Abstract
Langerhans cells (LCs) are antigen-presenting cells in the skin that play sentinel roles in host immune defense by secreting proinflammatory molecules and activating T cells. Here we studied the interaction of vaccinia virus with XS52 cells, a murine epidermis-derived dendritic cell line that serves as a surrogate model for LCs. We found that vaccinia virus productively infects XS52 cells, yet this infection displays an atypical response to anti-poxvirus agents. Whereas adenosine N1-oxide blocked virus production and viral protein synthesis during a synchronous infection, cytosine arabinoside had no effect at concentrations sufficient to prevent virus replication in BSC40 monkey kidney cells. Vaccinia virus infection of XS52 cells not only failed to elicit the production of various cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-6, IL-10, IL-12 p40, alpha interferon (IFN-α), and IFN-γ, it actively inhibited the production of proinflammatory cytokines TNF-α and IL-6 by XS52 cells in response to exogenous lipopolysaccharide (LPS) or poly(I:C). Infection with a vaccinia virus mutant lacking the E3L gene resulted in TNF-α secretion in the absence of applied stimuli. Infection of XS52 cells or BSC40 cells with the ΔE3L virus, but not wild-type vaccinia virus, triggered proteolytic decay of IκBα. These results suggest a novel role for the E3L protein as an antagonist of the NF-κB signaling pathway. ΔE3L-infected XS52 cells secreted higher levels of TNF-α and IL-6 in response to LPS and poly(I:C) than did cells infected with the wild-type virus. XS52 cells were productively infected by a vaccinia virus mutant lacking the K1L gene. ΔK1L-infected cells secreted higher levels of TNF-α and IL-6 in response to LPS than wild-type virus-infected cells. Vaccinia virus infection of primary LCs harvested from mouse epidermis was nonpermissive, although a viral reporter protein was expressed in the infected LCs. Vaccinia virus infection of primary LCs strongly inhibited their capacity for antigen-specific activation of T cells. Our results highlight suppression of the skin immune response as a feature of orthopoxvirus infection.
Poxviruses are dermatotropic DNA viruses that cause human diseases ranging in severity from a mild local skin eruption (molluscum contagiosum) to a catastrophic systemic illness signaled by a generalized pustular rash (smallpox). Poxviruses employ multiple strategies to evade the immune system, including (i) secretion of virus-encoded soluble cytokine receptors or cytokine analogs that act as molecular decoys to block the activity of host cytokines and (ii) elaboration of viral antagonists of the major intracellular signaling pathways that either trigger apoptosis, establish an antiviral state, or activate proinflammatory responses (reviewed in reference 44). Vaccinia virus, the laboratory prototype for the poxvirus family, has a 200-year history of intentional human infection via the skin for the purpose of smallpox prophylaxis. The stigma of successful vaccination is a localized pustular rash that, in uncomplicated cases, heals without either spreading to adjacent areas of skin or triggering serious systemic inflammation. Major complications of vaccination such as eczema vaccinatum are more common in individuals with abnormal skin immunity (50). Life-threatening progressive vaccinia virus occurs in patients with T-cell immunodeficiency (18). Molluscum contagiosum, a normally benign and self-limited skin infection of healthy children, is either more severe or more difficult to eradicate in children with atopic dermatitis and in immunocompromised patients (13, 17). These clinical correlations suggest that delineating the biology of natural poxvirus infections and the mechanisms underlying immunization via scarification (and ways to avoid or treat the complications of vaccination) would benefit from a better understanding of the interactions of poxviruses with the skin immune system.
Langerhans cells (LCs) are epidermal antigen-presenting dendritic cells (DCs) that play important roles in skin immune responses (3, 40). Derived from CD34+ progenitor cells in the bone marrow (28), they establish their residence in the basal and suprabasal layers of the epidermis. Whereas LCs in noninflamed skin are maintained by skin-resident hematopoietic precursors that self-renew in situ, skin injury results in loss of resident LCs and their replacement by circulating monocyte precursors (19, 36). LCs in the epidermis are immature, though capable of ingesting foreign antigens. Upon activation, under the influence of various cytokines and chemokines, they mature and migrate to regional lymph nodes, where they can present antigen and activate cognate naive T cells (4, 25, 34, 36).
Here we investigated whether and how LCs respond to vaccinia virus infection. In principle, LCs could serve as concerned bystanders in a cutaneous virus infection by responding to signals from infected epithelial cells and/or taking up viral antigens produced in neighboring cells. LCs can also be direct targets of virus infection. In the latter scenario, a virus infection of LCs could be either abortive or productive and either trigger maturation and migration of epidermal DCs and subsequent presentation of endogenous viral antigens to T cells in secondary lymphoid organs or else impede the skin immune response normally orchestrated by LCs. Depending on which case applies, LCs could either promote clearance of the virus infection and establishment of durable immunity or serve as a vehicle for spread of the infection within the host and a reservoir for persistence. In an example of the latter scenario, LCs are proposed to be the initial cellular targets during sexual transmission of human immunodeficiency virus and to play a key role in human immunodeficiency virus dissemination to T cells (reviewed in reference 29). However, in the case of herpes simplex virus infection of the skin or vaginal epithelium, LCs are not implicated in the ensuing T-cell response (2, 53).
It is not entirely clear how vaccinia virus is handled by antigen-presenting cells after human scarification. Norbury et al. (38) inoculated mouse footpads with a green fluorescent protein (GFP)-expressing vaccinia virus and documented the presence of virus-infected macrophages and DCs in the popliteal lymph nodes by 6 h postinfection. Their studies specifically implicated the virus-infected DCs in presenting viral antigen to naive CD8+ T cells. It was proposed that the DCs observed in the lymph node were infected at the site of inoculation and subsequently migrated via the lymphatics (38), but it is not known what proportion of these DCs are of epidermal origin.
Here we found that vaccinia virus replicates productively in a murine epidermis-derived DC line, XS52, a surrogate for LCs that responds to activating signals (52). An unexpected finding was that virus replication in XS52 cells displays idiosyncratic responses to known anti-poxvirus drugs, whereby virus production is blocked by adenosine N1-oxide (ANO) but not by cytosine arabinoside (araC). Vaccinia virus infection of XS52 cells does not trigger production of secreted cytokines but rather attenuates the inflammatory responses induced by the exogenous stimuli lipopolysaccharide (LPS) and poly(I:C). Partial remission of the virus inhibition of cytokine response by deletion of the vaccinia virus E3L or K1L gene suggests that vaccinia virus actively antagonizes innate immune signaling in XS52 cells. To translate these findings to a more physiological setting, we isolated primary LCs from mouse epidermis and found that vaccinia virus infection of primary LCs, though nonproductive, blocks their ability to present antigen to T cells.
MATERIALS AND METHODS
Cells.
The XS52 cell line (a kind gift of Arika Takashima, University of Texas Health Science Center, Dallas) was derived from BALB/c (H-2d) neonatal epidermis (52). XS cells were propagated in XS medium, composed of complete medium (CM) supplemented with 1 ng/ml recombinant granulocyte-macrophage colony-stimulating factor (Biosource) and culture supernatants from the skin-derived stromal NS cell line. CM is RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, 0.1 mM essential and nonessential amino acids, 2 mM l-glutamine, 1 mM sodium pyruvate, and 10 mM HEPES buffer. BSC40 cells (African green monkey kidney cells) were maintained in Dulbecco's modified Eagle's medium supplemented with 5% FBS. BHK-21 (baby hamster kidney) and RK13 (rabbit kidney) cells were cultured in modified Eagle's medium containing 10% FBS, 0.1 mM nonessential amino acids, and 50 μg/ml gentamicin. The HDK-1 cell line is a keyhole limpet hemocyanin (KLH)-specific CD4+ Th1 clone (47). These cells were cultured every 4 weeks with KLH-pulsed spleen cells and expanded by culturing in CM in the presence of murine interleukin-2 (IL-2; 200 U/ml; NIH). All cells were grown at 37°C in a 5% CO2 incubator.
Viruses.
