Abstract
Assembly of herpes simplex viruses (HSV) is a poorly understood process involving multiple redundant interactions between large number of tegument and envelope proteins. We have previously shown (G. E. Lee, G. A. Church, and D. W. Wilson, J. Virol. 77:2038-2045, 2003) that the virion host shutoff (Vhs) tegument protein is largely insoluble in HSV-infected cells and is also stably associated with membranes. Here we demonstrate that both insolubility and stable membrane binding are stimulated during the course of an HSV infection. Furthermore, we have found that the amino-terminal 42 residues of Vhs are sufficient to mediate membrane association and tegument incorporation when fused to a green fluorescent protein (GFP) reporter. Particle incorporation correlates with sorting to cytoplasmic punctate structures that may correspond to sites of HSV assembly. We conclude that the amino terminus of Vhs mediates targeting to sites of HSV assembly and to the viral tegument.
Herpes simplex virus (HSV) is a large DNA virus approximately 200 nm in diameter. The 150-kb double-stranded DNA genome of HSV is packaged within an icosahedral capsid which is surrounded by an amorphous proteinaceous layer called tegument. The tegument is a complex structure composed of more than 15 proteins (25). The virus is enclosed by a host cell-derived lipid envelope which contains multiple virally encoded glycoproteins.
The tegument proteins serve a variety of essential functions. Early in infection, they regulate viral and cellular gene expression. Later, the tegument proteins assemble with the capsid and envelope to form mature progeny virions. The innermost layer of tegument is believed to correspond to the largest tegument protein, VP1/2, the product of the UL36 gene, which directly contacts the capsid proteins, hence exhibiting icosahedral symmetry (33), and is also known to interact with the product of the UL37 gene (18, 19). In the outer layer of tegument, multiple interactions are thought to occur among the tegument proteins and between tegument proteins and cytoplasmic tails of envelope glycoproteins. Recently we have demonstrated interactions between Glycoprotein H (gH) and VP16 (14, 17) and gD and VP22 (4), while in pseudorabies virus, VP22 has been shown to interact with gE and gM (13). The tegument protein UL11 has also been shown to bind to UL16 (23). It is to be expected that many of the proteins forming the outer layer of tegument either need to associate with membrane proteins or are membrane associated themselves. Indeed, the tegument proteins VP22 (3), UL11 (1), UL51 (27), and Vhs (21) have all recently been found to be membrane associated. Moreover the tegument proteins UL51 (27), UL11 (22), and US2 (6), have been found to be modified with acyl or prenyl groups, a modification thought to be important for their localization to membranes.
The tegument protein Vhs (virion host shutoff) is a 58-kDa tegument phosphoprotein encoded by UL41. Early in infection, Vhs forms a complex with the cellular translation initiation factors eIF4H, -4B, and -4A (8, 12) to form an active RNase that indiscriminately cleaves mRNA to suppress host cell protein synthesis (10, 24). Upon initiation of viral protein synthesis, Vhs continues to cleave viral mRNA and hence ensures an efficient switch from early to late gene expression. However, at this time accumulating VP16 binds to Vhs (29) and suppresses its RNase activity (20). Vhs is conserved among alphaherpesviruses, suggesting a role in establishing infection in neuronal cells (30). In fact, the role of Vhs is critically important in pathogenesis as loss of UL41 impairs the virus' ability to establish infection and reactivate from latency in the trigeminal ganglia, brain, and cornea (31). UL41-null viruses are, however, viable in tissue culture, although they exhibit slower growth and yield lower titers than the wild type (16). There is emerging evidence that the impaired ability of Vhs mutant viruses to establish disease is due to their reduced effectiveness in disarming the host's immune system, particularly by interfering with production of interferons (9, 26).
In contrast to the more extensively studied RNase properties of Vhs, almost nothing is known about assembly of Vhs into tegument. We have previously reported that Vhs is largely insoluble in HSV-1-infected cells in the presence of a high salt concentration and Triton X-100 (21). Furthermore, a considerable proportion of Vhs is stably membrane associated, and some of it partitions into Triton X-100-resistant lipid complexes or lipid rafts, although Vhs in the mature virus is not raft associated (21). The primary amino acid sequence of Vhs does not reveal any amino-terminal signal sequence, an apparent membrane-spanning domain, or any known motifs for fatty acylation or isoprenylation. Hence the mechanism of association of Vhs with membranes remains unknown.
The aim of the present study was to better understand the membrane association properties of Vhs and its role in tegument assembly. We found that Vhs membrane association was largely HSV infection dependent, with some limited membrane association in the absence of infection, which could be eliminated by disrupting electrostatic interactions. By mutational analysis, we discovered that the amino-terminal 42 residues of Vhs are sufficient for both membrane association and tegument incorporation of Vhs. Hence, we have identified a 42-amino-acid sequence in Vhs that is capable of targeting fusion proteins to membranes at which HSV undergoes assembly and into the tegument of the particle itself.
(Data in this paper are from a thesis to be submitted in partial fulfillment of the requirements for the Degree of Doctor of Philosophy in the Graduate Division of Medical Sciences, Albert Einstein College of Medicine, Yeshiva University.)
MATERIALS AND METHODS
Cells and viruses.
COS and Vero cells were maintained in Dulbecco's modified Eagle,s medium supplemented with 1% penicillin-streptomycin (Gibco, Grand Island, NY) and either 10% fetal calf serum or 10% fetal bovine serum (Fisher Scientific, Suwanee, GA). The Vhs-null virus and HSV strains PAAR5, N138HA (16), and K26GFP (7) were all grown on Vero cells, and titers of virus were determined by plaque assay as previously described (5).
Generation of vhs mutants.
The UL41 gene was amplified from the genome of the HSV strain SC16 by PCR using upstream oligonucleotide 5′ GTATAAGCTTGTCGACATGGGTTTGTTCGGGATGATGAAG, which introduces a HindIII site upstream of the initiation codon, and downstream oligonucleotide 5′ GGACGGCGGCGGCTCGTCCCAGAATTTGGCCAG, which introduces a SacII site immediately before the stop codon. The PCR product was cloned into the HindIII and SacII sites of pEGFP-N1 (BD Biosciences Clontech, Palo Alto, CA). Subsequently three stop codons were inserted downstream of the SacII site. The Thr 214-to-Ile Vhs1 point mutation was introduced by mutagenic PCR using upstream primer 5′ ACGGTCGCGTACGTGTACACCACGGACATCGATCTCCTGTTGATGGGCTGT and downstream primer 5′ TAAAAGTACTTTAGTATATCG to generate a 120-bp fragment. The PCR product was inserted into wild-type Vhs using the sites BsiWI and ScaI. To prepare VhsHA, the hemagglutinin (HA) epitope tag was amplified by PCR and introduced at the carboxy terminal to Vhs using SacII and AgeI restriction sites.
The C-terminally-truncated mutant 274HA was prepared by amplifying the first 274-amino-acid coding region of Vhs1 using the oligonucleotides 5′ TTTAGTGAACCGTCAGATCC and 5′ GCGTAACCGCGGCACATCCTCCACGGAGGC, which introduces a SacII site downstream of the stop codon. D1+Δ274 was initially prepared as a deletion of the first 274-amino-acid region of Vhs, and then the amino-terminal domain 1 region (2) of Vhs was reintroduced. To do this, the last 212 codons of Vhs were amplified by PCR using the oligonucleotides 5′ CTGTAAGCTTGACATGCTGCGCGAATGTCACTGG and 5′ CGTCCCAGAATTTGGCCAGGACGTCCTTG. Subsequently the domain 1 region of Vhs was amplified using oligonucleotides 5′ CTAACTCGAGCTAGCGTCGACATGGGTTTGTTCGGG and 5′ CGTGTCAAGCTTCAACGTGTACATGACGTTCCACAG. This domain I region was inserted upstream of the fragment encoding amino acids 212 to 484 using the restriction sites XhoI and HindIII. The ΔSma mutant was created by digesting VhsHA with SmaI, which cuts vhs at codons 147 and 343, and then religating to create an in-frame fusion.
