Abstract
The vaccinia virus G9R gene (VACWR087) encodes a protein of 340 amino acids with the following structural features that are conserved in all poxviruses: a site for N-terminal myristoylation, 14 cysteines, and a C-terminal transmembrane domain. Previous studies showed that G9 is one of eight proteins associated in a putative entry-fusion complex. Our attempt to isolate a mutant without the G9R gene was unsuccessful, suggesting that it is essential for virus replication. To further investigate its role, we constructed a recombinant vaccinia virus in which G9R is regulated by addition of an inducer. Induced G9 protein was associated with mature infectious virions and could be labeled with a membrane-impermeant biotinylation reagent, indicating surface exposure. Omission of inducer reduced the infectious-virus yield by about 1.5 logs; nevertheless, all stages of virus morphogenesis appeared normal and extracellular virions were present on the cell surface. Purified virions assembled without inducer had a specific infectivity of less than 5% of the normal level and a comparably small amount of G9, whereas their overall polypeptide composition, including other components of the entry-fusion complex, was similar to that of virions made in the presence of inducer or of wild-type virions. G9-deficient virions bound to cells, but penetration of cores into the cytoplasm and early viral RNA synthesis were barely detected, and cell-cell fusion was not triggered by low pH. Of the identified components of the multiprotein complex, G9 is the sixth that has been shown to be required for entry and membrane fusion.
The mechanisms by which enveloped DNA viruses enter cells are poorly understood compared to those for many enveloped RNA viruses (17). Entry of the latter is typically mediated by one or two viral glycoproteins and involves virus attachment to the cell, activation of a fusion protein, and ultimately, merging of the viral and cellular membranes to allow entry of the genome and associated proteins. In contrast, three to four glycoproteins are required for entry of herpesviruses (35) and even more are needed for entry of vaccinia virus (VACV), the prototype poxvirus (26). Studies of VACV entry have been complicated by the existence of two infectious forms: the mature virion (MV), which contains a nucleoprotein core surrounded by a membrane containing more than 20 nonglycosylated proteins, and the extracellular virion (EV), which is essentially an MV surrounded by an additional membrane containing five proteins that are glycosylated and one that is not (9, 26, 34). The MV, which is extremely stable and can be liberated by cell lysis, is thought to mediate transmission between host animals, whereas the EV mediates cell-to-cell spread. There is evidence that the MV and EV bind differently to cells (39), consistent with their different outer membrane proteins. Binding of the MV to some cells appears to be due at least in part to three membrane proteins that can bind heparan or chondroitin sulfate (7, 19, 20, 23, 40), although individually, none of these proteins are essential. Recently, eight conserved VACV proteins were identified as components of a putative entry-fusion complex (32). Repression of the individual genes encoding five proteins of the complex (A28, A21, L5, H2, and A16) results in a conditional lethal phenotype (28, 31, 33, 36, 37). In each case, normal-looking MVs and EVs form under nonpermissive conditions but virus spread fails to occur. These noninfectious MVs can bind to cells, but the cores do not penetrate into the cytoplasm and cell-cell fusion cannot be induced by low pH. Furthermore, even though the entry-fusion proteins are components of the MV membrane, they are required for entry of the EV, suggesting that both forms of VACV use the same basic entry mechanism.
Of the eight proteins present in the entry-fusion complex, three (A16, G9, and J5) are related in structure, suggesting a common but distant evolutionary origin (30). Nevertheless, the presence of genes encoding each of these proteins in all sequenced poxviruses suggests that they have developed nonredundant functions. Previously, we showed that expression of A16 is required for entry and fusion. Here, we provide the first characterization of the G9 protein and show that it also is required for VACV entry and cell-cell fusion.
(This study was carried out at NIH to partially fulfill the Ph.D. thesis requirements of S. Ojeda at the University of Chile, Santiago, Chile.)
MATERIALS AND METHODS
Cells and viruses.
BS-C-1 cells (ATCC CCL-26) were grown in Eagle's minimum essential medium supplemented with 10% fetal bovine serum. Recombinant VACVs were derived from the Western Reserve (WR) strain. MVs were purified twice through a 36% sucrose cushion and banded once on a 25 to 40% sucrose gradient as described previously (16).
Antibodies.
Mouse monoclonal antibody (MAb) to L1 (42) was prepared from a hybridoma kindly provided by A. Schmaljohn. Rat monoclonal antibody to hemagglutinin (HA; clone 3F10) conjugated to horseradish peroxidase was from Roche Applied Sciences. Rabbit polyclonal antibodies to the following VACV proteins were used: anti-A4 (12), anti-A14 (3), anti-P4b/4b (R. Doms and B Moss, unpublished), anti-A21 and -L5 (36, 37), anti-A16 (28), and anti-H3 (10). Gretchen Nelson provided rabbit antiserum to A28. Rabbit antibody SC-7907 to proliferating cell nuclear antigen was from Santa Cruz Biotechnology.
Construction of recombinant viruses.
An inducible epitope-tagged G9 virus, vG9Li-HA, henceforth abbreviated vG9i, was derived from vT7lacOI, which contains an inducible copy of the bacteriophage T7 RNA polymerase gene and an Escherichia coli lac repressor gene inserted into the nonessential thymidine kinase gene locus (1). Standard overlap PCR was used to construct a DNA segment containing (i) the coding sequence of the G9R gene with an N-terminal HA tag under the control of the bacteriophage T7 promoter and an E. coli lac operator, (ii) the open reading frame (ORF) encoding enhanced green fluorescent protein (GFP) regulated by the VACV P11 late promoter, and (iii) sequences flanking G9R for homologous recombination with the VACV genome. The inducible G9R ORF was inserted in its natural site but in the opposite “L” orientation to avoid RNA polymerase read-through from neighboring genes. The PCR product was transfected into cells infected with vT7lacOI, and recombinant virus forming green fluorescent plaques was clonally purified. Relevant segments of the viral genome were amplified by PCR and sequenced to verify the construction.
