Abstract
Lymphoma induction and T-cell transformation by herpesvirus saimiri strain C488 depends on two viral oncoproteins, StpC and Tip. The major interaction partner of Tip is the protein tyrosine kinase Lck, a key regulator of T-cell activation. The Lck binding domain (LBD) of Tip comprises two interaction motifs, a proline-rich SH3 domain-binding sequence (SH3B) and a region with homology to the C terminus of Src family kinase domains (CSKH). In addition, biophysical binding analyses with purified Lck-SH2 domain suggest the phosphorylated tyrosine residue 127 of Tip (pY127) as a potential third Lck interaction site. Here, we addressed the relevance of the individual binding motifs, SH3B, CSKH, and pY127, for Tip-Lck interaction and for human T-cell transformation. Both motifs within the LBD displayed Lck binding activities and cooperated to achieve a highly efficient interaction, while pY127, the major tyrosine phosphorylation site of Tip, did not enhance Lck binding in T cells. Herpesvirus saimiri strain C488 recombinants lacking one or both LBD motifs of Tip lost their transforming potential on human cord blood lymphocytes. Recombinant virus expressing Tip with a mutation at position Y127 was still able to transform human T lymphocytes but, in contrast to wild-type virus, was strictly dependent on exogenous interleukin-2. Thus, the strong Lck binding mediated by cooperation of both LBD motifs was essential for the transformation of human T cells by herpesvirus saimiri C488. The major tyrosine phosphorylation site Y127 of Tip was particularly required for transformation in the absence of exogenous interleukin-2, suggesting its involvement in cytokine signaling pathways.
Herpesvirus saimiri (saimiriine herpesvirus type 2) causes fatal T-lymphoproliferative disorders in nonhuman primates and transforms T cells to permanent growth in vitro (reviewed in reference 25). Virus isolates are assigned to three subgroups, A, B, and C, according to pathogenic properties and sequence variations in a specific region of the viral genome (16, 53). A single open reading frame within this variable viral DNA segment was found to be essential for the oncogenic and in vitro-transforming phenotype of a herpesvirus saimiri subgroup A strain (57). Virus strains of subgroup C, which are unique in their ability to transform human T lymphocytes to stable antigen-independent growth in culture (5), carry two open reading frames at the corresponding genomic locus (6, 26). They are transcribed to a single bicistronic mRNA coding for StpC (for saimiri transformation-associated protein of subgroup C) and Tip (for tyrosine kinase-interacting protein) (7, 23, 26, 41). Deletions affecting the open reading frame for StpC and/or Tip within the viral genome of subgroup C strain 484 or 488 abolished viral transformation in vitro and pathogenicity in vivo (13, 20, 43, 52). Independently of the viral context, both viral oncoproteins induced tumors in transgenic mice; expression of StpC resulted in epithelial hyperplasia, while Tip caused peripheral T-cell lymphoma closely resembling the tumors in susceptible New World primates (56, 66). The effects of StpC are assigned to its interaction with the ubiquitous cellular proto-oncoprotein Ras and to its ability to activate the transcription factor NF-κB (12, 30, 38, 45, 55, 63). In accordance with the T-cell phenotype of the tumors in transgenic mice, Tip directly binds to the Src family tyrosine kinase Lck, which is a key regulator of T-cell activation (7, 37).
The strong interaction of Tip with Lck relies on the Lck binding domain (LBD), consisting of a 9-amino-acid motif with homology to the C termini of various Src family kinase domains (CSKH), a 20-amino-acid linker, and a 9-amino-acid proline-rich consensus sequence for SH3 domain binding (SH3B) (7, 39). In addition, a phosphotyrosyl peptide corresponding to tyrosine residue Y127 of Tip-C488, but not the one corresponding to Y114, was identified as a ligand for the SH2 domain of Lck in a biophysical assay (4). As the related positions of Tip-C484 (tyrosine residues Y72 and Y85, respectively) are substrates for Lck in transiently transfected 293T cells (32), phosphorylated Y85/Y127 might indeed represent a third Tip-Lck interaction site, but binding of full-length Tip to the Lck SH2 domain remains to be demonstrated.
Tip was also found to associate with signal transducers and activators of transcription 1 and 3 (STAT1/3) in cells expressing Lck (50). The interaction depends on tyrosine residue Y72 of Tip-C484, which is embedded in a STAT SH2 binding motif (YXPQ) conserved at position Y114 of Tip-C488 (32). These findings suggest that Tip, phosphorylated at position Y72/Y114 by Lck, binds to the SH2 domains of STAT1 and STAT3. Recruitment by Tip and subsequent phosphorylation by Lck provide an explanation for the observed activation of STAT1 and STAT3 in the presence of Tip and Lck (32, 42, 50, 51, 60). The implication of constitutively active STATs, especially STAT3, in growth regulation and oncogenesis in multiple cell types (8, 10) suggested a central role for Tip-induced STAT activity in viral T-cell transformation. However, recombinant herpesvirus saimiri C488 expressing Tip with a tyrosine-to-phenylalanine substitution at position 114 was able to transform primary human T lymphocytes in the absence of STAT1 or STAT3 activation (35). Thus, the essential function of Tip in lymphocyte transformation does not rely on Lck-mediated STAT1/3 phosphorylation.
Given the central role of Lck in T-cell activation, Tip was assumed to activate T-cell proliferation by inducing the kinase and thus mimicking T-cell receptor (TCR) ligation. In accordance with this hypothesis, several studies demonstrated a strong activation of Lck in the presence of Tip, which is achieved by cooperation of the CSKH and SH3B motifs within the LBD independently of regulatory tyrosine phosphorylation of Lck (32, 33, 42, 48, 51, 67). However, overexpression of Tip-C488 downregulated stimulation-induced cellular protein tyrosine phosphorylation in Jurkat T cells and partially reversed the phenotype of fibroblasts transformed by a constitutively active mutant of Lck (40). These effects were even enhanced when tyrosine residue Y114 in Tip-C488 was replaced by serine (Tip Y114S) (29). An explanation for these seemingly contradictory observations is provided by analyses of surface receptor levels on Tip-expressing T cells. As previously described for constitutively active Lck (19), Tip and, more prominently, Tip Y114S downregulate TCR/CD3 from the surfaces of T cells (29, 58, 59). Receptor modulation is caused by Lck-dependent endocytosis and involves a lysosomal 80-kDa protein binding to the C terminus of Tip, which targets the Lck-receptor complex to enlarged endosomal vesicles for subsequent degradation (58, 59). This process results in reduced levels of Lck and, by intracellular sequestration of signaling components, may account for the Tip-mediated block in signal transduction from the TCR to Zap70 (11). Another negative regulatory mechanism was described for lymphocytes transduced with Tip-expressing lentiviral vectors; in conjunction with Lck, Tip promotes Fas-mediated apoptosis (34). Taken together, overexpression of Tip in T cells appears to induce Lck activity, which in turn triggers feedback mechanisms that restrict cellular activation.
While all effects of Tip observed so far in transfected or transduced T cells depend on Lck, Duboise and colleagues (21) reported transformation and oncogenesis by herpesvirus saimiri C488 to be independent of Tip's ability to bind to the kinase. In their study, mutation of the SH3B motif was considered sufficient to abrogate Tip-Lck interaction, an assumption questioned by subsequent reports on residual Lck binding and activation by Tip mutated either at the SH3B or at the CSKH motif (31) and on a putative third Tip-Lck interaction site constituted by phosphorylation of tyrosine residue 127 (4).
To further analyze the role of the indiviual Tip-Lck interaction sites in viral T-cell transformation, we first tested all Tip mutants used for the ability to bind to Lck. Then, we introduced the same mutations into the viral genome and used the recombinant viruses to infect human cord blood lymphocytes (CBL). Both LBD motifs of Tip, CSKH and SH3B, were found to be required for growth transformation of human T cells, although their individual mutation did not completely abrogate Lck binding activity. Mutation of tyrosine residue Y127 of Tip did not reduce Lck binding in the presence of an intact LBD but interfered with the capability of recombinant virus to transform T cells in the absence of exogenous interleukin-2 (IL-2). Taken together, our data demonstrate that growth transformation by herpesvirus saimiri depends on a complex interaction of Tip with the T-cell tyrosine kinase Lck.
MATERIALS AND METHODS
Expression plasmids.
