indicated in most of these studies (6, 22, 25, 28, 57, 62).
Since the reactivity patterns of cross-reactive antibodies displayed a great degree of heterogeneity when tested against different flaviviruses, as well as TBE virus FP mutants (Table 2 and Fig. 2), it can be concluded that the epitopes recognized are not identical. Considering the relatively large size of an Fab footprint (600 to 900 Å) and the small number of conserved surface-exposed amino acids in the FP loop, it is indeed unlikely that these would constitute the only critical residues at the interaction site (Fig. 5). The specific reactivity patterns observed would instead be more compatible with a cluster of antibody binding sites that involve both highly conserved amino acids, as a prerequisite for cross-reactivity, and more variable residues. It can be envisaged that such differences in the relative contributions of conserved (FP loop) and surrounding variable amino acids might result not only in a whole range of epitopes with different levels of cross-reactivity, but also in different degrees of accessibility at the surfaces of infectious virions. Such a complex pattern could help to explain the characteristics of some partially cross-reactive antibodies described in the literature. For instance, some cross-reactive MAbs obtained by immunization with yellow fever virus were unable to neutralize JE and TBE viruses, and even in the homologous system could neutralize only certain strains of YFV and not others (6). In another study on the characterization of a cross-reactive humanized antibody derived from a chimpanzee infected with all four dengue virus serotypes (23, 24), it was shown that its binding was strongly affected by a mutation in the FP loop (Gly to Val at position 106), and its reactivities in a solid-phase three-layer ELISA with all four dengue virus serotypes were very similar. In neutralization, however, the antibody displayed a strong activity only against DEN type 1 and 2 viruses, significantly lower activities against DEN type 3 and 4 viruses, and very low activities against JE and Langat viruses, suggesting variations in the contributions of specificity- and function-determining surface-exposed residues in the different viruses.
Like virus neutralization, the phenomenon of antibody-mediated enhancement of infection requires antibody binding to the surfaces of infectious virions and has been described for broadly flavivirus cross-reactive antibodies, including MAb 4G2 (17, 28). This MAb was originally reported to have low neutralizing activity against DEN type 1 to 4 viruses but no activity against JE virus (22, 28), and in the present study, the specific activities against TBE and JE viruses were also very low. Both the low neutralizing activity and the enhancing activity might be explained by low-affinity binding to virions, as experimentally shown here for the interaction of 4G2 with TBE virus. Interestingly, infection enhancement of DEN type 2 virus by 4G2 was demonstrable only with certain virus strains and not with others (17, 28), suggesting that subtle structural differences can influence the capacity of infection by a given virus to be enhanced by a certain antibody. In this context, it is important to note that considerable variation in the use of the glycosylation site in domain I (Fig. 1) (Asn 154 in TBEV and 153 in DENV E) has been reported for different flaviviruses, including dengue virus (40) and WN virus (4). This carbohydrate forms a protective cover over the buried FP loop in the native E dimer structure (47, 48, 59) and thus probably contributes to its shielding at the surfaces of intact virions. In the absence of this carbohydrate, the accessibility of the cross-reactive site would be expected to be increased. Whether such an effect contributes to some of the virus- and strain-specific phenomena observed with flavivirus cross-reactive antibodies awaits experimental proof.
Originally, the broad cross-reactivity between all flaviviruses was revealed by HI assays with polyclonal sera (58). In these assays, the viral antigen is exposed to alkaline pH (9.0) during the preincubation step with antibody, followed by acidification, which is required for the interaction of the viral hemagglutinin with red blood cells (12). It is now known that both of these treatments lead to a disintegration of the E protein assemblies that are characteristic of native virions (2) (K. Stiasny, C. Koessl, J. Lepault, F. A. Rey, and F. X. Heinz, submitted for publication), thereby making the conserved FP loop accessible for antibody binding. It is important to emphasize that the E-protein-mediated hemagglutination of flaviviruses does not occur at neutral pH and is thus not correlated with receptor binding, as is the case with influenza virus (69). Instead, it reflects the interaction of E, presumably via the FP loop, with red blood cell membranes as a consequence of the acidic-pH-induced structural changes that are normally required for membrane fusion in endosomes (1, 5). Since cross-reactive nonneutralizing antibodies significantly contribute to the inhibition of hemagglutination, HI titers, in contrast to the situation with influenza virus, are not useful for estimating flavivirus neutralization activity. In the three-layer ELISA, the structural changes leading to the observed broad flavivirus cross-reactivities are presumably less well defined than those in the HI assay and are assumed to be primarily due to denaturation and/or disintegration effects caused by coating, washing procedures, and the use of detergent-containing buffers (10, 20, 67). The influences of different assay formats on the ELISA reactivities obtained, as shown in this work, are indeed striking. Most importantly, only low-avidity binding of cross-reactive antibodies could be shown in a four-layer ELISA with native virions, whereas high-avidity binding required assay configurations that led to partial disintegration and/or solubilization of the virion envelope (Table 5).
