Abstract
Fcγ receptor (FcγR)-mediated entry of infectious dengue virus immune complexes into monocytes/macrophages is hypothesized to be a key event in the pathogenesis of complicated dengue fever. FcγRIA (CD64) and FcγRIIA (CD32), which predominate on the surface of such dengue virus-permissive cells, were compared for their influence on the infectivity of dengue 2 virus immune complexes formed with human dengue virus antibodies. A signaling immunoreceptor tyrosine-based activation motif (ITAM) incorporated into the accessory γ-chain subunit that associates with FcγRIA and constitutively in FcγRIIA is required for phagocytosis mediated by these receptors. To determine whether FcγRIA and FcγRIIA activation functions are also required for internalization of infectious dengue virus immune complexes, we generated native and signaling-incompetent versions of each receptor by site-directed mutagenesis of ITAM tyrosine residues. Plasmids designed to express these receptors were transfected into COS-7 cells, and dengue virus replication was measured by plaque assay and flow cytometry. We found that both receptors mediated enhanced dengue virus immune complex infectivity but that FcγRIIA appeared to do so far more effectively. Abrogation of FcγRIA signaling competency, either by expression without γ-chain or by coexpression with γ-chain mutants, was associated with significant impairment of phagocytosis and of dengue virus immune complex infectivity. Abrogation of FcγRIIA signaling competency was also associated with equally impaired phagocytosis but had no discernible effect on dengue virus immune complex infectivity. These findings point to fundamental differences between FcγRIA and FcγRIIA with respect to their immune-enhancing capabilities and suggest that different mechanisms of dengue virus immune complex internalization may operate between these FcγRs.
The interaction between virus and antibody ordinarily leads to neutralization, but the infectivity of some antibody-coated viruses may be enhanced if susceptible cells bear Fcγ receptors (FcγR). This apparent paradox is of particular interest with respect to the dengue viruses: serious forms of dengue fever, manifested by heightened viremia levels and generalized microvascular leak syndromes (53), have been linked to enhanced infection of monocytes/macrophages by dengue virus immune complexes (10, 19). The nature of enhancing antibodies has been widely investigated using primary monocytes/macrophages or macrophage-like cell lines that express FcγR. Receptor properties that might affect immune enhancement, however, have received comparatively much less attention largely because heterogeneous FcγR display on such cells complicates the interpretation of experimental results.
FcγR comprise a multigene family of integral membrane glycoproteins that exhibit complex activation or inhibitory effects on cell functions after aggregation by complexed immunoglobulin G (IgG) (3, 34, 40, 45). Here, we are concerned with two activatory human FcγR of different classes and with distinctive but overlapping distribution among monocytes known to be permissive to dengue virus infection. The first, FcγRIA (CD64), is a 72-kDa protein found exclusively on antigen-presenting cells of macrophage and dendritic cell lineages, most of which are permissive to dengue virus replication (6, 23, 57). FcγRIA exhibits high affinity for monomeric IgG1 and exists bound to this immunoglobulin in vivo. The second, FcγRIIA (CD32), is a 40-kDa protein unique to humans and more broadly distributed among a variety of myelogenous cell types. It has low affinity for monomeric IgG, preferentially binding multivalent IgG (27). Each FcγR is composed of three domains: an extracellular domain of two (FcγRIIA) or three (FcγRIA) IgG-like domains, a short hydrophobic transmembrane region, and a cytoplasmic tail. A conserved immunoreceptor tyrosine-based activation motif (ITAM) links each FcγR to tyrosine kinase-activated signaling pathways that modulate cell metabolism and physical behavior when triggered by receptor clustering (5, 25, 49, 50). FcγRIA acquires this function by noncovalent association with the γ-chain subunit, a short (ca. 11-kDa) transmembrane ITAM-containing homodimer (22). FcγRIIA, unlike other Fc receptors and most immunoreceptors, incorporates the ITAM into its ligand binding chain.
Signal transduction triggered by ligand engagement is intimately involved in the phagocytosis of IgG-opsonized particles where the molecular details of FcγRIA and FcγRIIA signaling have been revealed in exquisite detail (8, 17, 18, 25, 49). A signaling requirement for the entry of infectious virus immune complexes following FcγR engagement is less certain and has been rarely studied. One view is that FcγR may facilitate the entry of dengue virus immune complexes by simply concentrating them onto a putative dengue virus receptor, in essence a passive effect that leads to internalization and infection perhaps uninfluenced by FcγR signal transduction (26). Conversely, evidence of differential immune enhancement levels among FcγR or for modulation of dengue virus immune complex infectivity by FcγR-triggered signaling would have important implications with respect to mechanisms of dengue neutralization and dengue fever pathogenesis.
