Abstract
When mice under the age of 5 to 6 days are infected, the FrCasE retrovirus induces a neurodegenerative disease leading to death within 1 to 2 months. We have recently reported that transient treatment with a neutralizing monoclonal antibody (MAb) shortly after infection, in addition to an expected immediate decrease in the viral load, also favors the development of a strong protective immune response that persists long after the MAb has been cleared. This observation may have important therapeutic consequences, as it suggests that MAbs might be used, not only as direct neutralizing agents, but also as immunomodulatory agents enabling patients to mount their own antiviral immune responses. We have investigated whether immunoglobulins from mothers who displayed a strong anti-FrCasE humoral response induced upon MAb treatment could affect both viremia and the immune systems of FrCasE-infected pups till adult age upon placental and/or breastfeeding transfer. The strongest effects, i.e., reduction in the viral load and induction of protective humoral antiviral responses, were observed upon breastfeeding alone and breastfeeding plus placental immunity transfer. However, placental transfer of anti-FrCasE antibodies was sufficient to both protect neonatally infected animals and help them initiate a neutralizing anti-FrCasE response. Also, administration of a neutralizing MAb to naive mothers during late gestation and breastfeeding could generate similar effects. Taken together, our data support the concept that passive immunotherapies during late gestation and/or breastfeeding might help retrovirally infected neonates prime their own protective immune responses, in addition to exerting an immediate antiviral effect.
The therapeutic potential of monoclonal antibodies (MAbs) is now well recognized: 19 have already been approved by the FDA for human use, and more than 400 others are being tested in humans, which makes them the largest class of engineered proteins used for medical applications (24). Although cancers and immune defects currently represent the major therapeutic targets, MAbs might also be used for the future treatment of infectious diseases. Thus, various neutralizing MAbs have already been considered for treating hepatitis B virus, hepatitis C virus, and human immunodeficiency virus (HIV) infections, which figure among the heaviest health burdens worldwide. In the specific case of HIV, several MAbs have already shown antiviral activity in vivo in a variety of adult- and neonatal-animal models and human volunteers (21). For hepatitis C virus and hepatitis B virus, MAbs that were initially shown to be safe and well tolerated and to display significant in vivo antiviral activity have been further developed for use in ongoing clinical trials (6).
Using a lethal-retroviral-infection model of immunocompetent mice, we recently reported that short passive immunization with a neutralizing MAb, in addition to an expected immediate decrease in the viral load, may help the endogenous immune system mount a long-term protective immune response. In brief, when newborn animals under the age of 5 to 6 days are inoculated, the FrCasE simple retrovirus first propagates in the periphery and then penetrates into the central nervous system. There, it causes a rapid noninflammatory spongiform degenerative disease, leading to the death of all mice within 1 to 2 months. In contrast, mice infected at an older age do not develop a neurological illness, but splenomegaly and leukemia were observed in 80% of them within 3 to 6 months postinfection, due to periphery-restricted replication of FrCasE. When newborn viremic animals are briefly treated (<15 days) with the neutralizing MAb 667 (IgG2a/κ), which recognizes the viral receptor-binding VRA domain of Env (7), shortly after infection (< 2 days), the animals survive and show no sign of any disease. This protection is due to both an immediate effect on the viral load and the development of a strong protective immune response capable of containing viral replication for more than 16 months (the duration of the experiments) following MAb clearance (13). Although the molecular and cellular bases underlying the immunomodulatory effect of MAb 667 have not yet been completely elucidated, a clear and essential contribution of the humoral response to antiviral protection has been demonstrated (13). If this is also the case in humans, this observation may have potentially important therapeutic consequences, as MAb treatments may help infected individuals develop their own antiviral immunity. The report that intensive treatment of juvenile simian immunodeficiency virus-infected macaques with anti-simian immunodeficiency virus hyperimmune serum immunoglobulins (Igs) accelerated the appearance of neutralizing antibodies, as assayed in vitro, in a fraction of the animals (14) suggests that this may be the case, at least in primates. However, although no virus challenge experiments were conducted to demonstrate that the antiviral response was actually protective in vivo, it is interesting that this immune response correlated with a delayed onset of the disease in some monkeys (14).
It is well established that humoral immunity can be passively transferred from mother to baby, prenatally across the placenta and postnatally through the colostrum and breast milk in rodents and humans, although the relative contributions of these two modes of transfer differ between the species (see Discussion for more details). Importantly, mother-to-baby transfer of immunity has been shown to confer protection against a variety of pathogens (4, 32). For example, in the specific case of viral infections, immunoglobulin transfer through milk has been shown to be beneficial against infections by respiratory syncytial virus in humans (8), feline immunodeficiency virus in kittens (3), and herpes simplex virus (HSV) in mice (35). In contrast to these relatively clear-cut situations, HIV represents a more complex system, as in addition to infections occurring in utero and upon delivery, breast milk constitutes another route of transmission of the virus (2, 16, 34). Nevertheless, broad neutralizing antibody responses in the mother's sera (28, 29) and antiviral secretory IgMs in the mother's milk during the lactation period seem to be associated with a lower risk of mother-to-infant virus transmission (34), although strict correlations between antibody levels, protection, and breast milk transmission have not yet been established (25, 26). Taken together with other data (31), these observations suggest that a challenge for neonatal antiviral therapy may not only be the development of efficient maternal-vaccination strategies (31), but also that of efficient passive immunotherapies during late gestation and/or early life.
A recent follow-up of neonates with perinatally acquired HIV type 1 infection suggested that the developing immune systems of children may exhibit greater plasticity than those of adults to contain escape variants appearing during continually evolving chronic infection (10, 11). This observation challenges the common idea that the mature adult immune system is systematically more efficient at counteracting infections and also indirectly lends support to the concept that passive immunotherapies during late gestation and/or early life might also help infected patients prime their own protective immune responses, in addition to exerting an immediate antiviral effect. As a first step toward testing this possibility, we resorted to the FrCasE neonatal-infection model and studied how immunoglobulins from mothers who either displayed natural anti-FrCasE humoral responses or were subjected to passive immunotherapy could affect both viremia and the immune systems of infected pups until adulthood following transplacental and/or breastfeeding immunity transfer.
MATERIALS AND METHODS
Virus stocks, cell lines, and monoclonal antibody production.
Culture supernatants of Mus dunni embryo fibroblasts transfected with the FrCasE proviral clone (23) were used as viral stocks (22). The anti-murine leukemia virus Env mouse 667 and 678 (20) and rat 83A25 MAbs (9) were prepared and purified from hybridoma cell culture supernatants and assayed as previously described (7).
Virus titers and 667 MAb neutralization activity assay.