It is not possible from our data to exclude the possibility that additional sites in E may be recognized by other types of broadly flavivirus cross-reactive antibodies, but it appears that the site involving the FP loop is quite dominant. All 12 of the MAbs tested in this study (which were raised against seven different flaviviruses from four different serocomplexes) (Table 1) recognized the same site, albeit with variations in fine specificity. Also, a significant proportion of cross-reactive antibodies in polyclonal dengue postinfection sera exhibited reduced binding with an FP loop mutant and therefore appeared to be specific for this site. The presence in postinfection human sera of broadly cross-reactive nonneutralizing antibodies that reacted much more strongly with disintegrated forms than with native virions suggests that cryptic sites become accessible and are presented to the immune system in the course of natural flavivirus infections. This is reminiscent of studies on the antibody responses to human immunodeficiency virus (54) and respiratory syncytial virus (64) in humans, as well as to lymphocytic choriomeningitis virus in mice (3), which have demonstrated that a significant proportion of the total of all antibodies elicited by these viruses consist of nonneutralizing antibodies directed to immature or disintegrated/denatured forms of protein antigens that in their native conformations would otherwise induce and bind neutralizing antibodies (29, 53, 56).
As with other viruses, there are several possibilities that can lead to the presentation of antigenic sites in the flavivirus E protein that are partially or completely inaccessible in the context of native infectious virions (29). These include the partial disintegration of mature or immature viral and subviral particles during the course of infection, the secretion of soluble forms of E from infected cells, and the release of incompletely processed and/or assembled E proteins from lysed cells. There are still many unresolved questions concerning the relative amounts, specificities, and functional activities of nonneutralizing antibodies generated as a result of flavivirus infection or immunization that need to be investigated in the future.
Acknowledgments
We thank Silvia Röhnke and Jutta Hutecek for their excellent technical assistance throughout the course of this work and Steven Allison for critical reading of the manuscript. We also thank Ernest A. Gould, John T. Roehrig, Herbert Schmitz, and Suwanna Sinsawaiwong-Sethawacharawanit for providing monoclonal antibodies and polyclonal sera. We are also grateful to Walter Holzer for help with virus production and to Irene Goerzer for help with the construction of the dendrogram.
This work was supported by the Austrian Science Fund (“Fonds zur Foerderung der wissenschaftlichen Forschung”), FWF project number P17035-B09.