The WR strain of vaccinia virus was propagated, and its titer was determined, in BSC40 monolayers at 37°C. The ΔE3L virus, which is the WR strain with the E3L gene deleted, was kindly provided by B. L. Jacobs (Arizona State University). It was propagated in BHK-21 cells, and its titer was determined on RK13 cells. The ΔK1L virus, which is the WR strain with the K1L gene deleted, was kindly provided by Joanna Shisler (University of Illinois). ΔK1L was grown, and its titer was determined, on BSC40 cells; ΔK1L was unable to replicate in RK13 cells, as reported previously (41). A recombinant vaccinia virus (NP-S-EGFP [where EGFP stands for enhanced GFP]) expressing a nucleus-localized EGFP reporter under the control of a vaccinia virus p7.5 promoter (38) was a gift of Jonathan Yewdell (NIH).
Mice.
Female BALB/c mice between 8 and 12 weeks of age were purchased from The Jackson Laboratory and used for preparation of epidermal cells and purification of primary epidermal LCs. These mice were maintained in the Weill Medical College of Cornell University animal facility. All procedures were performed with the consent of the Institutional Animal Use and Care Committee.
Materials.
Adenosine N1-oxide (ANO) was synthesized in the Organic Synthesis Core laboratory at Sloan-Kettering Institute. A 5-mg/ml stock solution of ANO dissolved in phosphate-buffered saline (PBS) was stored at −20°C. l-[35S]methionine (catalog number NEG/009L, 1,175 Ci/mmol) was purchased from Perkin-Elmer. AraC was purchased from Sigma. The proteasome inhibitor N-benzyloxycarbonyl-Ile-Glu(O-tert-butyl)-Ala-leucinal (PSI) was from Calbiochem.
One-step growth in cell culture.
XS52 cells were cultured overnight in CM prior to infection with vaccinia virus WR at a multiplicity of 3. The inoculum was removed after 30 min; the cells were washed twice with PBS and then overlaid with CM. The cells were harvested at 0, 4, 12, 24, and 48 h after 30 min of initial infection by scraping the cells into 3 ml of medium. After three cycles of freezing and thawing, the samples were sonicated and virus titers were determined by serial dilution and infection of BSC40 cell monolayers. Plaques were visualized by staining with 0.1% crystal violet in 20% ethanol.
Protein synthesis.
XS52 cells preincubated with 10 μg/ml ANO for 12 h were infected with vaccinia virus WR at a multiplicity of 10 for 30 min in the presence of ANO. Control infections were performed without ANO. The inoculum was removed, and the cells were washed twice with PBS and then overlaid with CM with or without ANO. At various times postinfection, the medium was removed and the cells were washed with methionine-free CM and then incubated for 30 min with fresh medium containing [35S]methionine at 30 μCi/ml (1,175 Ci/mmol) with or without ANO. The medium was removed thereafter, and the cells were lysed in situ by addition of 0.2 ml of 0.065 M Tris-HCl (pH 6.8)-2% sodium dodecyl sulfate (SDS)-5% β-mercaptoethanol-10% glycerol. Samples were heated at 100°C for 5 min and then analyzed by electrophoresis through a 12% polyacrylamide gel containing 0.1% SDS. Radiolabeled polypeptides were visualized by autoradiography of the dried gel.
Cytokine assays.
XS52 cells were infected with wild-type (WT), ΔE3L, or ΔK1L vaccinia virus at a multiplicity of 10 for 1 h or mock infected. The inoculum was removed, and the cells were washed with PBS twice and incubated with fresh CM. At various times postinoculation, supernatants were collected and stored at −80°C. Cytokine levels were measured by using enzyme-linked immunosorbent assay (ELISA) kits for tumor necrosis factor alpha (TNF-α), IL-6, IL-1β, alpha interferon (IFN-α), IFN-γ, IL-10, and IL-12 p40 from R&D Systems and for IFN-α from PBL Biomedical Laboratories according to the manufacturers' instructions.
Western blot analysis.
BSC40 cell monolayers were infected with WT or ΔE3L vaccinia virus at a multiplicity of 10 or mock infected. At various times postinoculation, the medium was removed and the cells were lysed on the plates by adding 100 μl of a solution containing 62.5 mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 50 mM dithiothreitol, and 0.01% bromophenol blue. The lysates were collected and sonicated for 10 to 15 s to reduce viscosity. Aliquots (20 μl) of the lysates were subjected to SDS-polyacrylamide gel electrophoresis (PAGE), and the polypeptides were transferred electrophoretically to a nitrocellulose membrane. The membranes were preincubated for 1 h with buffer containing 20 mM Tris-HCl (pH 7.6), 137 mM NaCl, 0.1% Tween 20, and 5% (wt/vol) nonfat dry milk and then probed with a 1:1,000 dilution of a rabbit polyclonal anti-IκBα antibody (Cell Signaling), followed by incubation with a 1:2,000 dilution of goat anti-rabbit immunoglobulin conjugated to horseradish peroxidase and horseradish peroxidase-conjugated anti-biotin antibody for the detection of biotinylated protein size markers (Cell Signaling). Anti-β-actin antibody was used as a loading control. The immunoreactive polypeptides were visualized with a chemiluminescence detection kit (Amersham).
Epidermal cell preparation and LC purification.
Epidermal cells were prepared by using a modification of a standard protocol (20, 43), and LCs were affinity purified as described by Seiffert et al. (45). Briefly, truncal skin samples of shaved and chemically depilated (Neet; Whitehall Laboratories) female BALB/c mice were removed and depleted of subcutaneous fat. The skin samples were floated, dermis side down, on a solution containing 0.5 U/ml dispase (Boehringer Mannheim) and 0.38% trypsin (Sigma) in PBS for 40 min at 37°C. Epidermal sheets were collected and dissociated in Hanks balanced salt solution (HBSS) with 2% heat-inactivated FBS for 20 min at room temperature under gentle agitation. The cells were then filtered through a 40-μm cell strainer (BD Biosciences) and washed in HBSS-2% FBS. The epidermal cells were incubated with a 1:50 dilution of anti-I-Ad antibody (BD PharMingen) on a rotator at 4°C for 30 min, followed by washing and subsequent incubation with Dynabeads M-450 coated with goat anti-mouse immunoglobulin G (Dynal AS) on a rotator at 4°C for 10 min. The I-A+ LCs were then purified by eight cycles of washing with HBSS-2% FBS and recovering cells with a Dynal MPC-2 magnetic particle concentrator (Dynal AS). This procedure yields a population of LCs with >95% purity as assessed by fluorescence-activated cell sorter analysis with double staining with FITC-conjugated anti-I-Ad antibody and propidium iodide (Sigma) to exclude dead cells.
Assay of antigen presentation by LCs to HDK-1 cells.
Purified LCs attached to antibody-coated magnetic beads were infected for 30 min at 37°C with vaccinia virus WR or ΔE3L at multiplicities of 0.1, 1, and 10. Virus was removed by washing the cells three times with CM using a Dynal MPC-2 magnetic particle concentrator. The infected cells and mock-infected control LCs were cultured in CM for 2 h and then incubated overnight with 100 μg/ml KLH (Sigma). The cells were washed three times to remove the KLH and then distributed among the wells of a 96-well round-bottom plate at 1 × 104 cells per well in CM containing 50 μM β-mercaptoethanol. HDK-1 cells were added at 5 × 104 cells per well. After coincubation of LCs and HDK-1 cells for 72 h, supernatants were collected and the IFN-γ concentration was determined by ELISA (R&D Systems).
RESULTS
Vaccinia virus replicates in the DC line XS52.
The XS52 cell line derived by Takashima and colleagues from BALB/c (H-2d) neonatal epidermis has been used as a surrogate for LCs. XS52 cells express markers characteristic of antigen-presenting cells and produce a variety of cytokines upon stimulation (52). XS52 cells were infected with vaccinia virus WR at a multiplicity of 3. The inoculum was removed after 30 min, and the cells were washed to remove unadsorbed virus. At various times postinfection, cells were harvested and virus titers were determined by plaque formation on BSC40 cells. The virus titer increased 200-fold over 48 h, indicating that vaccinia virus productively infected XS52 cells (Fig. 1A). To our knowledge, this is the first demonstration that vaccinia virus can replicate to a high titer in a DC. Previous studies reported abortive infections and induction of apoptosis in human monocyte-derived DCs exposed to WT vaccinia virus (14, 16). Canarypox virus was found to infect primate macrophage-derived immature DCs abortively and trigger apoptotic death of the infected cells (24).
