Abstract
The reasons for poor CD4+ T-cell recovery in some human immunodeficiency virus (HIV)-infected subjects despite effective highly active antiretroviral therapy (HAART) remain unclear. We recently reported that CXCR4-using (X4) HIV-1 could be gradually selected in cellular reservoirs during sustained HAART. Because of the differential expression of HIV-1 coreceptors CCR5 and CXCR4 on distinct T-cell subsets, the residual replication of R5 and X4 viruses could have different impacts on T-cell homeostasis during immune reconstitution on HAART. We examined this hypothesis and the mechanisms of CD4+ T-cell restoration by comparing the virological and immunological features of 15 poor and 15 good immunological responders to HAART. We found a high frequency of X4 viruses in the poor immunological responders. But the levels of intrathymic proliferation of the two groups were similar regardless of whether they were infected by R5 or X4 virus. The frequency of recent thymic emigrants in the poor immunological responders was also similar to that found in the good immunological responders, despite their reduced numbers of naïve CD4+ T cells. Our data, rather, suggest that the naïve T-cell compartment is drained by a high rate of mature naïve cell loss in the periphery due to bystander apoptosis or activation-induced differentiation. X4 viruses could play a role in the depletion of naïve T cells in poor immunological responders to HAART by triggering persistent T-cell activation and bystander apoptosis via gp120-CXCR4 interactions.
Highly active antiretroviral therapy (HAART) successfully controls human immunodeficiency virus type 1 (HIV-1) replication in most individuals, resulting in substantial immune restoration and decreased morbidity and mortality. However, the suppression of detectable HIV-1 viremia does not invariably result in a significant increase in CD4+ T cells. Five to 15% of HIV-infected patients still have a CD4+ T-cell counts of <200 cells/μl and are thus at risk of developing AIDS, despite being on HAART for several years (15, 23).
The mechanisms responsible for these persistently low CD4+ T-cell counts are not clear; they could be due to impaired reconstitution or to excessive destruction of CD4+ T cells. The recovery of peripheral CD4+ T-cell counts in patients on HAART depends mainly on a slow increase in naïve CD4+ T cells (3). Several studies have suggested that the thymus plays a role in immune reconstitution during HIV-1 infection, even in adults (12, 34, 36), but the extent to which it determines the regeneration of the peripheral CD4+ T-cell pool in patients on HAART remains unclear. Analyses of thymic activity are hampered by the difficulty of directly measuring thymic output. Most studies have measured the signal-joint (sj) T-cell receptor (TCR) excision circle (TREC) in blood T cells. This surrogate marker of thymic activity is a by-product of the TCRδ locus rearrangement within the TCRα locus (δRec-ψJα rearrangement) that occurs during thymopoiesis (5, 11, 12, 36, 44). However, TRECs persist in recent thymic emigrants (RTEs) as episomal DNA circles, do not replicate during mitosis, and are thus diluted during cell division (12). Hence, the TREC content of blood T cells depends not only on the thymic output alone but also on the turnover of TREC-bearing cells in the periphery (19). Recent studies have provided new tools for investigating thymic activity and the peripheral homeostasis of RTEs. One such tool is the measurement of the sj/βTREC ratio to assess thymic activity independently of peripheral events (10). The proliferation of immature thymocytes, a key determinant of thymic output (1), between the TCRβ chain and the δRec-ψJα rearrangements leads to the dilution of the DβJβTRECs before the generation of sjTREC. The sj/βTREC ratio is thus fixed in RTEs and is not influenced by downstream cell proliferation (10). The CD31 phenotype marker on peripheral-naïve CD4+ T cells can also be used to identify a CD31+-cell subset containing RTEs and a CD31−-cell subset of peripherally expanded naïve CD4+ T cells (24).
The more rapid death of mature CD4+ T cells in the periphery could also affect CD4+ T-cell reconstitution in patients on HAART (2, 5, 21). Low-level virus replication persists in most patients whose plasma virus loads are apparently suppressed by HAART (14, 29), probably because of sanctuary sites for virus replication. We recently reported that X4 variants could be gradually selected in cellular reservoirs during sustained HAART and that those patients on HAART who harbor predominantly X4 viruses tend to have lower CD4+ T-cell counts than those of patients harboring mainly R5 viruses (9). Residual replication of X4 viruses could interfere with both thymic activity and peripheral T-cell homeostasis, as thymocytes and naïve CD4+ T cells preferentially bear the CXCR4 coreceptor for HIV-1 (6, 41, 43).
We have examined this hypothesis and the mechanisms of immune reconstitution on HAART by comparing the virological and immunological features of two groups of patients, one having poor CD4+ T-cell restoration despite sustained virological responses and the second having good immunological and virological responses.
MATERIALS AND METHODS
Study subjects and samples.
Thirty HIV-1-infected patients, 15 of whom had poor immune reconstitutions (gain of <200 CD4 cells/μl) despite sustained optimal virological responses and 15 age-matched patients that had good immunological and virological responses (gain of >500 CD4 cells/μl), were enrolled at the Department of Infectious Diseases of Toulouse University Hospital, France. None of them had previously been studied for HIV-1 coreceptor usage. Patients receiving alpha interferon for hepatitis C virus infection or interleukin-2 immunotherapy were not included. All of the patients had sustained plasma virus loads of <200 copies/ml throughout follow-up. Samples (50 ml) of peripheral whole blood were collected from each patient, and Ficoll-separated peripheral blood mononuclear cells (PBMCs) were cryopreserved in liquid nitrogen. Studies of lymphocyte apoptosis were performed on fresh PBMCs. Cryopreserved samples taken at baseline before the start of suppressive HAART were used for retrospective analyses. Baseline plasma samples were available for 28 of the 30 patients, and PBMC samples were available for 13 of the 30 patients. This research was approved by the Institutional Review Boards of Toulouse University Hospital and the Pasteur Institute of Paris, France.
Cell sorting of naive T-cell subsets.
Polychromatic flow cytometry was used to sort the CD31+ and CD31− subsets of CD45RA+ CD27+ CD4+ T cells and the CD45RAbright CD27bright CD8+ T-cell subset from cryopreserved PBMCs. Combinations of CD3-fluorescein isothiocyanate (FITC), CD8-phycoerythrin Cy7 (PECy7), CD45RA-PECy5, CD27-PE (Becton Dickinson, Le Pont de Claix, France), CD4-allophycocyanin, CD45RA-FITC, CD27-PECy5, and CD31-PE monoclonal antibodies (MAbs) (Beckman Coulter, Villepinte, France) were employed. The subsets of naive T cells were purified on a MoFlo cell sorter (DakoCytomation, Trappes, France). The sorted cell populations were routinely better than 99% pure.
