Abstract
Dimethyl sulfoxide (DMSO) has been shown to induce the differentiation of primary hepatocytes in vitro. When actively dividing poorly differentiated human hepatoma-derived (Huh7) cells were cultured in the presence of 1% DMSO, cells became cytologically differentiated and transitioned into a nondividing state, characterized by the induction of hepatocyte-specific genes. Moreover, these cells were highly permissive for acute hepatitis C virus (HCV) infection, and persistent long term infection of these cultures could also be achieved. As HCV naturally replicates in highly differentiated nondividing human hepatocytes, this system may more accurately mimic the conditions under which HCV replicates in vivo than previous models using poorly differentiated rapidly dividing hepatoma cells.
Hepatitis C virus (HCV) is a positive-stranded RNA virus that causes acute and chronic hepatitis and hepatocellular carcinoma (15). A striking feature of HCV infection is that at least 70% of infections persist and cause chronic liver disease of various severities (1-3, 34). Although in vitro subgenomic and full-length replicon systems have yielded much insight into HCV translation and RNA replication in human hepatoma-derived Huh7 cells (16, 23, 33), our understanding of the rest of the HCV life cycle has been limited due to the lack of infectious cell culture models of HCV infection. Thanks to the recent identification of a full-length genotype 2a HCV genome (JFH-1) that replicates and produces infectious virus in cell culture (22, 39, 43) and the discovery that full-length RNA from an HCV genotype 1a genome (clone H77) containing cell culture-adaptive mutations is also infectious in vitro (20, 42), the entire HCV life cycle is now accessible for investigation. To date, HCV infection and replication in vitro have been studied in actively dividing asynchronous cultures of Huh7 cells or Huh7-derived cells that cannot respond to intracellular double-stranded RNA (43). Accordingly, these systems may not accurately mimic the events that occur during a natural HCV infection in vivo, as hepatocytes are naturally nondividing, differentiated (26), and fully responsive to double-stranded RNA (21). In order to enhance the physiological relevance of the existing HCV infection systems, we asked whether dimethyl sulfoxide (DMSO)-treated Huh7 cells that are highly differentiated and growth arrested are also permissive for HCV infection.
Effect of DMSO on Huh7 cells.
DMSO, a dipolar aprotic solvent, has been used extensively to induce or maintain differentiation of numerous primary or tumor cell lines (4-6, 8-10, 13, 14, 17-19, 27, 30, 35, 38, 44). The mechanism by which DMSO induces differentiation of certain cell types remains unclear; however, DMSO has been shown to affect cell membrane integrity (25), alter intracellular signaling processes (e.g., protein kinase C activity and integrin expression) (12, 24), and affect cellular alternative splicing (7), all of which may contribute to its potential to promote cell differentiation and alter cell proliferation. Thus, we examined the capacity of DMSO to induce a more differentiated state in Huh7 cells. Initially, we examined the morphological appearance and growth kinetics of Huh7 cells seeded in collagen-coated plates and continuously cultured in the presence of 1% DMSO for 20 days. As shown in Fig. 1A and B, Huh7 cells cultured in the presence of 1% DMSO formed tightly packed monolayers of mono- and binucleated cells displaying the typical pavement-like cytological features of primary hepatocytes. The cells also exhibited a low nucleus-to-cytoplasm ratio and contained multiple distinct nucleoli (Fig. 1B, inset). Although Huh7 cells cultured in the absence of DMSO initially formed tightly packed monolayers, by day 10 postseeding, monolayer integrity was compromised and cell death, measured by floating cells in the culture supernatant, was extensive (data not shown).
FIG. 1.