An attempt was made to delete the G9R ORF by using the VACV bacterial artificial chromosome (BAC) system (13, 14). The first step was to replace the G9R ORF in the VAC-BAC plasmid with the ampicillin resistance gene by recombination in E. coli. Ampicillin-resistant colonies were isolated and sequenced to verify the deletion of the G9R ORF. To rescue the mutated VAC-BAC virus, 1 μg of purified VAC-BACΔG9 DNA alone or with 300 ng of intact G9R DNA and flanking sequence was transfected into CV-1 cells that had been infected with 1 PFU per cell of helper fowlpox virus (FPV). After 7 days, cells were harvested and titers for VACV were determined by plaque assay with BS-C-1 cells, which are nonpermissive for FPV. Fluorescence microscopy was used to increase sensitivity for detection of plaques since the VAC-BAC encodes enhanced GFP.
Detergent extraction of purified virus.
Purified virions were extracted with 50 mM Tris (pH 7.4) containing 1% Nonidet P-40 detergent and 150 mM NaCl in the presence or absence of 50 mM dithiothreitol (DTT). The mixture was incubated for 1 h at 37°C, and insoluble and soluble materials were separated by centrifugation at 12,000 × g for 30 min.
Western blot analysis.
For Western blot analysis, virions or whole-cell lysates were solubilized in NuPage reduced loading sample buffer (Invitrogen) and heat denatured. Polypeptides were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 4 to 12% bis-Tris gels (Invitrogen) and transferred to a polyvinylidene fluoride membrane. Membranes were blocked in Tris-buffered saline with 5% nonfat dry milk and 0.05% Tween 20 and incubated with antibodies. Protein bands were visualized by chemiluminescence using West-Pico or Femto kits (Pierce).
Surface biotinylation of purified virions.
Purified virions were allowed to react with 10 mM sulfosuccinimidyl-2-(biotinamido)ethyl-1,3-dithiopropionate (sulfo-NHS-SS-biotin; Pierce) in phosphate-buffered saline (PBS) for 1 h at 4°C. The reaction was quenched with 100 mM glycine, and the virions were pelleted at 20,000 × g for 30 min. The virions were incubated in SDS-PAGE loading buffer for 5 min at 85°C and then loaded onto a NeutrAvidin gel column (Pierce). The column was washed, and the biotinylated proteins were released by incubating the gel with SDS-PAGE sample buffer with added DTT for 1 h at room temperature.
Confocal microscopy.
Infected HeLa cells were fixed in 4% paraformaldehyde in PBS, washed, and then permeabilized with 0.1% Triton X-100 for 10 min at room temperature. The cells were incubated with the primary antibody or Alexa Fluor 594 phalloidin diluted in 10% complement-inactivated fetal bovine serum and PBS for 1 h at 37°C. The cells were washed and incubated with the fluorophore-conjugated secondary antibody for 1 h at 37°C. DNA was stained with diamidino-2-phenylindole dihydrochloride (DAPI; Invitrogen). Images were collected using a Leica TCS-NT SP2 inverted confocal microscope.
Electron microscopy.
BS-C-1 cells were infected with virus at a multiplicity of 10 PFU per cell for 21 h, fixed with 2% glutaraldehyde, embedded in Epon resin, and viewed with an FEI-CM100 transmission electron microscope.
Northern blot analysis.
BS-C-1 cells were infected with 5 PFU per cell of VACV and harvested after 3 h. RNA was extracted with an RNeasy Mini kit (QIAGEN), and 3 μg of denatured RNA was separated by electrophoresis on a 1.2% glyoxal agarose gel (Ambion). The RNA was transferred to a nylon membrane (Amersham), and the blots were hybridized with an [α-32P]dCTP probe that had been labeled using a DECA prime kit (Ambion), washed, and subjected to autoradiography.
RESULTS
G9 is highly conserved throughout the poxvirus family.
The G9R ORF (VACWR087) is predicted to encode a 38.7-kDa protein with the following structural features that are conserved in all poxviruses: 14 cysteine residues, a site for N-terminal glycine myristoylation, and a C-terminal transmembrane domain (Fig. 1). A16 and J5 are structurally related to G9, although the sequence similarity is low (30). In addition, both G9 and A16 were shown to be myristoylated (25). No nonpoxvirus homologs of G9 were detected by a position-specific iterative BLAST search.
FIG. 1.
Multiple-sequence alignment of G9 orthologs. One representative sequence from each genus of Chordopoxvirinae and two from Entomopoxvirinae are included in the alignment. LSDV, lumpy skin disease virus (Capripoxvirus); YLDV, Yaba-like disease virus (Yatapoxvirus); SPV, swinepox virus (Suipoxvirus); MYX, myxoma virus (Leporipoxvirus); VAC, vaccinia virus (Orthopoxvirus); MCV, molluscum contagiosum (Molluscipoxvirus); FPV, fowlpox virus (Avipoxvirus); MSV, Melanoplus sanguinipes (Entomopoxvirus); AMV, Amsacta moorei (Entomopoxvirus). Invariant conserved cysteines are shown in white with a black background; other conserved amino acid residues are shown with a gray background.
G9 is required for efficient VACV replication.