Flow cytometry analysis.
Immunophenotypic analyses were performed by five-color flow cytometry from cryopreserved PBMCs using combinations of CD3-PECy7, CD3-energy coupled dye (ECD), CD4-ECD, CD45RA-PE, CD27-PECy5, CD31-FITC, CD38-PE, HLA-DR-FITC (Beckman-Coulter), CD8-PECy7, CD8-PerCP (Becton Dickinson), and Ki67-FITC (DakoCytomation) MAbs. Intracellular staining was performed after surface staining using Cytofix and Cytoperm reagents (Becton Dickinson). Flow cytometric acquisition (0.5 × 105 to 5 × 105 events) and analysis were performed on a Cytomics FC 500 driven by the RXP software package (Beckman Coulter).
Studies of lymphocyte apoptosis in vitro.
Spontaneous apoptosis was assayed on freshly isolated PBMCs that had been incubated in RPMI medium without stimulation for 24 h. The percentages of dying cells in CD4+ T-cell subsets were determined by five-color flow cytometry using 7-aminoactinomycin D (7-AAD) staining combined with CD3-PECy7, CD4-ECD, CD45RA-FITC, and CD27-PE MAb surface staining (Beckman Coulter), as described previously (30).
PCR amplification and cloning of HIV-1 env and analysis of sequence data.
Nested PCR was used to amplify a region spanning the V1-V2 and V3 regions of HIV-1 env from PBMCs. Both primary and nested PCRs were performed using the Expand High Fidelity Plus PCR system (Roche Diagnostics, Meylan, France). Primers are given in Table S1 in the supplemental material. The PCR products were cloned using a TOPO-TA cloning kit (Invitrogen, Cergy-Pontoise, France), and 15 to 20 molecular clones were sequenced for each patient. Phylogenetic analysis of V1-V3 sequences from the entire PCR products showed distinct clusters of sequences for each patient that excluded any possibility of sample contamination or mix-up (see Fig. S1 in the supplemental material). Multiple alignments were done with CLUSTALW 1.83. Molecular clones with an open reading frame (>85% of all clones sequenced) were selected for downstream analysis of coreceptor usage. We could not amplify V1-V3 env from PBMCs or obtain infectious recombinant viruses for subjects in each group who harbored non-B subtype viruses, whereas the 26 subjects successfully analyzed all harbored subtype B viruses (data not shown).
Determination of HIV-1 coreceptor usage.
The phenotype of HIV-1 coreceptor usage was determined by using a recombinant virus assay as described previously (45). Briefly, 293T cells were cotransfected with the V1-V3 env PCR products of molecular clones and the 43-ΔV vector (V1-V3 env-deleted pNL4.3) by the calcium phosphate method. The recombinant virus released from the transfected 293T cells into the supernatant was used to infect U373 indicator cells expressing CD4 and either CCR5 or CXCR4 coreceptors. These indicator cell lines carry an inducible long-terminal repeat-LacZ cassette that allows colorimetric assessment of virus infection by measuring HIV-1 Tat-induced β-Gal expression. Genotypic prediction of HIV-1 coreceptor usage was based on V3 amino acid sequence. X4 variants were predicted according to the presence of a K or R amino acid residue at one of V3 positions 11 and 25 and a net charge of at least +5 (13).
Cell-associated HIV-1 DNA quantification.
Multiplex PCR amplification was first performed on cell lysates for the HIV-1 gag region, together with the human CD3γ chain gene with outer primer pairs for 22 cycles. The HIV-1 gag and CD3γ amplicons were then quantified in parallel using material from the same first-round multiplex PCR amplification, by a nested real-time quantitative PCR, with inner primers and fluorescence resonance energy transfer probes on a LightCycler (Roche Diagnostics). The same serially diluted standard curve was used to measure both HIV-1 gag and CD3γ generated from the multiplex amplification of a plasmid in which one copy of the HIV-1 gag and CD3γ fragments had been cloned. This nested quantitative PCR assay detects one copy of HIV-1 DNA in 105 cells in patients harboring subtype B viruses. Experiments were performed in triplicate for PBMCs and in duplicate for sorted naive T cells. The primers and probes are shown in Table S1 in the supplemental material.
TRECs quantification.
The sjTREC (δRec-ψJα) and each of the 10 DβJβTRECs (Dβ1-Jβ1.1 to Dβ1-Jβ1.6 and Dβ2-Jβ2.1 to Dβ2-Jβ2.4) were measured by nested quantitative PCR (10). The sjTREC was quantified in triplicate, and each of the 10 DβJβTRECs was quantified in duplicate. The sum of the DβJβTREC frequencies is 1.3 times the sum of the 10 measured DβJβTREC frequencies (in order to extrapolate to the 13 principal DβJβTRECs). The sj/βTREC ratio is the sjTREC frequency divided by the sum of DβJβTREC frequencies.
Statistical analysis.
Quantitative and categorical variables were compared using the Wilcoxon rank sum test and the Fisher's exact test, respectively. Correlations between quantitative variables were estimated by calculating Spearman's rank correlation coefficients. The Wilcoxon matched pairs signed-rank test was used to compare the sjTREC contents and cellular virus loads in paired CD31+- and CD31−-cell subsets from each patient. All tests were two-sided, and P values of <0.05 were considered statistically significant. Statistical analyses were performed with Stata 8.2.
Nucleotide sequence accession numbers.
The sequences reported here were given GenBank accession numbers DQ136796 to DQ137123.
RESULTS
High frequency of CXCR4-using viruses in poor immunological responders.