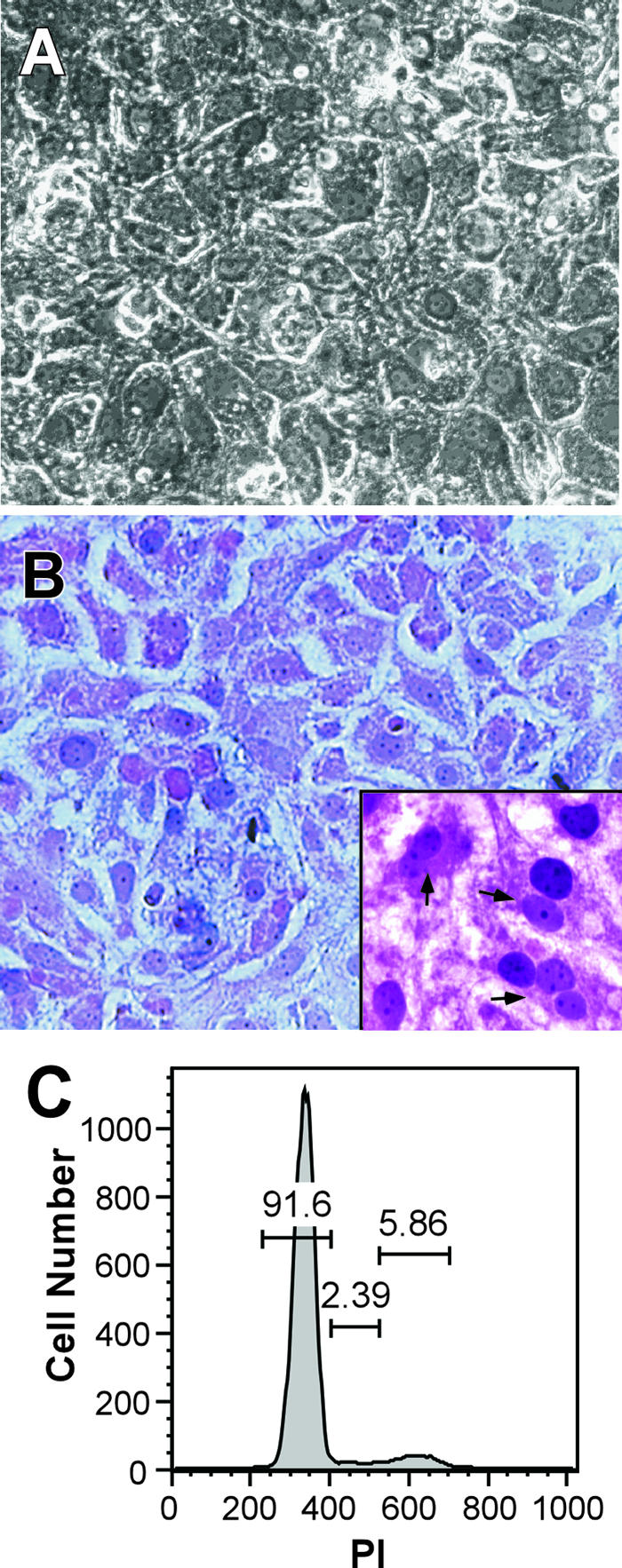
DMSO-induced differentiation of Huh7 cells. (A) Phase contrast micrograph of Huh7 cells cultured on BioCoat collagen-coated plates (Becton Dickinson, Franklin Lakes, NJ) in the presence of 1% DMSO (vol/vol) (Sigma-Aldrich, St. Louis, MO) and photographed 20 days after plating (magnification, ×200). (B) Hematoxylin and eosin staining of DMSO-treated Huh7 cells cultured in the presence of 1% DMSO for 20 days (magnification, ×400; inset magnification, ×800). Arrows indicate binucleated cells containing multiple distinct nucleoli. (C) Flow cytometric analysis of Huh7 cells cultured in the presence of 1% DMSO for 20 days. For cell cycle analysis, 1 × 105 cells were stained with 15 μg/ml propidium iodide (PI) (Boehringer Mannheim, Indianapolis, IN), in the presence of 0.25% NP-40 (Sigma-Aldrich) for 30 min, and analyzed using a FACSCalibur flow cytometer (Becton Dickinson), and data were analyzed using FloJo software (Tree Star, Inc., Ashland, OR).
The growth kinetics of the DMSO-treated Huh7 cells was evaluated in each of three wells up to day 20 post-DMSO treatment. During the first 6 days, cell numbers increased approximately 1.5-fold per day on average, after which the cells became growth arrested and remained nondividing for up to 20 days post-DMSO treatment (Fig. 2). Propidium iodide staining was performed, as described previously (11), in order to determine the cell cycle distribution of DMSO-treated Huh7 cells. As shown in Fig. 1C, approximately 92% of Huh7 cells cultured for 20 days in the presence of 1% DMSO were arrested in G0/G1 and, therefore, nondividing. As a reference, propidium iodide staining was also performed on cycling non-DMSO-treated Huh7 cells and approximately 63%, 13%, and 22% of the proliferating Huh7 cells were in G0/G1, S, and G2, respectively (data not shown).