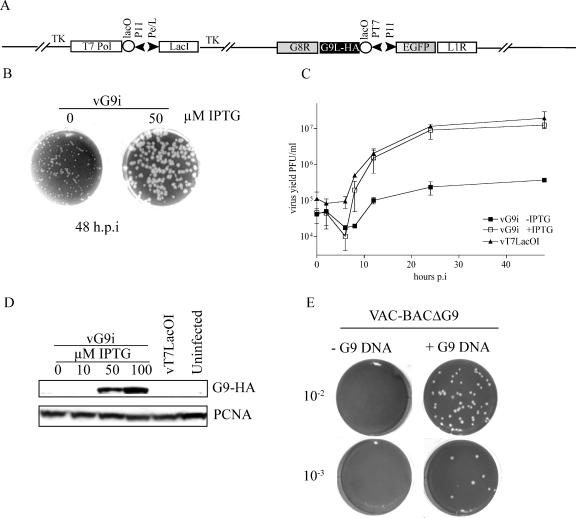
To determine the role of G9, we used a modification of previously described methods (41, 43) to construct a recombinant virus in which expression of G9 is regulated by an inducer. The mutant virus, called vG9i, contains (i) an N-terminal HA epitope-tagged G9R ORF regulated by the bacteriophage T7 RNA polymerase promoter and the E. coli lac operator, (ii) the E. coli lac repressor gene expressed constitutively by an early/late VACV promoter, and (iii) the bacteriophage T7 RNA polymerase gene controlled by a VACV late promoter and the lac operator. The stringency of the system depends on the presence of lac operator sequences located upstream of the T7 RNA pol and G9R ORFs (41). In addition, the orientation of the G9R ORF was reversed (to make it G9L) to prevent RNA polymerase read-through from neighboring genes (Fig. 2A). vG9i was constructed and propagated in the presence of IPTG (isopropyl-β-d-thiogalactopyranoside) in order to allow G9 expression. As a consequence of the presence of inducer, stocks of vG9i virus used for experimental infections contain G9 and only new G9 expression is repressed when cells are infected with such virus in the absence of IPTG. When BS-C-1 cells were infected with vG9i in the presence of 50 μM IPTG, plaques similar in size to those of the parental virus were formed, whereas much smaller plaques formed in the absence of inducer (Fig. 2B). Such small plaques could form by assembly of small numbers of infectious particles and their spread to neighboring cells. This interpretation was consistent with the results of one-step growth experiments. The yield of infectious vG9i was approximately 1.5 logs higher at the optimal IPTG concentration of 50 μM than in the absence of IPTG (Fig. 2C). A similar effect on virus yield was previously observed when the synthesis of A16 was repressed in the same manner (28). The induction of infectious virus formation by IPTG correlated with the effect of the inducer on G9 synthesis, as shown by Western blotting (Fig. 2D). Although not detected here, we will show later that very small amounts of G9 are made in the absence of inducer.
FIG. 2.
Correlation of G9 expression with virus replication. (A) Construction and characterization of a G9-inducible virus. The diagram shows relevant segments of the recombinant vG9i genome. T7 pol, bacteriophage T7 RNA polymerase; PT7, bacteriophage T7 promoter; lacO, E.coli lac operator; lacI, E.coli lac repressor; P11, vaccinia late promoter; Pe/l, P 7.5 early/late promoter; TK, thymidine kinase locus. (B) Plaque formation in the presence and absence of inducer. BS-C-1 monolayers were infected with the vG9i in the presence of 0 or 50 μM IPTG. At 48 h postinfection (h.p.i), the cells were stained with crystal violet. (C) Single-step virus yield in the presence and absence of inducer. vG9i or the parent virus vT7LacoOI was adsorbed to BS-C-1 cells (5 PFU/cell) in the presence or absence of IPTG for 60 min at 37°C. After 1 h, the residual inoculum was removed and the cells were washed and overlaid with medium with or without IPTG. Cells were then harvested at zero time or at the indicated times postinfection (p.i.). The crude lysates were treated with 125 μg/ml of trypsin for 30 min at 37°C to disperse virus particles. Virus yields were determined by plaque assay with 50 μM IPTG. Averages of data for two experiments with error bars are shown. (D) Western blot. BS-C-1 cells were infected with 5 PFU of vG9i in the presence of the indicated amounts of IPTG. After 24 h, whole-cell extracts were analyzed by Western blotting with an anti-HA MAb to detect G9 and anti-proliferating cell nuclear antigen (PCNA) rabbit antibody as a loading control. (E) Attempt to delete the G9R ORF. CV-1 cells were infected with FPV and transfected with the VAC-BACΔG9 plasmid. After 7 days, the cells were lysed and the presence or absence of infectious virus was determined by plaque assay. In parallel, another plate of CV-1 cells was infected with FPV and transfected with the VAC-BACΔG9 plasmid plus or minus DNA containing the G9 wild-type sequence and flanking region and assayed in the same manner.
The formation of small plaques and low but significant levels of replication in the absence of IPTG were likely due to the incomplete repression of G9 synthesis; nevertheless, we had to consider the alternative possibility that G9 is not absolutely essential. To investigate this question, we attempted to delete the gene by using a novel application of the VAC-BAC system in which the complete VACV genome is cloned in a bacterial artificial chromosome and can be rescued by FPV in mammalian cells (13, 14). The first step, construction of VAC-BACΔG9, in which the G9R ORF was replaced by the ampicillin resistance gene, was carried out with E. coli. CV-1 cells were then infected with helper FPV and transfected with VAC-BACΔG9. After 7 days, the cells were harvested and a plaque assay with BS-C-1 cells was performed to detect any VAC-BACΔG9 virus. However, no plaques were seen at the lowest dilution tested, even when viewed with a fluorescent microscope to detect individual cells expressing GFP, which was encoded in the VAC-BAC. To confirm that VAC-BACΔG9 had no additional mutations that would impair rescue, DNA containing the intact G9R gene and flanking region was transfected with VAC-BACΔG9 into cells infected with FPV. Under these conditions, recombination occurred between the VAC-BACΔG9 and the G9 DNA, resulting in the rescue of infectious virus (Fig. 2E). Our inability to isolate a deletion mutant indicated that G9 was essential or that the level of replication was too low for isolation by this method.
Association of G9 with the MV surface membrane.