The native tip open reading frame of herpesvirus saimiri C488 was transferred from plasmid p488PS (6) to the pFJ vector (39). Individual tyrosine-to-phenylalanine substitutions (Y114F, Y127F, and Y155F) were generated by site-directed mutagenesis using primers containing the desired mutation and the pFJ-Tip plasmid as a template. AU1 epitope-tagged tip sequences from plasmid pBKCMVAU-1-Tip (wild type [wt]), pBKCMVAU-1-Tip/ΔCSKH (deletion of 146EDLQSFLEK154), pBKCMVAU-1-Tip/mSH3B (175PTPPLPPRP183 mutated to ATAALAARA), or pBKCMVAU-1-Tip/ΔCSKHmSH3B (double mutant) (39) were transferred into the EcoRI and XhoI restriction sites of expression vector pcDNA3 (Invitrogen, Karlsruhe, Germany). For the expression of Tip with an amino-terminal hemagglutinin (HA) epitope, tip sequences were PCR amplified from plasmids pSTBlueStpCTip (35) or pSTBlueStpCTip-FFYF (pSTBlueStpCTip with tyrosine-to-phenylalanine substitutions at amino acid positions 94, 114, and 155 of Tip) with primers generating BglII and KpnI restriction sites and ligated into pcDNA-HA-MH (61).
The cDNA sequence encoding the full-length human lck tyrosine kinase (the plasmid was kindly provided by R. M. Perlmutter, Seattle, WA) was subcloned into the pFJ vector (39). The expression plasmid for the SH3 deletion mutant of Lck was generated by ligating the BsgI/MscI-cleaved pFJ construct with two complementary oligonucleotides (5′ ACCTGGTGG 3′ and 5′ CCACCAGGTTG 3′), thereby removing the coding sequences for amino acids 67 to 115.
Generation of recombinant viruses.
The herpesvirus saimiri C488 mutant HVS/Tip mSH3B (21) was obtained from J. U. Jung (Southborough, MA). All other virus recombinants were generated by a cosmid-based approach as described previously (35). The specific mutations in the LBD of the tip gene were introduced into pSTBlueStpCY114HN by insertion of XhoI-StuI fragments excised from the pBKCMVAU-1-Tip plasmids (39). Furthermore, tyrosine residue 127 was replaced by phenylalanine through PCR mutagenesis of plasmid pSTBlueStpCTip using the oligonucleotides y127f1 (5′-GAACAAGCTGTTCAAGTTTGTTAGC-3′) and y127f2 (5′-ACAACTTTTGAAGACGCAAGGGC-3′). Mutated Bst1107I fragments were reinserted into cosmid 331, which was then used to reconstitute replication-competent viral genomes as described previously (35).
DNA sequence analysis.
Nucleotide sequences of expression plasmids and virus recombination intermediates were determined with an ABI PRISM 3100 Genetic Analyzer according to the manufacturer's instructions (Applied Biosystems, Darmstadt, Germany). DNA sequence evaluation was done with the GAP4 software (Staden Package [14]).
Cell culture and transfection.
OMK cells (ATCC CRL1556) were used to propagate herpesvirus saimiri. The cells were cultivated in Dulbecco's modified Eagle's medium supplemented with glutamine (350 μg/ml), gentamicin (100 μg/ml), and 10% heat-inactivated fetal calf serum (FCS) (Biochrom, Berlin, Germany). Virus stocks were generated by infection of confluent OMK cells at a low multiplicity of infection.
Human lymphocyte cultures were grown in RPMI 1640 (Invitrogen) and Panserin 401 medium mixed at a ratio of 1:1 supplemented with 10% irradiated FCS (Pan Biotech, Aidenbach, Germany), glutamine (350 μg/ml), and gentamicin (100 μg/ml). Callithrix jacchus T-cell lines transformed by herpesvirus saimiri wild-type strain C488 (17) or by mutant HVS/Tip mSH3B, kindly provided by J. U. Jung (21), were maintained in a mixture of 50% CG medium (Vitromex, Selters, Germany) and 50% RPMI 1640 medium containing 10% FCS.
Jurkat clone E6-1 cells (ATCC TIB-152) were maintained in RPMI 1640 supplemented with 10% FCS. For transient transfection, 107 Jurkat cells were mixed with 30 to 95 μg plasmid DNA, electroporated by a single pulse of 250 V and 1,500 μF, and harvested after 24 to 48 h (EasyJect; Equibio).
COS-7 cells (27) were grown in Dulbecco's modified Eagle's medium containing 10% FCS. A DEAE-dextran procedure was used for transient transfection (62). Transfected COS-7 cells were grown for 24 to 48 h; washed twice with phosphate-buffered saline, pH 7.4 (PBS); harvested; and stored as cell pellets at −80°C.
Lymphocyte transformation.
Human CBL were isolated by selective sedimentation of erythrocytes for 45 min at 37°C in 5% dextran (molecular weight, 250,000) and 150 mM NaCl. Primary cells were washed with PBS and stimulated with 1 μg/ml phytohemagglutinin in cell culture medium. Ten units/ml exogenous IL-2 (Roche Diagnostics, Mannheim, Germany) was added after 24 h. On the next day, the cells were infected as described previously (24). Five days after infection, the cells were split into two cultures, and exogenous IL-2 was depleted from one of the cultures by centrifugation and washing of the cells. Cell culture densities were determined by automated cell counting (Micro Cell Counter F-300 [Sysmex, Norderstedt, Germany]; Beckman-Coulter [Krefeld, Germany] Z2). Growth transformation was assessed microscopically and by the observation of accelerated growth over a period of at least 3 months postinfection. Transformed T-cell lines were analyzed by PCR and restriction mapping to confirm the presence of the specific viral genotype.
Antibodies and antisera.
Rabbit antisera directed against Tip were kindly provided by J. U. Jung (Southborough, MA) and A. Y. Tsygankov (Philadelphia, PA) or generated by immunization with partially purified Tip (amino acids 1 to 226) (66). Rabbit polyclonal and mouse monoclonal anti-Lck were purchased from BD Biosciences (Heidelberg, Germany), and a guinea pig antiserum was generated by immunization with a glutathione S-transferase fusion protein of the Lck-unique domain (amino acids 1 to 61). Mouse monoclonal AU1 antibody was purchased from BAbCo (Richmond, CA). The CD4-specific mouse monoclonal antibody 16H5 was a generous gift of Frank Emmrich (Leipzig, Germany). Phosphotyrosine antibody 4G10 was obtained from Biomol (Hamburg, Germany). Horseradish peroxidase-conjugated secondary antibodies were purchased from DAKO (Hamburg, Germany) and Medac (Hamburg, Germany).
Immunoprecipitation and immunoblotting.
Cells were lysed in TNE (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 2 mM EDTA, 1% NP-40) supplemented with 1 mM sodium orthovanadate (Na3VO4), 5 mM NaF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin (Sigma-Aldrich, Taufkirchen, Germany). For immunoprecipitation, 1 μg purified antibody or 2 μl antiserum was added to a maximum of 1 mg total cellular proteins for at least 1 h at 4°C, followed by incubation with Staphylococcus aureus particles (Pansorbin cells; Calbiochem-Novabiochem, Bad Soden, Germany) or S. aureus particles preincubated with rabbit anti-mouse antibodies for 30 min at 4°C. The immunoprecipitates were washed four times in TNE and once in 10 mM Tris-HCl, pH 7.4. For immunoblotting, whole-cell lysates or precipitated proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (ImmobilonP; Millipore, Bedford, MA). The membranes were incubated for 1 h at room temperature or at 4°C overnight in blocking buffer (PBS, 0.1% Tween 20, 5% [wt/vol] nonfat dry milk powder), followed by incubation with primary antibody diluted in blocking buffer for 1 hour or overnight. Immunoblot detection was performed as described by the manufacturer of the enhanced-chemiluminescence system (ECL; Amersham Pharmacia Biotech, Freiburg, Germany) and documented by autoradiography or by a Fuji LAS-1000 chemiluminescence detection system (Raytest, Straubenhardt, Germany).
An alternative protocol was used for some experiments (see Fig. 4). Cells were lysed in 50 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM EDTA, 10 mM Na4P2O7, 10% glycerol, 1% Triton X-100 supplemented with inhibitors as indicated above. Immune complexes were precipitated using protein A-Sepharose CL-4B (Amersham Pharmacia Biotech, Freiburg, Germany) and washed with HNTG buffer (20 mM HEPES, pH 7.5, 150 mM NaCl, 0.1% Triton X-100, and 10% glycerol). After SDS-PAGE, the proteins were transferred to nitrocellulose membranes and NET-gelatin buffer (150 mM NaCl, 5 mM EDTA, pH 8.0, 50 mM Tris-HCl, pH 7.5, 0.05% Triton X-100, 2.5 g/liter gelatin) was used for all subsequent incubation and washing steps.