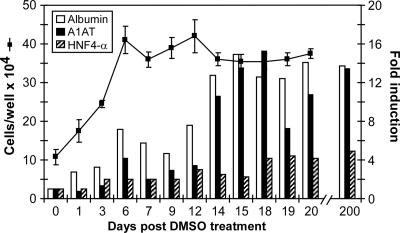
FIG. 2.
Growth kinetics and induction of hepatocyte-specific cellular genes in DMSO-treated Huh7 cells. For gene analysis, 8 × 104 Huh7 cells were plated in multiple wells of 12-well BioCoat collagen-coated plates. Medium was supplemented with 1% DMSO (vol/vol) at 24 h postseeding (when cells reached 95% confluence), and cells were cultured for an additional 20 days in the presence of DMSO. At indicated times post-DMSO treatment, triplicate wells were trypsinized and the average total cell number was calculated (line). Cells were additionally pelleted at 1,400 rpm for 5 min, and total cellular RNA was extracted (43) and analyzed by RT-QPCR (Bio-Rad, Hercules, CA) for human albumin, A1AT, and HNF4-α mRNA expression. GAPDH amplification was used as a normalization control, and the results are expressed as induction (n-fold) of gene expression in Huh7 cells post-DMSO treatment (days 1 to 20 and day 200) relative to that of control, non-DMSO-treated, Huh7 cells (day 0).
To characterize the differentiation status of the DMSO-treated Huh7 cells, we examined whether these cultures transcriptionally up-regulate the expression of human hepatocyte-specific cellular genes, as has been shown for primary hepatocytes cultured under the same conditions (5, 6, 8, 14, 17, 18, 27, 31). At indicated times post-DMSO treatment, total cellular RNA was extracted and the induction of hepatocyte-specific cellular genes was analyzed by quantitative, real-time reverse transcription-PCR (RT-QPCR) analysis as described previously (43). PCR amplification was performed on cDNA templates using primers specific for human albumin (sense, 5′CGCCTGAGCCAGAGATTTC3′; and antisense, 5′GCCCTGTCATCAGCACATTC3′), hepatocyte nuclear factor 4-α (HNF4-α) (sense, 5′ACATTCGGGCGAAGAAGATT3′; and antisense, 5′ACTTGGCCCACTCAACGAG3′), and α-1-antitrypsin (A1AT) (sense, 5′TGCTGCCCAGAAGACAGATA3′; and antisense, 5′GGCGGTATAGGCTGAAGG3′). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) amplification (43) was used as a normalization control. As illustrated in Fig. 2, human albumin and A1AT mRNA expression was induced 7- and 4-fold, respectively, in Huh7 cells as early as 6 days post-DMSO treatment and further increased to 13- and 11-fold, respectively, during the last part of DMSO treatment (days 14 to 20). Likewise, an average fourfold induction of the hepatocyte-specific transcription factor HNF4-α was also observed during the course of DMSO treatment (Fig. 2). Induction of HNF4-α was of considerable interest, as this transcription factor has been shown to be necessary for liver phenotypic expression in hepatocyte cultures (29). In addition, when RNA extracted from a normal chimpanzee liver biopsy specimen was analyzed, mRNA expression levels similar to the levels detected in the DMSO-treated Huh7 cells were observed (data not shown). Taken together, the results described above suggest that Huh7 cells cultured in the presence of DMSO become more differentiated, expressing characteristics associated with normal hepatocytes, including cytology, growth arrest, and increased induction of hepatocyte-specific cellular genes. Importantly, these cultures have maintained these cytological characteristics and gene expression profiles for at least 200 days without splitting (Fig. 2 and data not shown).
Productive HCV infection of growth-arrested DMSO-treated Huh7 cells.