FcγRIA and FcγRIIA have previously been shown to facilitate antibody-mediated dengue enhancement in human macrophage-like cells by using surrogate plaque assays to measure virus replication (20, 24) since dengue virus does not form plaques in such cells (38). Here, we have examined the relative efficiency with which each of these receptors individually enhances dengue virus immune complex infectivity and have inquired whether signal transduction competency plays a role. Our strategy for answering these fundamental questions surrounding the immune enhancement phenomenon involved the expression of native and mutant forms of human γ-chain/FcγRIA and FcγRIIA in dengue virus-permissive COS cells in which dengue virus immune enhancement was directly measured by conventional plaque assay. We found that the infectivities of dengue virus immune complexes were strikingly greater after the engagement of FcγRIIA than after that of FcγRIA and that signaling competency was required for optimally enhanced infectivity subserved by FcγRIA but apparently not for that subserved by FcγRIIA.
MATERIALS AND METHODS
Cells and dengue viruses.
African green monkey kidney-derived COS-7 (fibroblast) or Vero (epithelial) cells were grown in Dulbecco's modified Eagle's medium (DMEM) or MEM, respectively. THP-1 cells kindly provided by Melanie Wellington (University of Rochester, Rochester, NY) were grown in RPMI in stationary culture. C6/36 Aedes albopictus mosquito cells were grown at 28°C in MEM supplemented with sodium pyruvate and nonessential amino acids. Media were supplemented with fetal bovine serum, and cells were grown in a 5% CO2 atmosphere. Virulent strain 16681 dengue 2 virus (11) and strain New Guinea C (NGC) dengue 2 virus, attenuated by multiple passage in suckling mouse brain (41), were gifts of Walter Brandt and Tadeusz Kochel (Walter Reed Army Institute of Research, Washington, DC, and U.S. Naval Medical Research Center, Bethesda, MD, respectively). Each virus was propagated in mosquito cells and its titer determined by plaque assay in Vero cells.
Dengue virus antibodies.
Convalescent anti-dengue virus sera from Thai or Puerto Rican dengue fever patients, gifts from Eric Henchal (Armed Forces Institute for Medical Research, Bangkok, Thailand) and Gladys Sather (Centers for Disease Control, Puerto Rico), respectively, were pooled using equal amounts from each of six subjects; the pool exhibited broad dengue virus serotype neutralizing and hemagglutination-inhibiting activity. IgG1 mouse monoclonal antibodies (MAbs) (J. J. Schlesinger, unpublished) against dengue 2 virus NS1 (MAb 9A9) or envelope E protein (MAb 7E1) were used to detect dengue virus replication in plaque or flow cytometry assays, respectively. MAb 7E1 was purified by affinity chromatography (Pierce Chemicals) and labeled with Alexa 647 (Molecular Probes, Invitrogen Corp.) according to the manufacturers' instructions.
Construction of signaling-competent and signaling-incompetent γ-chain/FcγRIA or FcγRIIA vectors.
Human FcγRIA (30) and γ-chain (22) cDNA were generously provided by Clark L. Anderson (Ohio State University, Columbus, OH) and Jean-Pierre Kinet (Harvard University, Cambridge, MA), respectively. FcγRIIA (H131 allotype) (49) was provided by Jan G. J. van de Winkel (University Hospital Utrecht). To arrange for coordinated expression of FcγRIA with γ-chain, we used a PCR-based strategy to construct a bicistronic expression cassette in a pcDNA5/FLP recombination target (FRT) backbone that contained the coding sequences for γ-chain and FcγRIA in the upstream and downstream positions, respectively, separated by an internal ribosomal entry site derived from encephalomyocarditis virus and expressed under the control of the cytomegalovirus immediate-early promoter. Control constructs were generated by site-directed mutagenesis using standard methods (QuikChange II; Stratagene, La Jolla, CA) for inserting stop codons within γ-chain or FcγRIA. Similar methods were used to generate constructs that contained single, double, or triple ITAM tyrosine residue mutations in multiple permutations. FcγRIIA was also generated in the pcDNA5/FRT backbone in monocistronic form. Sequences for all constructs were verified by DNA sequence analysis.
Transient Fcγ receptor expression in COS cells.