Viral titers were determined using a focal immunofluorescence assay (FIA) (33). Dilutions of virus-containing samples were added to 25% confluent Mus dunni cell cultures in the presence of 8 μg/ml of Polybrene. Cell-to-cell spread of replication-competent retroviruses was allowed to proceed for 2 days, and focus-forming units (FFU) were visualized by indirect immunofluorescence using the 667 MAb and fluorescein isothiocyanate-conjugated rabbit anti-mouse Ig antiserum. For assaying the virus neutralization activities of mouse sera, 4 × 102 FrCasE FFU were diluted in a 1:1 ratio with serum samples previously diluted in phosphate-buffered saline (PBS) (0.15 M NaCl, 0.01 M Na phosphate, pH 7) and incubated at 37°C for 1 h. The mixtures were used to infect 2 × 104 cells cultured in 12-well plates overnight. The infection medium was replaced by fresh culture medium, and the cells were allowed to reach confluence, at which time FFU were scored as described above.
Virus infection and follow-up of mice.
Outbred Swiss mice were used in this study. Newborns were infected on day 3 using 100 μl of virus suspension containing 5 × 105 FFU/ml as previously described (13, 22). The mice were then examined for clinical signs of neurodegeneration daily until day 30 and weekly from then on. Blood was withdrawn from the retro-orbital sinus for viremia and anti-FrCasE serum immunoglobulin concentration assays. After clotting at room temperature for 15 min, blood samples were centrifuged at 6,000 × g for 15 min, and serum aliquots were stored at −20°C until required. For the virus challenge experiments, mice were injected intravenously with 300 μl of a FrCasE suspension containing 5 × 104 FFU/ml 30 weeks after the first infection. Blood samples were collected every 2 days during the first 2 weeks postchallenge to assay viremia and endogenous anti-FrCasE IgG concentrations. On day 18, the mice were sacrificed and the spleens were removed.
RNA purification, synthesis of cDNA, and reverse transcription (RT)-PCR analysis.
Total RNA from splenocytes was prepared using RNAzol as specified by the supplier (Eurobio), and cDNA synthesis was performed as previously described (13). The env cDNA was amplified by PCR using the following oligonucleotides: 5′-TCT TAT TCG TGA CAG GAG GGT T-3′ (sense) and 5′-ATA TGG AGG GTG GTT GTC TA-3′ (antisense). PCR amplification was carried out using a hot-start protocol (3 min at 94°C) in a final volume of 50 μl containing 2 μl of each cDNA, 50 pmol of each primer, 1.5 mM MgCl2, and 2.5 units of Taq I polymerase (Eurobio, Paris, France). Forty-five cycles (94°C for 3 min, 65°C for 45 s, and 72°C for 45 s) for Env and 25 for β-actin were followed by an elongation period of 10 min at 72°C. The nucleotide sequences of the amplification primers are available on request.
Flow cytometry analysis of infected splenocytes.
Two sets of flow cytometry experiments were always conducted in parallel using either 667 or the rat 83A25 MAb, which recognize different Env epitopes with no difference in the final outcomes (9). The spleens were dissociated in cell culture medium and washed once by centrifugation and resuspension in PBS. Red blood cells were eliminated by adding the ACK lysis buffer (Biowhittaker). White blood cells were recovered by centrifugation, washed twice in PBS, and resuspended in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. Samples of 5 × 105 cells were collected at room temperature for 1 h in the presence of 5 μg/ml of anti-Env MAb in 0.2% bovine serum albumin (BSA)-PBS, washed twice in 0.2% BSA-PBS, and incubated at room temperature with a secondary fluorescein isothiocyanate-conjugated rabbit anti-mouse IgG antibody for 1 h. The cells were washed twice in 0.2% BSA-PBS, resuspended in 500 μl of PBS, and analyzed using a FACScalibur flow cytometer (Becton Dickinson).
ELISA of anti-FrCasE antibodies.
The 667 MAb and anti-FrCasE serum antibodies were assayed as described previously (13). Whereas animals of sufficient size were bled at the retro-orbital sinus, the blood of pups was collected following sacrifice. Milk samples were directly withdrawn from the stomachs of sacrificed pups. After resuspension in PBS plus 0.1% Tween 40, the samples were first sonicated to fully solubilize the proteins and then used for enzyme-linked immunosorbent assay (ELISA). The 667 and 678 MAbs used as standards for anti-FrCasE IgG2a and IgG1 assays, respectively, were diluted in PBS plus 0.1% Tween 40 containing 1% BSA. Peroxidase-conjugated anti-mouse IgG2a and IgG1 rabbit antisera (Serotec) were used as secondary antibodies.
RESULTS
Endogenous anti-FrCasE immunoglobulins in the sera and milk of infected/667 MAb-treated mothers.
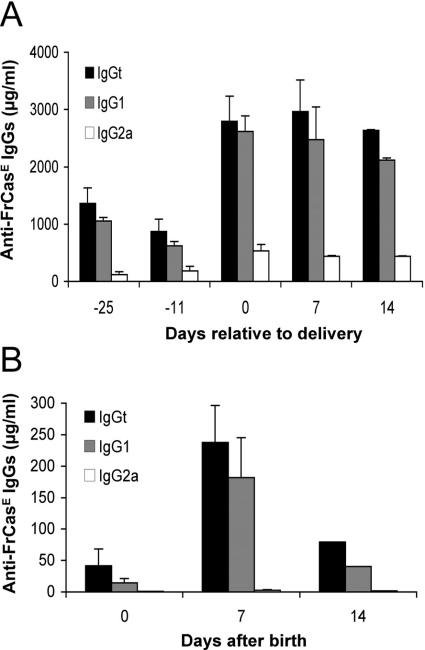
We have previously shown that mice infected neonatally by FrCasE and treated with the IgG2a/κ neutralizing MAb 667 for a few days (infected/treated animals) develop a sustained long-term protective immunity. This permits them to resist viral challenge, which is followed by the induction of a secondary immune response. Most often, the humoral arm of this response is predominantly of the IgG2a isotype, but it can occasionally be dominated by IgG1s (13). Here, as a first step, we followed anti-FrCasE antibody levels in both the sera and the milk of infected/treated mothers. Four-month-old infected/treated Swiss mice were mated, and their antiviral immune response was boosted upon intravenous reinoculation of FrCasE at midgestation (day 10 before birth). As our aforementioned passive immunotherapies were solely performed using a IgG2a/κ MAb (13), we resorted here to animals displaying a predominantly IgG1 antiviral response as a means to address the abilities of other isotypes to induce a protective immune response in infected pups. The data presented in Fig. 1A indicate that, upon FrCasE recall, the humoral response was rapidly stimulated with a serum level of FrCasE-specific total IgGs (IgGt) shifting from approximately 1 to 3 mg/ml on the day of delivery. Importantly, it was predominantly of the IgG1 isotype and persisted for at least 14 days, (i.e., during the whole breastfeeding period), at which point weaning began. Consistently, milk anti-FrCasE antibodies, which reached their maximal level (approximately 250 μg/ml) within 1 week postpartum, were of the IgG1 isotype (Fig. 1B).