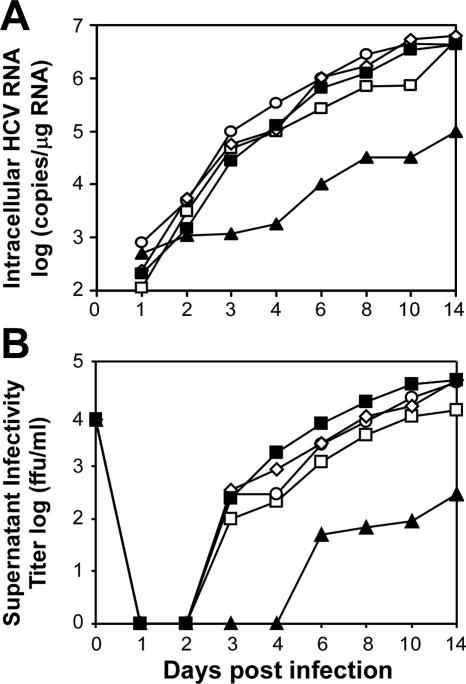
Several groups have indicated a strong relationship between HCV replication and cell confluence, demonstrating that high confluence or superconfluence of Huh7 cells negatively impacts HCV replication (28, 32, 41). Therefore, it was necessary to initially test whether the confluent, growth-arrested DMSO-treated cultures were permissive for HCV JFH-1 virus infection and replication. Following a 6-, 14-, or 20-day differentiation period in the presence of 1% DMSO, cultures were infected with HCV JFH-1 virus at a multiplicity of infection (MOI) of 0.01 focus forming units (FFU) per cell. The culture supernatant and RNA were harvested at various time points postinfection (p.i.), and the kinetics of viral RNA replication and production of infectious virus were determined as described previously (43). The data presented in Fig. 3 illustrate that not only are DMSO-treated Huh7 cells highly permissive for HCV JFH-1 infection but the kinetics and levels of viral RNA expansion and infectious virus production are similar to what is observed for non-DMSO-treated Huh7 cells infected under subconfluent conditions. To date, infection of Huh7 cells with JFH-1 virus has been carried out at subconfluence (22, 39, 43). Interestingly, when overconfluent non-DMSO-treated Huh7 cells were infected with the same inoculum, viral RNA replication (Fig. 3A) and the production of infectious virus in the supernatant (Fig. 3B) were severely impaired. A recent paper by Nelson and Tang demonstrates that cell superconfluence negatively affects HCV replicon replication, most likely by down-regulation of the de novo nucleoside biosynthetic pathway (28). Confluence and cell growth arrest, however, do not appear to limit HCV production in the DMSO-treated Huh7 cultures. Likewise, when Huh7 cells stably replicating either the subgenomic or the full-length JFH-1 replicon were treated with 1% DMSO and cultured for 20 days, no adverse effect on HCV RNA replication was observed by Northern blot analysis (data not shown). These data would suggest that Huh7 cells cultured in the presence of DMSO may not down-regulate the nucleoside biosynthetic pathway, in contrast to their non-DMSO-treated confluent counterparts. This hypothesis, however, remains to be tested.
FIG. 3.
HCV infection kinetics in DMSO-treated Huh7 cells. (A and B) Huh7 cells cultured for 6 (open square), 14 (open circle), or 20 (open diamond) days in the presence of 1% DMSO or subconfluent (closed squares) and confluent (closed triangles) non-DMSO-treated Huh7 cells were infected with JFH-1 HCV at an MOI of 0.01 FFU/cell, and the culture supernatant and intracellular RNA were collected at the indicated times postinfection. (A) Intracellular HCV RNA was analyzed by RT-QPCR and displayed as HCV RNA copies/μg total RNA. (B) Titers of supernatant infectivity, expressed as FFU/ml, were determined by indirect immunofluorescence analysis (Axiovert 200 fluorescence microscope; Zeiss, Germany) of 10-fold serially diluted culture supernatants on naïve Huh7 cells, using a 96-well plate format as described previously (43). A human monoclonal antibody with high avidity and specificity to HCV E2 (43) was used to detect positive foci (5 to 10 positive grouped cells constitute one focus). The anti-HCV E2 antibody was used in lieu of the previously reported (43) NS5A antibody, as it performs equally well in titer assays (data not shown).
Establishment of a persistent HCV infection in DMSO-treated Huh7 cells.