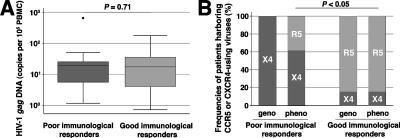
The poor immunological responders (n = 15) had a median increase in CD4+ T cells of +165 cells/μl in response to HAART, while the age-matched good immunological responders (n = 15) had an increase of +610 cells/μl at time of study. The median baseline CD4+ T-cell count was <200 cells/μl in both groups, although it was significantly lower in the poor immunological responders. All of the patients had been on HAART for a median of 84 months (interquartile range, 64 to 94 months), i.e., in a quasi-steady state, and had sustained plasma virus loads of <200 copies/ml throughout follow-up (Table 1). We measured the number of HIV-1 gag DNA copies in PBMCs because residual virus replication can persist on HAART and thus replenish the cellular reservoirs of HIV-1 (14, 29, 38). The virus loads in PBMCs from both groups were similar (P = 0.71) (Fig. 1A). We then looked for viruses that were using distinct coreceptors in the poor and good immunological responders. We used clonal analysis of PCR products from PBMCs to get a representative image of the virus population for each patient on HAART and characterized the coreceptor usage of the env molecular clones genotypically and phenotypically. Phenotypic analysis of env recombinant clonal viruses detected only R5 viruses in 11 of 13 good immunological responders, with some X4 or R5X4 dual-tropic viruses in the other two patients. In contrast, some X4 or R5X4 dual-tropic viruses were found in 8 of 13 poor immunological responders and R5 viruses alone were found in the other five (P < 0.05) (Fig. 1B). The phenotypic and genotypic analyses of coreceptor usage of env molecular clones were fully concordant, except for the five poor immunological responders having R5 phenotype viruses (the quasispecies from each patient is given in Fig. S2 in the supplemental material). Mutations in V3 that have been associated with CXCR4 use, such as an arginine residue at position 25 or an increased net charge of at least +5 (13), were found in viruses derived from these five poor immunological responders, although these clones were still of the R5 phenotype. In contrast, none of the R5 viruses from the good immunological responders had such “X4-like” genotypic determinants. We also retrospectively characterized env molecular clones obtained from plasma and/or PBMC samples taken before starting effective HAART. All but two of the patients in whom X4 or R5X4 viruses had been found in PBMCs on HAART already harbored closely related CXCR4-using viruses before they began HAART. The clones of viruses with an R5 phenotype but an “X4-like” genotype found in five poor immunological responders were also detected in pre-HAART quasispecies (data not shown).
TABLE 1.
Characteristics of the poor and good immunological responders
| Characteristic | Poor immunological responders (n = 15) | Good immunological responders (n = 15) | P |
|---|---|---|---|
| Agea | 48.7 (34-65) | 45.5 (30-63) | 0.43 |
| Duration of HIV-1 infection since diagnosisa | 11.3 (3-21) | 9.8 (6-15) | 0.24 |
| Baseline plasma HIV-1 RNA loadb | 5.0 (4.6-5.9) | 5.5 (4.9-5.8) | 0.38 |
| Baseline CD4+ T cellsc | 71 (43-114) | 155 (111-220) | <0.01 |
| CD4+ T cell gain from baselinec | |||
| After 6 mo of HAART | +68 (31-77) | +202 (156-230) | |
| After 12 mo of HAART | +79 (52-93) | +309 (254-341) | |
| After 24 mo of HAART | +96 (81-138) | +326 (256-466) | |
| After 48 mo of HAART | +112 (90-172) | +591 (451-675) | |
| Total increase at last follow-up | +165 (103-194) | +610 (430-822) |
Results shown as mean (range) years.
Results shown as median (interquartile range) log10 copies/ml.
Results shown as median (interquartile range) cells per microliter.
FIG. 1.
High frequency of CXCR4-using viruses in poor immunological responders. (A) Cell-associated HIV-1 DNA load in PBMCs. (B) Genotypic (geno) and phenotypic (pheno) characterization of HIV-1 isolates. Patients whose quasispecies included only pure R5 clones are classified as “R5,” and those with some pure X4 or R5X4 dual-tropic clones are classified as “X4.” Also see Fig. S2 in the supplemental material for an analysis of quasispecies in the two groups.
Similar levels of intrathymic proliferation in the poor and good immunological responders, regardless of the infection by R5 or X4 viruses.
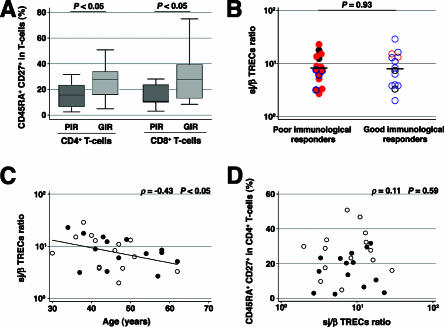
One of the main immunological hallmarks of the poor immunological responders was their severe losses of circulating naive T cells, defined as CD45RA+ CD27+ T cells, in both percentages (Fig. 2A) and absolute numbers (data not shown) of CD4+ and CD8+ T cells. As the level of thymic output is strongly determined by the intrathymic proliferation of precursor T cells following β selection (1, 42), we assayed the proliferation of immature thymocytes by measuring the sj/βTREC ratio in PBMCs (10) to determine whether differences in intrathymic proliferation could account for this depletion and the relationship with the presence of X4 viruses. The sj/βTREC ratios were similar in both groups (P = 0.93) and not correlated with infection by R5 or X4 viruses (Fig. 2B). The sj/βTREC ratio was negatively correlated with age (ρ = −0.43; P < 0.05) (Fig. 2C) but not with the percentage of naive CD4+ T cells (ρ = 0.11; P = 0.59) (Fig. 2D). We also retrospectively measured the sj/βTREC ratio before starting effective HAART in four poor and nine good immunological responders for whom baseline PBMC samples were available. There was a mean 2.4-fold increase in the sj/βTREC ratio in response to HAART, without any correlation with the increase in CD4+ T cells in response to HAART in both groups (data not shown).
FIG. 2.
Similar levels of intrathymic proliferation in poor and good immunological responders. (A) Percentage of the CD45RA+ CD27+ CD4+ and CD8+ T-cell populations of poor immunological responders (PIR) and good immunological responders (GIR). (B) Measure of the sj/βTREC ratio. Closed red circles, PIR harboring some X4 viruses; closed red circles with blue rings, PIR harboring R5 viruses with an “X4-like” genotype; gray circles, PIR in whom coreceptor usage could not be determined; open red circles, GIR harboring some X4 viruses; open blue circles, GIR harboring R5 viruses; open gray circles, GIR in whom coreceptor usage could not be determined. (C) Correlation between the sj/βTREC ratio and age. Closed circles, PIR; open circles, GIR. (D) Lack of correlation between the sj/βTREC ratio and the percentage of CD45RA+ CD27+ CD4+ T cells. Closed circles, PIR; open circles, GIR.
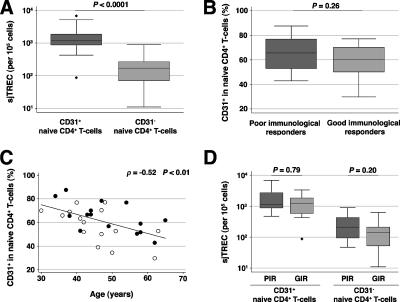
Similar frequencies of RTEs in the CD31+ naïve CD4+ T-cell subsets of the poor and good immunological responders.