FIG. 1.
Anti-FrCasE immunoglobulins in the sera and milk of infected/treated mothers. (A) Anti-FrCasE IgG concentrations in sera during gestation and breastfeeding. Four-month-old females infected with FrCasE, treated with the 667 MAb during the neonatal period, and displaying prominent IgG1 humoral immune responses were mated. Blood samples were collected at various times before and after delivery for ELISA of anti-FrCasE IgGt, IgG2a, and IgG1. The values are presented as the mean plus standard error of the mean (SEM). (B) Anti-FrCasE IgG concentrations in milk during the breastfeeding period. Milk was collected from the stomachs of two neonates nursed by infected/treated mothers for each time point, and anti-FrCasE IgGs were assayed by ELISA in triplicate. The values are presented as the mean plus SEM.
Short-term effects of placental and breastfeeding transfers of mother's antiviral IgGs in FrCasE-infected neonates.
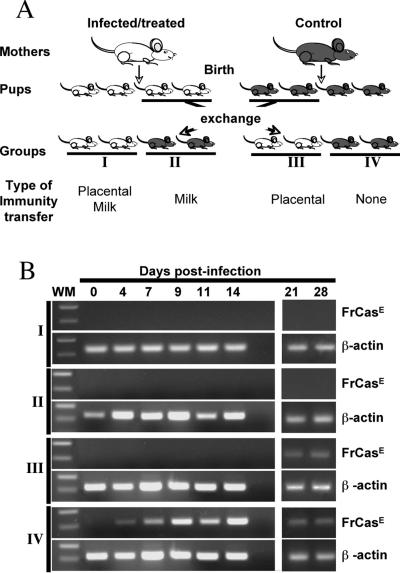
Secondly, we asked whether maternal immunity transferred either (i) prenatally, (ii) postnatally, or (iii) prenatally and postnatally could protect neonatally infected pups against FrCasE-induced diseases. To address this question, pups from two 4-month-old infected/treated mothers and two control nontreated/noninfected mothers of the same age synchronized for delivery were exchanged at the moment of delivery, i.e., prior to the start of breastfeeding, as follows: eight pups from each litter were kept with their natural mothers for nursing, whereas eight others were replaced by eight animals born to another type of mother. This allowed us to form four groups of 16 neonates each with regard to anti-FrCasE immunity transfer (Fig. 2A). Group I corresponded to neonates receiving placental plus breastfeeding transfer, group II to those receiving only breastfeeding transfer, group III to those receiving only placental transfer, and group IV to control neonates receiving neither placental nor breastfeeding immunity transfer. All neonates were infected with FrCasE 3 days after birth and surveyed for signs of illness, anti-FrCasE antibody levels, and viremia.
FIG. 2.
Experimental scheme and analysis of proviral DNA in splenocytes. (A) Experimental scheme. Two infected/treated and two control noninfected/nontreated mice were mated at the same time to ensure synchronized deliveries. Immediately after birth and before breastfeeding could occur, the pups were distributed as follows: eight neonates from each litter were kept with their natural mothers for nursing, whereas another eight were transferred to a foster mother in order to have animals born to infected/treated mice nursed by control mice and vice versa. All neonates were infected with FrCasE 3 days after birth and followed for signs of illness, anti-FrCasE antibodies, and viral propagation using several criteria (see the text). Analysis at early time points required the sacrifice of two animals per time point. (B) RT-PCR detection of FrCasE Env mRNA in the spleen. Two animals per group were sacrificed at the indicated times. The spleens were recovered and pooled for RNA preparation. Subgenomic FrCasE Env mRNA accumulation was assayed by RT-PCR as described in Materials and Methods. β-Actin was used as an internal invariant amplification standard. WM, molecular weight marker.
As expected, all animals from the control group (group IV) died from neurodegeneration within 4 to 5 weeks after infection and showed high plasma viremia (106 infectious particles/ml as assayed by FIA) (see Materials and Methods), as well as a high proportion of virus-infected splenocytes (44%, as assayed by flow cytometry analysis of retroviral Env-expressing cells) on day 28 after birth (Table 1). Importantly, none of them showed detectable anti-FrCasE antibody levels, in keeping with our previous report (13). In contrast, the animals from groups I to III survived and did not show the characteristic symptoms of FrCasE-induced neurodegenerative illness (ataxia, hind limb paralysis, and loss of weight) (see references 5 and 17 for more details) within the same time period. However, significant differences were observed between, on one hand, groups I and II (which were subjected to breastfeeding immunity transfer) and, on the other hand, group III (which only received placental immunity transfer) with regard to maternal anti-FrCasE antibody levels and FrCasE propagation.
TABLE 1.
Physiopathological analysis of groups I to IV by 1 month post-FrCasE infection
| Group | 28 days after birth
|
% Survival 35 days after birth | ||
|---|---|---|---|---|
| Symptoms | Viremiaa (FFu/ml) | Splenocyte infectionc (%) | ||
| I | None | NDb | 0.8 ± 0.4 | 100 |
| II | None | ND | 0.5 ± 0.3 | 100 |
| III | None | 8 × 102 ± 1.5 | 13.2 ± 7.4 | 100 |
| IV | Ataxia, paralysis | 1.3 × 106 ± 0.2 | 44 ± 2.6 | 0 |
Assayed by FIA from eight animals.
ND, not detectable. The limit of detection was 102 FFU/ml.
Flow cytometry assay of FrCasE Env-expressing spleen cells.
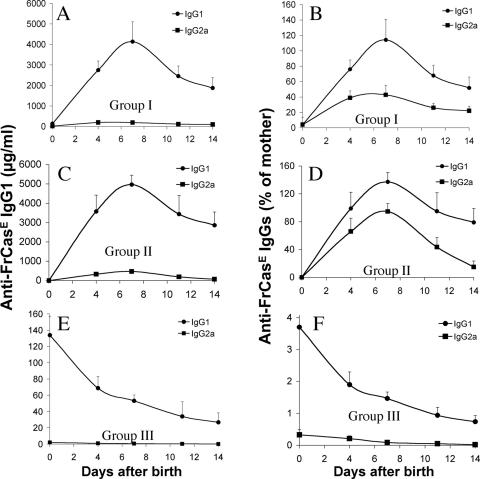
Two animals per group were sacrificed at various time points during the first month postinfection to assay plasma maternal anti-FrCasE IgG levels and FrCasE propagation. Time points later than 14 days were not considered for maternal-antibody assay, as our previous 667 MAb-based immunotherapy experiments (13) suggested that an endogenous immune response might arise after this time (see below) and bias the analysis. Anti-FrCasE IgG levels dramatically increased between day 0 and day 7 and slightly decreased thereafter during the weaning period in groups I and II (Fig. 3A and C). In contrast, they steadily decreased in group III from the day of birth to levels 100-fold lower than those of groups I and II within 1 week, as expected due to the limited life span of antibodies in vivo (Fig. 3A, C, and E). Comparison of anti-FrCasE immunoglobulin concentrations in sera from neonates and infected/treated mothers (Fig. 3B, D, and F) indicated that IgG1s were transferred more efficiently than IgG2a, whether the transfer was achieved via the placenta (a 3-fold factor) or milk (a 1.5- to 3-fold factor). Interestingly, FrCasE RNA could not be detected by RT-PCR in the spleens of group I, II, and III mice till day 14, whereas it was easily seen from day 4 onward in control group IV animals (Fig. 2B). Although this indicated a clear antiviral effect of maternal-immunity transfer during the period immediately following infection, regardless of the mode of IgG transmission, the analysis of animals at later time points gave more contrasting results. Thus, neither blood viremia (Table 1) nor viral RNA in the spleen (Fig. 2B) could be detected in group I and group II animals on day 28. Moreover, the fraction of FrCasE-infected splenocytes of group I and II animals sacrificed on day 28 was close to the limit of detection (Table 1). As for group III animals, viral RNA (Fig. 2B) was easily detectable in spleens from day 21 onward (Fig. 2B), and 13% of splenocytes were positive for Env expression on day 28 (Table 1). Moreover, significant viremia could be detected on day 28, although it was 2,000-fold weaker than that of group IV control mice (Table 1).