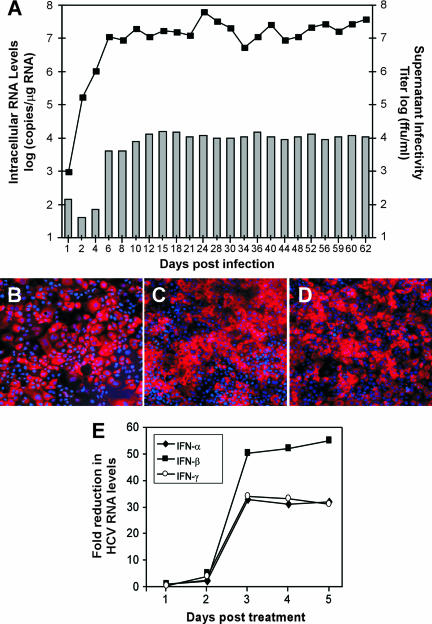
A significant benefit of the DMSO-treated Huh7 cell culture system is the capacity to maintain individual cultures for extended periods of time (up to 200 days) without splitting. As such, we sought to establish a long-term persistent HCV infection in these cultures and analyze HCV viral replication, virus production, and protein expression over an extended period of time. As illustrated in Fig. 4, when cells were infected (MOI of 0.01 FFU/ml) on day 20 post-DMSO treatment, intracellular HCV RNA and titers of supernatant infectivity progressively increased for 12 days, after which they remained stable for 63 days p.i. (Fig. 4A). Likewise, indirect immunofluorescence analysis indicated that at least 80% of the cells were positive for the HCV NS5A protein throughout the course of infection (Fig. 4B to D). The inability to detect NS5A protein expression in 100% of the cells could be attributed to the various degrees of staining intensities observed in this heterogeneous cell culture.
FIG. 4.
Establishment of a long-term persistent HCV infection in DMSO-treated Huh7 cells. Huh7 cells (8 × 104) were plated in multiple wells of 12-well BioCoat collagen-coated plates. Medium was supplemented with 1% DMSO (vol/vol) at 24 h postseeding and replenished every 3 days thereafter. At 20 days post-DMSO treatment, multiple wells were infected with JFH-1 HCV at an MOI of 0.01 FFU/cell and the culture supernatant and intracellular RNA were collected at the indicated times p.i. for up to 63 days. (A) Intracellular HCV RNA was analyzed by RT-QPCR and displayed as HCV RNA copies/μg total RNA (line). Titers of supernatant infectivity were determined for naïve Huh7 cells and are expressed as FFU/ml (bars). The data presented are representative of three independent experiments. (B to D) NS5A immunostaining of DMSO-treated Huh7 cells at days (B) 12, (C) 36, and (E) 62 p.i. (magnification, ×100) was performed as described in reference 43. Image brightness and contrast were adjusted using Adobe Photoshop (San Jose, CA). (E) HCV RNA replication in DMSO-treated Huh7 cells is sensitive to the effects of interferons. At 30 days p.i., DMSO-treated Huh7 cultures were treated with 100 U/ml of IFN-α, IFN-β, or IFN-γ (PBL Biomedical Laboratories, New Brunswick, NJ). On the indicated days posttreatment, total RNA was extracted and intracellular HCV RNA copies/μg of cellular RNA was quantitated by RT-QPCR. Reductions (n-fold) in HCV copy numbers were calculated as follows: number of intracellular HCV RNA copies per μg of cellular RNA in IFN-treated cultures/number of intracellular HCV RNA copies per μg of cellular RNA in diluent-treated cultures. The data presented are representative of three independent experiments.
To address the susceptibility of persistently infected DMSO-treated Huh7 cultures to known inhibitors of HCV replication, DMSO cultures infected with JFH-1 virus (MOI of 0.01 FFU/cell) for 30 days were treated with 100 U/ml of alpha interferon (IFN-α), IFN-β, or IFN-γ, and HCV RNA levels were determined (HCV and GAPDH RNA levels were quantified by RT-QPCR analysis) at 1, 2, 3, 4, and 5 days posttreatment. The results presented in Fig. 4E illustrate that HCV replication in persistently infected DMSO-treated Huh7 cell cultures is sensitive to the inhibitory effects of all three interferons tested. Notably, HCV RNA levels were reduced 33-, 50-, and 34-fold in cultures treated for 3 days with IFN-α, -β, and -γ, respectively, and this level of inhibition was maintained for up to 5 days posttreatment.
Attempts to establish persistent HCV infections in rapidly dividing cells have yielded mixed results. Unlike the stable persistent infection that can be readily established in DMSO-treated Huh7 cells (Fig. 4), in rapidly dividing Huh7 cells, a cytopathic effect is observed and HCV RNA and protein levels fluctuate by two or more orders of magnitude throughout the course of infection (J. Zhong and F. V. Chisari, unpublished data), challenging the physiological relevance of HCV infection in rapidly dividing Huh7 cells. Moreover, serum HCV RNA levels in most chronically HCV-infected chimpanzees typically do not fluctuate markedly (36, 37, 40), which more closely resembles the RNA profile observed for the HCV-infected DMSO-treated Huh7 cells (Fig. 4A).