Recent models of naïve T-cell homeostasis suggest that a variable, regulated proportion of RTEs become incorporated into the established naïve cell population (16). We used the marker CD31 to identify a subset enriched in RTEs within the naïve CD4+ T-cell population (24). The CD31+- and CD31−-naïve CD4+ T-cell subsets in the 15 poor and 15 good immunological responders were sorted by polychromatic flow cytometry. The frequencies of sjTREC in the CD31+- and CD31−-cell subsets of these patients confirmed that the CD31 marker delineates a TREC-rich cell subset among naïve CD4+ T cells (P < 0.0001) (Fig. 3A). The frequency of RTEs in CD31+ cells, measured by the frequency of sjTREC-bearing cells in the CD31+-naïve-cell subset, was correlated with the level of intrathymic proliferation, measured by the sj/βTREC ratio, which is a major determinant of the thymic output (ρ = 0.53; P < 0.01) (data not shown). The frequency of sjTREC in total PBMCs was also strongly correlated with the numbers of circulating CD31+-naïve CD4+ T cells (ρ = 0.74; P < 0.0001) (data not shown). These findings indicate that the CD31+-naïve-cell subset actually contained the majority of CD4+ RTEs. The frequency of CD31+-naïve cells in the poor immunological responders was similar to that found in the good immunological responders, despite their reduced numbers of naïve CD4+ T cells (P = 0.26) (Fig. 3B). It decreased constantly with age in both groups, in parallel with an expansion of the CD31−-cell subset (ρ = −0.52; P < 0.01) (Fig. 3C). The sjTREC frequencies in the CD31+- and CD31−-cell subsets of the poor immunological responders were similar to those of the good immunological responders (Fig. 3D). The finding of similar frequencies of TREC-rich RTEs in both groups, despite the reduced size of the naïve CD4+ T-cell pool in the poor immunological responders, may be due to a more rapid replacement of naïve CD4+ T cells and/or to increased survival of the CD4+ RTEs in these patients.
FIG. 3.
Similar frequencies of RTEs in the CD31+-naïve CD4+ T-cell subsets of the poor and good immunological responders. (A) sjTREC content of the CD31+- and CD31−-naive CD4+ T-cell subsets. *, statistically significant difference between groups. (B) Frequency of CD31+ cells in naive CD4+ T cells. (C) Correlation between the percentage of CD31+ cells among naive CD4+ T cells and age. Closed circles, PIR; open circles, GIR. (D) sjTREC content of the CD31+- and CD31−-naive CD4+ T-cell subsets.
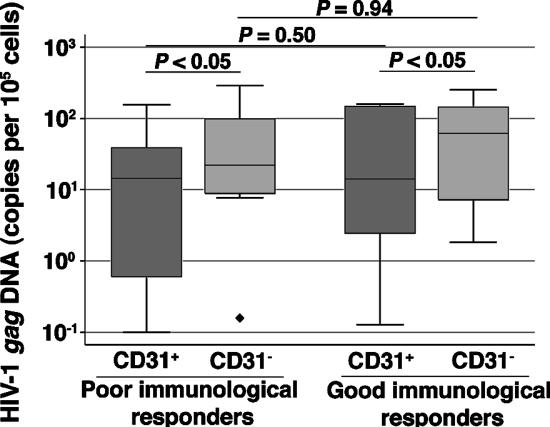
Similar levels of cellular virus loads in the naïve T cells of the poor and good immunological responders.
The infection of developing thymocytes could result in the export of infected RTEs to the periphery, or mature naïve CD4+ T cells may become infected in secondary lymphoid organs. We measured HIV-1 gag DNA in sorted CD31+- and CD31−-naïve CD4+ T cells and in CD45RA+ CD27+ CD8+ T cells in the poor and good immunological responders to determine which mechanism was predominant. The proportions of naïve CD4+ T cells containing HIV-1 DNA were similar in the poor and good immunological responders (Fig. 4), regardless of infection by R5 or X4 viruses (data not shown). The virus load was higher in CD31− cells, i.e., in peripherally expanded naïve cells, than in CD31+ cells in both groups (Fig. 4). It was correlated in both subsets with the cellular virus load in total PBMCs (for the CD31+ cells, ρ was 0.45 and P was <0.05; for the CD31− cells, ρ was 0.53 and P was <0.01) (data not shown). We amplified low levels of gag DNA in CD45RA+ CD27+ CD8+ T cells from only 5 of 26 patients (two poor and three good immunological responders; independent of infection with R5 or X4 viruses) (data not shown). However, even though the sorted cells were >99% pure, we cannot rule out the possibility of a contamination by a small proportion of other nonnaïve T-cell subsets. Thus, the infection of CD4+ CD8+ double-positive thymocytes, if it occurred, rarely resulted in the export of infected mature naïve CD8+ T cells to the periphery. The infection of naïve CD4+ T cells seems to occur primarily in the periphery, but the possibility that infection of mature thymocytes at a CD4+ CD8− single-positive stage could account for a significant proportion of infected cells in the CD31+-naïve CD4+ T-cell population in both groups cannot be excluded.
FIG. 4.
Similar levels of cellular virus load in naïve CD4+ T-cell subsets of the poor and good immunological responders.
Persistent T-cell activation in the poor immunological responders.
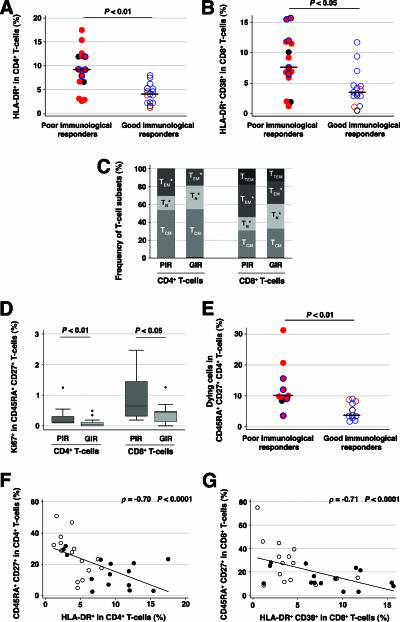
We investigated the possibility that persistent CD4+ T-cell lymphopenia in the poor immunological responders is related to greater T-cell activation and apoptosis than those in the good immunological responders. The activation of CD4+ and CD8+ T cells was greater in the poor immunological responders than it was in the good immunological responders, as assessed by the HLA-DR and CD38 markers (Fig. 5A and B). T-cell activation was increased in those poor immunological responders who harbored R5 viruses with an “X4-like” genotype, as it was in those who harbored X4 viruses, notably for the CD8+ T cells, but not in the two good immunological responders infected by X4 viruses. Immunophenotypic analysis of CD4+ and CD8+ T-cell subsets using CD45RA and CD27 markers revealed an increased proportion of effector memory T cells, concomitant to the depletion of naïve T cells, in the poor immunological responders (Fig. 5C). The proportion of CD45RA+ CD27+ CD4+ and CD8+ T cells bearing the Ki67 cell cycle marker in the poor immunological responders was greater than that in the good responders (Fig. 5D) and was correlated with the degree of T-cell activation (for the CD4+ T cells, ρ was 0.41 and P was <0.05; for the CD8+ T cells, ρ was 0.66 and P was <0.001) (data not shown). We also assessed the cell death rate of the naïve, central memory, and effector memory CD4+ T-cell subsets using 7-AAD staining. The rate at which CD4+ T cells died was mainly related to the percentage of effector memory cells among the CD4+ T cells (ρ = 0.86, P < 0.0001) (data not shown), but the cell death rate of naïve CD4+ T cells was greater in the poor immunological responders than in the good immunological responders (P < 0.01) (Fig. 5E). There was a strong negative correlation between T-cell activation and the size of the naïve CD4+ T-cell compartment in both percentage (ρ = −0.70, P < 0.0001) (Fig. 5F) and absolute numbers (ρ = −0.75, P < 0.0001) (data not shown). There was a similar negative correlation for the CD45RA+ CD27+ CD8+ T cells in both percentage (ρ = −0.71, P < 0.0001) (Fig. 5G) and absolute numbers (ρ = −0.67, P < 0.0001) (data not shown).