For in vitro translation experiments, Vhs1 was inserted into pcDNA3.1+ (Invitrogen) using the restriction sites HindIII and EcoRI. Green fluorescent protein (GFP) from pEGFP-C1 (BD Biosciences Clontech) was inserted into pcDNA3.1+ at the restriction sites NheI and BamHI.
D1(42)GFP was created by amplifying domain 1 of Vhs as described previously. Subsequently, the amplified product was inserted into XhoI- and HindIII-digested pEGFP-N1, hence creating a fusion protein with GFP. D1(42)DsRed2 was also created similarly by amplifying domain 1 of Vhs and inserting the product into pDsRed2-N1 (BD Biosciences Clontech).
Western blotting and antibodies.
Western blotting for Vhs was performed using anti-Vhs antibody as previously described (21). The following antibodies were obtained commercially: mouse anti-HA monoclonal (Roche, IN), rabbit anti-GFP polyclonal (eBioscience, San Diego, CA), mouse anti-GFP monoclonal (BD Biosciences, CA), mouse anti-VP16 monoclonal (Santa Cruz Biotechnology, Inc.), Alexa Fluor 488 goat anti-mouse immunoglobulin G (Molecular Probes, Eugene, OR), goat anti-mouse 10-nm gold-conjugated antibody (Electron Microscopy Sciences, Fort Washington, PA), Alkaline phosphatase-conjugated goat anti-rabbit (Chemicon, Pittsburgh, PA), and alkaline phosphatase-conjugated goat anti-mouse (Antibodies Incorporated, Davis, CA).
Subsequent to primary and secondary antibody incubation followed by washing in Tris-buffered saline (150 mM NaCl, 10 mM Tris-HCl [pH 7.4], 0.5% Tween 20), the membranes were immersed in alkaline Tris buffer (100 mM Tris-HCl [pH 9.5], 100 mM NaCl). Bound secondary antibodies were detected by nitroblue tetrazolium and BCIP (5-bromo-4-chloro-3-indolyl-phosphate) (Promega, Madison, WI), diluted, and used according to the manufacturer's instructions. Quantitation of bands was performed using Image J software (version 1.34s).
Transfection, infection, and preparation of PNS.
COS cells were transfected using the Lipofectamine PLUS reagent (Invitrogen/Life Technologies, Carlsbad, CA) following the manufacturer's protocol. Twenty-four hours after transfection, cells were infected with Vhs-null virus at a multiplicity of infection (MOI) of 10. Eighteen hours postinfection, the cells were washed twice with HBA (0.25 M sucrose, 2 mM MgCl2, 10 mM Tris-HCl [pH 7.6]) and finally resuspended in HBA with protease inhibitors (Complete Mini EDTA-free protease inhibitor cocktail tablets; Roche, Germany). Cells were lysed by passage through a 25G5/8 syringe. The postnuclear supernatant (PNS) was obtained after nuclei were pelleted by centrifugation at 2,000 × g for 10 min at 4°C.
In vitro translation of Vhs/GFP.
[35S]methionine-labeled Vhs and GFP were in vitro translated from pcDNA 3.1+ using TNT coupled reticulocyte lysate systems (Promega, WI). The in vitro-translated product was precleared by centrifugation at 12,500 × g for 20 min.
Isolation of detergent-insoluble complexes.
The PNS prepared as described above was treated with 1% Triton X-100 for 30 min on ice and then centrifuged at 100,000 × g for 30 min at 4°C to pellet insoluble materials, membrane fractions, and cytoskeletal elements. Proteins from the supernatant were trichloroacetic acid (TCA) precipitated, and both supernatant and pellet were analyzed by Western blotting.
Isolation of membrane and membrane-associated proteins.
A 2 M concentration of sucrose in TNE buffer (150 mM NaCl, 5 mM EDTA, 25 mM Tris-HCl [pH 7.4]) was added to the postnuclear supernatant of transfected and/or infected cells to achieve a final concentration of 1.4 M sucrose. This was overlaid with 1.2 M sucrose in TNE buffer followed by 0.25 M sucrose (HBA). This was subjected to centrifugation at 100,000 × g for 2 h at 4°C in a swinging bucket rotor. A membrane fraction was collected from the 0.25-1.2 M interface. The rest of the gradient was collected either as a single fraction or as three fractions. Proteins from each fraction were TCA precipitated before being analyzed by Western blotting.
Isolation of extracellular virions.
Extracellular virions were purified by a method adapted from Szilagyi and Cunningham (32). COS cells were transfected and infected as previously described. Eighteen hours postinfection, medium and cells were collected and the cells were removed by centrifugation at 1,200 × g for 15 min at 4°C. The virus was then pelleted from the medium by centrifugation at 23,000 × g for 2 h at 4°C. The viral pellet was resuspended in STE (10 mM Tris-HCl [pH 7.5], 50 mM NaCl, 5 mM EDTA) overnight at 4°C. The suspension was sonicated to disperse viral aggregates, and insoluble material was removed by pelleting at 10,000 × g for 5 min at 4°C. This was then gently layered on top of a 30-ml 5 to 15% Ficoll-400 gradient and subjected to centrifugation at 26,000 × g for 2 h at 4°C. Five fractions of 5 ml each and a final 6-ml fraction were collected, and each fraction was diluted six times in STE. The virus from each fraction was pelleted by centrifugation at 80,000 × g for 2 h at 4°C. The pellet was resuspended in STE and used for plaque assay as well as Western blotted for VP16 and GFP.
Immunocytochemistry.
COS cells were grown on coverslips in six-well plates. The cells were transfected as previously described and then infected with virus at an MOI of 10. Eighteen hours postinfection, medium was aspirated from the plates and the cells were washed twice in room temperature phosphate-buffered saline (PBS). The cells were then fixed for 10 min in 4% paraformaldehyde (Electron Microscopy Sciences) in PBS. The cells were again washed in PBS and permeabilized in 0.1% Triton X-100 in PBS for 10 min, washed twice in PBS, and then incubated with 1 mg/ml sodium borohydride for 15 min. The cells were again washed twice in PBS and blocked in NATS (20% newborn calf serum, 0.5% Tween 20 in PBS) for 30 min. The samples were incubated with anti-HA antibody (1:100) for 2 h, washed four times in PBS, and then incubated with Alexa Fluor 488-conjugated secondary antibody (1:300) for 1 h. The cells were finally washed four times in PBS, dried, and then mounted using a Prolong Antifade kit (Molecular Probes). Images were taken on an Olympus I ×81 microscope (Melville, NY) with ×60 N.A. 1.4 plano optics with a Cooke Sensicam QE air-cooled charge-coupled device camera. Images were collected with IPLab Spectrum 3.6.1 and analyzed, pseudocolored, and merged using NIH Image J (1.34s) software.
Immunogold electron microscopy.
COS cells were transfected and infected as previously described. Eighteen hours postinfection, medium was aspirated, and the cells were fixed using 4% paraformaldehyde plus 0.05% glutaraldehyde in 0.1 M sodium cacodylate buffer, dehydrated through a graded series of ethanol, and embedded in LR White resin (London Resin Company).