In summary, the DMSO system described herein allows for the establishment of persistent HCV infection in highly differentiated growth-arrested Huh7 cells, thereby permitting analysis of the biology of HCV infection in a physiologically relevant system that is not compromised by the variables inherent in asynchronous and actively dividing cell cultures. In addition, this system permits the testing of candidate antiviral agents in the context of a persistent HCV infection in cells whose metabolism is likely to approximate that of primary hepatocytes in vivo more closely than that of the less well-differentiated, rapidly dividing cell culture systems that are widely used at present.
Acknowledgments
We thank Dennis Burton (The Scripps Research Institute, La Jolla, CA) for providing the monoclonal anti-HCV E2 human antibody, Michael Houghton (Chiron, Emeryville, CA) for providing the polyclonal anti-NS5A rabbit antibody MS5, and Susan L. Uprichard and Stefan F. Wieland for helpful advice.
This study was supported by grant R01-CA108304 to F.V.C. and by an Institutional Ruth L. Kirchstein National Research Service Award (AI07354-15) fellowship from the National Institutes of Health to B.S.
Footnotes
This is manuscript number 18185-MEM from The Scripps Research Institute.
REFERENCES
- 1.Alter, H. J., and L. B. Seeff. 2000. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin. Liver Dis. 20:17-35. [DOI] [PubMed] [Google Scholar]
- 2.Alter, M. J. 1999. Hepatitis C virus infection in the United States. J. Hepatol. 31(Suppl. 1):88-91. [DOI] [PubMed] [Google Scholar]
- 3.Alter, M. J. 2002. Prevention of spread of hepatitis C. Hepatology 36:S93-S98. [DOI] [PubMed] [Google Scholar]
- 4.Aninat, C., A. Piton, D. Glaise, T. Le Charpentier, S. Langouet, F. Morel, C. Guguen-Guillouzo, and A. Guillouzo. 2006. Expression of cytochromes P450, conjugating enzymes and nuclear receptors in human hepatoma HepaRG cells. Drug Metab. Dispos. 34:75-83. [DOI] [PubMed] [Google Scholar]
- 5.Arterburn, L. M., J. Zurlo, J. D. Yager, R. M. Overton, and A. H. Heifetz. 1995. A morphological study of differentiated hepatocytes in vitro. Hepatology 22:175-187. [PubMed] [Google Scholar]
- 6.Azuma, H., T. Hirose, H. Fujii, S. Oe, K. Yasuchika, T. Fujikawa, and Y. Yamaoka. 2003. Enrichment of hepatic progenitor cells from adult mouse liver. Hepatology 37:1385-1394. [DOI] [PubMed] [Google Scholar]
- 7.Bolduc, L., B. Labrecque, M. Cordeau, M. Blanchette, and B. Chabot. 2001. Dimethyl sulfoxide affects the selection of splice sites. J. Biol. Chem. 276:17597-17602. [DOI] [PubMed] [Google Scholar]
- 8.Cable, E. E., and H. C. Isom. 1997. Exposure of primary rat hepatocytes in long-term DMSO culture to selected transition metals induces hepatocyte proliferation and formation of duct-like structures. Hepatology 26:1444-1457. [DOI] [PubMed] [Google Scholar]
- 9.Chang, H. H., P. Y. Oh, D. E. Ingber, and S. Huang. 2006. Multistable and multistep dynamics in neutrophil differentiation. BMC Cell Biol. 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung, W. M., W. W. Ng, and A. W. Kung. 2006. Dimethyl sulfoxide as an inducer of differentiation in preosteoblast MC3T3-E1 cells. FEBS Lett. 580:121-126. [DOI] [PubMed] [Google Scholar]
- 11.Ezhevsky, S. A., H. Nagahara, A. M. Vocero-Akbani, D. R. Gius, M. C. Wei, and S. F. Dowdy. 1997. Hypo-phosphorylation of the retinoblastoma protein (pRb) by cyclin D:Cdk4/6 complexes results in active pRb. Proc. Natl. Acad. Sci. USA 94:10699-10704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiore, M., and F. Degrassi. 1999. Dimethyl sulfoxide restores contact inhibition-induced growth arrest and inhibits cell density-dependent apoptosis in hamster cells. Exp. Cell Res. 251:102-110. [DOI] [PubMed] [Google Scholar]