FIG. 5.
Persistent T-cell activation in the poor immunological responders. Level of CD4+ (A) and CD8+ (B) T-cell activation. Closed red circles, PIR harboring some X4 viruses; closed red circles with a blue ring, PIR harboring R5 viruses with an “X4-like” genotype; closed gray circles, PIR in whom coreceptor usage could not be determined; open red circles, GIR harboring some X4 viruses; open blue circles, GIR harboring R5 viruses; open gray circles, GIR in whom coreceptor usage could not be determined. (C) Frequency of naïve T cells (TN, CD45RA+ CD27+), central memory cells (TCM, CD45RA− CD27+), effector memory cells (TEM, CD45RA− CD27−), and terminally differentiated effector memory cells (TTEM, CD45RA+ CD27−). The proportion of TTEM in the CD4+ T-cell subset was negligible and is thus not represented. *, statistically significant difference between the two groups. (D) Proportion of cells bearing the Ki67 cell cycle marker in CD45RA+ CD27+ CD4+ and CD8+ T cells. (E) Cell death rate in CD45RA+ CD27+ CD4+ T cells measured using 7-AAD staining. (F) Correlation between CD4+ T-cell activation and the percentage of CD45RA+ CD27+ CD4+ T cells. (G) Correlation between CD8+ T-cell activation and the percentage of CD45RA+ CD27+ CD8+ T cells. Closed circles, PIR; open circles, GIR.
DISCUSSION
Our investigation of the immunological and virological determinants of CD4+ T-cell restoration in poor and good immunological responders on sustained effective HAART suggests that the persistence of low CD4+ T-cell counts in poor immunological responders is due mainly to excessive destruction of mature CD4+ T cells in the periphery rather than to thymic dysfunction. Thymic production does not appear to be a limiting factor for long-term CD4+ T-cell recovery in patients on sustained HAART, since the levels of intrathymic proliferation assessed by the sj/βTREC ratio and RTE frequencies in the poor and good immunological responders were similar. Our results are in agreement with those of a recent study reporting high frequencies of sjTREC in the naïve CD4+ T cells of HIV-infected patients on HAART, in particular in patients with few naïve CD4+ T cells, low CD4 nadirs, and poor gains of CD4+ T cells in response to HAART (18). This may be due to enhanced thymic output and/or to increased incorporation of the RTEs into the established pool of naïve CD4+ T cells in these patients. In contrast, most previous studies indicating impaired thymic production in such patients have based their conclusions on the findings of either low TREC frequencies in CD4+ T cells, or reduced absolute numbers of sjTRECs per microliter of blood (5, 36, 44). The absolute number of sjTRECs per microliter of blood depends on only the net balance between the input and output of sjTREC-bearing cells in the blood compartment, whereas sjTREC frequencies are also affected by T-cell proliferation (19, 31). We found similar sjTREC frequencies in naïve CD4+ T cells of poor and good immunological responders, but because of lower CD4+ T-cell counts in the poor immunological responders, their absolute numbers of sjTRECs are lower than those in the good immunological responders. However, reduced absolute numbers of sjTRECs do not invariably reflect impaired thymic production, notably if sjTREC-bearing cells in the periphery die faster than the input of RTEs as probably occurs in the subjects we studied. Indeed, our data are consistent with an increased rate of naïve-cell death in the poor immunological responders but a thymic function similar to that found in the good immunological responders.
Our results, in agreement with those of other studies (2, 21), show that the frequency of naïve T cells is negatively correlated with the level of T-cell activation. Further investigation is necessary to determine why T-cell activation persists in poor immunological responders. This will include studies on the interactions between dendritic cells and T cells, T helper type 1/T helper type 2 differentiation, cytokine secretion patterns, and interactions between chemokines and T cells. We will also carefully examine naturally occurring regulatory T cells (Tregs) in the poor and good immunological responders, as it has been suggested that Tregs play a critical role in controlling immune activation during HIV and simian immunodeficiency virus infections (25, 26), although our preliminary data indicate no differences in the numbers of CD25high CD4+ T cells in the two groups (data not shown). The changes responsible for an increased susceptibility of naïve CD4+ T cells to apoptosis, such as reduced Bcl-2 expression (8), also require further investigation. The increased survival of both naïve and central memory CD4+ T cells has recently been reported to account for most of the increases in the number of CD4+ T cells in HIV-infected patients given interleukin-2 (27).
Our findings suggest a possible role for X4 viruses in the pathogenesis of poor immune reconstitution on HAART. X4 viruses could account for the depletion of the naïve T-cell pool by the impairment of the thymus output or by the destruction of mature naïve cells in the periphery. Thymocytes and naïve CD4+ T cells may be particularly susceptible to X4 viruses because CXCR4 is highly expressed on their surfaces (6, 41, 43), as is suggested by the infection of thymocytes with X4 viruses in vitro (41), and infection of rhesus macaques with recombinant simian-human immunodeficiency virus-expressing CXCR4-using envelopes in vivo (35, 40). Previous studies showed that immature thymocytes are highly sensitive to apoptosis following infection by X4 viruses, whereas mature thymocytes exhibit a high survival capacity to infection by X4 viruses, notably because of high levels of Bcl-2 (17). Mature thymocytes may thus represent a reservoir for X4 viruses and infected RTEs could be exported to the periphery (7, 41). However, we find that the levels of intrathymic proliferation and the proportions of CD31+-naïve T cells containing HIV-1 DNA in the poor and good immunological responders are similar, regardless of whether they are infected by R5 or X4 viruses. This suggests that the X4-infected poor immunological responders probably do not have significant levels of infection of their thymuses under sustained effective HAART.