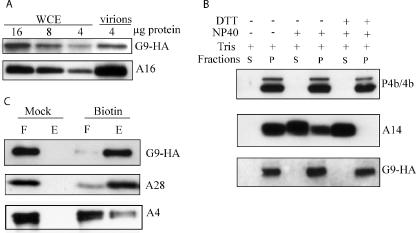
The previous isolation of G9 from infected cell membranes in complex with other entry-fusion proteins (32) suggests that G9 is also MV associated. However, we wanted to confirm the MV association of G9 more directly. Because highly specific antibody to G9 was unavailable, we took advantage of the HA epitope-tagged G9 in vG9i. Virions were purified from cells that were infected in the presence of IPTG and analyzed by Western blotting with an anti-HA MAb. We also analyzed whole-cell extracts in parallel. Relative to the amount of total protein applied to the polyacrylamide gel, G9 was enriched more than eightfold in MVs compared to that in the whole-cell extract (Fig. 3A). By comparison, A16 was enriched more than 16-fold (Fig. 3A). The relatively low percentage of G9 incorporated into MVs compared to that for A16 may be due to the higher than normal and slightly delayed expression that occurs with the inducible T7 promoter system.
FIG. 3.
Association of G9 protein with purified virions. (A) Whole-cell extracts (WCE) or sucrose gradient-purified virions from cells infected with vG9i in the presence of IPTG were analyzed by SDS-PAGE and Western blotting using anti-HA MAb. The amounts of total protein (4 to 16 μg) applied to the gel are indicated. (B) Extraction of G9 from purified virions. Virions prepared as in panel A were treated with 1% NP-40 with or without 50 mM DTT for 1 h at 37°C and separated into soluble (S) or insoluble (P) fractions. Proteins in both fractions were resolved by SDS-PAGE, followed by Western blotting with anti-A3 (P4b/4b) and anti-A14 polyclonal antibodies and anti-HA MAb for G9. (C) Biotinylation of surface proteins. Purified virions prepared as in panel A were treated or mock treated with sulfo-NHS-SS-biotin. Excess biotin was quenched, and the virions were recovered by sedimentation, disrupted with SDS and DTT, and allowed to bind to a NeutrAvidin column, which was then washed. The unbound flowthrough (F) and the bound and eluted (E) materials were resolved by SDS-PAGE and analyzed by Western blotting with anti-HA MAb and anti-A4 and anti-A28 polyclonal antibodies.
Membrane proteins are generally extractable form virions with NP-40 detergent. Accordingly, we treated the vG9i MVs with NP-40 detergent in the presence or absence of DTT at 37°C. However, no G9 was soluble under either condition (Fig. 3B). The previously characterized MV membrane and core proteins A14 and A3 (4b) were analyzed as controls. A14 was partially extracted with NP-40 alone and completely extracted with NP-40 plus DTT, whereas 4b was totally insoluble (Fig. 3B), as expected for membrane and core proteins, respectively. We had previously found that A16 resisted extraction with NP-40 but could be partially released when 150 mM NaCl was added (28). However, G9 was not solubilized with NP-40 in the presence (Fig. 3B) or absence (not shown) of 150 mM NaCl. The myristoylated glycine and the C-terminal transmembrane domain may contribute to the low solubilities of G9 and A16; however, L1 is easily extracted with NP-40 and it has a myristoyl residue and a C-terminal transmembrane domain.
We used a second method to investigate the membrane association of G9 that did not depend on its solubility in a nonionic detergent. MVs were purified from cells infected with vG9i in the presence of IPTG and biotinylated with sulfo-NHS-SS-biotin, a membrane-nonpermeating reagent. Subsequently, the excess biotin was quenched and the virions were collected by centrifugation and lysed by heating in SDS. Biotinylated and nonbiotinylated proteins were separated using NeutrAvidin beads, and the bound and unbound fractions were analyzed by Western blotting. In mock-treated MVs, G9 was associated with the unbound NeutrAvidin fraction, whereas in biotinylated MVs, G9 was almost entirely in the bound fraction (Fig. 3C). As controls, Western blotting was also carried out with antibody to A28, another component of the entry-fusion complex expected to be biotinylated, and with A4, a core protein that should not be biotinylated. The proportion of biotinylated A28 was similar to that of G9, whereas little A4 bound to the NeutrAvidin beads, indicating that the MVs were largely intact (Fig. 3C). We concluded that G9 is exposed on the surface of the MV membrane but that it is insoluble in nonionic detergent.
VACV morphogenesis was unaffected by repression of G9.
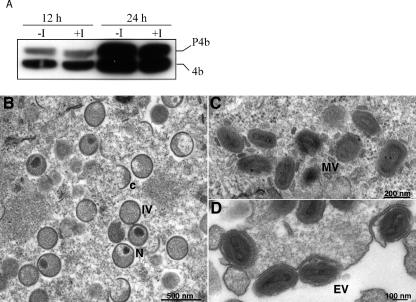
The processing of core protein precursors into their mature products is coordinated with MV morphogenesis, and interference with the latter prevents cleavage (27). We determined by Western blotting that the ratios of 4b to its protein precursor P4b were similar in the presence and absence of IPTG (Fig. 4A), consistent with normal morphogenesis.
FIG. 4.
Core protein processing and viral morphogenesis. (A) Cleavage of P4b. BS-C-1 cells were infected with 5 PFU per cell of vG9i in the absence or presence of 50 μM IPTG (I). After 12 and 24 h, whole-cell extracts were prepared and analyzed by SDS-PAGE and Western blotting with antibody to P4b/4b. (B, C, and D) Electron microscopy. BS-C-1 cells were infected with 10 PFU per cell of vG9i for 21 h in the absence of IPTG. Ultrathin sections were prepared and examined by transmission electron microscopy Abbreviations: C, crescent; IV, immature virion; N, immature virions with nucleoid. Magnification is indicated by bars.
Thin sections of BS-C-1 cells infected with vG9i in the presence or absence of IPTG were examined by transmission electron microscopy to directly assess the role of G9 in morphogenesis. Either with (not shown) or without (Fig. 4) IPTG, all stages of virion assembly were seen, including those with crescents and immature virions (Fig. 4B), MVs (Fig. 4C), and cell-associated EVs (Fig. 4D).
Characterization of vG9i MVs made in the absence of IPTG.