Samples on grids were blocked on the surface using 5% bovine serum albumin-1% cold water fish gelatin (BSA-c) for 2 h and then incubated overnight at 4°C with anti-GFP polyclonal antibody (eBioseciences) diluted 1:100 in PBS-0.1% BSA-c, pH 7.4. Labeling was performed with goat anti-mouse 10-nm gold-conjugated secondary antibodies for 2 h at 4°C, and the samples were finally stained with 8% uranyl acetate. Images were taken on a JEOL 1200EX transmission electron microscope at 80 kV.
RESULTS
Detergent insolubility of Vhs is largely infection dependent.
Previously we reported that Vhs in infected cells remains insoluble in detergent (TX-100) as well as in the presence of high NaCl concentrations (21) and was only partially solubilized when treated with both TX-100 and NaCl simultaneously. To further investigate the detergent-insoluble properties of Vhs, we tested whether this insolubilty was infection dependent. We expressed Vhs1—a T214I mutant with reduced RNase activity (11, 28)—in COS cells (as described in Materials and Methods) and subsequently infected the cells with a Vhs-null virus at an MOI of 10 PFU/cell. A control set of transfected cells was mock infected. Eighteen hours postinfection, PNS was prepared from both sets of cells and treated with TX-100 for 30 min on ice. Insoluble material was recovered by centrifugation at 100,000 × g. The supernatant was collected and TCA precipitated, and both the pellet and supernatant were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Vhs was detected by Western blotting using a Vhs antiserum as described previously (21).
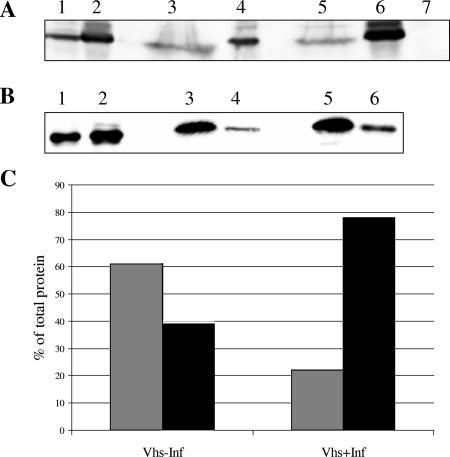
Vhs in the absence of infection was found to be expressed at lower levels than in the presence of infection (Fig. 1A, lanes 1 and 2). The reason for this observation is unknown. Although it maybe possible that Vhs in the absence of infection becomes degraded due to residual RNase activity in Vhs1, there is no evidence for this. It may also be possible that Vhs mRNA is translationally activated by a viral protein or that Vhs is stabilized when directed into the virion assembly pathway. The control GFP was expressed at similar levels in both the presence and absence of infection (Fig. 1B, lanes 1 and 2). In the absence of infection, Vhs was equally distributed between the supernatant and pellet (compare lane 3 containing supernatant and lane 4 containing pellet, Fig. 1A), while in the presence of infection, the majority of Vhs was insoluble (compare lane 5 containing supernatant and lane 6 containing pellet, Fig. 1A). Upon quantitation of the bands, 39% of Vhs was insoluble in the absence of infection, in contrast to 78% in the presence of infection (Fig. 1C). GFP was soluble in both the presence and absence of infection (lanes 3 and 4 containing supernatant and pellet, respectively, in the absence of infection and lanes 5 and 6 containing supernatant and pellet in the presence of infection, Fig. 1B). Hence the insolubility of Vhs was observed to be largely infection dependent.
FIG. 1.
Detergent insolubility of Vhs is largely infection dependent. COS cells were transfected with plasmids to express either Vhs1 or GFP and subsequently infected with Vhs-null virus or mock infected. The postnuclear supernatant was treated with Triton X-100 and subjected to centrifugation at 100,000 × g for 30 min. Proteins were TCA precipitated from the supernatant, and both pellet and supernatant were analyzed by Western blotting for Vhs or GFP. The distribution of (A) Vhs and (B) GFP in the detergent-soluble and -insoluble fractions is shown in the presence (lanes 2, 5, and 6) and absence (lanes 1, 3, and 4) of infection. Shown are results for total lysate (lanes 1 and 2), supernatant (lanes 3 and 5), and pellet (lanes 4 and 6). Lane 7 contained untransfected and mock-infected cell PNS demonstrating specificity of Vhs antisera. (C) Graphical representation of quantitation of the bands in panel A. Gray bars indicate supernatant, and black bars indicate pellet. −Inf, mock infected; +Inf, infected.
Membrane association of Vhs is also infection dependent.
The insoluble material collected in the pellet after a 100,000 × g centrifugation contains some membrane-associated proteins, cytoskeletal elements, and aggregates. We have previously shown that a significant amount of Vhs in infected cells (21) is stably associated with membranes. To investigate whether membrane association of Vhs was affected by other viral factors, Vhs1 was transfected into COS cells, one set of cells was infected and another set was mock infected. PNS was obtained from both set of cells, adjusted to final concentration of 1.4 M sucrose, and layered at the bottom of a sucrose density step gradient (described in Materials and Methods). Following centrifugation and fractionation, the membrane-containing fraction (the interface of the 0.25 M and 1.2 M sucrose layers) and the rest of the gradient were TCA precipitated, separated by 8% SDS-PAGE, and analyzed by immunoblotting for Vhs. GFP was expressed similarly as a negative control.
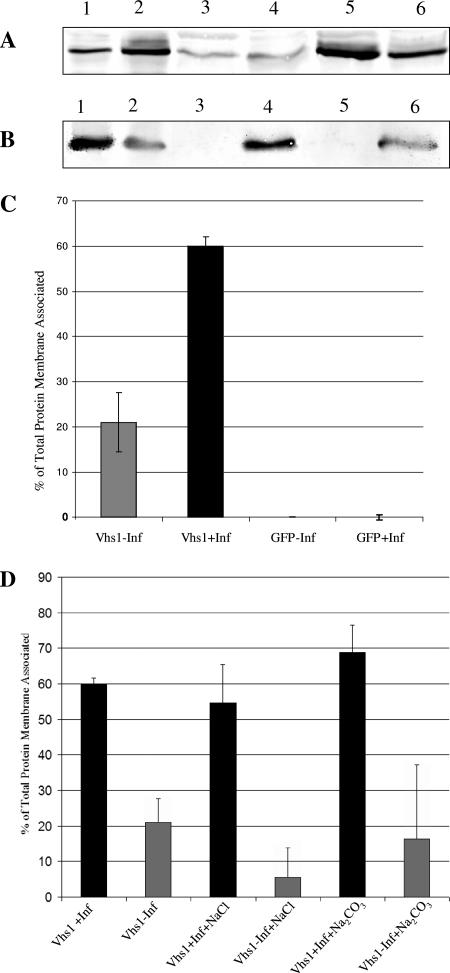
Vhs in the presence of infection (Fig. 2A, lane 5) was observed to be more efficiently membrane associated than Vhs expressed in uninfected cells (Fig. 2A, lane 3). GFP was not membrane associated under either condition (Fig. 2B, lanes 3 and 5). Vhs or GFP in both the membrane fraction and the rest of the gradient was quantitated, and the amount which was membrane associated was expressed as a percentage of the total protein present in the gradient. It was observed that 60% of total Vhs was membrane associated during infection, whereas only 21% was membrane associated in the absence of infection (Fig. 2C). These data are averages of three independent experiments. We conclude that membrane association of Vhs is largely dependent on HSV infection-specific factors.
FIG. 2.