Our data rather suggest that the naïve-T-cell compartment is drained by a high rate of mature naïve cell loss in the periphery. X4 viruses could account for the depletion of the naïve- T-cell pool by the direct killing of target cells or by bystander apoptosis and activation-induced differentiation. The low fraction of circulating naïve T cells harboring HIV-1 DNA does not exclude a higher frequency of X4-infected naïve cells in lymphoid tissues. But massive virus infection of target cells, either R5 infection of memory CD4+ T cells or X4 infection of naïve CD4+ T cells, occurs mainly in the setting of high-level virus replication, notably during primary HIV-1 infection (32, 33, 35). In contrast, the patients we studied were all receiving effective HAART for a median of 7 years, so they probably had low rates of virus replication in lymphoid tissues. Further investigations of lymphoid tissues from macaques infected by R5 or X4 simian-human immunodeficiency virus under HAART should be performed to clarify at what rate naïve CD4+ T cells are infected on HAART. X4 viruses could have damaged the naïve CD4+ T-cell pool before HAART, but our results suggest that the loss of naïve cells persists even after many years on effective HAART in the poor immunological responders, overwhelming thymic production. X4 viruses could play a role in this process by triggering persistent T-cell activation and bystander apoptosis via gp120-CXCR4 interactions, although we cannot entirely rule out the possibility that direct killing of infected naïve CD4+ T cells still occurs in lymphoid tissues despite HAART.
The residual replication of X4 viruses and the expression and/or secretion of HIV-1 proteins by persistently infected cells in patients on HAART could result in sufficient gp120 in the lymphoid microenvironment to trigger the activation and apoptosis of bystander uninfected T cells via CXCR4 (20, 37). X4 envelope glycoprotein could mimic the action of a chemokine on T cells that bear CXCR4 (4). Differences in residual X4 virus replication on HAART or other virus or host determinants could explain differences in T-cell activation and apoptosis between X4-infected poor and good immunological responders. Indirect causes of naïve cell loss could also explain why both CD4+- and CD8+-naïve T cells are depleted in the poor immunological responders. Moreover, our genotypic analysis of V3 env identified mutations related to CXCR4 coreceptor usage in all five poor immunological responders infected by viruses with an R5 phenotype. These mutations did not emerge during HAART since viruses with identical “X4-like” sequences already existed within pre-HAART quasispecies. We can speculate that these viruses could have sufficient mutations in V3 to enable them to interact with CXCR4 and thereby activate bystander T cells, even if these viruses could not efficiently infect target cells via CXCR4 and thus did not have an X4 phenotype. This model of X4-like pathogenicity of R5 “late” viruses might be consistent with studies reporting increased pathogenicity in R5-infected patients during late-stage disease (22, 28, 39).
Taken together, our data suggest that X4 viruses could play a role in the pathogenesis of poor immune reconstitution on HAART by triggering persistent T-cell activation and bystander apoptosis via gp120-CXCR4 interactions. This possibility should be taken into consideration when considering the benefits and risks of using CCR5 entry inhibitors in future therapeutic strategies.
Supplementary Material
Acknowledgments
We thank A. Louise and F.-E. L'Faqihi for technical assistance with flow cytometry, F. Mammano for providing the 43-ΔV vector, and M. Alizon for providing the U373 cell lines. We also thank C. Delourme, F. Balsarin, and M. Barone for assistance in monitoring the ANRS EP32 study.
P. Delobel received a doctoral fellowship from the College des Universitaires de Maladies Infectieuses et Tropicales and from the Pasteur Institute (CMIT-IP program). This study was supported by the Pasteur Institute and the French National Agency for AIDS Research (ANRS).
This work is dedicated to the memory of Nicole Israël, who died recently.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Almeida, A. R., J. A. Borghans, and A. A. Freitas. 2001. T cell homeostasis: thymus regeneration and peripheral T cell restoration in mice with a reduced fraction of competent precursors. J. Exp. Med. 194:591-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anthony, K. B., C. Yoder, J. A. Metcalf, R. DerSimonian, J. M. Orenstein, R. A. Stevens, J. Falloon, M. A. Polis, H. C. Lane, and I. Sereti. 2003. Incomplete CD4 T cell recovery in HIV-1 infection after 12 months of highly active antiretroviral therapy is associated with ongoing increased CD4 T cell activation and turnover. J. Acquir. Immune Defic. Syndr. 33:125-133. [DOI] [PubMed] [Google Scholar]
- 3.Autran, B., G. Carcelain, T. S. Li, C. Blanc, D. Mathez, R. Tubiana, C. Katlama, P. Debre, and J. Leibowitch. 1997. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 277:112-116. [DOI] [PubMed] [Google Scholar]
- 4.Balabanian, K., J. Harriague, C. Decrion, B. Lagane, S. Shorte, F. Baleux, J. L. Virelizier, F. Arenzana-Seisdedos, and L. A. Chakrabarti. 2004. CXCR4-tropic HIV-1 envelope glycoprotein functions as a viral chemokine in unstimulated primary CD4+ T lymphocytes. J. Immunol. 173:7150-7160. [DOI] [PubMed] [Google Scholar]
- 5.Benveniste, O., A. Flahault, F. Rollot, C. Elbim, J. Estaquier, B. Pedron, X. Duval, N. Dereuddre-Bosquet, P. Clayette, G. Sterkers, A. Simon, J. C. Ameisen, and C. Leport. 2005. Mechanisms involved in the low-level regeneration of CD4+ cells in HIV-1-infected patients receiving highly active antiretroviral therapy who have prolonged undetectable plasma viral loads. J. Infect. Dis. 191:1670-1679. [DOI] [PubMed] [Google Scholar]