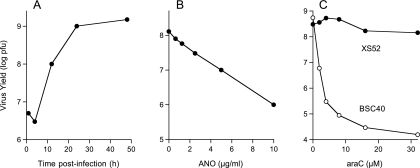
FIG. 1.
Productive vaccinia virus infection of XS52 cells and sensitivity to antiviral agents. (A) One-step growth. XS52 cells (6 × 106) were infected with vaccinia virus WR at a multiplicity of 3. The inoculum was removed after 30 min. Cells were harvested at the indicated times postinoculation. Virus yield (log PFU) was determined by titration on BSC40 cell monolayers. (B) Inhibition of virus replication by ANO. XS52 cells (1 × 106) were infected at a multiplicity of 3. The inoculum was removed after 30 min and replaced with medium containing ANO at the indicated concentrations. Cells were harvested at 30 h postinfection. Virus yield (log PFU) is plotted as a function of the ANO concentration. (C) AraC blocks vaccinia virus replication in BSC40 cells but not in XS52 cells. XS52 cells (3 × 106) and BSC40 cells (1 × 106) were infected at a multiplicity of 3. The inocula were removed after 30 min, and the cells were overlaid with medium containing araC or control medium with no araC. Cells were harvested at 30 h postinfection. Virus yield (log PFU) is plotted as a function of the araC concentration.
ANO inhibits vaccinia virus replication in XS52 cells.
ANO inhibits vaccinia virus replication in BSC40 cells by blocking the translation of viral early mRNAs without affecting host protein synthesis (27). ANO is proposed to exemplify a novel antiviral mechanism whereby selective incorporation of ANO into viral mRNAs by the vaccinia virus RNA polymerase poisons codon-anticodon base pairing. The selectivity of ANO as an anti-poxvirus agent suggested its use as a topical agent for treatment of the complications of smallpox vaccination and/or molluscum contagiosum infections. Here we tested whether ANO inhibits vaccinia virus replication in epidermal LCs.
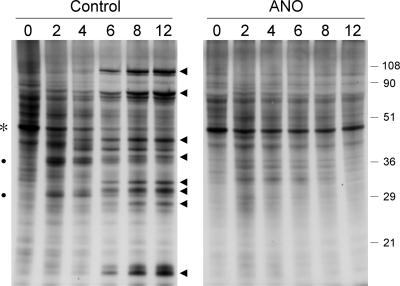
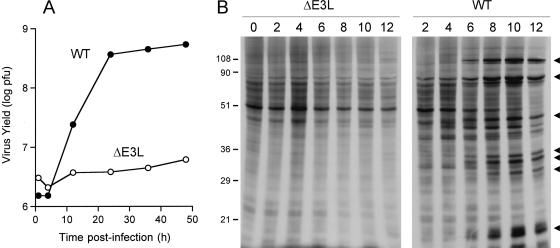
XS52 cells (1 × 106) were infected with vaccinia virus WR at a multiplicity of 3. The inoculum was removed after 30 min and replaced with fresh medium containing increasing concentrations of ANO. Cells were collected at 30 h postinoculation, and virus yield was determined by measuring plaque formation on BSC40 cells. We observed an ANO concentration-dependent decrement in virus yield, from 1.2 × 108 PFU in the drug-free control to 8 × 105 PFU in the presence of 10 μg/ml ANO (Fig. 1B). Protein synthesis during virus infection in the presence or absence of 10 μg/ml ANO was analyzed by pulse-labeling synchronously infected XS52 cells with [35S]methionine at 2, 4, 6, 8, and 12 h postinoculation and resolving the labeled polypeptides by SDS-PAGE (Fig. 2). Mock-infected XS52 cells were pulse-labeled and analyzed in parallel (time zero in Fig. 2). A typical temporal pattern of vaccinia virus gene expression was seen in the control infections, i.e., the appearance of novel early polypeptides at 2 to 4 h postinfection (indicated by the filled circles in Fig. 2) visible against a background of host protein synthesis, transition to the synthesis of distinctive late proteins by 6 to 12 h (indicated by the arrowheads in Fig. 2), and shutoff of host protein synthesis, also by 6 to 12 h postinfection, seen as loss of the prominent host polypeptide (denoted by the asterisk in Fig. 2). Preincubation of XS52 cells in ANO prior to infection had no effect on host protein synthesis (Fig. 2, compare lanes 0). No late viral protein synthesis was detected in the presence of ANO, and there was hardly any detectable early protein synthesis. Indeed, host protein synthesis continued unabated for at least 12 h postinfection (Fig. 2). Thus, ANO is as effective in inhibiting vaccinia virus replication and protein synthesis in this murine LC-like line as it is in BSC40 cells.
FIG. 2.
ANO inhibits viral protein synthesis in XS52 cells. XS52 cells (6 × 106) were pretreated with ANO (10 μg/ml) for 12 h and infected with vaccinia virus WR at a multiplicity of 10 in the presence of ANO or in the absence of the drug (Control). Cells were pulse-labeled for 30 min with [35S]methionine at 2, 4, 6, 8, and 12 h after removal of the inoculum. After the pulse, the medium was removed and the cells were lysed in situ with SDS. The labeled polypeptides were analyzed by SDS-PAGE. An autoradiograph of the gel is shown. Early viral polypeptides (indicated by filled circles) became visible at 2 to 4 h in the control infection against a background of host protein synthesis. The transition to the synthesis of late viral proteins (indicated by arrowheads) was evident at 6 to 12 h postinfection. Shutoff of host protein synthesis, also by 6 to 12 h postinfection, was evinced by decreased labeling of the prominent host polypeptide (denoted by the asterisk). The positions and sizes (in kilodaltons) of marker polypeptides are indicated on the right.
AraC fails to block vaccinia virus replication in XS52 cells.
AraC is a well-studied anti-poxvirus agent that blocks viral DNA replication catalyzed by vaccinia virus DNA polymerase (49). AraC inhibits vaccinia virus replication in a dose-dependent manner in BSC40 cells (Fig. 1C), suppressing virus yield by 10,000-fold at a concentration of 16 μM. However, we were surprised to find that araC had no effect on vaccinia virus replication in XS52 cells at concentrations of up to 32 μM (Fig. 1C). It is likely that XS52 cells either fail to accumulate a sufficient pool of intracellular araC or are defective in converting it into the triphosphate derivative, which is presumed to be the active compound that inhibits DNA replication. The atypical drug sensitivity of vaccinia virus replication in XS52 cells serves as a caveat to any presumptions that anti-poxvirus agents are equally effective in all target cells or tissues.
Vaccinia virus infection attenuates proinflammatory cytokine production in XS cells stimulated by LPS and poly(I:C).
LCs utilize Toll-like receptors (TLRs) that mediate responses to pathogen-associated molecular patterns conserved among microorganisms (1, 37). This recognition is instrumental in initiating innate immunity and regulating adaptive immunity. LPS from gram-negative bacteria acts on TLR4 to stimulate inflammatory responses. Poly(I:C), which mimics double-stranded RNA (dsRNA), acts on TLR3 to induce antiviral responses.
Given our finding that vaccinia virus replicates in XS52 cells, it was of immediate interest to evaluate whether the poxvirus infection induces an innate immune response. To do this, supernatants from synchronously infected XS52 cells were collected at various times postinoculation. We detected no secretion of IFN-α, IFN-γ, TNF-α, IL-1β, IL-6, IL-12 p40, or IL-10 by vaccinia virus-infected XS52 cells (Fig. 3 and 4 and data not shown), signifying either that the cultured LCs do not respond to vaccinia virus infection or that vaccinia virus actively suppresses the innate immune response.
FIG. 3.