FIG. 4.
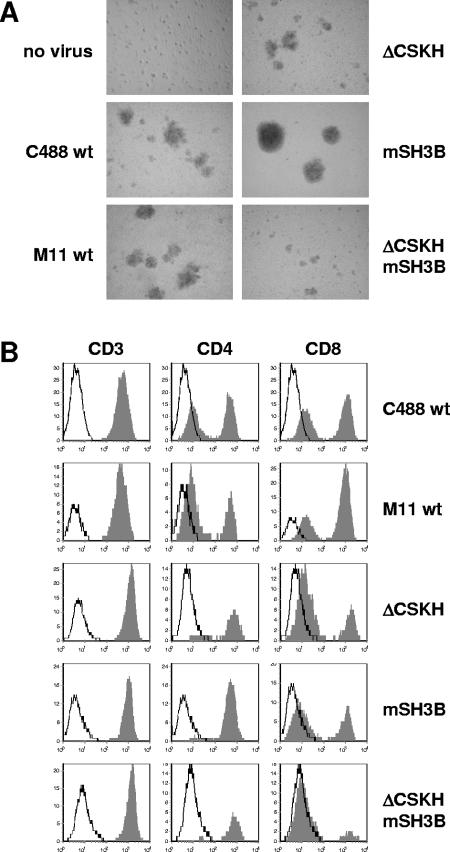
Phenotype of CBL cultures (donor 1749) infected with herpesvirus saimiri C488 (C488 wt) and recombinant viruses carrying wild-type sequences (M11 wt) or mutations within the Tip reading frame (ΔCSKH, mSH3B, and ΔCSKH mSH3B). (A) Morphology documented by photography 5 weeks postinfection (no virus, C488 wt, and M11 wt as in reference 35). (B) Surface expression of CD3, CD4, and CD8 antigens 6 weeks after infection. The histograms show fluorescence intensity in logarithmic scale on the x axis and cell numbers in linear scale on the y axis. The open graphs represent negative isotype controls, and the solid graphs represent specific staining.
In vitro protein kinase assay.
In vitro phosphotransferase reactions with immunoprecipitated proteins were performed as described previously (44) for 10 min at 25°C in 20 μl kinase buffer containing 1 μM ATP and 4 μCi/sample [γ-32P]ATP (6,000 Ci/mmol; Amersham Biosciences, Freiburg, Germany).
Flow cytometry.
Transformed human T cells were analyzed by flow cytometry with antibodies for T-cell surface epitopes on a FACScalibur flow cytometer according to standard protocols. The directly labeled monoclonal antibodies (Cy-Chrome, fluorescein-isothiocyanate, or phycoerythrin conjugated) were specific for CD3 (Leu-4), CD4 (Leu-3a), and CD8 (Leu-2a). Directly labeled isotype-matched monoclonal antibodies were used as controls (all antibodies were from BD Biosciences, Heidelberg, Germany).
RESULTS
Contributions of CSKH and SH3B motifs to Tip-Lck interaction.
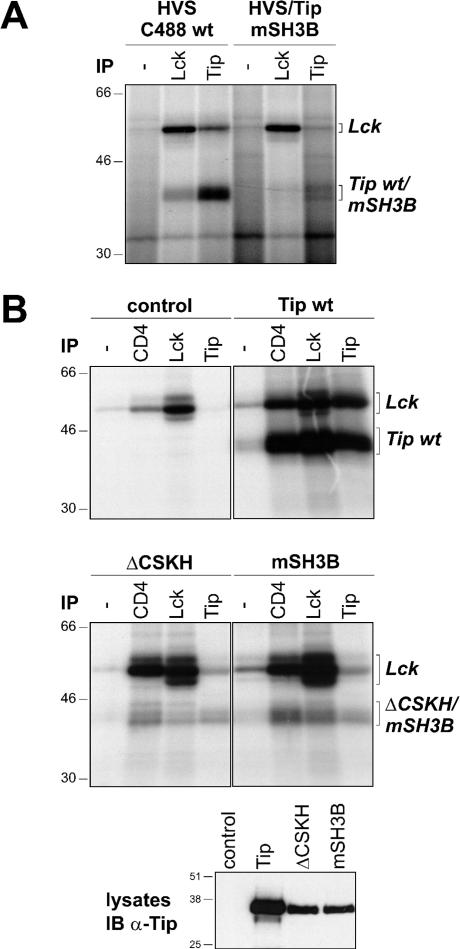
An early study indicated that binding of Tip to Lck depends on both the CSKH and the SH3B motifs and that mutation of either motif abrogates kinase interaction (39). Recombinant herpesvirus saimiri C488 mutated at the SH3B motif of Tip (HVS/Tip mSH3B) induced enhanced proliferation, suggesting that Tip-Lck interaction is not essential for T-cell growth transformation (21). However, a more recent study demonstrated residual Lck binding of Tip mutated at the SH3B motif when both proteins were coexpressed in 293 cells (31). To test for this residual binding in T cells, we infected monkey lymphocytes with C488 wild type or the mutant HVS/Tip mSH3B (21). Both viruses induced lymphocyte proliferation, and the cultures were expanded until sufficient material was available for further analysis. Lck and Tip immune complexes were precipitated from lysates of the infected cells and subjected to an in vitro kinase reaction. Phosphorylated proteins were separated by SDS-PAGE and visualized by autoradiography (Fig. 1A). Autophosphorylated Lck was detected equally in Lck precipitates from both cell lines and at a slightly reduced level upon precipitation of wild-type Tip. The intensity of the Lck band in the Tip mSH3B precipitate was hardly above background. Phosphorylated Tip was detectable in the Lck and Tip precipitates from wild-type-infected cells. However, signals above background were also observed upon infection with HVS/Tip mSH3B, suggesting that residual Lck binding and phosphorylation of the Tip mutant is also present in these transformed T cells.
FIG. 1.
Interaction of Tip wt, as well as mSH3B and ΔCSKH mutants, with Lck in T cells. (A) Lysates of monkey T cells transformed by wild-type herpesvirus saimiri C488 (HVS C488 wt) or by the recombinant virus HVS/Tip mSH3B were immunoprecipitated (IP) with rabbit anti-Lck (Lck) or anti-Tip (Tip) antiserum as indicated, and an immune complex kinase assay was performed. Precipitation without specific antiserum (−) was used as a control. (B) Jurkat T cells were transiently transfected with plasmids encoding AU1-tagged Tip (Tip wt) or the mutants Tip ΔCSKH (ΔCSKH) and Tip mSH3B (mSH3B) as indicated. The lysates were divided, and an immune complex kinase assay was performed using anti-CD4 (CD4), anti-Lck (Lck), or anti-AU1 (Tip) antibodies. Immunoprecipitation without specific antibodies (−) and nontransfected Jurkat cells (control) served as negative controls. Expression of Tip proteins was verified by Tip-specific immunoblotting on whole-cell lysates (IB α-Tip). The positions of Lck and Tip proteins are given on the right; molecular mass markers in kDa are on the left.
To further characterize LBD-dependent Lck-Tip interaction in lymphocytes, we transiently transfected Jurkat T cells with expression plasmids coding for Tip wild type or mutated at either the CSKH or the SH3B motif. CD4-, Lck-, and Tip-specific immune complex kinase assays revealed Lck interaction and phosphorylation of all Tip variants (Fig. 1B). The strongest signals were observed for the wild-type protein, but in spite of the reduced expression levels (Fig. 1B, bottom), relatively strong phosphorylation signals suggested residual Lck binding of both mutants in this T-cell system. However, the design of this assay did not allow discrimination between binding and phosphorylation of Tip and, due to high levels of endogenous Lck, may have been influenced by tyrosine phosphorylation of Tip in vivo.