FIG. 3.
Maternal anti-FrCasE immunoglobulins in sera of neonates after placental and/or breastfeeding immunity transfer. Anti-FrCasE IgG1s and IgG2as were assayed in the sera of group I (A and B), group II (C and D), and group III (E and F) animals. (A, C, and E) Concentrations of mothers' anti-FrCasE IgG1s and IgG2as in neonates' sera. The values are the mean ± standard error of the mean (SEM) of the data obtained from two animals sacrificed per time point. The maximal values measured for IgG2as in animals of groups I, II, and III were 195 ± 44.5, 473 ± 57, and 1 ± 0.7 μg/ml, respectively. (B, D, and F). Transfer efficiencies of anti-FrCasE IgG1s and IgG2as. The levels of immunoglobulins were calculated as percentages of immunoglobulins of the same isotype present in the mother's serum at the time of birth. The values are the mean ± SEM of the data obtained from two animals sacrificed per time point.
In summary, our data indicate that the low levels of maternal anti-FrCasE antibodies transferred exclusively across the placenta were not sufficient to eradicate neonatally inoculated FrCasE but could dramatically reduce systemic viral spread during the first 2 weeks after birth. This most likely prevented brain infection and, consequently, neurodegeneration. In contrast, immunity transfer via breastfeeding, as it permitted the supply of much higher amounts of maternal anti-FrCasE antibodies for a longer period of time, resulted in a stronger antiviral effect, as no virus was detectable 1 month postinfection.
Endogenous anti-FrCasE humoral responses in animals from groups I to III.
Mice from groups I to III were then followed up till week 30 postinfection for their health status, viral propagation, and a possible endogenous humoral anti-FrCasE immune response. Neither signs of neurodegeneration nor signs of leukemia, as indicated by normal hematocrits and absence of spleen swelling, were observed during that period of analysis. Consistently, viremia also could not be detected. In contrast, all mice developed anti-FrCasE antibodies, but with significant differences between the various groups.
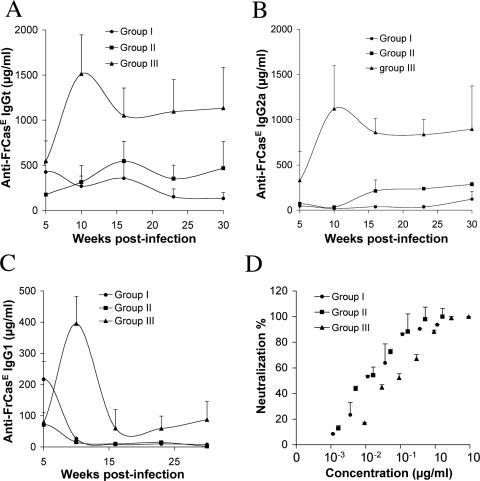
In the case of group III, the response, which was already high by week 5 postinfection, reached its maximal intensity by week 10 and, after a slight decrease, remained constant thereafter (Fig. 4A). Importantly, the higher concentration of anti-FrCasE antibodies at week 5 compared to that of day 14, as well as the fact that they were predominantly of the IgG2a isotype (Fig. 4B and C), i.e., of a different isotype from the maternal IgGs, unambiguously demonstrated that the anti-FrCasE antibodies were of endogenous origin. Interestingly, the humoral responses of group I and II animals, although sustained till the end of the analysis, were severalfold less intense than that of group III mice. The response of group I was the least intense (Fig. 4A). Moreover, the rise of anti-FrCasE IgG2as did not appear before week 10 in group II and week 23 in group I (Fig. 4B and C). The appearance of IgG2as clearly indicated the induction of endogenous antiviral responses in these two groups of animals. On the basis of our data, however, it was not possible to exclude the possibility that these responses were initiated later than in group I mice: in contrast to the transient accumulation of anti-FrCasE IgG1s seen between weeks 5 and 15 in group I sera, anti-FrCasE IgG1s decreased from weeks 5 to 10 in group II and III mice. It is therefore reasonable to assume that they corresponded to antibodies of maternal origin received in large amounts during breastfeeding and not to newly produced antibodies (Fig. 3). Finally, we compared the specific in vitro neutralization activities of anti-FrCasE antibodies generated by the various groups of mice. That of group III mice was 10-fold less than those of group I and II animals, with 50% inhibitory concentrations of 100 and 10 ng/ml, respectively, in the FIA used (Fig. 4D).
FIG. 4.
Anti-FrCasE immunoglobulin responses in group I to III mice to week 30 postinfection. (A) IgGts. Anti-FrCasE IgGts were assayed by ELISA in the sera of 12 animals per group from week 5 to week 30 postinfection. Assays were performed in triplicate, and the values are presented as the mean ± standard error of the mean. (B and C) Anti-FrCasE IgG2a and IgG1s, respectively. The same sera as in panel A were used for assay of anti-FrCasE IgG2as and IgG1s. (D) Neutralization activities of sera of group I to III mice at week 30. Neutralization activity was assayed by FIA as explained in Materials and Methods, and the results are expressed relative to the amount of anti-FrCasE IgGt assayed for panel A.
In conclusion, all mice generated a humoral anti-FrCasE response, whatever the transmission mode for maternal immunity. Qualitative and quantitative differences were nevertheless observed. Thus, the low levels of maternal IgGs transmitted transplacentally to the mice of group III were sufficient to rapidly induce a response stronger than in groups I and II. This response was, however, less neutralizing, at least according to an in vitro criterion. Moreover, the kinetics of appearance of anti-FrCasE IgG2as were different between the groups, with those of groups I and II being slower. This suggests differences in the solicitation of the mouse immune system, possibly due to differential propagation of the virus in the three groups of animals.