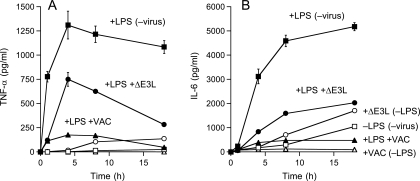
Effects of vaccinia virus infection on constitutive and LPS-induced cytokine production by XS52 cells. XS52 cells (6 × 106) were infected with WT WR or ΔE3L vaccinia virus at a multiplicity of 10 or mock infected. At 2 h after removal of the inoculum, LPS was added to the medium (where indicated) to a concentration of 1 μg/ml. The concentrations of secreted TNF-α (A) and IL-6 (B) in the medium at the indicated times thereafter were determined by ELISA. Each datum represents the average of three experiments with standard deviations shown.
FIG. 4.
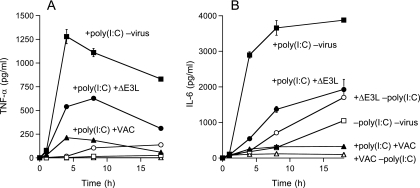
Effects of vaccinia virus infection on constitutive and poly(I:C)-induced cytokine production. XS52 cells (6 × 106) were infected with WT WR or ΔE3L vaccinia virus at a multiplicity of 10 or mock infected. At 2 h after removal of the inoculum, poly(I:C) was added to the medium (where indicated) to a concentration of 10 μg/ml. The concentrations of secreted TNF-α (A) and IL-6 (B) in the medium at the indicated times thereafter were determined by ELISA. Each datum represents the average of three experiments with standard deviations shown.
To investigate the latter scenario, we treated uninfected and vaccinia virus-infected XS52 cells with LPS or poly(I:C). Infected cells were washed after inoculation and overlaid with fresh medium for 2 h before they, and mock-treated control cells, were stimulated with 1 μg/ml LPS or 10 μg/ml poly(I:C). Supernatants were collected at various times thereafter for determination of TNF-α and IL-6 concentrations by ELISA. TNF-α and IL-6 are proinflammatory cytokines produced in the skin by both keratinocytes and LCs. They are important for the recruitment of lymphocytes and neutrophils. TNF-α has also been shown to be important for LC maturation and migration (5). Control XS52 cells responded to LPS by secreting TNF-α and IL-6; the TNF-α concentration peaked at 4 to 8 h poststimulation (Fig. 3A), while the IL-6 concentration peaked at 8 to 18 h (Fig. 3B). Vaccinia virus infection reduced TNF-α and IL-6 production by 80% and 90%, respectively (Fig. 3). Similar attenuations of TNF-α and IL-6 production by vaccinia virus infection were seen in XS52 cells stimulated with poly(I:C) (Fig. 4). These results indicate that virus inhibition of the proinflammatory response is not limited to a single TLR agonist.
Deletion of E3L partially restores proinflammatory cytokine production in infected XS52 cells.
E3L is one of the key IFN resistance determinants encoded by vaccinia virus (51). The E3L protein binds dsRNA avidly and blocks the activation of several cellular IFN-inducible antiviral enzymes, i.e., dsRNA-activated protein kinase PKR, 2′-5′ oligoadenylate synthetase, and RNase L. The ΔE3L virus, in which the gene for E3L is deleted, is avirulent in an animal model of lethal infection and displays a restricted host range for replication in cell culture. For example, ΔE3L replicates in BHK-21 and RK13 cells but not in HeLa or BSC40 cells (11). Here we found that ΔE3L was unable to replicate productively in XS52 cells infected at a multiplicity of 3 (Fig. 5A). A control infection verified that the cells used in this experiment were permissive for replication of WT vaccinia virus. Analysis of protein synthesis by [35S]methionine labeling of XS52 cells infected with ΔE3L at a multiplicity of 10 showed no evidence of progression to late viral protein synthesis over a 12-h period but also no evidence of global shutoff of host cell protein synthesis (Fig. 5B).
FIG. 5.
Nonproductive infection of XS52 cells by ΔE3L. (A) One-step growth. XS52 cells were infected with WT WR or ΔE3L vaccinia virus at a multiplicity of 3. The inoculum was removed after 30 min. Cells were harvested at the indicated times postinfection. Virus yield (log PFU) is plotted as a function of time postinfection. (B) Protein synthesis in virus-infected cells. XS52 cells (6 × 106) were infected with WT WR or ΔE3L vaccinia virus at a multiplicity of 10. At the indicated times postinfection, cells were pulse-labeled for 30 min with [35S]methionine as described in the legend to Fig. 2. Labeled polypeptides were analyzed by SDS-PAGE. An autoradiograph of the gel is shown. Late viral proteins are indicated by arrowheads. The positions and sizes (in kilodaltons) of marker polypeptides are indicated on the left.
The cytokine secretion profiles of ΔE3L-infected XS52 cells, with or without exposure to LPS and poly(I:C), are shown in Fig. 3 and 4. Whereas uninfected XS52 cells and cells infected with WT vaccinia virus failed to secrete TNF-α in the absence of LPS (Fig. 3A) or poly(I:C) (Fig. 4A), the ΔE3L-infected cells did secrete TNF-α, which accumulated to a concentration of 100 pg/ml after 18 h (Fig. 3A). Unstimulated, uninfected XS52 cells constitutively secreted IL-6, which accumulated to a concentration of ∼900 pg/ml after 18 h (Fig. 3B). Whereas the constitutive production of IL-6 was abolished by infection with WT vaccinia virus, cells infected with the ΔE3L virus secreted nearly twice as much IL-6 as the uninfected control cells (Fig. 3B and 4B). These results suggest that the ΔE3L infection is sensed by XS52 cells as an activating signal, the response to which is masked during an infection by WT vaccinia virus.
Other instructive findings were that LPS and poly(I:C) treatment of ΔE3L-infected XS52 cells resulted in even higher levels of secreted TNF-α than those seen in either the unstimulated, ΔE3L-infected cells or LPS-stimulated, WR-infected cells (Fig. 3A and 4A). Indeed, LPS- or poly(I:C)-triggered TNF-α production by ΔE3L-infected cells was ∼50% of the level observed for uninfected XS52 cells. An analogous enhancement of stimulated IL-6 secretion was observed in ΔE3L-infected cells versus cells infected with WT vaccinia virus (Fig. 3B and 4B). We interpret these results conservatively as signifying that full viral gene expression is required for maximum attenuation of the proinflammatory response in XS52 cells. Alternatively, the E3L protein itself might directly antagonize innate immune cell activation.
E3L is an antagonist of NF-κB activation.
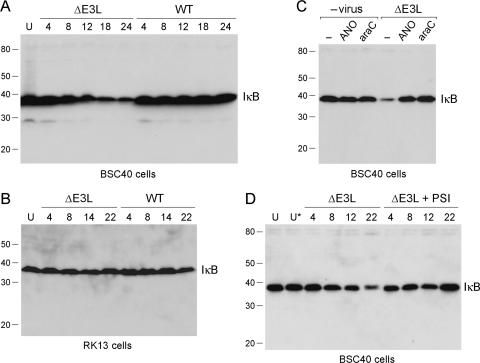
The transcription factor NF-κB is a master regulator of the proinflammatory response (1). NF-κB is kept in the off state by virtue of its association with IκBα. Activation of NF-κB by upstream signals entails phosphorylation of IκBα, which triggers IκBα ubiquitylation and then IκBα degradation. Depletion of IκBα allows NF-κB to enter the nucleus and turn on transcription of proinflammatory genes. Whereas poxviruses are known to encode proteins that inhibit intracellular signaling events involving NF-κB (6, 12, 22, 35, 39, 44, 46), there has been no report to date of such a role for E3L. Therefore, we queried whether infection with the ΔE3L virus activates the NF-κB pathway, as reflected by a change in the steady-state level of IκBα. Initial experiments performed with synchronously infected BSC40 cells showed that WT vaccinia virus had no apparent effect on the level of IκBα up to 24 h postinfection, as measured by Western blotting of whole-cell extracts (Fig. 6A). In contrast, ΔE3L triggered a decrease in IκBα levels at 12, 18, and 24 h postinfection (Fig. 6A). Probing the immunoblot with antibody to β-actin revealed equal loading in all lanes (not shown). A decline in IκBα was also observed in XS52 cells at 12, 18, and 24 h postinfection with ΔE3L vaccinia virus but not in XS52 cells infected with WT vaccinia virus (not shown). The late onset of IκBα decay after infection with ΔE3L correlated with the timing of TNF and IL-6 secretion by ΔE3L-infected XS52 cells in the absence of exogenous TLR agonists (Fig. 3). These findings imply a role for E3L as an antagonist of NF-κB signaling in BSC40 and XS52 cells. This function was not evident in RK13 cells, which are permissive for ΔE3L replication, insofar as infection with neither ΔE3L nor WT vaccinia virus triggered an analogous decline in IκBα levels in RK13 cells at up to 22 h postinfection (Fig. 6B).