Purified recombinant plasmids were transfected into COS cell monolayers by using standard methods (Lipofectamine 2000; Invitrogen, Carlsbad, CA). Cell cultures were trypsinized 48 h after transfection, washed with phosphate-buffered saline (PBS), and kept on ice for immediate use. FcγR expression was verified by rosette assay using sheep red blood cells (SRBC) opsonized with rabbit IgG anti-SRBC. The percentage of cells expressing FcγR was assessed by counting SRBC rosettes in a hemacytometer and by flow cytometry.
Flow cytometry.
THP-1 cells and COS transfectants were washed with PBS and stained with R-phycoerythrin (PE)-conjugated IgG1 monoclonal antibodies against human FcγRIA (CD64 MAb 10.1; eBioscences, San Diego, CA) or FcγRIIA (CD32 MAb AT10; Serotec, Raleigh, NC) using an R-PE-labeled IgG1 isotype control from the corresponding manufacturer. Stained cells were fixed with 1% paraformaldehyde and analyzed by FACSCalibur using CellQuest software (BD Immunocytometry Systems, Franklin Lakes, NJ); a minimum of 20,000 events was collected from each sample for analysis. The number of FcγRIA or FcγRIIA molecules expressed on the surface of COS transfectants and THP-1 cells was determined by a quantitative immunofluorescence method that employed standardized QuantiBRITE-PE beads (BD Pharmingen, San Jose, CA), following the manufacturer's instructions. Briefly, the fluorescent intensity of PE-labeled beads was used to establish a standard curve. The number of cell surface FcγRIA and/or FcγRIIA molecules per cell was then extrapolated from the standard after subtracting for background staining of the IgG1 isotype control. Dengue virus-infected COS cells were quantified by flow cytometry after direct staining of fixed permeabilized cells with Alexa 647-labeled MAb 7E1 against dengue 2 virus envelope E protein.
Western blotting.
COS cell transfectants were lysed in Tris saline buffer containing 1% NP-40 and protease inhibitors. Soluble proteins were reduced in Laemmli buffer and separated by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis for transfer to nitrocellulose membranes and immunoblotting with rabbit IgG anti-human γ-chain (Upstate Cell Signaling Solutions, Lake Placid, NY) and chemiluminescence-based detection (ECL; Amersham Biosciences, Piscataway, NJ). γ-Chain abundance was quantified by gel-scanning densitometry using ImageQuant version 5.2 software (Molecular Dynamics).
Measurement of opsonized particle binding and phagocytosis.
Sheep red blood cells were sensitized with a subagglutinating dilution of rabbit IgG anti-sheep RBC and incubated with transfected COS cells in suspension for 18 h at room temperature before being counted in a hemacytometer chamber; SRBC rosettes were expressed as the percentage of cells with at least three SRBC bound. Surface binding and phagocytosis of opsonized Candida albicans were distinguished by a previously described quantitative double-fluorescence method (55). Briefly, heat-killed yeast cells were stained with fluorescein isothiocyanate (FITC) and sensitized with rabbit antiserum. To measure phagocytosis, transfected COS cells were incubated with opsonized FITC-labeled yeast particles for 45 min at 4°C followed by low speed centrifugation and incubation for 45 min at 37°C before being counterstained with ethidium bromide. In parallel, mixtures of COS transfectants and yeast particles were centrifuged and incubated at 4°C to determine cell surface binding. Cell surface-bound yeast particles were counterstained yellow by ethidium bromide, but internalized FITC-stained yeast particles continued to fluoresce green since ethidium bromide cannot penetrate viable cells. The phagocytic activity of COS transfectants and THP-1 cells was expressed as the phagocytic index, the number of opsonized yeast particles ingested per 100 FcγR-expressing cells (25). Cell preparations were photographed at a magnification of ×40 with an Olympus BX41TF fluorescence microscope equipped with a digital camera using Qcapture 2.0 software. Images were prepared in Adobe Photoshop CS.
Measurement of dengue 2 virus replication by plaque assay.
Preformed dengue virus immune complexes were prepared by incubating mixtures of serially diluted virus or human pooled dengue virus antibody, in checkerboard fashion, for 75 min at 37°C before mixing with 2 × 105 trypsinized COS transfectants suspended in 24-well polystyrene cluster plates. After overnight incubation at 37°C, cell monolayers were washed with PBS and overlaid with 0.6% agarose (SeaKem GTG; FMC BioProducts, Rockland, ME). Agarose plugs were removed 3 days later, and cells were fixed with a 1:1 (vol/vol) acetone-methanol mixture. Dengue virus plaques developed with anti-dengue 2 virus NS1 MAb and a nickel-horseradish peroxidase-based detection method (Vectastain ABC kit; Vector Laboratories, Burlingame, CA), were counted with the aid of a 10× magnifying glass or by scanning the cluster plate into Adobe Photoshop CS for further magnification. In some assays, the addition of fresh medium to the washed COS transfectant monolayers substituted for the agarose overlay. At various times, supernatants were collected for virus titration in Vero cells and cell monolayers trypsinized for analysis by flow cytometry.