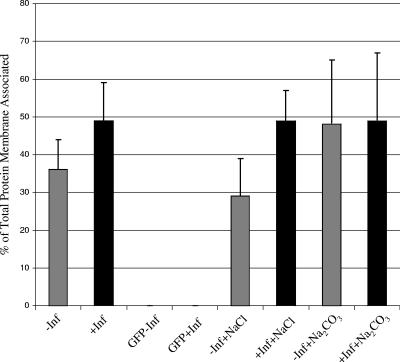
Membrane association of Vhs is largely infection dependent. (A and B) COS cells were transfected with plasmids to express either Vhs1 or GFP and subsequently infected with Vhs-null virus (+Inf) or mock infected (−Inf). The postnuclear supernatant was adjusted to 1.4 M sucrose and loaded at the bottom of a 1.4 M-1.2 M-0.25 M sucrose step gradient. Membranes and membrane-associated proteins at the 0.25 M-1.2 M sucrose interface and also the rest of the gradient were TCA precipitated, and analyzed by Western blotting for Vhs (A) or GFP (B). Lane 1, total lysate in absence of infection; lane 2, total lysate in the presence of infection; lane 3, membrane-associated protein in the absence of infection; lane 4, protein in the rest of the gradient in the absence of infection; lane 5, membrane-associated protein in the presence of infection; lane 6, protein in the rest of the gradient in the presence of infection. (C) Graphical representation of quantitation of the bands in panels A and B. Bands were quantitated by NIH Image J, and the amount of membrane associated is expressed as a percentage of the total. The results are the average of three independent experiments. (D) A similar experiment to those in panels A to C, but prior to gradient centrifugation, the samples were treated with either 0.5 M NaCl or 100 mM Na2CO3 as indicated. Results are the average of three independent experiments.
We have also previously (21) shown that Vhs in infected cells is stably membrane associated even after treatment with 100 mM Na2CO3, which removes most peripherally associated membrane proteins. To further understand the nature of membrane-associated Vhs in the presence and absence of infection, cell lysates were prepared exactly as for Fig. 2A, except that they were treated with 100 mM Na2CO3 or 0.5 M NaCl prior to centrifugation. It was observed that neither treatment had any effect on membrane association of Vhs in the presence of infection (Fig. 2D). However, membrane-bound Vhs in the absence of infection was very sensitive to NaCl treatment, whereas 100 mM Na2CO3 had no effect (Fig. 2B). This suggests that membrane association of Vhs in the absence of infection is mainly due to electrostatic interactions, whereas there are additional salt-resistant mechanisms that stimulate and stabilize membrane binding during a natural HSV infection.
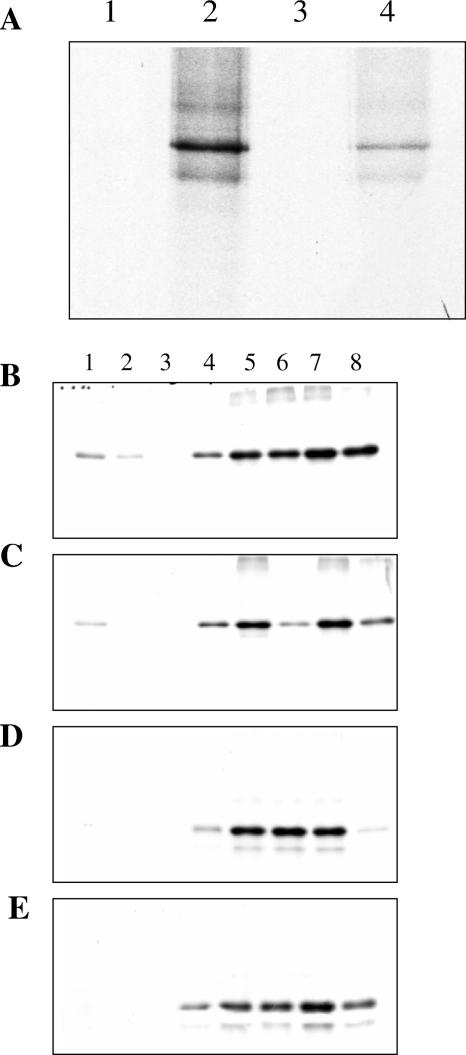
To further dissect the membrane association properties of Vhs, an assay was developed in which in vitro-translated Vhs1 or GFP was incubated with PNS obtained from infected and uninfected cells for 1 h at 37°C, followed by sucrose step gradient centrifugation as described above. It was observed that as expected, in the absence of added PNS, in vitro-translated Vhs1 does not partition into the low-density sucrose interface (Fig. 3A, lanes 1 and 3). Vhs1 with infected cell-derived PNS (Fig. 3B) floated to the membrane-containing fraction and did so slightly more efficiently than when mixed with PNS from uninfected cells (Fig. 3C). Control GFP was not membrane associated under either condition (Fig. 3D and E). It should be noted that these experiments were carried out under the optimal binding conditions determined below (Fig. 4). The amount of Vhs membrane association was quantitated, and 3% was membrane associated when mixed with infected-cell PNS and 2% was membrane associated when mixed with uninfected-cell PNS. The low efficiency can be attributed to the fact that this is an in vitro system where possibly many essential interactions and modifications necessary for Vhs membrane association have not been reconstituted.
FIG. 3.
Membrane association of in vitro-translated Vhs. [35S]methionine-labeled Vhs1 or GFP was prepared by in vitro translation. (A) In duplicate experiments, in vitro-translated Vhs1 was subjected to sucrose density flotation and the interface fraction (lanes 1 and 3) and the rest of the gradient (lanes 2 and 4) were analyzed by SDS-PAGE. (B to E) In vitro-translated Vhs1 (B and C) or GFP (D and E) was incubated with PNS obtained from infected (B and D) and uninfected (C and E) cells and subjected to sucrose density gradient flotation. Fractions were collected and analyzed by SDS-PAGE. Lane 1 corresponds to the membrane fraction, and lane 8 is the bottom of the gradient.
FIG. 4.
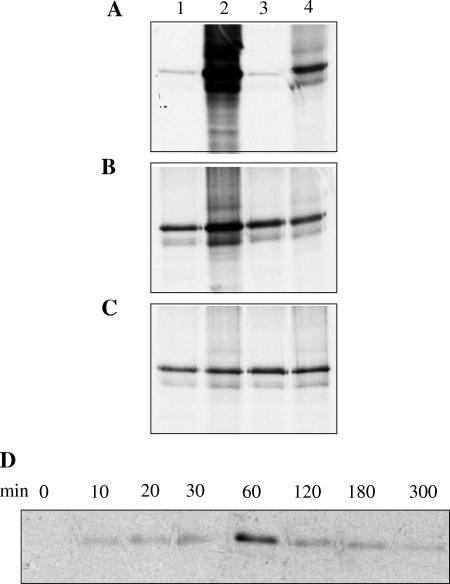
Determination of optimal conditions for membrane association of in vitro-translated Vhs. (A to C) In duplicate experiments, in vitro-translated Vhs1 was incubated with infected-cell PNS for 1 h at 4°C (A), room temperature (B), and 37°C (C) before being subjected to sucrose density gradient centrifugation. The membrane fractions (lanes 1 and 3) and the rest of the gradient (lanes 2 and 4) were analyzed by SDS-PAGE. (D) In vitro-translated Vhs1 was incubated with infected-cell PNS at 37°C for various times before separation of membranes by flotation. The membrane fractions obtained were analyzed by SDS-PAGE.