REFERENCES
- 1.Allison, S. L., J. Schalich, K. Stiasny, C. W. Mandl, and F. X. Heinz. 2001. Mutational evidence for an internal fusion peptide in flavivirus envelope protein E. J. Virol. 75:4268-4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison, S. L., J. Schalich, K. Stiasny, C. W. Mandl, C. Kunz, and F. X. Heinz. 1995. Oligomeric rearrangement of tick-borne encephalitis virus envelope proteins induced by an acidic pH. J. Virol. 69:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battegay, M., D. Moskophidis, H. Waldner, M. A. Brundler, W. P. Fung-Leung, T. W. Mak, H. Hengartner, and R. M. Zinkernagel. 1993. Impairment and delay of neutralizing antiviral antibody responses by virus-specific cytotoxic T cells. J. Immunol. 151:5408-5415. [PubMed] [Google Scholar]
- 4.Beasley, D. W., M. C. Whiteman, S. Zhang, C. Y. Huang, B. S. Schneider, D. R. Smith, G. D. Gromowski, S. Higgs, R. M. Kinney, and A. D. Barrett. 2005. Envelope protein glycosylation status influences mouse neuroinvasion phenotype of genetic lineage 1 West Nile virus strains. J. Virol. 79:8339-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bressanelli, S., K. Stiasny, S. L. Allison, E. A. Stura, S. Duquerroy, J. Lescar, F. X. Heinz, and F. A. Rey. 2004. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 23:728-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckley, A., and E. A. Gould. 1985. Neutralization of yellow fever virus studied using monoclonal and polyclonal antibodies. J. Gen. Virol. 66:2523-2531. [DOI] [PubMed] [Google Scholar]
- 7.Burke, D. S., and T. P. Monath. 2001. Flaviviruses, p. 1043-1125. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 8.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5:233-236. [DOI] [PubMed] [Google Scholar]
- 9.Burton, D. R., E. O. Saphire, and P. W. Parren. 2001. A model for neutralization of viruses based on antibody coating of the virion surface. Curr. Top. Microbiol. Immunol. 260:109-143. [DOI] [PubMed] [Google Scholar]
- 10.Butler, J. E. 2004. Solid supports in enzyme-linked immunosorbent assay and other solid-phase immunoassays. Methods Mol. Med. 94:333-372. [DOI] [PubMed] [Google Scholar]
- 11.Calisher, C. H., N. Karabatsos, J. M. Dalrymple, R. E. Shope, J. S. Porterfield, E. G. Westaway, and W. E. Brandt. 1989. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J. Gen. Virol. 70:37-43. [DOI] [PubMed] [Google Scholar]
- 12.Clarke, D. H., and J. Casals. 1958. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am. J. Trop. Med. Hyg. 7:561-573. [DOI] [PubMed] [Google Scholar]
- 13.Crill, W. D., and G. J. Chang. 2004. Localization and characterization of flavivirus envelope glycoprotein cross-reactive epitopes. J. Virol. 78:13975-13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeLano, W. L. 2002. The PyMOL molecular graphics system. DeLano Scientific, San Carlos, Calif.
- 15.De Madrid, A. T., and J. S. Porterfield. 1974. The flaviviruses (group B arboviruses): a cross-neutralization study. J. Gen. Virol. 23:91-96. [DOI] [PubMed] [Google Scholar]
- 16.Deriabin, P. G., and N. V. Loginova. 1995. A highly-productive attenuated variant of Japanese encephalitis virus. Vopr. Virusol. 40:265-268. [PubMed] [Google Scholar]
- 17.Diamond, M. S., D. Edgil, T. G. Roberts, B. Lu, and E. Harris. 2000. Infection of human cells by dengue virus is modulated by different cell types and viral strains. J. Virol. 74:7814-7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Earp, L. J., S. E. Delos, H. E. Park, and J. M. White. 2005. The many mechanisms of viral membrane fusion proteins. Curr. Top. Microbiol. Immunol. 285:25-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferlenghi, I., M. Clarke, T. Ruttan, S. L. Allison, J. Schalich, F. X. Heinz, S. C. Harrison, F. A. Rey, and S. D. Fuller. 2001. Molecular organization of a recombinant subviral particle from tick-borne encephalitis virus. Mol. Cell 7:593-602. [DOI] [PubMed] [Google Scholar]