Statistics.
Student's t test and analysis of variance were performed using MS Excel and SigmaStat software, v3.0 (SPSS, Inc.), respectively.
RESULTS
Signaling-competent and signaling-incompetent FcγRIA and FcγRIIA expressed in COS cells.
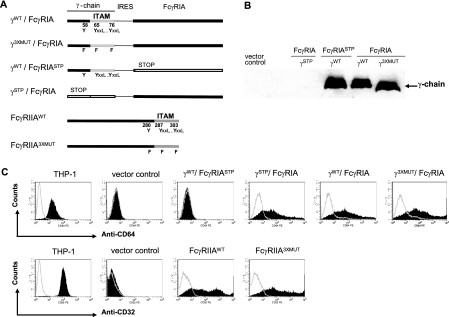
FcγRIIA exists in two functionally different allelic forms that are determined by a single His/Arg residue at position 131 (54); we selected the H131 form for the present studies because it, unlike the R131 form, efficiently binds IgG2 in addition to the other human IgG subclasses. The sequence for each gene construct was verified by comparing it with that published in GenBank. The FcγRIA and FcγRIIA gene constructs used in these experiments are presented in Fig. 1A. Earlier investigations that defined a γ-chain signaling requirement for FcγRIA-mediated phagocytosis by COS transfectants have employed separate vectors or FcγRIA-γ-chain chimeras to express these genes (17, 25). To ensure uniform FcγRIA and γ-chain coexpression in transfected cells, we incorporated the respective genes into a bicistronic vector, with γ-chain being inserted upstream of FcγRIA so that FcγRIA transfectants detected by rosette formation with opsonized particles or by flow cytometry using anti-CD64 monoclonal antibodies were also sure to contain γ-chain. This gene arrangement would be predicted to result in expression of γ-chain in excess of FcγRIA (roughly by a factor of 2), presumably due to the inherent inefficiency of ribosomal entry (13, 29). A stoichiometric γ-chain excess was desired since each transmembrane FcγRIA monomer associates with a γ-chain dimer to form the functional complex. It also served to increase our confidence that cells exhibiting surface expression of FcγRIA by flow cytometry would also likely contain γ-chain which is largely intracellular and therefore cannot be detected by this method without permeabilization. Stop codons were inserted into the FcγRIA or γWT-chain sequence of bicistronic constructs (Fig. 1A) to provide control vectors. The γ-chain cytoplasmic tail incorporates three tyrosine residues: one (Y58) upstream of the ITAM and two (Y65 and Y76) in the ITAM. The FcγR IIA cytoplasmic tail has an analogous tyrosine residue distribution (upstream Y280; ITAM Y287 and Y303). Earlier molecular dissection of phagocytosis indicated that the upstream non-ITAM tyrosine residue also contributed to this function (14, 18, 31). To ensure the abrogation of the signaling competency of the γ-chain/FcγRIA and FcγRIIA constructs, we therefore mutated the three potentially activating tyrosine residues of each receptor (γ3XMUT/FcγRIA and FcγRIIA3XMUT). γ-Chain expression was assessed by Western blotting (Fig. 1B); equivalence of γ-chain abundance among the γ-chain transfectants was confirmed by scanning densitometry. Flow cytometry was used to verify and to measure FcγR expression and to determine the number of FcγR molecules on the cell surface. THP-1 cells, a human monocyte line that constitutively expresses FcγRIA and FcγRIIA exclusively (9), were used as a control (Fig. 1C and Table 1). The percentages of FcγRIA- and FcγRIIA-expressing COS cells were comparable within the panel of transfectants (Table 1) and were at least two- to threefold higher than those previously obtained using a DEAE-dextran transfection method (25, 42). THP-1 cells expressed ∼5,500 FcγRIA and ∼58,000 FcγRIIA surface molecules per cell, amounts that are in agreement with published data (9). The number of FcγRIA or FcγRIIA molecules expressed on COS cells was not affected by Tyr-to-Phe mutations in the associated γ-chain or FcγRIIA cytoplasmic tails, respectively. The average number of cell surface FcγRIIA molecules (∼43,000) was greater than that of FcγRIA molecules associated with γ-chain (∼30,000), but this difference was not statistically significant (P > 0.10; two-tailed t test). Remarkably, the number of surface FcγRIA molecules was ∼50% higher (P < 0.03; two-tailed t test) when this receptor was associated with γ-chain than when it was expressed without it.