To further characterize membrane association, in vitro-translated Vhs1 was incubated with infected-cell-derived PNS for 1 h on ice, at room temperature or at 37°C. It was observed that efficient membrane association failed to occur at 4°C (Fig. 4A) but did occur at room temperature and at 37°C (Fig. 4B and C, respectively). Incubation of Vhs with infected-cell-derived PNS for various times followed by sucrose density step gradient centrifugation revealed some membrane binding after as early as 10 min but maximal membrane association after 1 h. Longer times, however, decreased membrane association (Fig. 4D). It is unknown why membrane association decreases with longer incubation. It may be possible that either Vhs itself or some membrane-associated receptor is losing activity over time. Hence the optimum conditions for membrane association were determined to be 1 h at 37°C. These in vitro studies suggest that membrane association of Vhs is optimal at physiological temperatures.
The N-terminal 42 amino acids of Vhs are sufficient for membrane association.
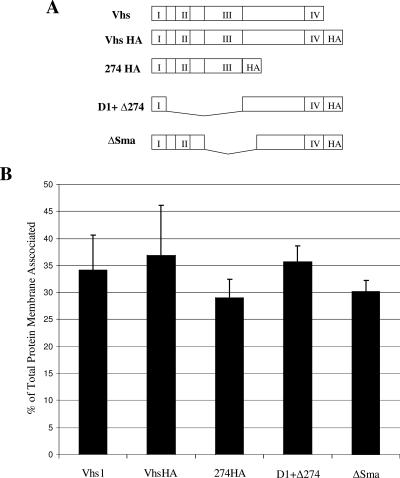
To attempt to identify which specific regions of Vhs were important for membrane association, several mutant forms of Vhs1 were created, as illustrated in Fig. 5A. Four domains (marked as I, II, III, and IV) have been found to be conserved among Vhs in different alphaherpesviruses (2). The Vhs1 mutant 274HA lacks all residues after amino acid 274 and thus retains only domains I, II, and III. A second mutant, ΔSma, carries a deletion from amino acids 147 to 343 and retains domains I, II, and IV only. The third mutant, D1+ Δ274, carries a deletion from amino acids 42 to 274 and hence retains domains I and IV. All of the mutants carried a carboxy-terminal HA tag. Upon transfection, each of these mutants expressed similar amounts of protein (data not shown).
FIG. 5.
Membrane association of Vhs mutants. (A) Schematic representation of Vhs1 mutants. I, II, III, and IV represent conserved domains of Vhs. (B) Graphical representation of membrane association of Vhs1 mutants in the presence of infection. The results are the average of three independent experiments.
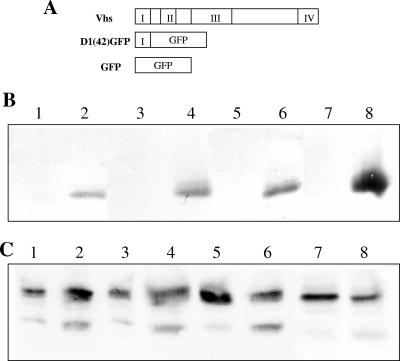
Upon transfection of COS cells to express these mutants and subsequent infection, sucrose density centrifugation was performed as before to separate membrane-bound proteins. Four fractions were collected, and the amount of Vhs membrane associated was expressed as a percentage of the total protein in the gradient. It was observed that all three mutants behaved similarly to wild-type Vhs (Fig. 5B). We noted that there was only one common region in all of these mutants: i.e., the first 42 amino acids of conserved domain 1. To investigate whether these 42 amino acids are sufficient for membrane association of Vhs, a GFP fusion containing the first 42 amino acids of Vhs, termed D1(42)GFP, was generated (Fig. 6A). When D1(42)GFP was transfected into COS cells and membrane association in the presence of infection was tested, it was found to be efficiently membrane associated (Fig. 6C, lanes 5 and 7). The control GFP was not membrane associated (Fig. 6B). Hence the first 42 residues of Vhs are sufficient for membrane association. Membrane association of D1(42)GFP in the absence of infection was also tested. D1(42)GFP was found to be membrane associated in the absence of infection, although less efficiently than in the presence of infection (Fig. 6C, lanes 1 and 3), reflecting similar properties to full-length Vhs. Quantitation of the amount of D1(42)GFP membrane associated in the presence and absence of infection is graphically represented in Fig. 7.
FIG. 6.
Membrane association of D1(42)GFP in the presence and absence of infection. (A) Schematic representation of the D1(42)GFP construct used to analyze domain 1 of Vhs. (B and C) Membrane association, in duplicate, of (B) GFP or (C) D1(42)GFP in the presence (lanes 5 to 8) and absence (lanes 1 to 4) of infection. Lanes 1, 3, 5, and 7 represent the membrane fraction, whereas the corresponding rest of the gradient is in lanes 2, 4, 6, and 8.
FIG. 7.
Membrane association of D1(42)GFP after NaCl or Na2CO3 treatment. Graphical representation of D1(42)GFP membrane association in the presence (+Inf) or absence (−Inf) of infection when treated with either 0.5 M NaCl or 100 mM Na2CO3. Results are the average of two independent experiments. Lanes labeled GFP correspond to membrane association of a GFP control.
To test whether the amino-terminal 42 residues of Vhs reproduce the biochemical characteristics of full-length Vhs binding, cell lysates of D1(42)GFP-expressing cells in both the presence and absence of infection were treated with NaCl or Na2CO3 as described previously. It was observed that, similar to full-length Vhs, membrane association of D1(42)GFP in the presence of infection was not diminished by either treatment. However, in the absence of infection, NaCl treatment reduced membrane association slightly. Na2CO3 treatment, however, had no effect (Fig. 7). We conclude that the first 42 residues of Vhs are sufficient to confer the biochemical properties of Vhs-membrane association upon a GFP reporter.
Colocalization of D1(42)DsRed2 with endogenous Vhs.
We wished to test whether the biochemical membrane association properties of D1(42)GFP meant that it was also subjected to the same intracellular localization as wild-type Vhs. For reasons of antibody availability, these studies were conducted using a red variant of D1(42) made by fusing the amino-terminal 42 residues of Vhs with DsRed2. COS cells were grown on coverslips and transfected to express D1(42)DsRed2 and infected with the HSV strain N138HA (16). The N138HA virus has an HA tag in its vhs gene which is known not to affect its function (16), and hence Vhs could be detected subsequently by immunostaining. Control cells were infected with PAAR5, which expresses wild-type Vhs and hence should not be stained with anti-HA antibody. Eighteen hours postinfection, the cells were fixed in 4% paraformaldehyde and immunostained for HA as described in Material and Methods.