Absence of detectable viremia and stimulation of the antiviral humoral response in FrCasE-challenged animals of groups I to III.
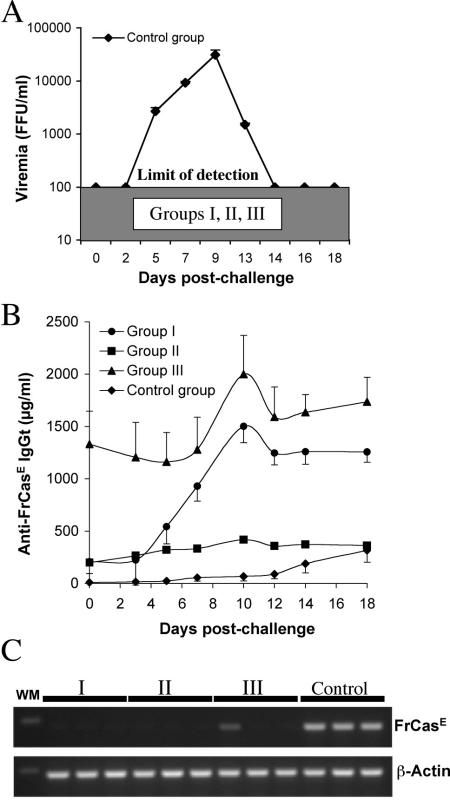
Next, we tested whether mice from groups I to III could resist an FrCasE challenge 30 weeks after the first infection. To this end, the virus was administered to three mice from each group, as well as to three naive age-matched control animals, and serum samples were collected at different times for 18 days to assay both viremia and anti-FrCasE antibodies. High, but transient, virus titers were measured in control animals, whereas no viremia was detected in any of the mice from groups I to III (Fig. 5A). A slow and modest rise in anti-FrCasE IgGs was observed in control animals on and after day 14 postchallenge, which contrasted with a fast and robust increase in those from groups I and III (Fig. 5B). This suggested a normally occurring primary immune response in the control group and a strong response in the other two. IgG2a was the main antibody subclass contributing to these responses in all animals, whether they were subjected to a challenge or a primary infection (data not shown). Mice from group II showed a lower increase (30 to 40%) of anti-FrCasE antibodies than those of groups I and III after virus challenge. Whether this was due to a more protective preexisting anti-FrCasE immunity in these animals, however, could not be studied. All mice were sacrificed after 18 days for RT-PCR analysis of FrCasE Env mRNA expression in the spleen. No signal was detected in the challenged mice from groups I to III, except for one animal in group III, which showed a weak signal (Fig. 5C). In contrast, strong signals were seen in the three control mice (Fig. 5C). In conclusion, our data support the idea that animals from groups I to III all resisted reinfection by FrCasE due to a preexisting antiviral immune response, which was clearly restimulated, albeit more strongly in groups I and III than in group II.
FIG. 5.
Viremia, anti-FrCasE immunoglobulins, and FrCasE RNA in challenged group I to III mice. (A) Viremia. FrCasE was administered to three mice from each group and three control animals in week 30. Serum samples were collected at different times for 18 days, and viremia was assayed by FIA. (B) Assay of anti-FrCasE IgGs. IgGts were assayed by ELISA. The values are presented as the mean of the values obtained for each mouse of each group ± standard error of the mean. (C) FrCasE Env mRNA in the spleen. The presence of Env mRNA was assayed by RT-PCR in total spleen RNAs from animals sacrificed 18 days postinfection. β-Actin was used as an internal standard of amplification.
Administration of the 667 MAb to mothers during gestation and the breastfeeding period protects FrCasE-infected neonates.
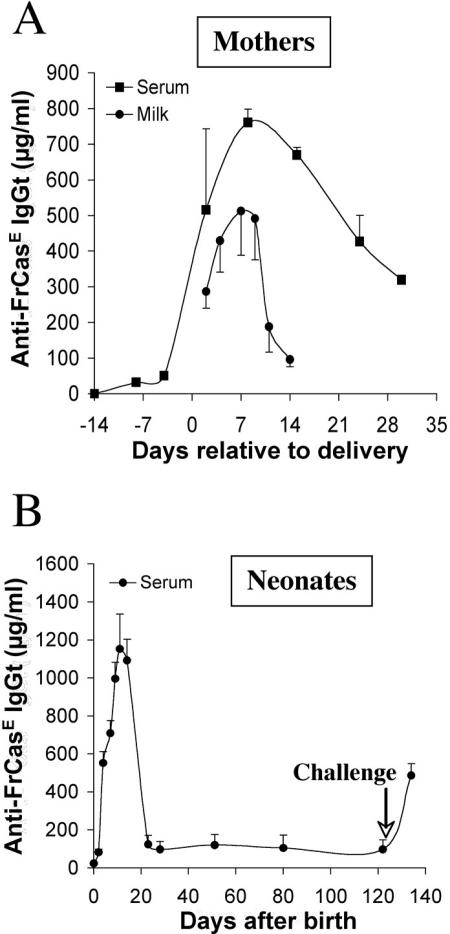
After having demonstrated that placental and/or breastfeeding immunity transfer of a natural and polyclonal anti-FrCasE response could protect neonatally infected pups and help them mount a long-term endogenous protective immune response, we addressed whether a neutralizing MAb could do the same and thereby show some therapeutic interest. To this end, the 667 MAb was administered to naive noninfected/nontreated mothers during a period extending from 14 days before to 10 days after delivery, and newborns were infected with FrCasE on day 3 after birth, maintained with their natural mothers, and surveyed for disease signs, viremia, and development of an endogenous immune response. The data presented in Fig. 6A indicate that the levels of 667 in the mother's serum were already high at the moment of delivery, continued to increase till day 6, and decreased thereafter due to antibody turnover. Consistently, 667 levels in the mother's milk roughly paralleled those in the neonates' sera, with a return to basal level by day 15, i.e., after weaning (Fig. 6A and B). All animals survived and showed no signs of neurodegeneration and no viremia during the 20-week follow-up. In contrast, they displayed a clear anti-FrCasE humoral response, with plasma anti-FrCasE Ig concentrations between 1,000 and 1,200 μg/ml (Fig. 6B). This response was essentially of the IgG2a isotype (not shown), showed a high in vitro neutralizing activity (as assayed by FIA at week 16 postinfection [data not shown]), and prevented viremia after inoculation with FrCasE on week 17 (as assayed by FIA for 3 weeks postchallenge [data not shown]). Moreover, the fivefold increase in anti-FrCasE Ig concentrations observed a few days after virus challenge confirmed the presence of a strong memory response in these animals (Fig. 6B). Thus, our data indicate that placental and/or breastfeeding transfer of a monoclonal antibody administered to a mother during the neonatal period can protect mice from infection by FrCasE and permit them to mount a protective immune response.