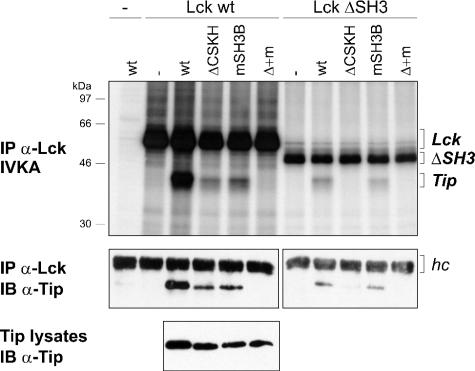
To test for the relative roles of the SH3B and CSKH motifs in Lck interaction independently of the tyrosine phosphorylation of Tip that occurs upon coexpression of both proteins, we used an in vitro binding assay. For this purpose, COS-7 cells were individually transfected with Tip wt, ΔCSKH, mSH3B, or ΔCSKH mSH3B, as well as Lck wt and Lck ΔSH3. Lysates of transfected cells were adjusted for both equal total cellular protein content and equal Tip or Lck levels by the addition of lysates from nontransfected COS-7 cells. The binding of Tip and its mutants was assessed by Lck immunoprecipitation from mixed lysates, followed by an in vitro kinase assay and immunoblot detection of coprecipitated Tip proteins (Fig. 2). A correlation between phosphorylation and coprecipitation of Tip was observed in all samples. Therefore, phosphorylation of Tip and its mutants appeared to depend only on Lck binding and not the presence of specific residues within the CSKH or SH3B motifs. Wild-type Tip binding and phosphorylation were detected in Lck and to a lesser extent in Lck ΔSH3 precipitates, indicating that SH3-independent binding was detectable in this system. Relative to wild-type Tip, binding of the mutants Tip ΔCSKH and Tip mSH3B to wild-type Lck was reduced, demonstrating the modular nature and the cooperation of the LBD motifs. Interaction of Tip ΔCSKH with wild-type Lck, but not with the SH3 deletion mutant, indicated that SH3-independent binding relied on the CSKH motif, while comparable binding of Tip wild type and mSH3B to Lck ΔSH3 confirmed the SH3 specificity of the SH3B motif. The involvement of additional Tip sequences in Lck interaction under these experimental conditions was excluded by the double mutant Tip ΔCSKH mSH3B, which was devoid of any Lck binding activity.
FIG. 2.
In vitro binding of Tip wt, ΔCSKH, mSH3B, and ΔCSKH mSH3B to Lck. Lysates (500 μl/300 μg each) of untreated COS-7 cells (−) or COS-7 cells transfected with expression plasmids coding for Lck (Lck wt), SH3-deficient Lck (Lck ΔSH3), Tip (wt), Tip ΔCSKH (ΔCSKH), Tip mSH3B (mSH3B), or the double mutant Tip ΔCSKH mSH3B (Δ + m) were mixed as indicated. Lck was immunoprecipitated from the mixtures with specific rabbit antibodies (IP α-Lck). The precipitates were divided into two aliquots and subjected to an in vitro kinase assay (IVKA) and Tip-specific immunoblotting (IB α-Tip). The presence of Tip in the lysates (12 μg total cellular protein/lane) was verified by immunoblotting. The positions of Lck and Tip proteins are given on the right; molecular mass markers are on the left. hc, immunoglobulin heavy chain.
Transformation of human T cells by recombinant viruses expressing Tip LBD mutants.
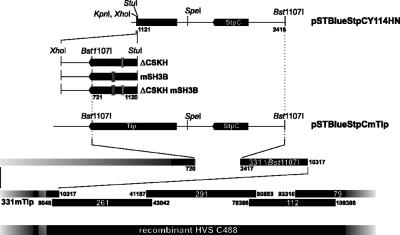
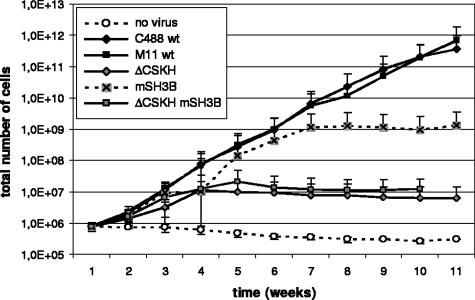
We next wanted to study the relative roles of the cooperating LBD motifs, CSKH and SH3B, in the transformation of human T lymphocytes by herpesvirus saimiri C488. Therefore, mutations within the LBD were introduced into the viral genome by a well established, cosmid-based procedure (Fig. 3) (35, 68). The resulting recombinant viruses encoded the same Tip mutants as the expression plasmids used in the previous experiments and were accordingly designated herpesvirus saimiri C488 ΔCSKH, mSH3B, and ΔCSKH mSH3B. The mutant C488 mSH3B encodes the same proline-to-alanine substitutions in Tip as HVS/Tip mSH3B (21). Human CBL were infected with wild-type C488, cosmid-generated wild-type M11 control virus, or the recombinant Tip-mutated viruses and monitored for growth transformation as detailed in Materials and Methods. While uninfected control cells were not growing during the observation period, all infected cultures initially displayed spontaneous proliferation (see Fig. 5). Morphological differences among the cultures were documented 5 weeks postinfection, when most of the control cells were dead. Cultures infected with wild-type or ΔCSKH viruses contained aggregates of viable cells. These aggregates were much larger in the cultures infected with the mSH3B mutant virus. The double-mutant herpesvirus saimiri C488 ΔCSKH mSH3B induced smaller aggregates, and these cultures contained large amounts of cell debris (Fig. 4A). Subsequent fluorescence-activated cell sorter analyses identified cells from all infected cultures as T cells expressing CD3, as well as CD4 and/or CD8, at their surfaces (Fig. 4B). Cultures infected with virus mutants lacking the CSKH motif stopped growing approximately 4 weeks after infection, while cells infected with the mSH3B virus ceased to proliferate only after 7 to 8 weeks (Fig. 5). These data suggest that viruses with mutations in the LBD of Tip retain their capability for initial T-cell stimulation, which is extended in the presence of the CSKH motif. However, none of the LBD mutant viruses supported long-term T-cell proliferation. Thus, growth transformation of human T lymphocytes by herpesvirus saimiri C488 depended on Tip-Lck interaction via both the SH3B and CSKH motifs. Mutation of either of these motifs resulted in loss of the transforming capacity.
FIG. 3.
Construction of recombinant herpesvirus saimiri C488 ΔCSKH, mSH3B, and ΔCSKH mSH3B. Mutated Tip-coding DNA fragments were introduced into plasmid pSTBlueStpCY114HN. The altered Bst1107I fragments from pSTBlue were then reinserted into the Bst1107I-digested cosmid 331ΔBst1107I. The resulting cosmids were linearized and cotransfected, together with cosmids 261, 291, 112, and 79, into permissive OMK cells. Recombinant virus from the culture supernatant was amplified by infection of OMK cells.
FIG. 5.
T-cell transformation assay with herpesvirus saimiri C488 wild type (C488 wt and M11 wt), ΔCSKH, mSH3B, and ΔCSKH mSH3B. Stimulated human CBL were infected and cultured in the presence of exogenous IL-2. Proliferation was monitored by automated cell counting, and total cell numbers were calculated as outlined in Materials and Methods. The median values and standard deviation (error bars) of each time point were determined from three independent donors. C488 wt, wild-type isolate; M11 wt, wild type reconstituted from cosmids.
Tyrosine phosphorylation and Lck interaction of Tip mutated at the conserved tyrosine residues.