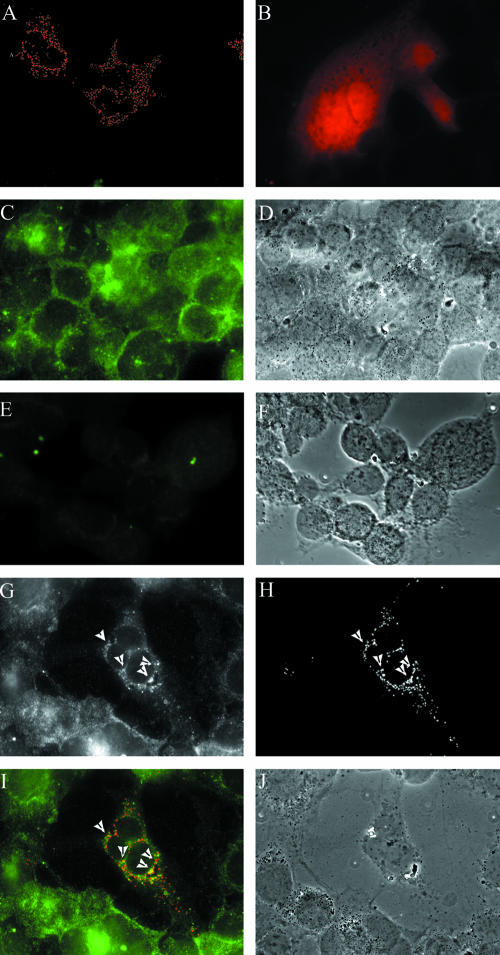
As can be seen in Fig. 8, D1(42)Dsred2 displayed punctate structures in the cytoplasm (Fig. 8A) with hardly any fluorescence present in the nucleus. In contrast, DsRed2 alone had diffuse staining throughout the cytoplasm and nucleus (Fig. 8B). Vhs in N138HA-infected cells was visible in the cytoplasm (Fig. 8C) as both diffuse and punctate staining. The PAAR5-infected control cells (Fig. 8E) showed no staining, demonstrating that all of the staining seen in panel C is Vhs specific. In cells expressing D1(42)DsRed2 and infected with N138HA virus, visualization of Vhs (Fig. 8G) and D1(42)DsRed2 (Fig. 8H) revealed areas of colocalization (arrowheads in panels G and H and yellow regions in merged image, panel I). Interestingly, D1(42)DsRed2 colocalized with punctate Vhs, not the diffuse population of Vhs. This will be discussed later.
FIG. 8.
D1(42)DsRed2 colocalizes with endogenous Vhs. COS cells grown on coverslips were either transfected with D1(42)DsRed2 (A and G to J) or DsRed2 (B) or were mock transfected (C to F) and subsequently infected with virus strain N138HA (C, D, and G to J) or PAAR5 (E and F). The cells were fixed in 4% paraformaldehyde and immunostained for HA as described in Materials and Methods. The images were merged and pseudocolored using Image J software. Panels G and H are the separate green and red channel images of the same field, which has been merged in panel I. Panels D, F, and J are the phase-contrast images of panels C, E, and I, respectively.
The first 42 amino acids of Vhs cause GFP to cofractionate with HSV.
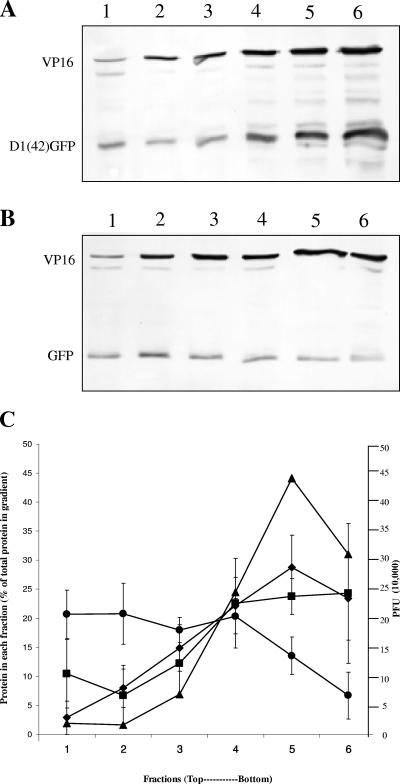
We wished to test whether colocalization of D1(42)DsRed2 with endogenous Vhs and its ability to bind membranes correlated with its assembly into HSV particles. We transfected COS cells to express D1(42)GFP or GFP and subsequently infected them with Vhs-null virus. Eighteen hours postinfection, cells were scraped and taken up along with the media. Virus particles were isolated from the media by a method adapted from Szilagyi et al. (32) using Ficoll gradient ultracentrifugation. Six fractions were collected (as described in Materials and Methods), and the titer of material from each fraction was determined by plaque assay and analyzed by immunoblotting for GFP and VP16.
The distribution of VP16 and D1(42)GFP peaked towards the bottom of the gradient (Fig. 9A), whereas GFP mainly stayed at the top of the gradient (Fig. 9B). As shown in Fig. 9C, the plaque assay titer peaked at fraction 5. Similarly upon quantitation of the Western blots, VP16 also peaked in fraction 5, consistent with this portion of the gradient containing the majority of the viral particles. The profile of D1(42)GFP closely followed that of VP16 and the viral titer, whereas the GFP control was quite different. Most GFP was found at the top of the gradient and trailed off towards the bottom. These results suggest that the first 42 residues of Vhs are sufficient for targeting of GFP into the viral particle.
FIG. 9.
D1(42)GFP cofractionates with extracellular virions. Extracellular virus was isolated from D1(42)GFP or GFP-transfected and infected cells by a method adapted from Szilagyi and Cunningham (32 [described in Materials and Methods]). Six fractions were collected, the titer was determined for PFU, and the fractions were analyzed by Western blotting. (A and B) Western blot of VP16 and D1(42)GFP (A) and VP16 and GFP (B) in each fraction. Lane 1 represents the top of the gradient (fraction 1), and lane 6 represents the bottom (fraction 6). (C) The amount of VP16, D1(42)GFP, or GFP was quantitated in each fraction. Data from three independent experiments are plotted, representing PFU (triangles), VP16 (diamonds), D1(42)GFP (squares), and GFP (circles).
Immunogold electron microscopy to confirm assembly of D1(42)GFP into the HSV particle.
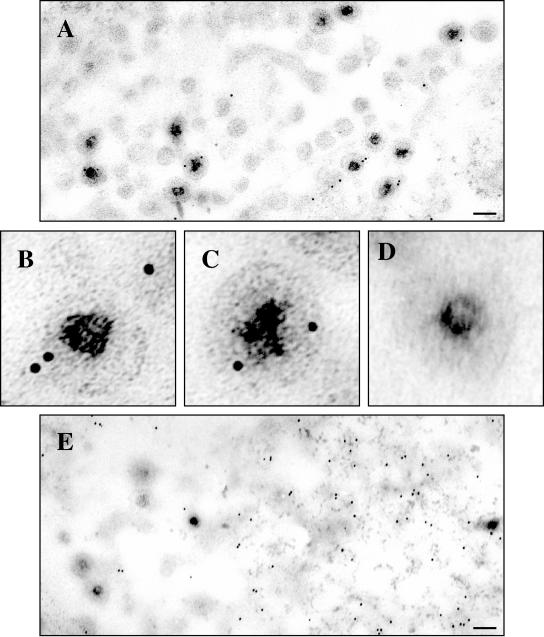
D1(42)GFP or GFP was expressed in COS cells and subsequently infected with vhs-null virus. The cells were fixed and sectioned for immunogold electron microscopy, and GFP was detected with 10-nm gold-conjugated antibodies. As a positive control, COS cells were infected with HSV strain K26GFP (7), which is known to incorporate a VP26-GFP fusion protein into the viral capsid.