FIG. 6.
Abortive ΔE3L infection triggers degradation of IκBα. (A) BSC40 cells were infected with WT WR or ΔE3L vaccinia virus at a multiplicity of 10. Whole-cell lysates were prepared from infected cells at 4, 8, 12, 18, and 24 h postinfection and also from uninfected control cells (lane U). The lysate proteins were separated by SDS-PAGE and then transferred to a membrane, which was immunoblotted with a rabbit polyclonal anti-IκBα antibody. The positions and sizes (kilodaltons) of coeloectrophoresed marker polypeptides are indicated on the left. The position of the immunoreactive IκBα protein is indicated at the right. (B) RK13 cells were infected with WT WR or ΔE3L vaccinia virus at a multiplicity of 10. Whole-cell lysates were prepared from infected cells at 4, 8, 14, and 22 h postinfection and also from uninfected control cells (lane U). The lysate proteins were separated by SDS-PAGE. A Western blot assay with rabbit polyclonal anti-IκBα antibody is shown (C). BSC40 cells were preincubated with control medium (lane −) or medium containing either ANO (10 μg/ml) or araC (8 μM) for 8 h prior to infection with ΔE3L virus at a multiplicity of 10. Virus-infected cells and mock-infected control monolayers exposed to ANO or araC were washed and incubated in fresh medium with or without ANO or araC. Whole-cell lysates were prepared at 16 h postinfection and subjected to SDS-PAGE and immunoblotting with anti-IκBα antibody. (D) BSC40 cells were infected with ΔE3L virus at a multiplicity of 10. After removal of the inoculum, the cell were overlaid with control medium or medium containing the proteasome inhibitor PSI at 0.125 μM. Lysates were prepared from ΔE3L-infected cells at 4, 8, 12, and 22 h postinfection and from uninfected cells maintained for an equivalent time in control medium (lane U) or medium containing 0.125 μM PSI (lane U*). A Western blot assay with anti-IκBα antibody is shown.
The decay of IκBα in ΔE3L-infected BSC40 cells was blocked by treatment of the cells with either ANO or araC (Fig. 6C). Neither drug affected the steady-state level of IκBα in mock-infected BSC40 cells (Fig. 6C). These results show that vaccinia virus activation of the NF-κB pathway in the absence of E3L depends on the synthesis of viral early proteins (the step blocked by ANO) and the onset of viral DNA replication (inhibited by araC). It is conceivable that the synthesis of the intermediate class of viral mRNAs, which requires the onset of DNA replication, is the inciting event in signaling via NF-κB when E3L is absent. Vaccinia virus postreplicative mRNAs are transcribed from overlapping segments of both genomic DNA strands and are therefore capable of forming dsRNA during a virus infection. E3L binds dsRNA and attenuates intracellular responses to dsRNA. Additional experiments showed that the decay of IκBα in ΔE3L-infected BSC40 cells was prevented by the proteasome inhibitor PSI (Fig. 6D; +PSI), implying that activation of NF-κB in the absence of E3L occurs via the canonical route of proteolytic digestion of IκBα by the proteasome.
Deletion of K1L partially restores LPS-induced cytokine production in infected XS52 cells.
The vaccinia virus K1L gene is a determinant of host range. The ΔK1L virus, a mutant of vaccinia virus strain WR in which the K1L gene is deleted, displays a restricted host range for replication in cell culture, whereby ΔK1L replicates in monkey kidney cells but not in rabbit kidney RK13 cells (41). The K1L protein is composed of several ankyrin repeats; an alanine cluster mutation in one of the ankyrin repeats phenocopies the host range defect of a ΔK1L deletion mutant (7). The K1L protein has been reported to inhibit activation of NF-κB by preventing degradation of IκBα (46). Indeed, abortive infection of RK13 cells with the ΔK1L virus activates NF-κB whereas infection with WT vaccinia virus does not (46). Infection of HeLa cells with the highly attenuated NYVAC strain of vaccinia virus (in which the gene for K1L and many other viral genes are deleted) results in NF-κB activation, whereas WT WR infection does not (21). Yet, NF-κB activation in NYVAC-infected HeLa cells was blocked when the host cells were transfected with a plasmid expressing K1L (21). In light of these findings, we queried whether the ΔK1L virus replicates in XS52 cells and what effect it might have on cytokine production.
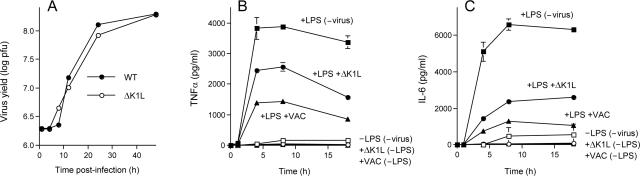
We found that ΔK1L was able to replicate productively in XS52 cells; the kinetics of accumulation of infectious ΔK1L progeny virus and the final virus yield were similar to those of WT vaccinia virus WR (Fig. 7A). The TNF-α and IL-6 secretion profiles of ΔK1L-infected XS52 cells, with or without exposure to LPS, are shown in Fig. 7B and C, respectively, along with those of cells infected in parallel with WT vaccinia virus and uninfected controls. WT vaccinia virus infection reduced LPS-stimulated TNF-α and IL-6 production in this experiment by 63% and 80%, respectively. This effect was partially attenuated in ΔK1L-infected XS52 cells, where the peak levels of secreted TNF-α and IL-6 were 34% and 64% lower than the values observed in the uninfected control cells. Thus, deleting K1L had an effect similar to that of an E3L deletion in partially restoring LPS responsiveness in vaccinia virus-infected XS52 cells. Because the ΔK1L virus is replication competent in XS52 cells, we surmise that the extent of inhibition of cytokine production by vaccinia virus is not a trivial effect of virus inhibition of host protein synthesis but rather an active process that depends on several viral gene products, including, but not limited to, E3L and K1L. A noteworthy distinction between the ΔK1L and ΔE3L viruses in XS52 cells is that infection with ΔK1L did not trigger cytokine production in the absence of LPS. Thus, in a permissive host cell, K1L is not uniquely required to suppress proinflammatory signals generated by the virus itself (presumably via NF-κB). Consistent with this idea, we observed no decay in IκBα levels in BSC40 cells productively infected with the ΔK1L virus (not shown).
FIG. 7.
Productive replication of the ΔK1L virus in XS52 cells and effects of K1L deletion on LPS-induced cytokine production. (A) XS52 cells (6 × 106) were infected with WT WR or ΔK1L vaccinia virus at a multiplicity of 3. The inoculum was removed after 30 min. Cells were harvested at the indicated times postinoculation. Virus yield (log PFU) was determined by titration on BSC40 cell monolayers. (B and C) XS52 cells (6 × 106) were infected with WT WR or ΔK1L vaccinia virus at a multiplicity of 10 or mock infected. At 2 h after removal of the inoculum, LPS was added to the medium (where indicated) to a concentration of 1 μg/ml. The concentrations of secreted TNF-α (A) and IL-6 (B) in the medium at the indicated times thereafter were determined by ELISA. Each datum represents the average of three experiments with standard deviations shown.
Vaccinia virus infection inhibits antigen presentation by primary LCs.