FIG. 6.
Pre- and postnatal treatment of mothers with the 667 MAb and follow-up of 667 accumulation in neonates. The 667 MAb (30 μg/injection) was administered intraperitoneally twice a week to two 4-month-old females during a period extending from 14 days before to 10 days after delivery. Newborns were infected with FrCasE 3 days after birth and were nursed by their mothers without any additional treatment. (A) 667 levels in the sera and milk of treated mothers. 667 MAb levels were determined by ELISA in serum samples from mothers collected at different times and in milk recovered from the stomachs of two nursed pups sacrificed for this purpose per time point. (B) 667 levels in the sera of nursed pups. The 667 MAb was assayed in the sera of the same pups used for panel A. After day 14, serum 667 was assayed from blood samples withdrawn at various times. The values are the results of two experiments performed in triplicate and are presented as the mean ± standard error of the mean.
DISCUSSION
Relative protection efficiencies conferred by placental and breastfeeding transfers of maternal antiretroviral immunity.
We have previously reported that the transient treatment of FrCasE-infected neonates with the 667 neutralizing MAb shortly after infection favors the development of a strong, long-lasting protective immune response with an essential humoral contribution (13). The mechanisms underlying this response are not yet clear. They may include at least two nonexclusive mechanisms: (i) blunting of viral infection by the MAb, which both prevents the development of neurodegeneration and allows the endogenous immune system time to react, and (ii) acceleration and/or strengthening of the immune response by virus/MAb immune complexes via the stimulation of infected/treated professional antigen-presenting cells (APCs) (discussed in reference 13). Concerning the containment of viral replication, it is noteworthy that IgG2a is the most efficient antibody isotype at fixing complement, at binding Fc receptors on macrophages and NK cells, and at mediating antibody-dependent cellular cytotoxicity and opsonization. It is therefore unlikely that the sole effect of 667 in vivo is virus neutralization (discussed in references 7 and 13).
Here, we have examined whether such an acquired anti-FrCasE immunity could be maternally transmitted via the placenta and/or breastfeeding to generate a long-term immune response comparable to that occurring upon 667 treatment of infected neonates. Our data show that this is the case, as regardless of the immunity transmission mode, all animals escaped neurodegeneration, remained healthy with no detectable sign of leukemia, developed an endogenous anti-FrCasE humoral response, and showed a clear antiviral response in a challenge experiment, which was accompanied by an easily detectable secondary immune response.
Although both mice that received maternal antiviral immunity via the placenta and those that received immunity via breastfeeding did not develop pathology and were able to mount secondary antiviral responses, significant differences were still observed. Thus, (i) FrCasE was not eradicated in the former group, whereas it remained undetectable in the latter; (ii) serum anti-FrCasE IgGs of the former group of animals accumulated in larger amounts; and (iii) they were less neutralizing than those of the latter group. It is reasonable to assume that the differences in levels of maternally transmitted antiviral antibodies were responsible for differential stimulation of the endogenous immune system by influencing the quantity, nature, and duration of presentation of viral antigens. Thus, the much smaller amounts of maternal antiviral antibodies received in the case of placental transfer permitted longer exposure to nonneutralized propagating virus particles. In contrast, a larger fraction of virus particles engaged in immune complexes may have led to a stronger stimulation of APCs of breastfed animals, albeit for a shorter period due to faster viremia control. Further studies are still necessary to formally demonstrate the latter point. It is, however, worth noting that introduction of viruses into hosts with preexisting antiviral antibodies is known to result in the formation of antigen-antibody complexes in a variety of settings and that high maternal antibody-antigen ratios are important for optimal activation of neonatal APCs. This activation could be a key element for induction of an adult-like Th1 response in early life characterized, in particular, by a prominent IgG2a humoral contribution (31).
The observation that protection against a neonatally inoculated murine retrovirus could be achieved by maternal antibodies is not entirely new. Saha et al. (27) have already suggested that protection efficiency may be related to the levels of antiviral antibodies, using ts1, a thermosensitive mutant of the Moloney murine leukemia virus TB strain that induces fatal immunodeficiencies and neurological disorders. These experiments, however, significantly differed from ours on several points. First, maternal immunity was naturally acquired upon infection by ts1 in adulthood and was not induced by MAb-based immunotherapy of infected pups, which most likely triggers a more optimal antiviral response (13). Second, the authors did not investigate whether the mothers' antiviral response was isotype polarized, in contrast to our study, which showed that a predominant IgG1-type maternal immunity can lead to a predominant IgG2a response in infected neonates. Third, even though all the animals did not develop disease after ts1 infection, as in our experiments, only a fraction of them were protected from neurodegeneration upon placental immunity transfer only (although the authors acknowledged that some form of protection may have been conferred by antibody-rich colostrum before neonates born of immune mothers were moved to nonimmune foster mothers). The better protective effect observed in our study, therefore, is suggestive of a stronger antiviral response generated in the case of mothers infected and treated with a neutralizing MAb during the neonatal period. Finally, the authors did not study whether infected neonates, after blunting of viral replication by maternal antibodies, developed a protective immune response capable of both containing the virus after infection and blocking virus propagation in a later virus challenge, as reported here for FrCasE. Determining whether this would be the case would be interesting, as it would indicate that our observations could be extended to both other retroviruses and other means of induction of antiviral immune responses.
Generation and nature of the anti-FrCasE response in infected animals receiving maternal immunity.
In our previous work (13), we reported that the majority of mice infected neonatally by FrCasE and treated with the 667 IgG2a/κ MAb for a few days developed a sustained long-term protective humoral immunity dominated by IgG2as. Here, we have taken advantage of the few mice that developed a dominant anti-FrCasE IgG1 response as a first approach to test whether induction of the protective response could be induced by isotypes other than IgG2a. Our data support this possibility, as all pups that received maternal antiviral immunity, whether via the placenta or via breastfeeding, remained healthy and developed their own anti-FrCasE responses. Even though IgG2as were transferred to pups less efficiently than IgG1s, further work with anti-FrCasE MAbs of various isotypes is still necessary to demonstrate this point formally, as a significant fraction of antibodies transmitted by the mothers, in addition to being polyclonal, were IgG2as. We are currently developing such MAbs, which will be used in direct passive immunotherapy of infected neonates, as in our previous study (13), as well as in indirect therapy of pregnant mice, as described here.
It is noteworthy that all mice that received either natural anti-FrCasE antibodies or the 667 MAb from their mothers developed a dominant anti-FrCasE IgG2a response. This is interesting for at least two reasons. First, as already mentioned, IgG2a is the most potent isotype for complement cell lysis, for binding to Fc receptors found on a variety of cells of the immune system, and for antibody-dependent cell-mediated cytotoxicity. This makes it the antibody type with the highest activity against both viruses of various sorts and infected cells (see reference 13 for references). Second, the presence of high concentrations of IgG2as is suggestive of Th1-biased helper T-cell responses that are usually also contributed by cytotoxic-T-lymphocyte responses. It will, therefore, be interesting in future experiments to determine whether such cellular responses directed against FrCasE-infected cells have been generated and have participated in virus containment in protected animals. As mice born of immune mothers but nursed by nonimmune foster mothers showed a weaker antiviral effect than those nursed by their biological mothers, another point of interest will be to determine whether such a cytotoxic-T-lymphocyte response might be attenuated, or absent, in the former group of animals.