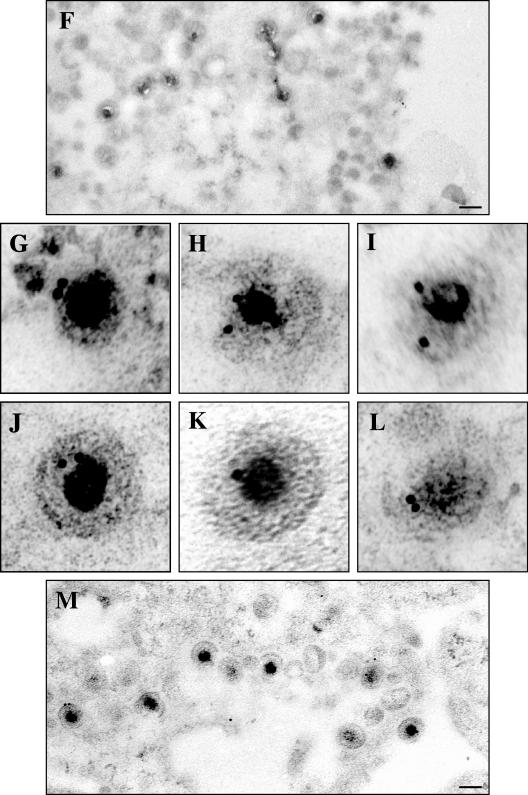
As expected, we saw many virus particles in the cytoplasm labeled with gold in the K26GFP-infected cells (Fig. 10A to C). Cells which had been transfected to express GFP and then infected with Vhs-null virus displayed gold scattered throughout the cytoplasm, which was not associated with virus particles (Fig. 10D and E). In cells expressing D1(42)GFP and subsequently infected, we observed numerous examples of cytoplasmic virus particles labeled with gold (Fig. 10G to M). The no-primary-antibody control (Fig. 10F) represents the specificity of the antibody. Only cytoplasmic particles were studied in each case to facilitate comparison with our earlier immunocytochemical studies (Fig. 8).
FIG.10.
Immunogold electron microscopy confirms assembly of D1(42)GFP into the HSV particle. COS cells were transfected to express D1(42)GFP or GFP and subsequently infected with vhs-null virus. As a positive control, mock-transfected COS cells were infected with K26GFP virus. The cells were fixed and processed for electron microscopy as described in Materials and Methods. GFP and D1(42)GFP were detected using an anti-GFP antibody followed by a secondary antibody conjugated to 10-nm gold particles. (A to C) Cells infected with K26GFP virus. (D and E) Cells transfected with GFP and then infected. (F to M) Cells transfected with D1(42)GFP and then subsequently infected. Panel F represents a control to which no primary antibody was added. Scale bars in panels A, E, F, and M indicate 0.11 μm. Panels B to D and G to L have been enlarged fivefold relative to panels A, E, F, and M.
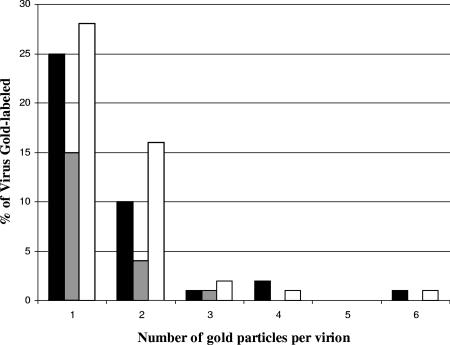
We counted viruses labeled with one to six particles of gold in each of the samples. Totals of 79, 114, and 129 viruses were counted for D1(42)GFP, GFP, and K26GFP, respectively. The distribution of the number of gold particles is graphically represented in Fig. 11. A high number of viruses labeled with one gold particle in cells transfected with GFP is probably due to the ubiquitous distribution of GFP, which is evident in Fig. 8B and 10E. In GFP-expressing cells, far fewer virions were found associated with multiple gold particles. Hence upon elimination of nonspecific gold incorporation by counting only those viruses with two or more gold particles, 18% of the viruses in D1(42)GFP-transfected cells, 16% of the viruses in K26GFP-infected cells, and 4% of the viruses in GFP-transfected cells were positive. Thus it appears that the amino-terminal 42 residues of Vhs are sufficient to mediate incorporation of GFP into the HSV particle, presumably the tegument.
FIG. 11.
Graphical representation of percentage of viruses labeled with one to six gold particles when infection proceeded in the presence of plasmid-expressed D1(42)GFP (black bars) or GFP (gray bars) or during infection by the virus strain K26GFP (white bars). These data are from a total of 79, 114, and 129 virus particles, respectively.
DISCUSSION
In the present study, we have extended our earlier analysis of membrane association properties of the tegument protein Vhs. We have observed that detergent insolubility and membrane association are largely HSV infection dependent. A small fraction of Vhs was seen to associate with membranes in the absence of infection, but unlike infection-dependent binding, it could be eliminated by washing the membranes with NaCl. It is interesting that similar results were reported for the membrane association of the tegument component VP22 in the absence of infection (3). Similar binding characteristics were observed between Vhs and membranes in vitro. Interestingly optimal binding required an hour's incubation at 37°C. Whether this is because Vhs must undergo some biochemical modification to become membrane associated is currently being investigated. We are also currently trying to identify viral candidates that could be responsible for enhanced Vhs membrane association during infection. One possible candidate could be gH, as previous results from our laboratory have shown gH to interact with VP16 (14, 17), which in turn interacts with Vhs (29). It is also possible that higher levels of Vhs expression during infection drive more efficient membrane association due to mass action effects.
Through a combination of deletion analysis and preparation of GFP fusion proteins, we have identified a 42-amino-acid region at the N terminus of Vhs that is sufficient for membrane association, intracellular trafficking, and particle incorporation. These 42 residues lie in conserved domain 1 of many alphaherpesvirus Vhs molecules, including those of HSV-2, cercopithecine herpesviruses 1 and 2, bovine herpesvirus 2, suid herpesvirus 1, pseudorabies virus, and equine herpesvirus 4. In contrast, the C-terminal conserved region of VP22 has been identified as important for membrane association, virion incorporation, and interaction with VP16 (3, 15). We observed that D1(42)GFP had similar properties to full-length Vhs. D1(42)GFP was more efficiently and stably associated with membranes from infected rather than uninfected cells, and membrane binding in the absence of infection could be disrupted by NaCl treatment. The biochemical membrane binding data correlated with the observation that D1(42)DsRed2 colocalized with a subpopulation of virally expressed Vhs. We propose that partial colocalization is a consequence of the fact that full-length Vhs has a variety of functions: it is an RNase, binds to cellular translation factors, binds to VP16, and also must be assembled into tegument. We propose that the first 42 amino acids mediate tegument incorporation and that other functions of Vhs have been eliminated. Hence the regions of colocalization that we see are either mature viral particles or regions where viral assembly is taking place. Consistent with this, immunogold electron microscopy confirmed the incorporation of D1(42)GFP into the viral particle. Since our immunocytochemical studies suggest that the subcellular localization of D1(42)DsRed2 is similar in both infected and uninfected cells, our current working model is that the amino terminus of Vhs is sufficient for the correct subcellular localization of the protein, but during the course of an infection additional HSV-encoded factors stimulate or stabilize membrane binding and guide Vhs into the viral assembly pathway. This is consistent both with the increased membrane association and steady-state level of Vhs during an infection. Nevertheless, at the moment this is highly speculative.
It is also interesting to note that as a further test of importance of domain 1 in membrane association and tegument incorporation, a Vhs mutant lacking the first 42 amino acids was created. This mutant failed to be expressed (data not shown), indicating that the first conserved domain of Vhs also plays a role in the stability of the protein.
A small domain able to direct molecules into the tegument could be useful in the field of drug delivery and may also enable the tegument targeting of factors able to disrupt HSV assembly. Further work in our laboratory is being carried out to identify the smallest sequence capable of directing efficient targeting into the HSV tegument.
Acknowledgments
This work was supported by NIH grants RO1AI38265 and RO1CA80723 to D.W.W.
The HSV Vhs-null, PAAR5, and N138HA strains were kind gifts from James Smiley, and strain K26GFP was kindly provided by Prashant Desai. We thank Lily Huang for excellent technical assistance.