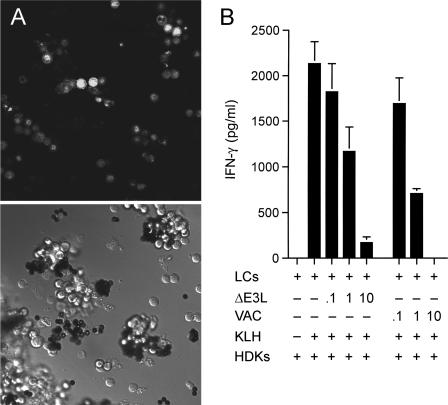
Fresh primary LCs were obtained from mouse epidermis and purified away from epithelial cells (to >95% enrichment) by using anti-I-Ad antibody to make the LCs adsorb to magnetic beads (45). Initial experiments showed that WT vaccinia virus was unable to replicate productively in these affinity-purified primary mouse LCs (data not shown). However, infection of the primary LCs with a recombinant virus that expresses a nucleus-localized GFP under the control of a vaccinia virus p7.5 promoter did result in the appearance of fluorescent nuclei in most of the cells at 24 h postinfection (Fig. 8A), indicating that vaccinia virus abortively infects LCs. Green fluorescent nuclei were detected as early as 6 h postinfection (data not shown). To probe what the functional impact of abortive infection might be on LC function, we compared the ability of mock-infected and virus-infected LCs to elicit antigen-specific activation of T cells.
FIG. 8.
Abortive vaccinia virus infection of primary LCs inhibits their antigen presentation function. (A) LCs were purified from female BALB/c mouse epidermis with anti-I-Ad antibody, followed by incubation with magnetic beads coated with goat anti-mouse immunoglobulin G antibody. They were infected with vaccinia virus NP-S-EGFP at a multiplicity of 10. At 24 h postinfection, the cells were imaged with a Zeiss laser scanning confocal microscope. The bright-field image is shown in the bottom half of the panel. Green fluorescence of the same field is shown in the top half of the panel. (B) Antigen presentation. Purified primary LCs were infected with vaccinia virus WR or ΔE3L at a multiplicity of 0.1, 1, or 10 or mock infected. Cells bound to magnetic beads were washed to remove unadsorbed virus, incubated overnight with the KLH antigen, and then washed to remove KLH. LCs (1 × 104) were then coincubated with HDK-1 cells (5 × 104) for 72 h. The IFN-γ concentration in the medium was measured by ELISA. Each datum represents the average of three antigen presentation assays with standard deviations shown.
Purified LCs attached to antibody-coated magnetic beads were infected with either WT or ΔE3L vaccinia virus at a multiplicity of 0.1, 1, or 10 or mock infected. Cells were concentrated with an external magnet, and the supernatant containing the unadsorbed virus was removed. The cells were resuspended in fresh medium and subjected to several rounds of magnetic concentration and washing to deplete the residual virus. At 3 h postinfection, the cells were incubated overnight with the test antigen, KLH, followed by concentration and washing to remove residual KLH. The antigen-primed LCs were then incubated with HDK-1 cells, which are a KLH-specific CD4+ Th1 T-cell line. The uninfected primary LCs were able to present KLH peptides to HDK-1 cells and stimulate them to produce IFN-γ (Fig. 8B). In the absence of KLH or LCs, the HDK-1 cells failed to produce IFN-γ (Fig. 8B and data not shown). Vaccinia virus infection resulted in multiplicity-dependent attenuation of antigen presentation by the LCs. The T-cell IFN-γ response was inhibited completely at a multiplicity of 10, whereas IFN-γ secretion was reduced by 60% at a multiplicity of 1 (Fig. 8B). Infection with ΔE3L had only slightly less inhibitory effects on the antigen presentation activity of the primary LCs, reducing IFN-γ production to 8% of the control value at a multiplicity of 10.
DISCUSSION
The present study contributes to our understanding of the interface of poxviruses with the skin immune system by demonstrating that (i) vaccinia virus replicates productively in an epidermal LC cell line, XS52; (ii) virus infection of XS52 cells suppresses their ability to produce TNF-α and IL-6 in response to stimulation by LPS and poly(I:C); (iii) loss of the E3L dsRNA binding protein causes infection of XS52 cells to abort at a stage prior to the onset of late gene expression, which results in induction of TNF-α secretion by ΔE3L-infected cells and significant amelioration of the suppressive effects of WT virus on LPS- or poly(I:C)-induced cytokine production; (iv) loss of the K1L protein, which has no effect on virus replication in XS52 cells, also partially reverses the virus inhibition of LPS-induced cytokine production; and (v) abortive infection of primary epidermal LCs by WT vaccinia virus inhibits their ability to present antigen and activate T cells.
These findings extend previous studies of poxvirus interactions with DCs, most of which have focused on monocyte-derived or bone marrow-derived DCs. Whereas WT vaccinia virus or vaccinia virus recombinants have been reported to infect such DCs abortively (14, 16, 26), we found that the XS52 cells line supports virus vaccinia virus replication to a high titer. The kinetics of virus production during a synchronous infection of XS52 cells and the temporal pattern of viral protein synthesis are similar to those seen in BSC40 cells, which are a standard laboratory host for vaccinia virus. It appears that adaptation of XS52 cells to continuous culture endows them with a permissiveness for vaccinia virus infection that is not found in primary LCs harvested freshly from mouse epidermis, which we find are infected abortively. The latter result agrees with the recent report by Liu et al. (33) that vaccinia virus abortively infects primary LCs from human skin. In our study and theirs, there is evidence that at least some early viral gene expression occurs in vaccinia virus-infected primary LCs, which echoes the findings that early gene expression is detectable in monocyte-derived DCs abortively infected with vaccinia virus (14, 16, 26).
The immune effector functions of XS52 cells and primary skin LCs are actively suppressed by vaccinia virus infection. Vaccinia virus-infected XS52 cells are strongly attenuated in their response to LPS and poly(I:C), which signal cytokine production via the TLR4 and TLR3 pathways, respectively. Recent studies have shown that vaccinia virus and other mammalian poxviruses encode several proteins that inhibit the intracellular phase of TLR signaling, especially events involving NF-κB (6, 9, 12, 22, 35, 39, 44, 46, 48). The present study implicates E3L and K1L as contributors to the interdiction of immune signaling pathways in XS52 cells by vaccinia virus.
The vaccinia virus E3L protein binds avidly to dsRNA (10, 23). E3L blocks IFN induction and antagonizes the IFN-regulated antiviral enzymes dsRNA-activated protein kinase PKR and RNase L (42, 51). Deletion of E3L sensitizes vaccinia virus replication to IFN in permissive RK13 cells and results in a host range phenotype whereby ΔE3L cannot replicate in HeLa or BSC40 cells (11). Abortive infection with ΔE3L triggers apoptosis of HeLa cells, apparently in response to dsRNA accumulation after the onset of late viral transcription (30). We find here that ΔE3L abortively infects XS52 cells. However, the restriction point in XS52 cells appears to differ from that reported for HeLa cells, where ΔE3L triggers a complete shutoff of the synthesis of both viral and host proteins by 6 h postinfection (31). ΔE3L-infected XS52 cells continue to synthesize host proteins for at least 12 h without any apparent synthesis of viral late proteins. We suspect that some early viral genes are expressed in ΔE3L-infected XS52 cells, insofar as the abortive infection triggers secretion of TNF-α and IL-6, which are not produced during infection with WT vaccinia virus. Moreover, ΔE3L is less effective than WT vaccinia virus in attenuating the production of TNF-α and IL-6 by XS52 cells in response to LPS and poly(I:C), which might be pertinent to its prospects as a safer vaccine for smallpox prophylaxis and inducing mucosal immunity against other pathogens (8).
The activation of cytokine production in ΔE3L-infected cells in the absence of exogenous stimuli is apparently a consequence of virus activation of the NF-κB pathway through the canonical mechanism of IκBα degradation. Decay of IκBα is observed at late times in ΔE3L-infected BSC40 or XS52 cells but not when these cells are infected with WT vaccinia virus. These results suggest a novel role for E3L as an antagonist of NF-κB signaling. This new function imputed to E3L is context dependent, insofar as a permissive infection of RK13 cells with ΔE3L did not result in decay of IκBα, suggesting that other viral proteins (e.g., K1L) might suffice to block IκBα turnover in RK13 cells. We find that K1L plays a role in viral attenuation of the LPS cytokine response in XS52 cells because deletion of K1L increases TNF-α and IL-6 secretion after LPS treatment.