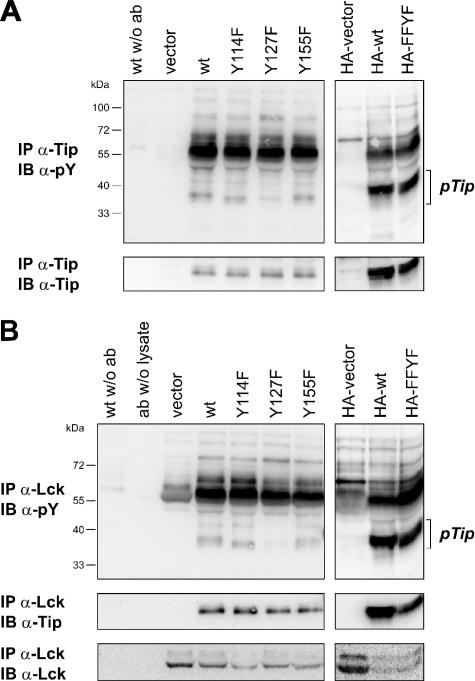
An additional Tip-Lck interaction site was supported by biophysical binding assays with Tip phosphopeptides, in which the phosphorylated tyrosine residue 127 of Tip-C488 was identified as a high-affinity ligand for the SH2 domain of Lck (4). The corresponding tyrosine residue 85 of Tip-C484 had been reported to be phosphorylated by Lck (32), but its role in kinase interaction and in T-cell transformation has not been analyzed. To test for the relevance of Tip's tyrosine phosphorylation, which we had consciously excluded in our in vitro binding assay (Fig. 2), Jurkat T cells were transiently transfected with plasmids coding for wild-type or mutated Tip-C488. The variants Tip Y114F, Tip Y127F, and Tip Y155F carry individual phenylalanine substitutions at the three conserved tyrosine residues of Tip (Fig. 6, left); in the HA-tagged mutant FFYF, only the tyrosine residue at position 127 is conserved (Fig. 6, right). Tip was immunoprecipitated from the lysates of these cells and analyzed for tyrosine phosphorylation by immunoblotting (Fig. 6A). Tyrosine-phosphorylated Tip was detected in the lysates of all Tip-transfected cells. A strong reduction relative to the respective wild-type Tip was observed for mutant Y127F, but not for mutant FFYF, indicating that residue Y127 represents the major tyrosine phosphorylation site of Tip in Jurkat T cells. A minor difference in the migration of Tip Y114F observed in the phosphotyrosine blot, but not in Tip-specific immunoblots, suggests a Y114-dependent modification that remains to be characterized. Phosphoproteins coprecipitated with Tip, including a major band comigrating with Lck at 55 to 60 kDa, showed no difference with respect to the tyrosine mutants of Tip. Lck immune complexes contained equal amounts of wild-type and mutant Tip, but tyrosine phosphorylation was again specifically diminished on Tip Y127F (Fig. 6B). Differences in Lck-associated phosphoproteins and in the phosphorylation status of Lck were observed in cells transfected with Tip versus vector plasmids, but not among Tip and its mutants. These experiments indicate that the major tyrosine phosphorylation site of Tip, which had been described as a potential ligand for Lck SH2, does not enhance Tip-Lck interaction in the presence of an intact LBD.
FIG. 6.
Lck binding and tyrosine phosphorylation of Tip Y/F mutants in T cells. Jurkat T cells were transfected with plasmids coding for Tip (wt), individual point mutants of Tip (Y114F, Y127F, and Y155F), HA-tagged Tip (HA-wt), or HA-tagged Tip with Y/F mutations at positions 94, 114, and 155 (HA-FFYF). The cells were harvested and lysed 24 h after transfection. (A) Tip was immunoprecipitated (IP) with a specific rabbit antiserum. (B) A guinea pig antiserum was used to precipitate Lck. Tyrosine-phosphorylated proteins were detected by immunoblotting (IB) with the monoclonal antibody 4G10. The membranes were reprobed with the Tip-specific rabbit serum and with a monoclonal antibody directed against Lck. Immunoprecipitations without antibody (w/o ab) or without cell lysate (ab w/o lysate) served as controls. pY, phosphotyrosine; pTip, tyrosine-phosphorylated Tip.
Transformation of human T cells by a virus coding for the Tip mutant Y127F.
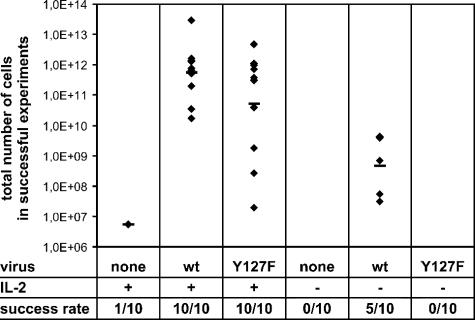
Although no influence on Lck binding was detected in our previous assay, tyrosine phosphorylation of Tip might well have an impact on T-cell transformation by herpesvirus saimiri C488, e.g., by affecting Lck function or by changing the composition of Tip-Lck complexes. Therefore, we constructed a herpesvirus saimiri recombinant coding for Tip Y127F instead of the wild-type protein. Mutant virus was used for in vitro transformation assays with human cord blood lymphocytes, along with wild-type virus as a positive control and uninfected cells as a negative control. Cells were cultured and monitored for 15 weeks (Fig. 7). In the presence of exogenous IL-2 (left three columns), all infections with both wild-type and Y127F virus resulted in cell proliferation. An approximately 10-fold reduction of total cell numbers was observed for the mutant virus; however, overall deviations showed an even wider range. The presence of live cells in 1 out of 10 uninfected negative controls suggested that the cutoff for efficient transformation should be at an extrapolated cell number of 107 by the end of the observation period. Indeed, all cells proliferating in response to viral infection exceeded this limit. Taken together, these data indicated that recombinant herpesvirus saimiri C488-Y127F was still capable of transforming human T cells to antigen-independent growth. To test for more subtle differences, the transformation assay included parallel samples that were not treated with exogenous IL-2 (Fig. 7, right three columns). Under these conditions, 5 out of 10 samples infected with wild-type virus yielded proliferating cell lines by the end of the observation period. In contrast, no transformed cell lines were established with the Y127F mutant virus when exogenous IL-2 was omitted from the culture medium. Thus, transformation by wild-type herpesvirus saimiri is possible in the absence of exogenous IL-2 but is enhanced by its addition. The strict dependence of the mutant virus on IL-2 supplementation suggests a link between IL-2 signaling pathways and the major tyrosine phosphorylation site at position 127 in Tip, which may contribute to virus-induced T-cell proliferation.
FIG. 7.
T-cell transformation assay with reconstituted herpesvirus saimiri wild-type (wt) and the virus expressing Tip with a mutation at position 127 (Y127F). Stimulated human CBL from 10 different donors were infected, and proliferation in the presence or absence of IL-2 was monitored by automated cell counting. An experiment was considered successful when viable cells were still present 15 weeks after infection when total cell numbers were determined as outlined in Materials and Methods. Diamonds, cell numbers of individual samples; horizontal lines, geometrical mean values.
DISCUSSION
The Src family tyrosine kinase Lck is an essential transducer of signals initiated by ligation of the T-cell receptor complex. The discovery of its interaction with the oncoprotein Tip of herpesvirus saimiri subgroup C strains prompted speculations about the role of Lck in viral T-cell transformation and oncogenesis (7, 49). However, a first study with recombinant herpesvirus saimiri C488 expressing a Tip mutant impaired in Lck binding indicated that kinase interaction is not required for immortalization of common marmoset (Callithrix jacchus) lymphocytes in vitro or for induction of lymphoma in this species (21). The mutation in the virus used in this study affected only the SH3B, but not the CSKH, motif in the LBD of Tip so that residual Lck engagement by the encoded Tip mutant was still possible (31). Furthermore, an interaction of phosphorylated tyrosine residue Y127 of Tip with the SH2 domain of Lck had not been taken into consideration (4).
We now asked what was the role of the individual Tip-Lck interaction sites in growth transformation of primary human T cells, a feature unique to herpesvirus saimiri subgroup C strains. Although residual Lck binding was observed when either the CSKH or SH3B sequence was mutated, both motifs in the LBD of Tip were found to be essential for transformation in this system. The enhanced initial proliferation of cells infected with the mSH3B recombinant virus suggests a specific activating function, most likely mediated by binding of the CSKH motif to the kinase domain of Lck. A contribution of Lck-SH2 interaction to Tip-Lck binding was not detectable in our assay system, but mutation of the respective tyrosine residue Y127 abrogated transformation in the absence of exogenous IL-2. Thus, in contrast to published data on monkey cells (21), each of the Lck binding sites of Tip was required to transform human T cells in vitro. The discrepancy may be due, at least in part, to the cell systems used; human T cells are susceptible to herpesvirus saimiri subgroup C strains only (5), while common marmosets and their isolated lymphocytes are also targeted by subgroup A and, to a certain extent, subgroup B strains (16, 18, 64). This possibility is supported by the failure to transform human T cells with mutant C488 mSH3B (Fig. 5), as well as with HVS/Tip mSH3B, generated by J. U. Jung and coworkers (21).
The differences in the susceptibilities of human and monkey T cells may be linked to differences in the regulation of Lck and other Src family kinases. This is most obvious in the squirrel monkey, the natural host of herpesvirus saimiri, where T-cell stimulation does not result in Lck activation and the virus persists without causing disease (25, 28). In contrast, common marmosets (Callithrix jacchus) and cottontop tamarins (Saguinus oedipus) are sensitive to herpesvirus saimiri subgroup A and B strains, whose oncoproteins interact with the SH2 domain of Src (12, 36, 46) but seem to be unable to replace StpC and Tip in human T-cell transformation (A. Ensser, unpublished data). In contrast, such a substitution is achieved by Tio, the oncoprotein of herpesvirus ateles, which interacts with the SH2 and SH3 domains of Src family kinases (1, 2). Transformation of human cells by Tio recombinant viruses depends on both the SH3 binding motif and the tyrosine phosphorylation site in Tio (3). Thus, in the marmoset system, a weak Tip-Lck interaction via one Lck-interacting domain of Tip may be sufficient for growth transformation in culture and lymphoma induction in vivo, while growth regulation of human T cells requires a complex interaction of viral oncoproteins with Src family kinases, like Lck.