FIG. 1.
Structure and expression of γ-chain/FcγRIA complex and FcγRIIA versions in COS transfectants. (A) The order of γ-chain and FcγRIA genes in the bicistronic construct ensured that FcγRIA-expressing COS cells also expressed the γ-chain (γWT/FcγRIA). An encephalomyocarditis virus-derived internal ribosomal entry site (IRES) drives the internal initiation of the FcγRIA gene. Other genes are expressed under the control of a cytomegalovirus immediate-early promoter. Stop codons inserted into the FcγRIA or γWT-chain sequence of bicistronic constructs provided control vectors. FcγRIIA was cloned into the same pcDNA5/FRT to generate a monocistronic construct (FcγRIIAWT and FcγRIIA3XMUT). A consensus Kozak sequence was introduced upstream of the γ-chain and FcγRIIA genes. Tyrosine residue positions in ITAMs of γ-chain and FcγRIIA are numbered, starting from the +1 start. (B) Solubilized lysates prepared from 2.5 × 105 cells of each COS transfectant were electrophoresed and subjected to Western blotting using a monospecific rabbit serum against human γ-chain (22). (C) PE-labeled CD32 (MAb AT10) or CD64 (MAb 10.1) monoclonal antibodies and PE-labeled mouse IgG1 were used to measure the proportions of COS transfectants expressing the respective FcγR. The THP-1 human macrophage cell line served as a control. Results are representative of five or six determinations for FcγRIA transfectants and three determinations for FcγRIIA transfectants (Table 1).
TABLE 1.
Expression of human FcγRIA (CD64) and FcγRIIA (CD32) in COS-7 cells analyzed by flow cytometry
| FcγR | % of positive cells (mean ± SD) | No. of molecules/cell (mean ± SD) | No. of determinations |
|---|---|---|---|
| CD64 | |||
| THP-1 | 94 ± 4 | 5,487 ± 3,840 | 9 |
| Vector control | 0 | 0 | 7 |
| γWT | 0 | 0 | 3 |
| FcγRIA | 75 ± 5 | 17,031 ± 5,771a | 5 |
| γWT/FcγRIA | 79 ± 6 | 28,453 ± 8,530 | 6 |
| γ3XMUT/FcγRIA | 84 ± 5 | 31,227 ± 10,872 | 6 |
| CD32 | |||
| THP-1 | 94 ± 4 | 57,644 ± 9,610 | 3 |
| Vector control | 0 | 0 | 3 |
| FcγRIIAWT | 59 ± 12 | 41,435 ± 8,138 | 3 |
| FcγRIIA3XMUT | 66 ± 13 | 44,561 ± 8,389b | 3 |
P < 0.03 (FcγRIA versus γWT/FcγRIA or γ3XMUT/FcγRIA).
P > 0.10 (FcγRIIA versus γWT/FcγRIA or γ3XMUT/FcγRIA).
FcγR surface expression and signaling competency verified by binding and phagocytosis of opsonized yeast particles.
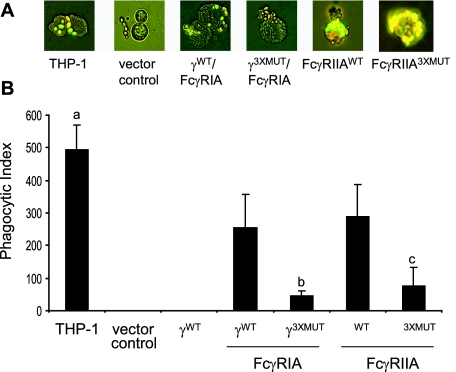
Signaling-incompetent FcγRIA or FcγRIIA transfectants bind opsonized SRBC but are not efficiently phagocytic (17, 25, 48). If properly constructed, our γWT/FcγRIA and FcγRIIAWT COS transfectants were expected to perform both functions, whereas transfectants appropriately mutated or expressing FcγRIA without γ-chain were expected to only bind IgG-coated particles. We measured binding and phagocytosis of IgG-opsonized yeast particles by COS cells that expressed FcγRIA or FcγRIIA to verify receptor functional activity and used phagocytic THP-1 cells as a control. Fluorescence microscopy has been validated as an accurate method for estimating phagocytosis (25). To simultaneously measure FcγR-mediated binding and phagocytosis, we adopted a quantitative double- fluorescence method that employed IgG-opsonized, FITC-stained Candida albicans particles and ethidium bromide counterstaining to distinguish between THP-1 cell surface-bound and internalized particles (55). Figure 2A illustrates the appearance of surface-bound (yellow) or internalized (green) fluorescent-stained yeast particles after incubation with THP-1 cells or COS cells that expressed γ/FcγRIA or FcγRIIA. COS cells transfected with the control empty vector or γWT/FcγRIASTP did not bind opsonized particles. In accord with receptor expression measured by flow cytometry (Table 1), more THP-1 cells (∼90%) bound opsonized yeast particles than did the FcγR transfectants, where the levels were similar (50 to 60%). Comparable results were obtained with opsonized SRBC (data not shown). COS cells expressing FcγR versions that were predicted to be signaling-competent (γWT/FcγRIA or FcγRIIAWT) exhibited significantly greater phagocytic capacity than did cells expressing the respective signaling-incompetent versions (γ3XMUT/FcγRIA or FcγRIIA3XMUT) (Fig. 2B).