The findings here that vaccinia virus attenuates immune signaling in epidermis-derived DCs resonate with previous studies with abortively infected monocyte-derived DCs, where WT vaccinia virus inhibited maturation of DCs exposed to proinflammatory cytokines or poly(I:C) and consequently inhibited their ability to activate T cells (16, 26). Also, Li et al. (32) reported that WT vaccinia virus infection of human bone marrow-derived DCs, macrophages, and B cells decreased MHC class II-restricted antigen presentation to CD4+ T cells. It is noteworthy that whereas WT vaccinia virus infection of monocyte-derived DCs fails to trigger their maturation, infection with attenuated modified vaccinia virus Ankara activates DCs to secrete proinflammatory cytokines (15), a situation analogous to our observation that infection with ΔE3L triggers cytokine production by epidermal DCs.
The permissiveness of XS52 cells for vaccinia virus replication provides a valuable model for future studies of the interface of poxviruses with skin immune cells. Systematic testing of other single-gene knockout viruses and viruses with multigene deletions should aid in identifying additional viral mediators of the suppression of cytokine production. XS52 cells should also prove useful in evaluating antiviral drugs as agents for treatment of poxvirus skin infections and complications of vaccination. We show that infection of these LC-like cells is not sensitive to an anti-poxvirus agent, araC, that is widely used in laboratory studies to arrest viral DNA replication. However, ANO emerges as a more plausible agent for topical treatment of poxvirus infections because it retains potency in XS52 cells.
Acknowledgments
We thank Eric Pamer and Alan Houghton for constructive comments on the manuscript and Katia Manova for assistance with confocal imaging of virus-infected primary LCs.
L.D. is the recipient of a Dermatologist Investigator Research Fellowship and a Physician Scientist Career Development Award from the Dermatology Foundation. S.S. is an American Cancer Society Research Professor.
REFERENCES
- 1.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. [DOI] [PubMed] [Google Scholar]
- 2.Allan, R. S., C. M. Smith, G. T. Belz, A. L. van Lint, L. M. Wakim, W. R. Heath, and F. R. Carbone. 2003. Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science 301:1925-1928. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 4.Bennett, C. L., E. van Rijn, S. Jung, K. Inaba, R. M. Steinman, M. L. Kapsenberg, and B. E. Clausen. 2005. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J. Cell Biol. 169:569-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berthier-Vergnes, O., F. Bermond, V. Flacher, C. Massacrier, D. Schmitt, and J. Peguet-Navarro. 2005. TNF-α enhances phenotypic and functional maturation of human epidermal Langerhans cells and induces IL-12 p40 and IP-10/CXCL-10 production. FEBS Lett. 579:3660-3668. [DOI] [PubMed] [Google Scholar]
- 6.Bowie, A., E. Kiss-Toth, J. A. Symons, G. L. Smith, S. K. Dower, and L. A. J. O'Neill. 2000. A46R and A52R from vaccinia virus are antagonists of host IL-1 and Toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 97:10162-10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley, R. R., and M. Terajima. 2005. Vaccinia virus K1L protein mediates host-range function in RK-13 cells via ankyrin repeat and may interact with a cellular GTPase-activating protein. Virus Res. 114:104-112. [DOI] [PubMed] [Google Scholar]
- 8.Brandt, T., M. C. Heck, S. Viyaysri, G. M. Jentarra, J. M. Cameron, and B. L. Jacobs. 2005. The N-terminal domain of the vaccinia virus E3L-protein is required for neurovirulence, but not induction of a protective immune response. Virology 333:263-270. [DOI] [PubMed] [Google Scholar]
- 9.Camus-Bouclainvillem, C., L. Fiette, S. Bouchiha, B. Pignolet, D. Counor, C. Filipe, J. Gelfi, and F. Messud-Petit. 2004. A virulence factor of myxoma virus colocalizes with NF-κB in the nucleus and interferes with inflammation. J. Virol. 78:2510-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, H., J. C. Watson, and B. L. Jacobs. 1992. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 89:4825-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang, H., L. H. Uribe, and B. L. Jacobs. 1995. Rescue of vaccinia virus lacking the E3L gene by mutants of E3L. J. Virol. 69:6605-6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiPerna, G., J. Stack, A. G. Bowie, A. Boyd, G. Kotwal, A. Zhang, S. Arvikar, E. Latz, K. A. Fitzgerald, and W. L. Marshall. 2004. Poxvirus protein N1L targets the I-κB kinase complex, inhibits signaling to NF-κB by the tumor necrosis factor superfamily of receptors, and inhibits NF-κB and IRF3 signaling by Toll-like receptors. J. Biol. Chem. 279:36570-36578. [DOI] [PubMed] [Google Scholar]
- 13.Dohill, M. A., P. Lin, J. Lee, A. W. Lucky, A. S. Paller, and L. F. Eichenfeld. 2006. The epidemiology of molluscum contagiosum in children. J. Am. Acad. Dermatol. 54:47-54. [DOI] [PubMed] [Google Scholar]
- 14.Drillien, R., D. Spehner, A. Bohbot, and D. Hanau. 2000. Vaccinia virus-related events and phenotypic changes after infection of dendritic cells derived from human monocytes. Virology 268:471-481. [DOI] [PubMed] [Google Scholar]
- 15.Drillien, R., D. Spehner, and D. Hanau. 2004. Modified vaccinia virus Ankara induces moderate activation of human dendritic cells. J. Gen. Virol. 85:2167-2175. [DOI] [PubMed] [Google Scholar]
- 16.Engelmayer, J., M. Larsson, M. Subklewe, A. Chahroudi, W. I. Cox, R. M. Steinman, and N. Bhardwaj. 1999. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J. Immunol. 163:6762-6768. [PubMed] [Google Scholar]
- 17.Euvrard, S., J. Kanitakis, P. Cochat, F. Cambazard, and A. Claudy. 2001. Skin diseases in children with organ transplants. J. Am. Acad. Dermatol. 44:932-939. [DOI] [PubMed] [Google Scholar]
- 18.Fulginiti, V. A., A. Papier, M. Lane, J. M. Neff, and D. A. Henderson. 2003. Smallpox vaccination: a review, part II. Adverse events. Clin. Infect. Dis. 37:251-271. [DOI] [PubMed] [Google Scholar]
- 19.Ginhoux, F., F. Tacke, V. Angeli, M. Bogunovic, M. Loubeau, X. M. Dai, E. R. Stanley, G. J. Randolph, and M. Merad. 2006. Langerhans cell arise from monocytes in vivo. Nat. Immunol. 7:223-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granstein, R. D., M. Askari, D. Whitaker, and G. F. Murphy. 1987. Epidermal cells in activation of suppressor lymphocytes: further characterization. J. Immunol. 138:4055-4062. [PubMed] [Google Scholar]
- 21.Guerra, S., L. A. López-Fernandez, A. Pascual-Montano, J. L. Nájera, A. Zaballos, and M. Esteban. 2006. Host response to the attenuated poxvirus vector NYVAC: upregulation of apoptotic genes and NF-κB-responsive genes in infected HeLa cells. J. Virol. 80:985-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harte, M. T., I. R. Haga, G. Maloney, P. Gray, P. C. Reading, N. W. Bartlett, G. L. Smith, A. Bowie, and L. A. J. O'Neill. 2003. The poxvirus protein A52R targets Toll-like receptor signaling to suppress host defense. J. Exp. Med. 197:343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho, C. K., and S. Shuman. 1996. Physical and functional characterization of the double-stranded RNA binding protein encoded by the vaccinia virus E3 gene. Virology 217:272-284. [DOI] [PubMed] [Google Scholar]