- 13.Glebe, D., A. Berting, S. Broehl, H. Naumann, R. Schuster, N. Fiedler, T. K. Tolle, S. Nitsche, M. Seifer, W. H. Gerlich, and S. Schaefer. 2001. Optimised conditions for the production of hepatitis B virus from cell culture. Intervirology 44:370-378. [DOI] [PubMed] [Google Scholar]
- 14.Hino, H., C. Tateno, H. Sato, C. Yamasaki, S. Katayama, T. Kohashi, A. Aratani, T. Asahara, K. Dohi, and K. Yoshizato. 1999. A long-term culture of human hepatocytes which show a high growth potential and express their differentiated phenotypes. Biochem. Biophys. Res. Commun. 256:184-191. [DOI] [PubMed] [Google Scholar]
- 15.Hoofnagle, J. H. 2002. Course and outcome of hepatitis C. Hepatology 36:S21-S29. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda, M., M. Yi, K. Li, and S. M. Lemon. 2002. Selectable subgenomic and genome-length dicistronic RNAs derived from an infectious molecular clone of the HCV-N strain of hepatitis C virus replicate efficiently in cultured Huh7 cells. J. Virol. 76:2997-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isom, H. C., T. Secott, I. Georgoff, C. Woodworth, and J. Mummaw. 1985. Maintenance of differentiated rat hepatocytes in primary culture. Proc. Natl. Acad. Sci. USA 82:3252-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isom, I., I. Georgoff, M. Salditt-Georgieff, and J. E. Darnell, Jr. 1987. Persistence of liver-specific messenger RNA in cultured hepatocytes: different regulatory events for different genes. J. Cell Biol. 105:2877-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang, J., N. Kojima, L. Guo, K. Naruse, M. Makuuchi, A. Miyajima, W. Yan, and Y. Sakai. 2004. Efficacy of engineered liver tissue based on poly-l-lactic acid scaffolds and fetal mouse liver cells cultured with oncostatin M, nicotinamide, and dimethyl sulfoxide. Tissue Eng. 10:1577-1586. [DOI] [PubMed] [Google Scholar]
- 20.Kanda, T., A. Basu, R. Steele, T. Wakita, J. S. Ryerse, R. Ray, and R. B. Ray. 2006. Generation of infectious hepatitis C virus in immortalized human hepatocytes. J. Virol. 80:4633-4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanford, R. E., B. Guerra, H. Lee, D. R. Averett, B. Pfeiffer, D. Chavez, L. Notvall, and C. Bigger. 2003. Antiviral effect and virus-host interactions in response to alpha interferon, gamma interferon, poly(I)-poly(C), tumor necrosis factor alpha, and ribavirin in hepatitis C virus subgenomic replicons. J. Virol. 77:1092-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindenbach, B. D., M. J. Evans, A. J. Syder, B. Wolk, T. L. Tellinghuisen, C. C. Liu, T. Maruyama, R. O. Hynes, D. R. Burton, J. A. McKeating, and C. M. Rice. 2005. Complete replication of hepatitis C virus in cell culture. Science 309:623-626. [DOI] [PubMed] [Google Scholar]
- 23.Lohmann, V., F. Korner, J. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 24.Makowske, M., R. Ballester, Y. Cayre, and O. M. Rosen. 1988. Immunochemical evidence that three protein kinase C isozymes increase in abundance during HL-60 differentiation induced by dimethyl sulfoxide and retinoic acid. J. Biol. Chem. 263:3402-3410. [PubMed] [Google Scholar]
- 25.Melkonyan, H., C. Sorg, and M. Klempt. 1996. Electroporation efficiency in mammalian cells is increased by dimethyl sulfoxide (DMSO). Nucleic Acids Res. 24:4356-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michalopoulos, G. K., and M. C. DeFrances. 1997. Liver regeneration. Science 276:60-66. [DOI] [PubMed] [Google Scholar]
- 27.Mitaka, T., T. Mizuguchi, F. Sato, C. Mochizuki, and Y. Mochizuki. 1998. Growth and maturation of small hepatocytes. J. Gastroenterol. Hepatol. 13(Suppl.):S70-S77. [PubMed] [Google Scholar]