The conserved binding of Tip and related viral oncoproteins to SH2 domains of Src family kinases suggests a functional relevance for this type of interaction. The role of the putative SH2 binding site of Tip, tyrosine residue 127, in human T-cell transformation under IL-2-free conditions indicated an influence on IL-2 signaling pathways, which had already been suggested for wild-type Tip in earlier studies (47, 49). However, as combined mutations of Y127 with CSKH or SH3B were not tested, additional functions of Tip Y127 may have been masked in our analyses. In addition, residues surrounding Tip Y127, as well as the CSKH and SH3B motifs, are well conserved among different herpesvirus saimiri subgroup C isolates (22, 28) and, except for the CSKH motif, in herpesvirus ateles (2, 3). The implicit selective pressure for all Lck interaction sites points to an essential function in the natural host whose nature remains enigmatic.
The major question raised by our findings addresses the effectors acting downstream of the Tip-Lck complex in human T-cell transformation. As detailed in the introduction, STAT activation by Tip appeared to be a promising candidate. We therefore performed transformation experiments with a recombinant virus expressing the Tip mutant Y114F, which is no longer able to induce STAT1 and STAT3 activity, and found that abrogation of STAT induction by Tip rather enhances the growth of human T cells transformed by herpesvirus saimiri C488 (35). An explanation for the increased proliferation rates might be provided by enhanced binding and activation of Lck by Tip carrying a tyrosine-to-serine mutation at position 114 (29). However, we did not detect any differences in Lck binding for Tip Y114F (Fig. 6). Furthermore, a Tip Y114 phosphopeptide exhibited no significant affinity for the SH2 domain of Lck (4). Taken together, current data do not provide evidence for an essential role of Tip Y114 or STAT1/3 activation in human T-cell transformation by herpesvirus saimiri C488.
Another effect of Tip that has to be considered for its involvement in viral transformation is the downregulation of surface receptor molecules. High levels of stable or transient Tip expression in Jurkat T cells, but not in an Lck-negative Jurkat variant, led to modulation of the TCR-CD3 complex and of the coreceptor CD4 (40, 59). This process is enhanced when tyrosine residue 114 of Tip is replaced by serine and involves binding of Tip to the lysosomal targeting protein p80 (58, 59). However, the lower levels of Tip expression in herpesvirus saimiri C488-transformed cells do not seem to be sufficient for overt receptor downregulation. Surface expression of CD4 is normal, and CD3 is only modestly reduced in lymphocytes infected with herpesvirus saimiri C488 wild type compared to transformation-incompetent mutants (Fig. 4). Another minor decrease in CD3 expression is observed in C488-Y114F cells relative to wild-type transformed cells (35). Therefore, only a small fraction of TCR/CD3 complexes is likely to be endocytosed and degraded in virus-transformed lymphocytes and may not have a detectable impact on Lck expression levels and on basal, as well as inducible, cellular protein tyrosine phosphorylation, which was strongly reduced upon Tip overexpression (29, 40, 58, 59). Likewise, the Tip-induced block in signaling from Lck to ZAP70 (11) may not affect all TCR-CD3 complexes on the surfaces of transformed cells. These assumptions are supported by one of the most intriguing properties of human T-cell clones transformed by herpesvirus saimiri C488, the maintenance of antigen specificity and responsiveness (9, 15, 65). The transformed T-cell clones also display increased basal levels of cellular protein tyrosine phosphorylation relative to their noninfected parental lines (67). In conclusion, Tip-mediated downregulation of TCR expression and signaling is not likely to be essential for T-cell transformation.
Modulation of the TCR-CD3 complex from the surfaces of Jurkat T cells is an Lck-dependent process that may be induced by the expression of constitutively active Lck (19). Therefore, TCR-CD3 downregulation by Tip indicates Lck activation in T cells expressing the viral oncoprotein. While negative regulatory downstream effects seem to mask this activation in T cells overexpressing Tip, it has been demonstrated in several other cellular and in vitro systems (31, 42, 48, 51, 67). Dependent on the presence of both the CSKH or SH3B motifs, Tip is able to activate Lck in an in vitro assay system even when the regulatory tyrosine residues Y394 and Y505 of Lck are mutated (33). This novel type of Lck regulation by simultaneous binding of both LBD motifs may result in an altered substrate specificity of the kinase, contributing to the abrogation of ZAP70 phosphorylation (11). More importantly, this dysregulation may link Tip-bound Lck to alternative downstream effectors, which may account for the substitution of ZAP70 function by herpesvirus saimiri C488 (54). Therefore, the characterization of substrates specific for Tip-complexed Lck will be an important step to identify cellular proteins mediating the essential functions downstream of Tip-Lck interaction.
In summary, our data provide evidence that a complex interaction between the viral oncoprotein Tip and the T-cell tyrosine kinase Lck is required for transformation of human T cells by herpesvirus saimiri C488. However, known downstream targets of Tip, STAT activation, TCR modulation, and blocking of ZAP70 activation, can be excluded as mediators of the transforming effects. Consequently, future investigations will have to address in more detail the Lck signal transduction pathways in herpesvirus saimiri-transformed human T cells.
Acknowledgments
We are grateful to Jens-Christian Albrecht for critical reading of the manuscript and to J. U. Jung (New England Primate Research Center, Harvard Medical School, Southborough, MA), R. M. Perlmutter (University of Washington, Seattle, WA), A. Y. Tsygankov (Temple University, Philadelphia, PA), and F. Emmrich (Leipzig, Germany) for recombinant virus, plasmids, and antibodies.
This project was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 466 and Graduiertenkolleg 1071), the Interdisciplinary Center for Clinical Research (IZKF) (Genesis, Diagnostics and Therapy of Inflammation Processes) at the Friedrich-Alexander-Universität Erlangen-Nürnberg, the Academy of Sciences and Literature (Mainz), the Wilhelm Sander-Stiftung, and the German-Israeli Foundation (project 674/2000).