Therapeutic interest of treating mothers with antiviral MAbs during pregnancy and breastfeeding.
Our observation that administration of the 667 MAb to pregnant mice and during breastfeeding fully protects infected pups from developing any illness and permits the induction of a protective immune response raises two questions with regard to a possible human application: can such an approach be of interest to combat human neonatal infections by infected mothers, and how must the treatments be designed? As neutralizing MAbs against HIV are available, and as neutralization of simian immunodeficiency virus has been well deciphered in animal models, HIV should be considered the first target for such an approach. Indeed, passive-immunotherapy trials with a combination of carefully selected MAbs with HIV-neutralizing properties have already been conducted. Despite the fact that two of the MAbs used (2F5 and 4E10) were later shown to display some anti-self reactivity, as they also recognize the phospholipid cardiolipin (15), encouraging outcomes were seen in humans and impressive protection of primates was obtained, especially in the context of neonatal infection (12, 19, 26). Treatment of pregnant macaque females with combinations of anti-HIV MAbs was even reported in one study (1). The experimental setting was, however, considerably different from ours, and whether MAbs might have affected the endogenous antiviral immune responses of infected newborns was not considered. Whatever the case, the application of MAb-based immunotherapy/immunoprophylaxis to humans may be complicated by the fact that mother-to-child transmission can occur, not only at delivery, but also late in utero and by breastfeeding. In the case of intrapartum infection, it can be expected that immunotherapy of the mother during late gestation would be beneficial to both the mother herself and, via MAb transplacental transfer, to her child. However, as systemic transfer of breast milk antibodies does not occur in humans, breastfed babies of infected mothers could be protected more efficiently by direct administration of MAbs rather than by administration of these MAbs to the lactating mothers. Further analysis, first in monkeys, will be necessary to establish whether passive immunotherapy of lactating mothers sufficiently reduces the risk of mucosal transmission of viruses to breastfed infants. As for the neonates, who would accumulate the MAbs received from their treated mothers during pregnancy and those administered directly after birth, it would be interesting to determine whether this two-step treatment would help them mount a long-lasting protective antiviral response in the case of contamination, as observed for FrCasE (reference 13 and this work). This is particularly important, since a follow-up of neonatally HIV type 1-infected infants has recently shown that the immune system of young children is unexpectedly reactive for generating efficient and evolving antiviral responses (11). In addition, two other points must be taken into consideration. First, the antibody compositions of colostrums and milk differ between species (18). For example, IgGs constitute the prominent antibody class in rodent milk and colostrum, whereas they are not abundant in human milk, in which secretory IgA is the main isotype and serves as a first-line defense in mucosal areas (34). Second, the relative contributions of placental and breastfeeding transfer of maternal humoral immunity are not equivalent in humans and mice. Thus, in rodents, the transfer of IgGs via breastfeeding is quantitatively more important than via the placenta (as illustrated here for anti-FrCasE antibodies in Fig. 3), in contrast to primates, due to differences in placentation (30, 32). This indicates that, on the one hand, in humans, the highest transmission efficiency of the therapeutic IgG-type MAbs should be achieved during late pregnancy and not during breastfeeding and, on the other hand, the therapeutic strategy offering the best chances of both success and safety, with regard to contamination by milk, may be immunotherapy of mothers during late pregnancy, followed by direct administration of the MAbs to neonates.
Acknowledgments
M.P.'s Laboratory is an “Equipe labelisée” funded by the Ligue Nationale contre le Cancer. This work has also been supported by grants from the ARC.
We are grateful to our colleagues J. Hernandez, H.-A. Michaud, P. Van de Perre, and V. Kalatzis for helpful discussions and critical readings of the manuscript.
REFERENCES
- 1.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 2.Bulterys, M., and P. Lepage. 1998. Mother-to-child transmission of HIV. Curr. Opin. Pediatr. 10:143-150. [DOI] [PubMed] [Google Scholar]
- 3.Chappuis, G. 1998. Neonatal immunity and immunisation in early age: lessons from veterinary medicine. Vaccine 16:1468-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cleary, T. G. 2004. Human milk protective mechanisms. Adv. Exp. Med. Biol. 554:145-154. [DOI] [PubMed] [Google Scholar]
- 5.Czub, S., W. P. Lynch, M. Czub, and J. L. Portis. 1994. Kinetic analysis of spongiform neurodegenerative disease induced by a highly virulent murine retrovirus. Lab. Investig. 70:711-723. [PubMed] [Google Scholar]
- 6.Dagan, S., and R. Eren. 2003. Therapeutic antibodies against viral hepatitis. Curr. Opin. Mol. Ther. 5:148-155. [PubMed] [Google Scholar]
- 7.Dreja, H., L. Gros, S. Villard, E. Bachrach, A. Oates, C. Granier, T. Chardes, J. C. Mani, M. Piechaczyk, and M. Pelegrin. 2003. Monoclonal antibody 667 recognizes the variable region A motif of the ecotropic retrovirus CasBrE envelope glycoprotein and inhibits Env binding to the viral receptor. J. Virol. 77:10984-10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Englund, J., W. P. Glezen, and P. A. Piedra. 1998. Maternal immunization against viral disease. Vaccine 16:1456-1463. [DOI] [PubMed] [Google Scholar]
- 9.Evans, L. H., R. P. Morrison, F. G. Malik, J. Portis, and W. J. Britt. 1990. A neutralizable epitope common to the envelope glycoproteins of ecotropic, polytropic, xenotropic, and amphotropic murine leukemia viruses. J. Virol. 64:6176-6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feeney, M. E. 2004. HIV and children: the developing immune system fights back. West Indian Med. J. 53:359-362. [PubMed] [Google Scholar]