- 28.Nelson, H. B., and H. Tang. 2006. Effect of cell growth on hepatitis C virus (HCV) replication and a mechanism of cell confluence-based inhibition of HCV RNA and protein expression. J. Virol. 80:1181-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oda, H., K. Nozawa, Y. Hitomi, and A. Kakinuma. 1995. Laminin-rich extracellular matrix maintains high level of hepatocyte nuclear factor 4 in rat hepatocyte culture. Biochem. Biophys. Res. Commun. 212:800-805. [DOI] [PubMed] [Google Scholar]
- 30.Pagan, R., A. Sanchez, I. Martin, M. Llobera, I. Fabregat, and S. Vilaro. 1999. Effects of growth and differentiation factors on the epithelial-mesenchymal transition in cultured neonatal rat hepatocytes. J. Hepatol. 31:895-904. [DOI] [PubMed] [Google Scholar]
- 31.Pasquetto, V., S. F. Wieland, S. L. Uprichard, M. Tripodi, and F. V. Chisari. 2002. Cytokine-sensitive replication of hepatitis B virus in immortalized mouse hepatocyte cultures. J. Virol. 76:5646-5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pietschmann, T., V. Lohmann, G. Rutter, K. Kurpanek, and R. Bartenschlager. 2001. Characterization of cell lines carrying self-replicating hepatitis C virus RNAs. J. Virol. 75:1252-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Randall, G., and C. M. Rice. 2001. Hepatitis C virus cell culture replication systems: their potential use for the development of antiviral therapies. Curr. Opin. Infect. Dis. 14:743-747. [DOI] [PubMed] [Google Scholar]
- 34.Saito, I., T. Miyamura, A. Ohbayashi, H. Harada, T. Katayama, S. Kikuchi, Y. Watanabe, S. Koi, M. Onji, Y. Ohta, et al. 1990. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 87:6547-6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoehr, S. A., and H. C. Isom. 2003. Gap junction-mediated intercellular communication in a long-term primary mouse hepatocyte culture system. Hepatology 38:1125-1135. [DOI] [PubMed] [Google Scholar]
- 36.Su, A. I., J. P. Pezacki, L. Wodicka, A. D. Brideau, L. Supekova, R. Thimme, S. Wieland, J. Bukh, R. H. Purcell, P. G. Schultz, and F. V. Chisari. 2002. Genomic analysis of the host response to hepatitis C virus infection. Proc. Natl. Acad. Sci. USA 99:15669-15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villa, P., P. Arioli, and A. Guaitani. 1991. Mechanism of maintenance of liver-specific functions by DMSO in cultured rat hepatocytes. Exp. Cell Res. 194:157-160. [DOI] [PubMed] [Google Scholar]
- 39.Wakita, T., T. Pietschmann, T. Kato, T. Date, M. Miyamoto, Z. Zhao, K. Murthy, A. Habermann, H. G. Krausslich, M. Mizokami, R. Bartenschlager, and T. J. Liang. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11:791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wieland, S. F., and F. V. Chisari. 2005. Stealth and cunning: hepatitis B and hepatitis C viruses. J. Virol. 79:9369-9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Windisch, M. P., M. Frese, A. Kaul, M. Trippler, V. Lohmann, and R. Bartenschlager. 2005. Dissecting the interferon-induced inhibition of hepatitis C virus replication by using a novel host cell line. J. Virol. 79:13778-13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yi, M., A. Rodrigo, R. A. Villanueva, D. L. Thomas, T. Wakita, and S. M. Lemon. 2006. Production of infectious genotype 1a hepatitis C virus (Hutchinson strain) in cultured human hepatoma cells. Proc. Natl. Acad. Sci. USA 103:2310-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong, J., P. Gastaminza, G. Cheng, S. Kapadia, T. Kato, D. R. Burton, S. F. Wieland, S. L. Uprichard, T. Wakita, and F. V. Chisari. 2005. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. USA 102:9294-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zvibel, I., A. S. Fiorino, S. Brill, and L. M. Reid. 1998. Phenotypic characterization of rat hepatoma cell lines and lineage-specific regulation of gene expression by differentiation agents. Differentiation 63:215-223. [DOI] [PubMed] [Google Scholar]