- 6.Bleul, C. C., L. Wu, J. A. Hoxie, T. A. Springer, and C. R. Mackay. 1997. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc. Natl. Acad. Sci. USA 94:1925-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks, D. G., S. G. Kitchen, C. M. Kitchen, D. D. Scripture-Adams, and J. A. Zack. 2001. Generation of HIV latency during thymopoiesis. Nat. Med. 7:459-464. [DOI] [PubMed] [Google Scholar]
- 8.David, D., H. Keller, L. Nait-Ighil, M. P. Treilhou, M. Joussemet, B. Dupont, B. Gachot, J. Maral, and J. Theze. 2002. Involvement of Bcl-2 and IL-2R in HIV-positive patients whose CD4 cell counts fail to increase rapidly with highly active antiretroviral therapy. AIDS 16:1093-1101. [DOI] [PubMed] [Google Scholar]
- 9.Delobel, P., K. Sandres-Saune, M. Cazabat, C. Pasquier, B. Marchou, P. Massip, and J. Izopet. 2005. R5 to X4 switch of the predominant HIV-1 population in cellular reservoirs during effective highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 38:382-392. [DOI] [PubMed] [Google Scholar]
- 10.Dion, M. L., J. F. Poulin, R. Bordi, M. Sylvestre, R. Corsini, N. Kettaf, A. Dalloul, M. R. Boulassel, P. Debre, J. P. Routy, Z. Grossman, R. P. Sekaly, and R. Cheynier. 2004. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity 21:757-768. [DOI] [PubMed] [Google Scholar]
- 11.Douek, D. C., M. R. Betts, B. J. Hill, S. J. Little, R. Lempicki, J. A. Metcalf, J. Casazza, C. Yoder, J. W. Adelsberger, R. A. Stevens, M. W. Baseler, P. Keiser, D. D. Richman, R. T. Davey, and R. A. Koup. 2001. Evidence for increased T cell turnover and decreased thymic output in HIV infection. J. Immunol. 167:6663-6668. [DOI] [PubMed] [Google Scholar]
- 12.Douek, D. C., R. D. McFarland, P. H. Keiser, E. A. Gage, J. M. Massey, B. F. Haynes, M. A. Polis, A. T. Haase, M. B. Feinberg, J. L. Sullivan, B. D. Jamieson, J. A. Zack, L. J. Picker, and R. A. Koup. 1998. Changes in thymic function with age and during the treatment of HIV infection. Nature 396:690-695. [DOI] [PubMed] [Google Scholar]
- 13.Fouchier, R. A., M. Groenink, N. A. Kootstra, M. Tersmette, H. G. Huisman, F. Miedema, and H. Schuitemaker. 1992. Phenotype-associated sequence variation in the third variable domain of the human immunodeficiency virus type 1 gp120 molecule. J. Virol. 66:3183-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furtado, M. R., D. S. Callaway, J. P. Phair, K. J. Kunstman, J. L. Stanton, C. A. Macken, A. S. Perelson, and S. M. Wolinsky. 1999. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N. Engl. J. Med. 340:1614-1622. [DOI] [PubMed] [Google Scholar]
- 15.Grabar, S., V. Le Moing, C. Goujard, C. Leport, M. D. Kazatchkine, D. Costagliola, and L. Weiss. 2000. Clinical outcome of patients with HIV-1 infection according to immunologic and virologic response after 6 months of highly active antiretroviral therapy. Ann. Intern. Med. 133:401-410. [DOI] [PubMed] [Google Scholar]
- 16.Grossman, Z., B. Min, M. Meier-Schellersheim, and W. E. Paul. 2004. Concomitant regulation of T-cell activation and homeostasis. Nat. Rev. Immunol. 4:387-395. [DOI] [PubMed] [Google Scholar]
- 17.Guillemard, E., M. T. Nugeyre, L. Chene, N. Schmitt, C. Jacquemot, F. Barre-Sinoussi, and N. Israel. 2001. Interleukin-7 and infection itself by human immunodeficiency virus 1 favor virus persistence in mature CD4+CD8−CD3+ thymocytes through sustained induction of Bcl-2. Blood 98:2166-2174. [DOI] [PubMed] [Google Scholar]
- 18.Harris, J. M., M. D. Hazenberg, J. F. Poulin, D. Higuera-Alhino, D. Schmidt, M. Gotway, and J. M. McCune. 2005. Multiparameter evaluation of human thymic function: interpretations and caveats. Clin. Immunol. 115:138-146. [DOI] [PubMed] [Google Scholar]
- 19.Hazenberg, M. D., S. A. Otto, J. W. Cohen Stuart, M. C. Verschuren, J. C. Borleffs, C. A. Boucher, R. A. Coutinho, J. M. Lange, T. F. Rinke de Wit, A. Tsegaye, J. J. van Dongen, D. Hamann, R. J. de Boer, and F. Miedema. 2000. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nat. Med. 6:1036-1042. [DOI] [PubMed] [Google Scholar]
- 20.Hazenberg, M. D., S. A. Otto, D. Hamann, M. T. Roos, H. Schuitemaker, R. J. de Boer, and F. Miedema. 2003. Depletion of naive CD4 T cells by CXCR4-using HIV-1 variants occurs mainly through increased T-cell death and activation. AIDS 17:1419-1424. [DOI] [PubMed] [Google Scholar]
- 21.Hunt, P. W., J. N. Martin, E. Sinclair, B. Bredt, E. Hagos, H. Lampiris, and S. G. Deeks. 2003. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J. Infect. Dis. 187:1534-1543. [DOI] [PubMed] [Google Scholar]
- 22.Karlsson, I., J. C. Grivel, S. S. Chen, A. Karlsson, J. Albert, E. M. Fenyo, and L. B. Margolis. 2005. Differential pathogenesis of primary CCR5-using human immunodeficiency virus type 1 isolates in ex vivo human lymphoid tissue. J. Virol. 79:11151-11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufmann, G. R., L. Perrin, G. Pantaleo, M. Opravil, H. Furrer, A. Telenti, B. Hirschel, B. Ledergerber, P. Vernazza, E. Bernasconi, M. Rickenbach, M. Egger, and M. Battegay. 2003. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch. Intern. Med. 163:2187-2195. [DOI] [PubMed] [Google Scholar]