- 11.Feeney, M. E., Y. Tang, K. Pfafferott, K. A. Roosevelt, R. Draenert, A. Trocha, X. G. Yu, C. Verrill, T. Allen, C. Moore, S. Mallal, S. Burchett, K. McIntosh, S. I. Pelton, M. A. St John, R. Hazra, P. Klenerman, M. Altfeld, B. D. Walker, and P. J. Goulder. 2005. HIV-1 viral escape in infancy followed by emergence of a variant-specific CTL response. J. Immunol. 174:7524-7530. [DOI] [PubMed] [Google Scholar]
- 12.Ferrantelli, F., R. A. Rasmussen, K. A. Buckley, P. L. Li, T. Wang, D. C. Montefiori, H. Katinger, G. Stiegler, D. C. Anderson, H. M. McClure, and R. M. Ruprecht. 2004. Complete protection of neonatal rhesus macaques against oral exposure to pathogenic simian-human immunodeficiency virus by human anti-HIV monoclonal antibodies. J. Infect. Dis. 189:2167-2173. [DOI] [PubMed] [Google Scholar]
- 13.Gros, L., H. Dreja, A. L. Fiser, M. Plays, M. Pelegrin, and M. Piechaczyk. 2005. Induction of long-term protective antiviral endogenous immune response by short neutralizing monoclonal antibody treatment. J. Virol. 79:6272-6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haigwood, N. L., D. C. Montefiori, W. F. Sutton, J. McClure, A. J. Watson, G. Voss, V. M. Hirsch, B. A. Richardson, N. L. Letvin, S. L. Hu, and P. R. Johnson. 2004. Passive immunotherapy in simian immunodeficiency virus-infected macaques accelerates the development of neutralizing antibodies. J. Virol. 78:5983-5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haynes, B. F., J. Fleming, E. W. St Clair, H. Katinger, G. Stiegler, R. Kunert, J. Robinson, R. M. Scearce, K. Plonk, H. F. Staats, T. L. Ortel, H. X. Liao, and S. M. Alam. 2005. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 308:1906-1908. [DOI] [PubMed] [Google Scholar]
- 16.John, G. C., and J. Kreiss. 1996. Mother-to-child transmission of human immunodeficiency virus type 1. Epidemiol. Rev. 18:149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch, W. P., S. Czub, F. J. McAtee, S. F. Hayes, and J. L. Portis. 1991. Murine retrovirus-induced spongiform encephalopathy: productive infection of microglia and cerebellar neurons in accelerated CNS disease. Neuron 7:365-379. [DOI] [PubMed] [Google Scholar]
- 18.Manz, R. A., A. E. Hauser, F. Hiepe, and A. Radbruch. 2005. Maintenance of serum antibody levels. Annu. Rev. Immunol. 23:367-386. [DOI] [PubMed] [Google Scholar]
- 19.Mc Cann, C. M., R. J. Song, and R. M. Ruprecht. 2005. Antibodies: can they protect against HIV infection? Curr. Drug Targets Infect. Disord. 5:95-111. [DOI] [PubMed] [Google Scholar]
- 20.McAtee, F. J., and J. L. Portis. 1985. Monoclonal antibodies specific for wild mouse neurotropic retrovirus: detection of comparable levels of virus replication in mouse strains susceptible and resistant to paralytic disease. J. Virol. 56:1018-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakowitsch, S., H. Quendler, H. Fekete, R. Kunert, H. Katinger, and G. Stiegler. 2005. HIV-1 mutants escaping neutralization by the human antibodies 2F5, 2G12, and 4E10: in vitro experiments versus clinical studies. AIDS 19:1957-1966. [DOI] [PubMed] [Google Scholar]
- 22.Pelegrin, M., M. Marin, A. Oates, D. Noel, R. Saller, B. Salmons, and M. Piechaczyk. 2000. Immunotherapy of a viral disease by in vivo production of therapeutic monoclonal antibodies. Hum. Gene Ther. 11:1407-1415. [DOI] [PubMed] [Google Scholar]
- 23.Portis, J. L., S. Czub, C. F. Garon, and F. J. McAtee. 1990. Neurodegenerative disease induced by the wild mouse ecotropic retrovirus is markedly accelerated by long terminal repeat and Gag-Pol sequences from nondefective Friend murine leukemia virus. J. Virol. 64:1648-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reichert, J. M., C. J. Rosensweig, L. B. Faden, and M. C. Dewitz. 2005. Monoclonal antibody successes in the clinic. Nat. Biotechnol. 23:1073-1078. [DOI] [PubMed] [Google Scholar]
- 25.Rychert, J., and A. M. Amedee. 2005. The antibody response to SIV in lactating rhesus macaques. J. Acquir. Immune Defic. Syndr. 38:135-141. [DOI] [PubMed] [Google Scholar]
- 26.Safrit, J. T., R. Ruprecht, F. Ferrantelli, W. Xu, M. Kitabwalla, K. Van Rompay, M. Marthas, N. Haigwood, J. R. Mascola, K. Luzuriaga, S. A. Jones, B. J. Mathieson, and M. L. Newell. 2004. Immunoprophylaxis to prevent mother-to-child transmission of HIV-1. J. Acquir. Immune Defic. Syndr. 35:169-177. [DOI] [PubMed] [Google Scholar]
- 27.Saha, K., D. Hollowell, and P. K. Wong. 1994. Mother-to-baby transfer of humoral immunity against retrovirus-induced neurologic disorders and immunodeficiency. Virology 198:129-137. [DOI] [PubMed] [Google Scholar]
- 28.Scarlatti, G., J. Albert, P. Rossi, V. Hodara, P. Biraghi, L. Muggiasca, and E. M. Fenyo. 1993. Mother-to-child transmission of human immunodeficiency virus type 1: correlation with neutralizing antibodies against primary isolates. J. Infect. Dis. 168:207-210. [DOI] [PubMed] [Google Scholar]
- 29.Scarlatti, G., T. Leitner, V. Hodara, E. Halapi, P. Rossi, J. Albert, and E. M. Fenyo. 1993. Neutralizing antibodies and viral characteristics in mother-to-child transmission of HIV-1. Aids 7(Suppl. 2):S45-S48. [DOI] [PubMed] [Google Scholar]
- 30.Shah, U., B. L. Dickinson, R. S. Blumberg, N. E. Simister, W. I. Lencer, and W. A. Walker. 2003. Distribution of the IgG Fc receptor, FcRn, in the human fetal intestine. Pediatr. Res. 53:295-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siegrist, C. A. 2001. Neonatal and early life vaccinology. Vaccine 19:3331-3346. [DOI] [PubMed] [Google Scholar]
- 32.Simister, N. E. 2003. Placental transport of immunoglobulin G. Vaccine 21:3365-3369. [DOI] [PubMed] [Google Scholar]
- 33.Sitbon, M., J. Nishio, K. Wehrly, D. Lodmell, and B. Chesebro. 1985. Use of a focal immunofluorescence assay on live cells for quantitation of retroviruses: distinction of host range classes in virus mixtures and biological cloning of dual-tropic murine leukemia viruses. Virology 141:110-118. [DOI] [PubMed] [Google Scholar]
- 34.Van de Perre, P. 2003. Transfer of antibody via mother's milk. Vaccine 21:3374-3376. [DOI] [PubMed] [Google Scholar]
- 35.Yorty, J. L., and R. H. Bonneau. 2004. Prenatal transfer of low amounts of HSV-specific antibody protects newborn mice against HSV infection during acute maternal stress. Brain Behav. Immun. 18:15-23. [DOI] [PubMed] [Google Scholar]