- 24.Ignatius, R., M. Marovich, E. Mehlhop, L. Villamide, K. Mahnke, W. I. Cox, F. Isdell, S. S. Frankel, J. R. Miscola, R. M. Steinman, and M. Popo. 2000. Canarypox virus-induced maturation of dendritic cells is mediated by apoptotic cell death and tumor necrosis factor alpha secretion. J. Virol. 74:11329-11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakob, T., J. Ring, and M. C. Udey. 2001. Multistep navigation of Langerhans/dendritic cells in and out of the skin. J. Allergy Clin. Immunol. 108:688-696. [DOI] [PubMed] [Google Scholar]
- 26.Jenne, L., C. Hauser, J. F. Arrighi, J. H. Saurat, and A. W. Hügin. 2000. Poxvirus as a vector to transduce human dendritic cells for immunotherapy: abortive infection but reduced APC function. Gene Ther. 7:1575-1583. [DOI] [PubMed] [Google Scholar]
- 27.Kane, E. M., and S. Shuman. 1995. Adenosine N1-oxide inhibits vaccinia virus replication by blocking translation of viral early mRNAs. J. Virol. 69:6352-6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz, S. I., K. Tamaki, and D. H. Sachs. 1979. Epidermal Langerhans cells are derived from cells originating in the bone marrow. Nature 282:324-326. [DOI] [PubMed] [Google Scholar]
- 29.Kawamura, T., S. E. Kurtz, A. Blauvelt, and S. Shimada. 2005. The role of Langerhans cells in the sexual transmission of HIV. J. Dermatol. Sci. 40:147-155. [DOI] [PubMed] [Google Scholar]
- 30.Kibler, K. V., T. Shors, K. B. Perkins, C. C. Zeman, M. P. Banaszak, J. Biesterfeldt, J. O. Langland, and B. L. Jacobs. 1997. Double-stranded RNA is a trigger for apoptosis in vaccinia virus-infected cells. J. Virol. 71:1992-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langland, J. O., and B. L. Jacobs. 2002. The role of the PKR-inhibitory genes, E3L and K3L, in determining vaccinia virus host range. Virology 299:133-141. [DOI] [PubMed] [Google Scholar]
- 32.Li, P., N. Wang, D. Zhou, C. S. K. Yee, C. H. Chang, R. R. Brutkiewicz, and J. S. Blum. 2005. Disruption of MHC class II-restricted antigen presentation by vaccinia virus. J. Immunol. 175:6481-6488. [DOI] [PubMed] [Google Scholar]
- 33.Liu, L., Z. Xu, R. C. Fuhlbrigge, V. Pena-Cruz., J. Lieberman, and T. S. Kupper. 2005. Vaccinia virus induces strong immunoregulatory cytokine production in healthy human epidermal keratinocytes: a novel strategy for immune evasion. J. Virol. 79:7363-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayerova, D., E. A. Parke, L. S. Bursch, O. A. Odumade, and K. A. Hogquist. 2004. Langerhans cells activate naïve self-antigen-specific CD8 T cells in the steady state. Immunity 21:391-400. [DOI] [PubMed] [Google Scholar]
- 35.McCoy, S. L., S. E. Kurtz, C. J. MacArthur, D. R. Trune, and S. H. Hefeneider. 2005. Identification of a peptide derived from vaccinia virus A52R protein that inhibits cytokine secretion in response to TLR-dependent signaling and reduces in vivo bacterial-induced inflammation. J. Immunol. 174:3006-3014. [DOI] [PubMed] [Google Scholar]
- 36.Merad, M., M. G. Manz, H. Karsunky, A. Wagers, W. Peters, I. Charo, I. L. Weissman, J. G. Cyster, and E. G. Engelman. 2002. Langerhans cell renew in the skin throughout life under steady-state conditions. Nat. Immunol. 3:1135-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitsui, H., T. Watanabe, H. Saeki, K. Mori, H. Fujita, Y. Tada, A. Asahina, K. Nakamura, and K. Tamaki. 2004. Differential expression and function of Toll-like receptors in Langerhans cells: comparison with splenic dendritic cells. J. Investig. Dermatol. 122:95-102. [DOI] [PubMed] [Google Scholar]
- 38.Norbury, C. C., D. Malide, J. S. Gibbs, J. R. Bennink, and J. W. Yewdell. 2002. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat. Immunol. 3:265-271. [DOI] [PubMed] [Google Scholar]
- 39.Oie, K. L., and D. J. Pickup. 2001. Cowpox virus and other members of the orthopoxvirus genus interfere with the regulation of NF-κB activation. Virology 288:175-187. [DOI] [PubMed] [Google Scholar]
- 40.Palucka, K., and J. Banchereau. 2002. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr. Opin. Immunol. 14:420-431. [DOI] [PubMed] [Google Scholar]
- 41.Ramsey-Ewing, A. L., and B. Moss. 1996. Complementation of a vaccinia virus host-range K1L gene deletion by the nonhomologous CP77 gene. Virology 222:75-86. [DOI] [PubMed] [Google Scholar]
- 42.Romano, P. R., F. Zhang, S.-L. Tan, M. T. Barcia-Barrio, M. G. Katze, T. E. Dever, and A. G. Hinnebusch. 1998. Inhibition of double-stranded RNA-dependent protein kinase PKR by vaccinia virus E3: role of complex formation and the E3 N-terminal domain. Mol. Cell. Biol. 18:7304-7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuler, G., and R. M. Steinman. 1985. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J. Exp. Med. 161:526-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seet, B. T., J. B. Johnston, C. R. Brunetti, J. W. Barrett, H. Everett, C. Cameron, J. Sypula, S. H. Nazarian, A. Lucas, and G. McFadden. 2003. Poxviruses and immune evasion. Annu. Rev. Immunol. 21:377-423. [DOI] [PubMed] [Google Scholar]
- 45.Seiffert, K., J. Hosoi, H. Torii, H. Ozawa, W. Ding, K. Campton, J. A. Wagner, and R. D. Granstein. 2002. Catecholamines inhibit the antigen-presenting capability of epidermal Langerhans cells. J. Immunol. 168:6128-6135. [DOI] [PubMed] [Google Scholar]
- 46.Shisler, J. L., and X. L. Jin. 2004. The vaccinia virus K1L gene product inhibits host NF-κB activation by preventing IκBα degradation. J. Virol. 78:3553-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simon, J. C., P. D. Cruz, P. R. Bergstresser, and R. E. Tigelaar. 1990. Low dose ultraviolet B-irradiated Langerhans cells preferentially activate CD4+ cells of the T helper 2 subset. J. Immunol. 145:2087-2091. [PubMed] [Google Scholar]
- 48.Stack, J., I. R. Haga, M. Schröder, N. W. Bartlett, G. Maloney, P. C. Reading, K. A. Fitzgerald, G. L. Smith, and A. G. Bowie. 2005. Vaccinia virus protein A46R targets multiple Toll-like-interleukin-1 receptor adaptors and contributes to virulence. J. Exp. Med. 201:1007-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taddie, J. A., and P. Traktman. 1993. Genetic characterization of the vaccinia virus DNA polymerase: cytosine arabinoside resistance requires a variable lesion conferring phosphonoacetate resistance in conjunction with an invariant mutations localized to the 3′-5′ exonuclease domain. J. Virol. 67:4323-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wollenberg, A., and R. Engler. 2004. Smallpox, vaccination and adverse reactions to smallpox vaccine. Curr. Opin. Allergy Clin. Immunol. 4:271-275. [DOI] [PubMed] [Google Scholar]
- 51.Xiang, Y., R. C. Condit, S. Vijaysri, B. Jacobs, B. R. G. Williams, and R. H. Silverman. 2002. Blockade of interferon induction and action by the E3L double-stranded RNA binding proteins of vaccinia virus. J. Virol. 76:5251-5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu, S., P. R. Bergstresser, and A. Takashima. 1995. Phenotypic and functional heterogeneity among murine epidermal-derived dendritic cell clones. J. Investig. Dermatol. 105:831-836. [DOI] [PubMed] [Google Scholar]
- 53.Zhao, X., E. Deak, K. Soderberg, M. Linehan, D. Spezanno, J. Zhu, D. M. Knipe, and A. Iwasaki. 2003. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus 2. J. Exp. Med. 197:153-162. [DOI] [PMC free article] [PubMed] [Google Scholar]