FIG. 2.
Binding and phagocytosis of opsonized C. albicans by COS cells expressing FcγRIA or FcγRIIA. Rabbit IgG-sensitized, fluorescein isothiocyanate-stained yeast particles were incubated with COS cells expressing signal-competent (γWT/FcγRIA and FcγRIIAWT) or signal-incompetent (γ3XMUT/FcγRIA, γSTP/FcγRIA, and FcγRIIA3XMUT) FcγR. COS cells expressing γ-chain only or transfected with the pcDNA5/FRT vector served as controls. Phagocytosis by human macrophage-like THP-1 cells that express both FcγR was measured in parallel in each experiment. Binding and phagocytosis of opsonized C. albicans were measured using a quantitative double-fluorescence technique that employed ethidium bromide to selectively stain cell-bound (yellow) but not internalized (green) fluorescein isothiocyanate-stained yeast particles (see Materials and Methods). (A) Immunofluorescent photomicrographs (×40) of FcγR and control cells incubated with opsonized yeast particles. (B) Phagocytosis is expressed as the phagocytic index, the number of internalized yeast particles per 100 FcγR-expressing COS cells. P values: a, P < 0.02 (THP-1 versus γWT/FcγRIA or FcγRIIAWT); b, P < 0.03 (γ3XMUT/FcγRIA versus γWT/FcγRIA); c, P < 0.003 (FcγRIIA3XMUT versus FcγRIIAWT). Results are the means and standard deviations for three individual experiments with FcγRIA and four individual experiments with FcγRIIA, performed in duplicate.
Collectively, our results indicated that the FcγR and γ-chain genes of interest were properly constructed, correctly expressed, and functional with respect to binding and internalization of IgG-opsonized particles. Having confirmed that these properties of a professional macrophage were conferred on COS cells, we next investigated their interaction with dengue virus immune complexes.
Dengue virus immune complex infectivity is greater in COS cells expressing FcγRIIA than in those expressing FcγRIA.
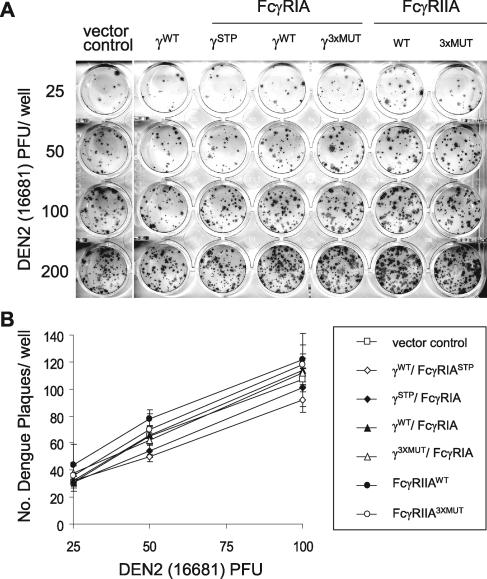
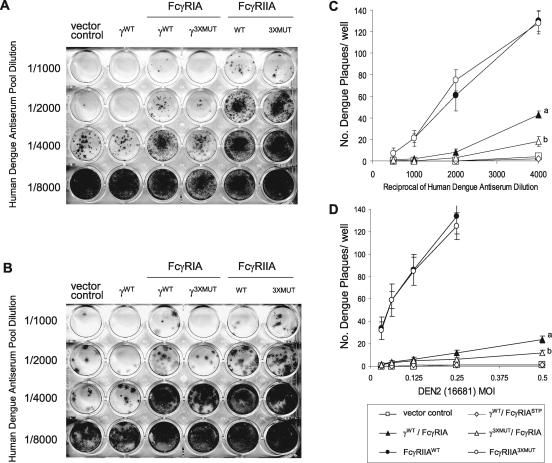
Pooled human anti-dengue virus convalescent-phase sera with broad dengue virus serotype-neutralizing and hemagglutination-inhibiting capacity were used to prepare infectious dengue 2 virus immune complexes for presentation to the respective COS FcγR transfectants. This polyclonal serum pool, prepared from serologically screened American and Asian dengue fever patients, likely represents broad dengue virion antigenic specificity and IgG subclass diversity (47), so that any differences in results among COS transfectants should confidently reflect behavior specific to the respective FcγR. We used two strains of dengue 2 virus to prepare immune complexes: (i) a virulent strain, 16681, isolated from a patient with dengue hemorrhagic fever/shock syndrome during a South Asian epidemic that was marked by a high prevalence of complicated dengue fever (11) and (ii) the prototypic attenuated strain, New Guinea C (NGC) (41). We measured the infectivities of preformed dengue 2 virus immune complexes in FcγRIA- or FcγRIIA-expressing COS cells by a conventional flavivirus plaque reduction neutralization assay method performed by infecting cells in suspension (32). Cells were thus continuously exposed to virus immune complexes during the initial monolayer formation. Strain 16681 dengue 