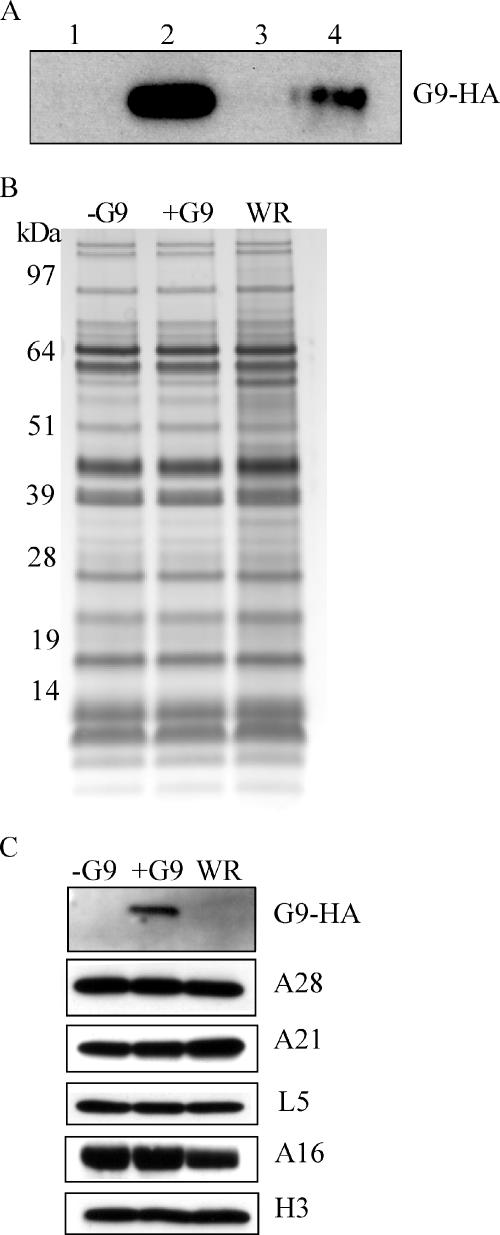
As previously noted, vG9i was always propagated in the presence of IPTG so that the virus used for all infections thus far contained G9; consequently, only the effects of repressing de novo G9 synthesis were studied. It was important, however, to compare the properties of vG9i virus made in the absence of IPTG (called −G9 virus, although, as will be seen, it is not totally lacking G9) with that made in the presence of IPTG (called +G9 virus). In each case, MVs were purified by sedimentation twice through a sucrose cushion and banded once on a sucrose gradient. Specific infectivity, defined as the ratio of PFU to virus particles estimated from the optical density at 260 nm (OD260), was more than 20-fold higher for +G9 virions than for −G9 virions. This residual infectivity was explained by the presence of a small amount of G9 in the virions. Although G9 could barely be detected when equal amounts of −G9 and +G9 virions were used for Western blotting, the protein was readily detected when six times more −G9 than +G9 virus was analyzed (Fig. 5A). Despite the deficiency of G9, the two virus preparations could not be distinguished from each other or from wild-type VACV WR by silver staining polypeptides following SDS-PAGE (Fig. 5B). Furthermore, Western blotting with available antibodies to other entry-fusion proteins demonstrated the presence of similar relative amounts of A28, A21, L5, and A16 as well as an additional membrane protein, H3, in all three MV preparations (Fig. 5C). Note that at this loading, the small amount of G9 was not detected in −G9 virions; G9 was not detected in wild-type VACV WR because of the absence of the HA epitope tag (Fig. 5C).
FIG. 5.
Polypeptide composition of purified virions. (A) SDS-PAGE and Western blotting with anti-HA MAb of G9 from purified virions made in the presence (+G9) or absence (−G9) of IPTG. Lanes 1, 2, and 3 were loaded with equal amounts of WR, +G9, and −G9 virions, respectively, and lane 4 with six times more +G9 virions. (B) Equal numbers of purified −G9, +G9, and VACV WR virus particles were analyzed by SDS-PAGE and silver staining. The mobilities of standard proteins of the indicated masses are shown on the left. (C) SDS-PAGE and Western blotting with polyclonal antibodies to A28, A21, L5, A16, and H3 from purified −G9 and +G9 virions. G9 was probed with anti-HA MAb as in panel A.
MVs deficient in G9 are defective in cell entry.
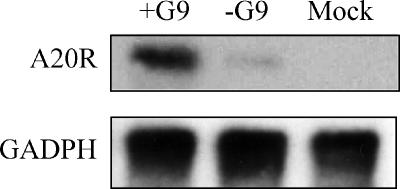
The normal appearance and polypeptide composition of MVs deficient in G9 and the physical association of G9 with other entry-fusion proteins strongly suggest that G9 has a role in entry. We used two different methods to compare the entries of +G9 and −G9 MVs. The first depended on the presence of the viral RNA polymerase and other transcription factors in the virus core, which allows viral mRNA synthesis to occur soon after penetration into the cytoplasm. Cells were infected with purified vG9i MVs made in the presence or absence of IPTG. After 3 h of incubation in the presence of AraC to prevent viral DNA replication and late gene expression, total RNA was extracted. The mRNAs were resolved by electrophoresis in a denaturing agarose gel, transferred to a membrane, and hybridized to a 32P-labeled DNA probe that was complementary to the transcript of the early A20R gene. An intense band corresponding in size to A20 mRNA was detected in cells infected with +G9 virus, whereas that mRNA was barely detected in cells infected with −G9 virus (Fig. 6). Glyceraldehyde-3-phosphate dehydrogenase mRNA, used as a loading control, indicated that equivalent amounts of cytoplasmic mRNA were present in each sample (Fig. 6).
FIG. 6.
Analysis of early transcription in cells infected with purified −G9 and +G9 virions. BS-C-1 cells were mock infected or infected with 5 PFU per cell of +G9 virions or the equivalent OD260 of −G9 virions in the presence of AraC. Total RNA was extracted at 3 h after infection. Northern blot analysis was carried out using [α-32P]dCTP-labeled DNA probes complementary to the VACV A20R and GADPH cellular transcripts. Radioactivity was detected with a phosphorimager.