- 24.Kimmig, S., G. K. Przybylski, C. A. Schmidt, K. Laurisch, B. Mowes, A. Radbruch, and A. Thiel. 2002. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J. Exp. Med. 195:789-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinter, A. L., M. Hennessey, A. Bell, S. Kern, Y. Lin, M. Daucher, M. Planta, M. McGlaughlin, R. Jackson, S. F. Ziegler, and A. S. Fauci. 2004. CD25+CD4+ regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4+ and CD8+ HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J. Exp. Med. 200:331-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kornfeld, C., M. J. Ploquin, I. Pandrea, A. Faye, R. Onanga, C. Apetrei, V. Poaty-Mavoungou, P. Rouquet, J. Estaquier, L. Mortara, J. F. Desoutter, C. Butor, R. Le Grand, P. Roques, F. Simon, F. Barre-Sinoussi, O. M. Diop, and M. C. Muller-Trutwin. 2005. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J. Clin. Investig. 115:1082-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacs, J. A., R. A. Lempicki, I. A. Sidorov, J. W. Adelsberger, I. Sereti, W. Sachau, G. Kelly, J. A. Metcalf, R. T. Davey, Jr., J. Falloon, M. A. Polis, J. Tavel, R. Stevens, L. Lambert, D. A. Hosack, M. Bosche, H. J. Issaq, S. D. Fox, S. Leitman, M. W. Baseler, H. Masur, M. Di Mascio, D. S. Dimitrov, and H. C. Lane. 2005. Induction of prolonged survival of CD4+ T lymphocytes by intermittent IL-2 therapy in HIV-infected patients. J. Clin. Investig. 115:2139-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwa, D., J. Vingerhoed, B. Boeser, and H. Schuitemaker. 2003. Increased in vitro cytopathicity of CC chemokine receptor 5-restricted human immunodeficiency virus type 1 primary isolates correlates with a progressive clinical course of infection. J. Infect. Dis. 187:1397-1403. [DOI] [PubMed] [Google Scholar]
- 29.Lafeuillade, A., L. Chollet, G. Hittinger, N. Profizi, O. Costes, and C. Poggi. 1998. Residual human immunodeficiency virus type 1 RNA in lymphoid tissue of patients with sustained plasma RNA of <200 copies/ml. J. Infect. Dis. 177:235-238. [DOI] [PubMed] [Google Scholar]
- 30.Lecoeur, H., E. Ledru, M. C. Prevost, and M. L. Gougeon. 1997. Strategies for phenotyping apoptotic peripheral human lymphocytes comparing ISNT, annexin-V and 7-AAD cytofluorometric staining methods. J. Immunol. Methods 209:111-123. [DOI] [PubMed] [Google Scholar]
- 31.Lewin, S. R., R. M. Ribeiro, G. R. Kaufmann, D. Smith, J. Zaunders, M. Law, A. Solomon, P. U. Cameron, D. Cooper, and A. S. Perelson. 2002. Dynamics of T cells and TCR excision circles differ after treatment of acute and chronic HIV infection. J. Immunol. 169:4657-4666. [DOI] [PubMed] [Google Scholar]
- 32.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148-1152. [DOI] [PubMed] [Google Scholar]
- 33.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 34.McCune, J. M., R. Loftus, D. K. Schmidt, P. Carroll, D. Webster, L. B. Swor-Yim, I. R. Francis, B. H. Gross, and R. M. Grant. 1998. High prevalence of thymic tissue in adults with human immunodeficiency virus-1 infection. J. Clin. Investig. 101:2301-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishimura, Y., C. R. Brown, J. J. Mattapallil, T. Igarashi, A. Buckler-White, B. A. Lafont, V. M. Hirsch, M. Roederer, and M. A. Martin. 2005. Resting naive CD4+ T cells are massively infected and eliminated by X4-tropic simian-human immunodeficiency viruses in macaques. Proc. Natl. Acad. Sci. USA 102:8000-8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nobile, M., R. Correa, J. A. Borghans, C. D'Agostino, P. Schneider, R. J. De Boer, and G. Pantaleo. 2004. De novo T-cell generation in patients at different ages and stages of HIV-1 disease. Blood 104:470-477. [DOI] [PubMed] [Google Scholar]
- 37.Popovic, M., K. Tenner-Racz, C. Pelser, H. J. Stellbrink, J. van Lunzen, G. Lewis, V. S. Kalyanaraman, R. C. Gallo, and P. Racz. 2005. Persistence of HIV-1 structural proteins and glycoproteins in lymph nodes of patients under highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 102:14807-14812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramratnam, B., J. E. Mittler, L. Zhang, D. Boden, A. Hurley, F. Fang, C. A. Macken, A. S. Perelson, M. Markowitz, and D. D. Ho. 2000. The decay of the latent reservoir of replication-competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged anti-retroviral therapy. Nat. Med. 6:82-85. [DOI] [PubMed] [Google Scholar]
- 39.Repits, J., M. Oberg, J. Esbjornsson, P. Medstrand, A. Karlsson, J. Albert, E. M. Fenyo, and M. Jansson. 2005. Selection of human immunodeficiency virus type 1 R5 variants with augmented replicative capacity and reduced sensitivity to entry inhibitors during severe immunodeficiency. J. Gen. Virol. 86:2859-2869. [DOI] [PubMed] [Google Scholar]
- 40.Reyes, R. A., D. R. Canfield, U. Esser, L. A. Adamson, C. R. Brown, C. Cheng-Mayer, M. B. Gardner, J. M. Harouse, and P. A. Luciw. 2004. Induction of simian AIDS in infant rhesus macaques infected with CCR5- or CXCR4-utilizing simian-human immunodeficiency viruses is associated with distinct lesions of the thymus. J. Virol. 78:2121-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmitt, N., L. Chene, D. Boutolleau, M. T. Nugeyre, E. Guillemard, P. Versmisse, C. Jacquemot, F. Barre-Sinoussi, and N. Israel. 2003. Positive regulation of CXCR4 expression and signaling by interleukin-7 in CD4+ mature thymocytes correlates with their capacity to favor human immunodeficiency X4 virus replication. J. Virol. 77:5784-5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swainson, L., S. Kinet, N. Manel, J. L. Battini, M. Sitbon, and N. Taylor. 2005. Glucose transporter 1 expression identifies a population of cycling CD4+ CD8+ human thymocytes with high CXCR4-induced chemotaxis. Proc. Natl. Acad. Sci. USA 102:12867-12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor, J. R., Jr., K. C. Kimbrell, R. Scoggins, M. Delaney, L. Wu, and D. Camerini. 2001. Expression and function of chemokine receptors on human thymocytes: implications for infection by human immunodeficiency virus type 1. J. Virol. 75:8752-8760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teixeira, L., H. Valdez, J. M. McCune, R. A. Koup, A. D. Badley, M. K. Hellerstein, L. A. Napolitano, D. C. Douek, G. Mbisa, S. Deeks, J. M. Harris, J. D. Barbour, B. H. Gross, I. R. Francis, R. Halvorsen, R. Asaad, and M. M. Lederman. 2001. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. AIDS 15:1749-1756. [DOI] [PubMed] [Google Scholar]
- 45.Trouplin, V., F. Salvatori, F. Cappello, V. Obry, A. Brelot, N. Heveker, M. Alizon, G. Scarlatti, F. Clavel, and F. Mammano. 2001. Determination of coreceptor usage of human immunodeficiency virus type 1 from patient plasma samples by using a recombinant phenotypic assay. J. Virol. 75:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.