2 virus produced small (<1 mm), relatively homogeneous, and sharply defined plaques in COS cells, whereas those formed by the NGC strain were larger (2 mm) and more irregular. The efficiencies of dengue 2 virus plaque formation, in the absence of antibodies, were comparable among the FcγR and control (empty vector, γWT/FcγRIASTP) transfectants (Fig. 3). Figure 4 shows the relative infectivities of strain 16681 (panel A) and NGC (panel B) dengue 2 virus immune complexes, respectively, in signaling-competent (γWT/FcγRIA and FcγRIIAWT) or signaling-incompetent (γ3XMUT/FcγRIA and FcγRIIA3XMUT) COS transfectants. COS cells transfected with the pcDNA5/FRT “empty” vector or with γ-chain only (γWT/FcγRIASTP) served as controls. In 10 such experiments performed in duplicate or triplicate, the infectivities of partially neutralized dengue virus immune complexes were enhanced in both γ/FcγRIA- and FcγRIIA-expressing COS cells, but this effect was consistently and strikingly greater in FcγRIIA than in FcγRIA transfectants. The abrogation of FcγRIA signaling competency by mutation of all γ-chain cytoplasmic-tail Tyr residues led to reduced dengue virus immune complex infectivity, but the mutation of the analogous FcγRIIA cytoplasmic tyrosine residues had no apparent effect. We observed no difference between the two dengue virus strains with respect to the degrees of enhanced immune complex infectivity among the FcγR transfectants. To further compare the relative importance of signal transduction capacity for FcγRIA- and FcγRIIA-mediated enhancement, we presented immune complexes formed with a range of antibody and dengue 2 virus concentrations to COS cells expressing the respective native and mutant receptors (Fig. 4C and D). We used strain 16681 dengue 2 virus for these experiments because its distinctive plaque morphology allowed for more-precise counting than did strain NGC, and we adjusted the antibody and virus concentrations such that the numbers of plaques produced were in a range that permitted comparative counting among the respective COS transfectants in the same assay. Enhanced immune complex infectivity was observed only in FcγR-expressing cells (independent of receptor signaling competency) compared to what was found for control transfectants. Dengue virus immune complex infectivity was significantly greater in FcγRIIA- than in FcγRIA-expressing COS cells over the range of virus and antibody concentrations examined. Complete abrogation of signaling competency significantly diminished FcγRIA-enhanced infection but, remarkably, had no discernible effect on immune complex infectivity enhanced by FcγRIIA engagement.
FIG. 3.
Efficiencies of plaquing are comparable among COS transfectants. COS transfectants were infected with serial twofold (25 to 200) PFU dengue 2 virus (16681) in the absence of antibody. (A) Plaques were detected by indirect immunostaining with a dengue 2 virus NS1-specific monoclonal antibody. (B) The efficiencies of virus plaque formation were comparable (P = 0.17) among FcγRIA, FcγRIIA, and control (empty vector, γWT/FcγRIASTP) COS transfectants at each virus MOI. There were too many plaques to count at the 200-PFU input. Results are representative of three experiments performed in quadruplicate.
FIG. 4.