- 20.Friguet, B., L. Djavadi-Ohaniance, and M. E. Goldberg. 1984. Some monoclonal antibodies raised with a native protein bind preferentially to the denatured antigen. Mol. Immunol. 21:673-677. [DOI] [PubMed] [Google Scholar]
- 21.Funk, G. A., A. D. Barbour, H. Hengartner, and U. Kalinke. 1998. Mathematical model of a virus-neutralizing immunglobulin response. J. Theor. Biol. 195:41-52. [DOI] [PubMed] [Google Scholar]
- 22.Gentry, M. K., E. A. Henchal, J. M. McCown, W. E. Brandt, and J. M. Dalrymple. 1982. Identification of distinct antigenic determinants on dengue-2 virus using monoclonal antibodies. Am. J. Trop. Med. Hyg. 31:548-555. [DOI] [PubMed] [Google Scholar]
- 23.Goncalvez, A. P., R. Men, C. Wernly, R. H. Purcell, and C. J. Lai. 2004. Chimpanzee Fab fragments and a derived humanized immunoglobulin g1 antibody that efficiently cross-neutralize dengue type 1 and type 2 viruses. J. Virol. 78:12910-12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goncalvez, A. P., R. H. Purcell, and C. J. Lai. 2004. Epitope determinants of a chimpanzee Fab antibody that efficiently cross-neutralizes dengue type 1 and type 2 viruses map to inside and in close proximity to fusion loop of the dengue type 2 virus envelope glycoprotein. J. Virol. 78:12919-12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gould, E. A., A. Buckley, N. Cammack, A. D. Barrett, J. C. Clegg, R. Ishak, and M. G. Varma. 1985. Examination of the immunological relationships between flaviviruses using yellow fever virus monoclonal antibodies. J. Gen. Virol. 66:1369-1382. [DOI] [PubMed] [Google Scholar]
- 26.Gould, E. A., A. Buckley, S. Higgs, and S. Gaidamovich. 1989. Antigenicity of flaviviruses. Arch. Virol. Suppl. 1:137-152. [Google Scholar]
- 27.Guirakhoo, F., F. X. Heinz, and C. Kunz. 1989. Epitope model of tick-borne encephalitis virus envelope glycoprotein E: analysis of structural properties, role of carbohydrate side chain, and conformational changes occurring at acidic pH. Virology 169:90-99. [DOI] [PubMed] [Google Scholar]
- 28.Halstead, S. B., C. N. Venkateshan, M. K. Gentry, and L. K. Larsen. 1984. Heterogeneity of infection enhancement of dengue 2 strains by monoclonal antibodies. J. Immunol. 132:1529-1532. [PubMed] [Google Scholar]
- 29.Hangartner, L., R. M. Zinkernagel, and H. Hengartner. 2006. Antiviral antibody responses: the two extremes of a wide spectrum. Nat. Rev. Immunol. 6:231-243. [DOI] [PubMed] [Google Scholar]
- 30.Harrison, S. C. 2005. Mechanism of membrane fusion by viral envelope proteins. Adv. Virus Res. 64:231-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinz, F. X. 1986. Epitope mapping of flavivirus glycoproteins. Adv. Virus Res. 31:103-168. [DOI] [PubMed] [Google Scholar]
- 32.Heinz, F. X., and S. L. Allison. 2003. Flavivirus structure and membrane fusion. Adv. Virus Res. 59:63-97. [DOI] [PubMed] [Google Scholar]
- 33.Heinz, F. X., R. Berger, O. Majdic, W. Knapp, and C. Kunz. 1982. Monoclonal antibodies to the structural glycoprotein of tick-borne encephalitis virus. Infect. Immun. 37:869-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heinz, F. X., M. S. Collett, R. H. Purcell, E. A. Gould, C. R. Howard, M. Houghton, R. J. M. Moormann, C. M. Rice, and H.-J. Thiel. 2000. Family Flaviviridae, p. 859-878. In M. H. V. Van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy. Seventh report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 35.Heinz, F. X., and C. Kunz. 1981. Homogeneity of the structural glycoprotein from European isolates of tick-borne encephalitis virus: comparison with other flaviviruses. J. Gen. Virol. 57:263-274. [DOI] [PubMed] [Google Scholar]