REFERENCES
- 1.Albrecht, J. C., B. Biesinger, I. Müller-Fleckenstein, D. Lengenfelder, M. Schmidt, B. Fleckenstein, and A. Ensser. 2004. Herpesvirus ateles Tio can replace herpesvirus saimiri StpC and Tip oncoproteins in growth transformation of monkey and human T cells. J. Virol. 78:9814-9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrecht, J. C., U. Friedrich, C. Kardinal, J. Koehn, B. Fleckenstein, S. M. Feller, and B. Biesinger. 1999. Herpesvirus ateles gene product Tio interacts with nonreceptor protein tyrosine kinases. J. Virol. 73:4631-4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrecht, J. C., I. Müller-Fleckenstein, M. Schmidt, B. Fleckenstein, and B. Biesinger. 2005. Tyrosine phosphorylation of the Tio oncoprotein is essential for transformation of primary human T cells. J. Virol. 79:10507-10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer, F., E. Hofinger, S. Hoffmann, P. Rösch, K. Schweimer, and H. Sticht. 2004. Characterization of Lck-binding elements in the herpesviral regulatory Tip protein. Biochemistry 43:14932-14939. [DOI] [PubMed] [Google Scholar]
- 5.Biesinger, B., I. Müller-Fleckenstein, B. Simmer, G. Lang, S. Wittmann, E. Platzer, R. C. Desrosiers, and B. Fleckenstein. 1992. Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc. Natl. Acad. Sci. USA 89:3116-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biesinger, B., J. J. Trimble, R. C. Desrosiers, and B. Fleckenstein. 1990. The divergence between two oncogenic herpesvirus saimiri strains in a genomic region related to the transforming phenotype. Virology 176:505-514. [DOI] [PubMed] [Google Scholar]
- 7.Biesinger, B., A. Y. Tsygankov, H. Fickenscher, F. Emmrich, B. Fleckenstein, J. B. Bolen, and B. M. Bröker. 1995. The product of the herpesvirus saimiri open reading frame 1 (tip) interacts with T cell-specific kinase p56lck in transformed cells. J. Biol. Chem. 270:4729-4734. [DOI] [PubMed] [Google Scholar]
- 8.Bowman, T., R. Garcia, J. Turkson, and R. Jove. 2000. STATs in oncogenesis. Oncogene 19:2474-2488. [DOI] [PubMed] [Google Scholar]
- 9.Bröker, B. M., A. Y. Tsygankov, I. Müller-Fleckenstein, A. H. Guse, N. A. Chitaev, B. Biesinger, B. Fleckenstein, and F. Emmrich. 1993. Immortalization of human T cell clones by herpesvirus saimiri. Signal transduction analysis reveals functional CD3, CD4, and IL-2 receptors. J. Immunol. 151:1184-1192. [PubMed] [Google Scholar]
- 10.Bromberg, J., and J. E. Darnell, Jr. 2000. The role of STATs in transcriptional control and their impact on cellular function. Oncogene 19:2468-2473. [DOI] [PubMed] [Google Scholar]
- 11.Cho, N. H., P. Feng, S. H. Lee, B. S. Lee, X. Liang, H. Chang, and J. U. Jung. 2004. Inhibition of T cell receptor signal transduction by tyrosine kinase-interacting protein of herpesvirus saimiri. J. Exp. Med. 200:681-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi, J. K., S. Ishido, and J. U. Jung. 2000. The collagen repeat sequence is a determinant of the degree of herpesvirus saimiri STP transforming activity. J. Virol. 74:8102-8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou, C. S., M. M. Medveczky, P. Geck, D. Vercelli, and P. G. Medveczky. 1995. Expression of IL-2 and IL-4 in T lymphocytes transformed by herpesvirus saimiri. Virology 208:418-426. [DOI] [PubMed] [Google Scholar]
- 14.Dear, S., and R. Staden. 1991. A sequence assembly and editing program for efficient management of large projects. Nucleic Acids Res. 19:3907-3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Carli, M., S. Berthold, H. Fickenscher, I. M. Fleckenstein, M. M. D'Elios, Q. Gao, R. Biagiotti, M. G. Giudizi, J. R. Kalden, and B. Fleckenstein. 1993. Immortalization with herpesvirus saimiri modulates the cytokine secretion profile of established Th1 and Th2 human T cell clones. J. Immunol. 151:5022-5030. [PubMed] [Google Scholar]
- 16.Desrosiers, R. C., A. Bakker, J. Kamine, L. A. Falk, R. D. Hunt, and N. W. King. 1985. A region of the herpesvirus saimiri genome required for oncogenicity. Science 228:184-187. [DOI] [PubMed] [Google Scholar]
- 17.Desrosiers, R. C., and L. A. Falk. 1982. Herpesvirus saimiri strain variability. J. Virol. 43:352-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desrosiers, R. C., D. P. Silva, L. M. Waldron, and N. L. Letvin. 1986. Nononcogenic deletion mutants of herpesvirus saimiri are defective for in vitro immortalization. J. Virol. 57:701-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Oro, U., M. S. Vacchio, A. M. Weissman, and J. D. Ashwell. 1997. Activation of the Lck tyrosine kinase targets cell surface T cell antigen receptors for lysosomal degradation. Immunity 7:619-628. [DOI] [PubMed] [Google Scholar]
- 20.Duboise, S. M., J. Guo, S. Czajak, R. C. Desrosiers, and J. U. Jung. 1998. STP and Tip are essential for herpesvirus saimiri oncogenicity. J. Virol. 72:1308-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duboise, S. M., H. Lee, J. Guo, J. K. Choi, S. Czajak, M. Simon, R. C. Desrosiers, and J. U. Jung. 1998. Mutation of the Lck-binding motif of Tip enhances lymphoid cell activation by herpesvirus saimiri. J. Virol. 72:2607-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ensser, A., M. Thurau, S. Wittmann, and H. Fickenscher. 2003. The genome of herpesvirus saimiri C488 which is capable of transforming human T cells. Virology 314:471-487. [DOI] [PubMed] [Google Scholar]
- 23.Fickenscher, H., B. Biesinger, A. Knappe, S. Wittmann, and B. Fleckenstein. 1996. Regulation of the herpesvirus saimiri oncogene stpC, similar to that of T-cell activation genes, in growth-transformed human T lymphocytes. J. Virol. 70:6012-6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fickenscher, H., and B. Fleckenstein. 1998. Growth transformation of human T cells. Methods Microbiol. 25:573-602. [Google Scholar]
- 25.Fleckenstein, B., and R. C. Desrosiers. 1982. Herpesvirus saimiri and herpesvirus ateles, p. 253-332. In B. Roizman (ed.), The herpesviruses. Plenum Publishing Corp., New York, N.Y.
- 26.Geck, P., S. A. Whitaker, M. M. Medveczky, and P. G. Medveczky. 1990. Expression of collagenlike sequences by a tumor virus, herpesvirus saimiri. J. Virol. 64:3509-3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gluzman, Y. 1981. SV40-transformed simian cells support the replication of early SV40 mutants. Cell 23:175-182. [DOI] [PubMed] [Google Scholar]
- 28.Greve, T., G. Tamguney, B. Fleischer, H. Fickenscher, and B. M. Bröker. 2001. Downregulation of p56(lck) tyrosine kinase activity in T cells of squirrel monkeys (Saimiri sciureus) correlates with the nontransforming and apathogenic properties of herpesvirus saimiri in its natural host. J. Virol. 75:9252-9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo, J., M. Duboise, H. Lee, M. Li, J. K. Choi, M. Rosenzweig, and J. U. Jung. 1997. Enhanced downregulation of Lck-mediated signal transduction by a Y114 mutation of herpesvirus saimiri Tip. J. Virol. 71:7092-7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo, J., K. Williams, S. M. Duboise, L. Alexander, R. Veazey, and J. U. Jung. 1998. Substitution of ras for the herpesvirus saimiri STP oncogene in lymphocyte transformation. J. Virol. 72:3698-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartley, D. A., K. Amdjadi, T. R. Hurley, T. C. Lund, P. G. Medveczky, and B. M. Sefton. 2000. Activation of the Lck tyrosine protein kinase by the herpesvirus saimiri Tip protein involves two binding interactions. Virology 276:339-348. [DOI] [PubMed] [Google Scholar]
- 32.Hartley, D. A., and G. M. Cooper. 2000. Direct binding and activation of STAT transcription factors by the herpesvirus saimiri protein Tip. J. Biol. Chem. 275:16925-16932. [DOI] [PubMed] [Google Scholar]
- 33.Hartley, D. A., T. R. Hurley, J. S. Hardwick, T. C. Lund, P. G. Medveczky, and B. M. Sefton. 1999. Activation of the Lck Tyrosine-protein kinase by the binding of the Tip protein of herpesvirus saimiri in the absence of regulatory tyrosine phosphorylation. J. Biol. Chem. 274:20056-20059. [DOI] [PubMed] [Google Scholar]
- 34.Hasham, M. G., and A. Y. Tsygankov. 2004. Tip, an Lck-interacting protein of herpesvirus saimiri, causes Fas- and Lck-dependent apoptosis of T lymphocytes. Virology 320:313-329. [DOI] [PubMed] [Google Scholar]
- 35.Heck, E., D. Lengenfelder, M. Schmidt, I. Müller-Fleckenstein, B. Fleckenstein, B. Biesinger, and A. Ensser. 2005. T-cell growth transformation by herpesvirus saimiri is independent of STAT3 activation. J. Virol. 79:5713-5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hör, S., A. Ensser, C. Reiss, K. Ballmer-Hofer, and B. Biesinger. 2001. Herpesvirus saimiri protein StpB associates with cellular Src. J. Gen. Virol. 82:339-344. [DOI] [PubMed] [Google Scholar]