REFERENCES
- 1.Baines, J. D., R. J. Jacob, L. Simmerman, and B. Roizman. 1995. The herpes simplex virus 1 UL11 proteins are associated with cytoplasmic and nuclear membranes and with nuclear bodies of infected cells. J. Virol. 69:825-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berthomme, H., B. Jacquemont, and A. Epstein. 1993. The pseudorabies virus host-shutoff homolog gene: nucleotide sequence and comparison with alphaherpesvirus protein counterparts. Virology 193:1028-1032. [DOI] [PubMed] [Google Scholar]
- 3.Brignati, M. J., J. S. Loomis, J. W. Wills, and R. J. Courtney. 2003. Membrane association of VP22, a herpes simplex virus type 1 tegument protein. J. Virol. 77:4888-4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi, J. H., C. A. Harley, A. Mukhopadhyay, and D. W. Wilson. 2005. The cytoplasmic tail of herpes simplex virus envelope glycoprotein D binds to the tegument protein VP22 and to capsids. J. Gen. Virol. 86:253-261. [DOI] [PubMed] [Google Scholar]
- 5.Church, G. A., and D. W. Wilson. 1997. Study of herpes simplex virus maturation during a synchronous wave of assembly. J. Virol. 71:3603-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clase, A. C., M. G. Lyman, T. del Rio, J. A. Randall, C. M. Calton, L. W. Enquist, and B. W. Banfield. 2003. The pseudorabies virus Us2 protein, a virion tegument component, is prenylated in infected cells. J. Virol. 77:12285-12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai, P., and S. Person. 1998. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J. Virol. 72:7563-7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doepker, R. C., W.-L. Hsu, H. A. Saffran, and J. R. Smiley. 2004. Herpes simplex virus virion host shutoff protein is stimulated by translation initiation factors eIF4B and eIF4H. J. Virol. 78:4684-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duerst, R. J., and L. A. Morrison. 2004. Herpes simplex virus 2 virion host shutoff protein interferes with type I interferon production and responsiveness. Virology 322:158-167. [DOI] [PubMed] [Google Scholar]
- 10.Everly, D. N., Jr., P. Feng, I. S. Mian, and G. S. Read. 2002. mRNA degradation by the virion host shutoff (Vhs) protein of herpes simplex virus: genetic and biochemical evidence that Vhs is a nuclease. J. Virol. 76:8560-8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everly, D. N., Jr., and G. S. Read. 1997. Mutational analysis of the virion host shutoff gene (UL41) of herpes simplex virus (HSV): characterization of HSV type 1 (HSV-1)/HSV-2 chimeras. J. Virol. 71:7157-7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng, P., D. N. Everly, Jr., and G. S. Read. 2005. mRNA decay during herpes simplex virus (HSV) infections: protein-protein interactions involving the HSV virion host shutoff protein and translation factors eIF4H and eIF4A. J. Virol. 79:9651-9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuchs, W., B. G. Klupp, H. Granzow, C. Hengartner, A. Brack, A. Mundt, L. W. Enquist, and T. C. Mettenleiter. 2002. Physical interaction between envelope glycoproteins E and M of pseudorabies virus and the major tegument protein UL49. J. Virol. 76:8208-8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross, S. T., C. A. Harley, and D. W. Wilson. 2003. The cytoplasmic tail of Herpes simplex virus glycoprotein H binds to the tegument protein VP16 in vitro and in vivo. Virology 317:1-12. [DOI] [PubMed] [Google Scholar]
- 15.Hafezi, W., E. Bernard, R. Cook, and G. Elliott. 2005. Herpes simplex virus tegument protein VP22 contains an internal VP16 interaction domain and a C-terminal domain that are both required for VP22 assembly into the virus particle. J. Virol. 79:13082-13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones, F. E., C. A. Smibert, and J. R. Smiley. 1995. Mutational analysis of the herpes simplex virus virion host shutoff protein: evidence that vhs functions in the absence of other viral proteins. J. Virol. 69:4863-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamen, D. E., S. T. Gross, M. E. Girvin, and D. W. Wilson. 2005. Structural basis for the physiological temperature dependence of the association of VP16 with the cytoplasmic tail of herpes simplex virus glycoprotein H. J. Virol. 79:6134-6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klupp, B. G., W. Fuchs, H. Granzow, R. Nixdorf, and T. C. Mettenleiter. 2002. Pseudorabies virus UL36 tegument protein physically interacts with the UL37 protein. J. Virol. 76:3065-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klupp, B. G., H. Granzow, E. Mundt, and T. C. Mettenleiter. 2001. Pseudorabies virus UL37 gene product is involved in secondary envelopment. J. Virol. 75:8927-8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam, Q., C. A. Smibert, K. E. Koop, C. Lavery, J. P. Capone, S. P. Weinheimer, and J. R. Smiley. 1996. Herpes simplex virus VP16 rescues viral mRNA from destruction by the virion host shutoff function. EMBO J. 15:2575-2581. [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, G. E., G. A. Church, and D. W. Wilson. 2003. A subpopulation of tegument protein vhs localizes to detergent-insoluble lipid rafts in herpes simplex virus-infected cells. J. Virol. 77:2038-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loomis, J. S., J. B. Bowzard, R. J. Courtney, and J. W. Wills. 2001. Intracellular trafficking of the UL11 tegument protein of herpes simplex virus type 1. J. Virol. 75:12209-12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loomis, J. S., R. J. Courtney, and J. W. Wills. 2003. Binding partners for the UL11 tegument protein of herpes simplex virus type 1. J. Virol. 77:11417-11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu, P., F. E. Jones, H. A. Saffran, and J. R. Smiley. 2001. Herpes simplex virus virion host shutoff protein requires a mammalian factor for efficient in vitro endoribonuclease activity. J. Virol. 75:1172-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mettenleiter, T. C. 2006. Intriguing interplay between viral proteins during herpesvirus assembly or: the herpesvirus assembly puzzle. Vet. Microbiol. 113:163-169. [DOI] [PubMed] [Google Scholar]
- 26.Murphy, J. A., R. J. Duerst, T. J. Smith, and L. A. Morrison. 2003. Herpes simplex virus type 2 virion host shutoff protein regulates alpha/beta interferon but not adaptive immune responses during primary infection in vivo. J. Virol. 77:9337-9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nozawa, N., T. Daikoku, T. Koshizuka, Y. Yamauchi, T. Yoshikawa, and Y. Nishiyama. 2003. Subcellular localization of herpes simplex virus type 1 UL51 protein and role of palmitoylation in Golgi apparatus targeting. J. Virol. 77:3204-3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Read, G. S., and N. Frenkel. 1983. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of α (immediate early) viral polypeptides. J. Virol. 46:498-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smibert, C. A., B. Popova, P. Xiao, J. P. Capone, and J. R. Smiley. 1994. Herpes simplex virus VP16 forms a complex with the virion host shutoff protein vhs. J. Virol. 68:2339-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, T. J., L. A. Morrison, and D. A. Leib. 2002. Pathogenesis of herpes simplex virus type 2 virion host shutoff (vhs) mutants. J. Virol. 76:2054-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strelow, L. I., and D. A. Leib. 1995. Role of the virion host shutoff (vhs) of herpes simplex virus type 1 in latency and pathogenesis. J. Virol. 69:6779-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szilagyi, J. F., and C. Cunningham. 1991. Identification and characterization of a novel non-infectious herpes simplex virus-related particle. J. Gen. Virol. 72:661-668. [DOI] [PubMed] [Google Scholar]
- 33.Zhou, Z. H., D. H. Chen, J. Jakana, F. J. Rixon, and W. Chiu. 1999. Visualization of tegument-capsid interactions and DNA in intact herpes simplex virus type 1 virions. J. Virol. 73:3210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]