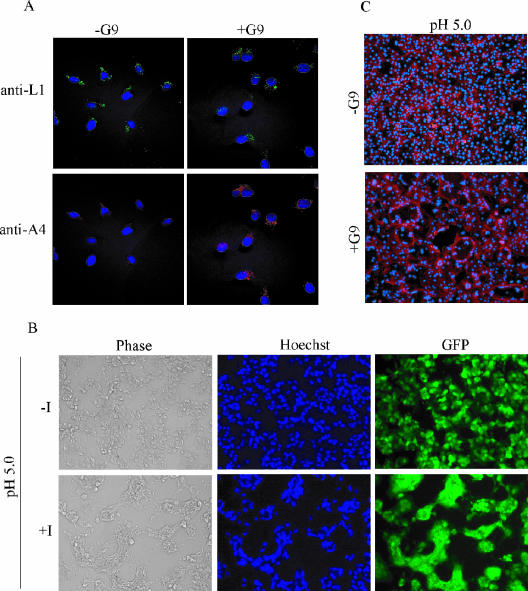
The second procedure, based on antibody detection of uncoated cores in the cytoplasm (38), is more specific for the entry step but less sensitive than measurement of early transcription. HeLa cells were incubated with purified −G9 and +G9 virions at 4°C to allow binding. After being washed to remove unbound and loosely bound virions, the cells were incubated for 2 h at 37°C to allow penetration. This step was done in the presence of cycloheximide, a protein synthesis inhibitor, to prevent the dissolution of cytoplasmic cores and cytopathic effects. A MAb to the L1 MV membrane protein was used to detect virions on the cell surface and antibody to the A4 core protein to detect intracellular cores. Both +G9 and −G9 MVs were bound to cells, indicating no defect in attachment, but there were numerous cores of +G9 virus in the cytoplasm but very few of −G9 virus (Fig. 7A).
FIG. 7.
Virus binding, entry, and induced cell-cell fusion. (A) Detection of extracellular virions and intracellular cores. HeLa cells were infected with 5 PFU per cell of +G9 virions or the equivalent OD260 of −G9 virions in the presence of 300 μg of cycloheximide per ml for 1 h at 4°C and then for 2 h at 37°C. At this time, the cells were washed, fixed, and permeabilized with 0.1% Triton X-100 and then incubated with anti-L1 mouse MAb and anti-A4 rabbit polyclonal antibody followed by fluorescein isothiocyanate-conjugated goat anti-mouse (green) and rhodamine red-X-conjugated goat anti-rabbit (red) antibodies, respectively. DNA was visualized by DAPI staining, and optical sections were obtained by confocal microscopy. (B) Fusion from within. BS-C-1 cells were infected with 2 PFU per cell of vG9i in the absence (-I) or presence (+I) of 50 μM IPTG for 18 h at 37°C followed by a brief exposure to pH 5.0 or 7.4 buffer. The buffer was replaced with regular medium, and the incubation continued for 3 h. The cells were stained with Hoechst dye and visualized by phase and epifluorescence microscopy for DNA and GFP expression. (C) Fusion from without. BS-C-1 monolayers were incubated with 200 PFU per cell of +G9 virions or the equivalent OD260 of −G9 virions at 4°C for 1 h. Unabsorbed and loosely bound virions were removed by washing, and the cells were briefly treated with pH 5.0 or 7.4 buffer at 37°C and then incubated in regular medium containing 300 μg of cycloheximide per ml for 3 h. The cells were fixed and stained with DAPI and Alexa Fluor 594-phalloidin to visualize DNA and the actin cytoskeleton, respectively.
G9 is required to induce low-pH-triggered cell-cell fusion.
VACV-infected cells form syncytia after brief exposure to low pH (15, 18). This phenomenon, called fusion from within, is dependent on the presence of EVs on the infected cell surface (4) and those components of the entry-fusion complex that have been studied thus far (28, 31, 33, 36, 37). To determine whether G9 expression is required for fusion from within, BS-C-1 cells were infected with vG9i in the presence or absence of IPTG for 21 h, briefly exposed to low pH, and then incubated for an additional 3 h in normal medium. As shown in Fig. 7B, cells infected in the presence of IPTG formed large, multinucleated syncytia whereas cells infected in the absence of IPTG did not. The explanation as to why vG9i can form small plaques but not syncytia in the absence of IPTG is probably due to the large number of fusion-competent virus particles needed for the latter, whereas single infectious particles are sufficient for virus spread to form plaques.
Formation of syncytia can also be triggered by briefly lowering the pH immediately after adding a high multiplicity of MVs to cells (18). This phenomenon, called fusion from without, also requires the previously studied entry-fusion proteins. To determine whether G9 is required for fusion from without, 200 PFU of +G9 virions or the equivalent number of −G9 virions were added per cell. The cells were then briefly treated with pH 5.0 or 7.0 buffer and incubated in regular medium for 3 h in the presence of cycloheximide to prevent cytopathic effects. Extensive cell-cell fusion was observed only in monolayers infected with +G9 virions and exposed to low pH (Fig. 7C). Thus, G9 is required for VACV-induced fusion from within and without.
DISCUSSION
Approximately 20 proteins with transmembrane domains are associated with MVs. Our finding that G9 is an MV protein is in agreement with a recent mass spectroscopy analysis of the entire MV proteome (8). The labeling of G9 in purified MVs with a membrane-nonpermeating biotinylation reagent demonstrated that G9 is exposed on the surface. G9, however, could not be extracted with NP-40 detergent in the presence or absence of DTT and 150 mM NaCl. Previously, we found that the A16 protein, which is structurally related to G9, could be partially solubilized in the presence of NP-40 but only if DTT was omitted and 150 mM NaCl added (28). Both proteins were more efficiently solubilized from the membrane fraction of infected cells, which would presumably contain IV membranes, than from purified virions. Similarly, the complex of entry-fusion proteins was more efficiently isolated from infected cell membranes than from virions (T. Senkevich, personal communication). Since each of the entry-fusion proteins analyzed thus far is in the MV membrane, some rearrangement or change in membrane composition apparently occurs during virion maturation.