- 36.Heinz, F. X., C. W. Mandl, H. Holzmann, C. Kunz, B. A. Harris, F. Rey, and S. C. Harrison. 1991. The flavivirus envelope protein E: isolation of a soluble form from tick-borne encephalitis virus and its crystallization. J. Virol. 65:5579-5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinz, F. X., K. Stiasny, G. Puschner-Auer, H. Holzmann, S. L. Allison, C. W. Mandl, and C. Kunz. 1994. Structural changes and functional control of the tick-borne encephalitis virus glycoprotein E by the heterodimeric association with protein prM. Virology 198:109-117. [DOI] [PubMed] [Google Scholar]
- 38.Holzmann, H., M. Kundi, K. Stiasny, J. Clement, P. McKenna, C. Kunz, and F. X. Heinz. 1996. Correlation between ELISA, hemagglutination inhibition, and neutralization tests after vaccination against tick-borne encephalitis. J. Med. Virol. 48:102-107. [DOI] [PubMed] [Google Scholar]
- 39.Holzmann, H., K. Stiasny, H. York, F. Dorner, C. Kunz, and F. X. Heinz. 1995. Tick-borne encephalitis virus envelope protein E-specific monoclonal antibodies for the study of low pH-induced conformational changes and immature virions. Arch. Virol. 140:213-221. [DOI] [PubMed] [Google Scholar]
- 40.Johnson, A. J., F. Guirakhoo, and J. T. Roehrig. 1994. The envelope glycoproteins of dengue 1 and dengue 2 viruses grown in mosquito cells differ in their utilization of potential glycosylation sites. Virology 203:241-249. [DOI] [PubMed] [Google Scholar]
- 41.Kielian, M., and F. A. Rey. 2006. Virus membrane-fusion proteins: more than one way to make a hairpin. Nat. Rev. Microbiol. 4:67-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuhn, R. J., W. Zhang, M. G. Rossmann, S. V. Pletnev, J. Corver, E. Lenches, C. T. Jones, S. Mukhopadhyay, P. R. Chipman, E. G. Strauss, T. S. Baker, and J. H. Strauss. 2002. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 44.Lanciotti, R. S., J. T. Roehrig, V. Deubel, J. Smith, M. Parker, K. Steele, B. Crise, K. E. Volpe, M. B. Crabtree, J. H. Scherret, R. A. Hall, J. S. Mackenzie, C. B. Cropp, B. Panigrahy, E. Ostlund, B. Schmitt, M. Malkinson, C. Banet, J. Weissman, N. Komar, H. M. Savage, W. Stone, T. McNamara, and D. J. Gubler. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286:2333-2337. [DOI] [PubMed] [Google Scholar]
- 45.Mackenzie, J. S., D. J. Gubler, and L. R. Petersen. 2004. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 10:S98-S109. [DOI] [PubMed] [Google Scholar]
- 46.Mandl, C. W., F. X. Heinz, and C. Kunz. 1988. Sequence of the structural proteins of tick-borne encephalitis virus (western subtype) and comparative analysis with other flaviviruses. Virology 166:197-205. [DOI] [PubMed] [Google Scholar]
- 47.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2003. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. USA 100:6986-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2005. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J. Virol. 79:1223-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mukhopadhyay, S., B. S. Kim, P. R. Chipman, M. G. Rossmann, and R. J. Kuhn. 2003. Structure of West Nile virus. Science 302:248. [DOI] [PubMed] [Google Scholar]
- 50.Mukhopadhyay, S., R. J. Kuhn, and M. G. Rossmann. 2005. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 3:13-22. [DOI] [PubMed] [Google Scholar]
- 51.Nabel, G. J. 2002. HIV vaccine strategies. Vaccine 20:1945-1947. [DOI] [PubMed] [Google Scholar]
- 52.Nybakken, G. E., T. Oliphant, S. Johnson, S. Burke, M. S. Diamond, and D. H. Fremont. 2005. Structural basis of West Nile virus neutralization by a therapeutic antibody. Nature 437:764-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parren, P. W., D. R. Burton, and Q. J. Sattentau. 1997. HIV-1 antibody—debris or virion? Nat. Med. 3:366-367. [DOI] [PubMed] [Google Scholar]
- 54.Parren, P. W., I. Mondor, D. Naniche, H. J. Ditzel, P. J. Klasse, D. R. Burton, and Q. J. Sattentau. 1998. Neutralization of human immunodeficiency virus type 1 by antibody to gp120 is determined primarily by occupancy of sites on the virion irrespective of epitope specificity. J. Virol. 72:3512-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parren, P. W., J. P. Moore, D. R. Burton, and Q. J. Sattentau. 1999. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS 13(Suppl. A):S137-S162. [PubMed] [Google Scholar]