- 37.Isakov, N., and B. Biesinger. 2000. Lck protein tyrosine kinase is a key regulator of T-cell activation and a target for signal intervention by herpesvirus saimiri and other viral gene products. Eur. J. Biochem. 267:3413-3421. [DOI] [PubMed] [Google Scholar]
- 38.Jung, J. U., and R. C. Desrosiers. 1995. Association of the viral oncoprotein STP-C488 with cellular Ras. Mol. Cell. Biol. 15:6506-6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jung, J. U., S. M. Lang, U. Friedrich, T. Jun, T. M. Roberts, R. C. Desrosiers, and B. Biesinger. 1995. Identification of Lck-binding elements in Tip of herpesvirus saimiri. J. Biol. Chem. 270:20660-20667. [DOI] [PubMed] [Google Scholar]
- 40.Jung, J. U., S. M. Lang, T. Jun, T. M. Roberts, A. Veillette, and R. C. Desrosiers. 1995. Downregulation of Lck-mediated signal transduction by Tip of herpesvirus saimiri. J. Virol. 69:7814-7822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung, J. U., J. J. Trimble, N. W. King, B. Biesinger, B. W. Fleckenstein, and R. C. Desrosiers. 1991. Identification of transforming genes of subgroup A and C strains of herpesvirus saimiri. Proc. Natl. Acad. Sci. USA 88:7051-7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kjellen, P., K. Amdjadi, T. C. Lund, P. G. Medveczky, and B. M. Sefton. 2002. The herpesvirus saimiri Tip484 and Tip488 proteins both stimulate Lck tyrosine protein kinase activity in vivo and in vitro. Virology 297:281-288. [DOI] [PubMed] [Google Scholar]
- 43.Knappe, A., C. Hiller, M. Thurau, S. Wittmann, H. Hofmann, B. Fleckenstein, and H. Fickenscher. 1997. The superantigen-homologous viral immediate-early gene ie14/vsag in herpesvirus saimiri-transformed human T cells. J. Virol. 71:9124-9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lang, S. M., A. J. Iafrate, C. Stahl-Hennig, E. M. Kuhn, T. Nisslein, F. J. Kaup, M. Haupt, G. Hunsmann, J. Skowronski, and F. Kirchhoff. 1997. Association of simian immunodeficiency virus Nef with cellular serine/threonine kinases is dispensable for the development of AIDS in rhesus macaques. Nat. Med. 3:860-865. [DOI] [PubMed] [Google Scholar]
- 45.Lee, H., J. K. Choi, M. Li, K. Kaye, E. Kieff, and J. U. Jung. 1999. Role of cellular tumor necrosis factor receptor-associated factors in NF-κB activation and lymphocyte transformation by herpesvirus saimiri STP. J. Virol. 73:3913-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee, H., J. J. Trimble, D. W. Yoon, D. Regier, R. C. Desrosiers, and J. U. Jung. 1997. Genetic variation of herpesvirus saimiri subgroup A transforming protein and its association with cellular Src. J. Virol. 71:3817-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lund, T., M. M. Medveczky, P. Geck, and P. G. Medveczky. 1995. A herpesvirus saimiri protein required for interleukin-2 independence is associated with membranes of transformed T cells. J. Virol. 69:4495-4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lund, T., M. M. Medveczky, and P. G. Medveczky. 1997. Herpesvirus saimiri Tip-484 membrane protein markedly increases p56lck activity in T cells. J. Virol. 71:378-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lund, T., M. M. Medveczky, P. J. Neame, and P. G. Medveczky. 1996. A herpesvirus saimiri membrane protein required for interleukin-2 independence forms a stable complex with p56lck. J. Virol. 70:600-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lund, T. C., R. Garcia, M. M. Medveczky, R. Jove, and P. G. Medveczky. 1997. Activation of STAT transcription factors by herpesvirus saimiri Tip-484 requires p56lck. J. Virol. 71:6677-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lund, T. C., P. C. Prator, M. M. Medveczky, and P. G. Medveczky. 1999. The Lck binding domain of herpesvirus saimiri Tip-484 constitutively activates Lck and STAT3 in T cells. J. Virol. 73:1689-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Medveczky, M. M., P. Geck, J. L. Sullivan, D. Serbousek, J. Y. Djeu, and P. G. Medveczky. 1993. IL-2 independent growth and cytotoxicity of herpesvirus saimiri-infected human CD8 cells and involvement of two open reading frame sequences of the virus. Virology 196:402-412. [DOI] [PubMed] [Google Scholar]
- 53.Medveczky, P., E. Szomolanyi, R. C. Desrosiers, and C. Mulder. 1984. Classification of herpesvirus saimiri into three groups based on extreme variation in a DNA region required for oncogenicity. J. Virol. 52:938-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meinl, E., T. Derfuss, R. Pirzer, N. Blank, D. Lengenfelder, A. Blancher, F. Le Deist, B. Fleckenstein, and C. Hivroz. 2001. Herpesvirus saimiri replaces ZAP-70 for CD3- and CD2-mediated T cell activation. J. Biol. Chem. 276:36902-36908. [DOI] [PubMed] [Google Scholar]
- 55.Merlo, J. J., and A. Y. Tsygankov. 2001. Herpesvirus saimiri oncoproteins Tip and StpC synergistically stimulate NF-κB activity and interleukin-2 gene expression. Virology 279:325-338. [DOI] [PubMed] [Google Scholar]
- 56.Murphy, C., C. Kretschmer, B. Biesinger, J. Beckers, J. Jung, R. C. Desrosiers, H. K. Müller-Hermelink, B. W. Fleckenstein, and U. Rüther. 1994. Epithelial tumours induced by a herpesvirus oncogene in transgenic mice. Oncogene. 9:221-226. [PubMed] [Google Scholar]
- 57.Murthy, S. C., J. J. Trimble, and R. C. Desrosiers. 1989. Deletion mutants of herpesvirus saimiri define an open reading frame necessary for transformation. J. Virol. 63:3307-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park, J., N. H. Cho, J. K. Choi, P. Feng, J. Choe, and J. U. Jung. 2003. Distinct roles of cellular Lck and p80 proteins in herpesvirus saimiri Tip function on lipid rafts. J. Virol. 77:9041-9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park, J., B. S. Lee, J. K. Choi, R. E. Means, J. Choe, and J. U. Jung. 2002. Herpesviral protein targets a cellular WD repeat endosomal protein to downregulate T lymphocyte receptor expression. Immunity 17:221-233. [DOI] [PubMed] [Google Scholar]
- 60.Reiss, C., G. Niedobitek, S. Hör, R. Lisner, U. Friedrich, W. Bodemer, and B. Biesinger. 2002. Peripheral T-cell lymphoma in herpesvirus saimiri-infected tamarins: tumor cell lines reveal subgroup-specific differences. Virology 294:31-46. [DOI] [PubMed] [Google Scholar]
- 61.Schäfer, A., D. Lengenfelder, C. Grillhösl, C. Wieser, B. Fleckenstein, and A. Ensser. 2003. The latency-associated nuclear antigen homolog of herpesvirus saimiri inhibits lytic virus replication. J. Virol. 77:5911-5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seed, B., and A. Aruffo. 1987. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc. Natl. Acad. Sci. USA 84:3365-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sorokina, E. M., J. J. Merlo, Jr., and A. Y. Tsygankov. 2004. Molecular mechanisms of the effect of herpesvirus saimiri protein StpC on the signaling pathway leading to NF-κB activation. J. Biol. Chem. 279:13469-13477. [DOI] [PubMed] [Google Scholar]
- 64.Szomolanyi, E., P. Medveczky, and C. Mulder. 1987. In vitro immortalization of marmoset cells with three subgroups of herpesvirus saimiri. J. Virol. 61:3485-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weber, F., E. Meinl, K. Drexler, A. Czlonkowska, S. Huber, H. Fickenscher, I. Müller-Fleckenstein, B. Fleckenstein, H. Wekerle, and R. Hohlfeld. 1993. Transformation of human T-cell clones by herpesvirus saimiri: intact antigen recognition by autonomously growing myelin basic protein-specific T cells. Proc. Natl. Acad. Sci. USA 90:11049-11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wehner, L. E., N. Schröder, K. Kamino, U. Friedrich, B. Biesinger, and U. Rüther. 2001. Herpesvirus saimiri Tip gene causes T-cell lymphomas in transgenic mice. DNA Cell Biol. 20:81-88. [DOI] [PubMed] [Google Scholar]
- 67.Wiese, N., A. Y. Tsygankov, U. Klauenberg, J. B. Bolen, B. Fleischer, and B. M. Bröker. 1996. Selective activation of T cell kinase p56lck by herpesvirus saimiri protein Tip. J. Biol. Chem. 271:847-852. [DOI] [PubMed] [Google Scholar]
- 68.Wieser, C., D. Stumpf, C. Grillhösl, D. Lengenfelder, S. Gay, B. Fleckenstein, and A. Ensser. 2005. Regulated and constitutive expression of anti-inflammatory cytokines by nontransforming herpesvirus saimiri vectors. Gene Ther. 12:395-406. [DOI] [PubMed] [Google Scholar]