VACV MV membrane proteins can be divided into two functional groups, those required for assembly and structure and those that participate in virus entry and membrane fusion. Here, we have shown that G9 belongs to the latter class, which includes at least five other membrane proteins, namely, A16, A21, A28, H2, and L5 (28, 31, 33, 36, 37). In each case, conditional null mutants produce normal-looking MVs and EVs that can bind to cells but are unable to penetrate or induce membrane fusion. These six proteins, as well as two others (G3 and J5) that are still uncharacterized, are conserved in all sequenced poxviruses and associate in an entry-fusion complex (32). There appear to be at least two mechanisms for activation of VACV fusion: binding of MVs to an unknown receptor or receptors on the plasma membrane, allowing neutral pH fusion (2, 5, 6), or via an internalization mechanism (11, 29) that is mimicked by low-pH-induced syncytium formation (15, 18). There is no evidence that the EV membrane is directly involved in membrane fusion, though it may affect attachment and internalization (21, 22, 24, 38).
There is variation among the effects of repressing the expression of VACV genes with the inducible system, which may depend on position effects of the target gene within the genome and the amount of the protein needed to fulfill its role in replication. In the case of G9, some infectious virus was made in the absence of inducer, even though we reversed the orientation of the ORF in order to prevent read-through from neighboring genes. The infectivity correlated with small but detectable amounts of G9 in purified virions. Nevertheless, this result could also be interpreted as indicating that G9 is important but not absolutely essential. To evaluate the latter possibility, we tried to delete G9 by using a novel approach that has worked well in other cases (A. Domi, unpublished). The method depends on the ability of FPV to rescue the VACV genome cloned in a bacterial artificial chromosome (13). In the present case, we deleted the G9R ORF from the cloned genome but were unable to demonstrate the rescue of infectious VACV unless DNA containing G9R was also transfected, even though this required an additional recombination step. Thus, either G9 expression is essential or deletion of the gene severely impairs infectivity, preventing us from isolating mutants. A similar ambiguity exists with respect to the A16L gene (28). Regardless, the phenotypes of the inducible G9 and A16 mutants were similar to those of the other entry-fusion proteins.
In conclusion, an unprecedented number of proteins are required for poxvirus entry and membrane fusion. Why poxviruses require so many proteins to accomplish what other viruses do perfectly well with one or two is an intriguing question.
Acknowledgments
Nelson Gretchen and Alan Townsley kindly provided antibodies specific for A28, L5, and A21. We thank Andrea Weisberg for the electron microscopy, Norman Cooper for cell culture assistance, and Juraj Kabat for confocal microscopy assistance.
The research was supported by the Intramural Research Program of the NIAID of the NIH.
REFERENCES
- 1.Alexander, W. A., B. Moss, and T. R. Fuerst. 1992. Regulated expression of foreign genes in vaccinia virus under the control of bacteriophage T7 RNA polymerase and the Escherichia coli lac repressor. J. Virol. 66:2934-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong, J. A., D. H. Metz, and M. R. Young. 1973. The mode of entry of vaccinia virus into L cells. J. Gen. Virol. 21:533-537. [DOI] [PubMed] [Google Scholar]
- 3.Betakova, T., E. J. Wolffe, and B. Moss. 1999. Regulation of vaccinia virus morphogenesis: phosphorylation of the A14L and A17L membrane proteins and C-terminal truncation of the A17L protein are dependent on the F10L protein kinase. J. Virol. 73:3534-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasco, R., and B. Moss. 1991. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-dalton outer envelope protein. J. Virol. 65:5910-5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter, G. C., M. Law, M. Hollinshead, and G. L. Smith. 2005. Entry of the vaccinia virus intracellular mature virion and its interactions with glycosaminoglycans. J. Gen. Virol. 86:1279-1290. [DOI] [PubMed] [Google Scholar]
- 6.Chang, A., and D. H. Metz. 1976. Further investigations on the mode of entry of vaccinia virus into cells. J. Gen. Virol. 32:275-282. [DOI] [PubMed] [Google Scholar]
- 7.Chung, C.-S., J.-C. Hsiao, Y.-S. Chang, and W. Chang. 1998. A27L protein mediates vaccinia virus interaction with cell surface heparin sulfate. J. Virol. 72:1577-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung, C. S., C. H. Chen, M. Y. Ho, C. Y. Huang, C. L. Liao, and W. Chang. 2006. Vaccinia virus proteome: identification of proteins in vaccinia virus intracellular mature virion particles. J. Virol. 80:2127-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Condit, R. C., N. Moussatche, and P. Traktman. In a nutshell: structure and assembly of the vaccinia virion. Adv. Virus Res., in press. [DOI] [PubMed]
- 10.da Fonseca, F. G., A. Weisberg, E. J. Wolffe, and B. Moss. 2000. Characterization of the vaccinia virus H3L envelope protein: topology and posttranslational membrane insertion via the C-terminal hydrophobic tail. J. Virol. 74:7508-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dales, S., and R. Kajioka. 1964. The cycle of multiplication of vaccinia virus in Earle's strain L cells. I. Uptake and penetration. Virology 24:278-294. [DOI] [PubMed] [Google Scholar]
- 12.Demkowicz, W. E., J. S. Maa, and M. Esteban. 1992. Identification and characterization of vaccinia virus genes encoding proteins that are highly antigenic in animals and are immunodominant in vaccinated humans. J. Virol. 66:386-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domi, A., and B. Moss. 2002. Cloning the vaccinia virus genome as a bacterial artificial chromosome in Escherichia coli and recovery of infectious virus in mammalian cells. Proc. Natl. Acad. Sci. USA 99:12415-12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domi, A., and B. Moss. 2005. Engineering of a vaccinia virus bacterial artificial chromosome in Escherichia coli by bacteriophage lambda-based recombination. Nat. Methods 2:95-97. [DOI] [PubMed] [Google Scholar]