- 56.Parren, P. W., P. Poignard, H. J. Ditzel, R. A. Williamson, and D. R. Burton. 2000. Antibodies in human infectious disease. Immunol. Res. 21:265-278. [DOI] [PubMed] [Google Scholar]
- 57.Peiris, J. S., J. S. Porterfield, and J. T. Roehrig. 1982. Monoclonal antibodies against the flavivirus West Nile. J. Gen. Virol. 58:283-289. [DOI] [PubMed] [Google Scholar]
- 58.Porterfield, J. S. 1975. The basis of arbovirus classification. Med. Biol. 53:400-405. [PubMed] [Google Scholar]
- 59.Rey, F. A., F. X. Heinz, C. Mandl, C. Kunz, and S. C. Harrison. 1995. The envelope glycoprotein from tick-borne encephalitis virus at 2 Å resolution. Nature 375:291-298. [DOI] [PubMed] [Google Scholar]
- 60.Roehrig, J. T. 1982. Development of an enzyme-linked immunosorbent assay for the identification of arthropod-borne togavirus antibodies. J. Gen. Virol. 63:237-240. [DOI] [PubMed] [Google Scholar]
- 61.Roehrig, J. T. 2003. Antigenic structure of flavivirus proteins. Adv. Virus Res. 59:141-175. [DOI] [PubMed] [Google Scholar]
- 62.Roehrig, J. T., R. A. Bolin, and R. G. Kelly. 1998. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology 246:317-328. [DOI] [PubMed] [Google Scholar]
- 63.Roehrig, J. T., J. H. Mathews, and D. W. Trent. 1983. Identification of epitopes on the E glycoprotein of Saint Louis encephalitis virus using monoclonal antibodies. Virology 128:118-126. [DOI] [PubMed] [Google Scholar]
- 64.Sakurai, H., R. A. Williamson, J. E. Crowe, J. A. Beeler, P. Poignard, R. B. Bastidas, R. M. Chanock, and D. R. Burton. 1999. Human antibody responses to mature and immature forms of viral envelope in respiratory syncytial virus infection: significance for subunit vaccines. J. Virol. 73:2956-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schalich, J., S. L. Allison, K. Stiasny, C. W. Mandl, C. Kunz, and F. X. Heinz. 1996. Recombinant subviral particles from tick-borne encephalitis virus are fusogenic and provide a model system for studying flavivirus envelope glycoprotein functions. J. Virol. 70:4549-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schibli, D. J., and W. Weissenhorn. 2004. Class I and class II viral fusion protein structures reveal similar principles in membrane fusion. Mol. Membr. Biol. 21:361-371. [DOI] [PubMed] [Google Scholar]
- 67.Schwab, C., and H. R. Bosshard. 1992. Caveats for the use of surface-adsorbed protein antigen to test the specificity of antibodies. J. Immunol. Methods 147:125-134. [DOI] [PubMed] [Google Scholar]
- 68.Sissons, J. G., and M. B. Oldstone. 1980. Antibody-mediated destruction of virus-infected cells. Adv. Immunol. 29:209-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69:531-569. [DOI] [PubMed] [Google Scholar]
- 70.Solomon, T., and M. Mallewa. 2001. Dengue and other emerging flaviviruses. J. Infect. 42:104-115. [DOI] [PubMed] [Google Scholar]
- 71.Stiasny, K., S. L. Allison, J. Schalich, and F. X. Heinz. 2002. Membrane interactions of the tick-borne encephalitis virus fusion protein E at low pH. J. Virol. 76:3784-3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whitton, J. L., M. K. Slifka, F. Liu, A. K. Nussbaum, and J. K. Whitmire. 2004. The regulation and maturation of antiviral immune responses. Adv. Virus Res. 63:181-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang, W., P. R. Chipman, J. Corver, P. R. Johnson, Y. Zhang, S. Mukhopadhyay, T. S. Baker, J. H. Strauss, M. G. Rossmann, and R. J. Kuhn. 2003. Visualization of membrane protein domains by cryo-electron microscopy of dengue virus. Nat. Struct. Biol. 10:907-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang, Y., W. Zhang, S. Ogata, D. Clements, J. H. Strauss, T. S. Baker, R. J. Kuhn, and M. G. Rossmann. 2004. Conformational changes of the flavivirus E glycoprotein. Structure 12:1607-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]