- 15.Doms, R. W., R. Blumenthal, and B. Moss. 1990. Fusion of intra- and extracellular forms of vaccinia virus with the cell membrane. J. Virol. 64:4884-4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Earl, P. L., N. Cooper, S. Wyatt, B. Moss, and M. W. Carroll. 1998. Preparation of cell cultures and vaccinia virus stocks, p. 16.16.1-16.16.3. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. John Wiley and Sons, New York, N.Y. [Google Scholar]
- 17.Earp, L. J., S. E. Delos, H. E. Park, and J. M. White. 2005. The many mechanisms of viral membrane fusion proteins. Curr. Top. Microbiol. Immunol. 285:25-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong, S. C., C. F. Lai, and M. Esteban. 1990. Vaccinia virus induces cell fusion at acid pH and this activity is mediated by the N-terminus of the 14-kDa virus envelope protein. Virology 178:81-91. [DOI] [PubMed] [Google Scholar]
- 19.Hsiao, J. C., C. S. Chung, and W. Chang. 1998. Cell surface proteoglycans are necessary for A27L protein-mediated cell fusion: identification of the N-terminal region of A27L protein as the glycosaminoglycan-binding domain. J. Virol. 72:8374-8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsiao, J. C., C. S. Chung, and W. Chang. 1999. Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. J. Virol. 73:8750-8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichihashi, Y. 1996. Extracellular enveloped vaccinia virus escapes neutralization. Virology 217:478-485. [DOI] [PubMed] [Google Scholar]
- 22.Law, M., G. C. Carter, K. L. Roberts, M. Hollinshead, and G. L. Smith. 2006. Ligand-induced and non-fusogenic dissolution of a viral membrane. Proc. Natl. Acad. Sci. USA 103:5989-5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin, C. L., C. S. Chung, H. G. Heine, and W. Chang. 2000. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol. 74:3353-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Locker, J. K., A. Kuehn, S. Schleich, G. Rutter, H. Hohenberg, R. Wepf, and G. Griffiths. 2000. Entry of the two infectious forms of vaccinia virus at the plasma membrane is signaling-dependent for the IMV but not the EEV. Mol. Biol. Cell 11:2497-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin, K. H., D. W. Grosenbach, C. A. Franke, and D. E. Hruby. 1997. Identification and analysis of three myristylated vaccinia virus late proteins. J. Virol. 71:5218-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moss, B. 2006. Poxvirus entry and membrane fusion. Virology 344:48-54. [DOI] [PubMed] [Google Scholar]
- 27.Moss, B., and E. N. Rosenblum. 1973. Protein cleavage and poxvirus morphogenesis: tryptic peptide analysis of core precursors accumulated by blocking assembly with rifampicin. J. Mol. Biol. 81:267-269. [DOI] [PubMed] [Google Scholar]
- 28.Ojeda, S., T. G. Senkevich, and B. Moss. 2006. Entry of vaccinia virus and cell-cell fusion require a highly conserved cysteine-rich membrane protein encoded by the A16L gene. J. Virol. 80:51-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Payne, L. G., and E. Norrby. 1978. Adsorption and penetration of enveloped and naked vaccinia virus particles. J. Virol. 27:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senkevich, T. G., E. V. Koonin, J. J. Bugert, G. Darai, and B. Moss. 1997. The genome of molluscum contagiosum virus: analysis and comparison with other poxviruses. Virology 233:19-42. [DOI] [PubMed] [Google Scholar]
- 31.Senkevich, T. G., and B. Moss. 2005. Vaccinia virus H2 protein is an essential component of a complex involved in virus entry and cell-cell fusion. J. Virol. 79:4744-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senkevich, T. G., S. Ojeda, A. Townsley, G. E. Nelson, and B. Moss. 2005. Poxvirus multiprotein entry-fusion complex. Proc. Natl. Acad. Sci. USA 102:18572-18577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senkevich, T. G., B. M. Ward, and B. Moss. 2004. Vaccinia virus entry into cells is dependent on a virion surface protein encoded by the A28L gene. J. Virol. 78:2357-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith, G. L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 83:2915-2931. [DOI] [PubMed] [Google Scholar]
- 35.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 77:10179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Townsley, A., T. G. Senkevich, and B. Moss. 2005. The product of the vaccinia virus L5R gene is a fourth membrane protein encoded by all poxviruses that is required for cell entry and cell-cell fusion. J. Virol. 79:10988-10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Townsley, A., T. G. Senkevich, and B. Moss. 2005. Vaccinia virus A21 virion membrane protein is required for cell entry and fusion. J. Virol. 79:9458-9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanderplasschen, A., M. Hollinshead, and G. L. Smith. 1998. Intracellular and extracellular vaccinia virions enter cells by different mechanisms. J. Gen. Virol. 79:877-887. [DOI] [PubMed] [Google Scholar]
- 39.Vanderplasschen, A., and G. L. Smith. 1997. A novel virus binding assay using confocal microscopy: demonstration that intracellular and extracellular vaccinia virions bind to different cellular receptors. J. Virol. 71:4032-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vazquez, M. I., and M. Esteban. 1999. Identification of functional domains in the 14-kilodalton envelope protein (A27L) of vaccinia virus. J. Virol. 73:9098-9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward, G. A., C. K. Stover, B. Moss, and T. R. Fuerst. 1995. Stringent chemical and thermal regulation of recombinant gene expression by vaccinia virus vectors in mammalian cells. Proc. Natl. Acad. Sci. USA 92:6773-6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolffe, E. J., S. Vijaya, and B. Moss. 1995. A myristylated membrane protein encoded by the vaccinia virus L1R open reading frame is the target of potent neutralizing monoclonal antibodies. Virology 211:53-63. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, Y., and B. Moss. 1991. Inducer-dependent conditional-lethal mutant animal viruses. Proc. Natl. Acad. Sci. USA 88:1511-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]