Infectivity of the virulent strain 16681 or attenuated strain New Guinea C dengue 2 virus immune complex is enhanced in COS cells that express FcγRIA or FcγRIIA. Virus-antibody complexes, prepared with serially diluted human dengue virus antiserum and dengue 2 virus (16681 [A] and NGC [B]), were added to signaling-competent (γWT/FcγRIA and FcγRIIAWT) or signaling-incompetent (γ3xMUT/FcγRIA; FcγRIIA3xMUT) Fc receptor-expressing COS cells. COS cells expressing γ-chain only (γWT/FcγRIASTP) or cells transfected with the empty pcDNA5/FRT vector served as negative controls. Plaques were detected by indirect immunostaining with a dengue 2 virus NS1-specific monoclonal antibody. Results are representative of 10 individual experiments performed in duplicate or triplicate. (C) Dengue 2 virus (16681) immune complexes were prepared by incubating virus at a single MOI (0.025) with serially diluted human dengue virus antiserum. (D) Immune complexes prepared with a single antibody dilution (1/1,000) and serial virus MOIs were added to signaling-competent (γWT/FcγRIA and FcγRIIAWT) or signaling-incompetent (γ3XMUT/FcγRIA and FcγRIIA3XMUT) Fc receptor-expressing COS cells. Cells expressing γ-chain only (γWT/FcγRIASTP) or those transfected with the pcDNA5/FRT vector served as negative controls. Plaques were detected by indirect immunostaining with a dengue 2 virus NS1-specific monoclonal antibody. For FcγRIIA, plaques corresponding to an MOI of 0.5 were too numerous to count. P values were determined using a two-tailed t test. a, γWT/FcγRIA versus γ3XMUT/FcγRIA; b, γ3XMUT/FcγRIA versus γWT/FcγRIASTP and vector controls. For panel C: a, P < 0.001; b, P < 0.03. For panel D: a, P < 0.005; b, P < 0.007. Results are the means and standard deviations for an experiment performed in quadruplicate and are representative of three individual experiments performed in triplicate or quadruplicate.
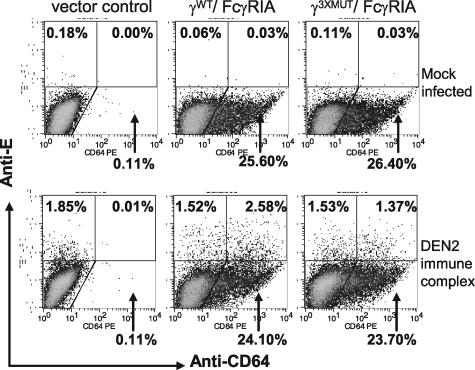
The modulating effect of a signaling-competent γ-chain on FcγRIA-mediated infection was consistent and significant but relatively small (∼2-fold) in this series of experiments. Therefore, to further verify the effect, we performed three additional experiments. First, we used flow cytometry as an independent method for comparing dengue virus immune complex infectivities between COS cells that expressed FcγRIA associated with a signaling-competent or a signaling-incompetent γ-chain (Fig. 5). COS transfectants were mixed with preformed dengue virus immune complexes or were mock infected in suspension. After overnight incubation, cell monolayers were washed to remove residual virus, and the medium was replenished. Two days later (5 days following transfection), cell monolayers were trypsinized and cells were stained with fluorescence-labeled MAbs against either FcγRIA (CD64) or dengue virion envelope E glycoprotein. The proportions of γWT/FcγRIA- and γ3XMUT/FcγRIA-expressing cells were essentially equivalent (ca. 25%) but substantially lower than when infection was initiated, 2 days after transfection (ca. 80%) (Table 1), reflecting expression decay and the possibly preferential growth of the nonexpressing cell population. The proportions of infected cells detected among non-FcγRIA-expressing cells in the three COS transfectants were also roughly equivalent (1.52% to 1.85%). In contrast, the proportion of dengue virus infected cells expressing γWT/FcγRIA was approximately twofold greater than that of cells expressing γ3XMUT/FcγRIA. This observation was highly consistent with results obtained by direct plaque assay.
FIG. 5.
Infection of signaling-competent and -incompetent, FcγRIA-expressing COS cells measured by flow cytometry. COS cells expressing γWT/FcγRIA or γ3XMUT/FcγRIA and control vector COS transfectants were infected with preformed dengue virus immune complexes (MOI, 0.25; dengue virus antiserum dilution, 1/4,000) or mock infected and analyzed by flow cytometry 2 days postinfection with Alexa 647-labeled anti-dengue 2 virus envelope E protein (7E1) or PE-labeled anti-CD64 monoclonal antibodies, respectively.
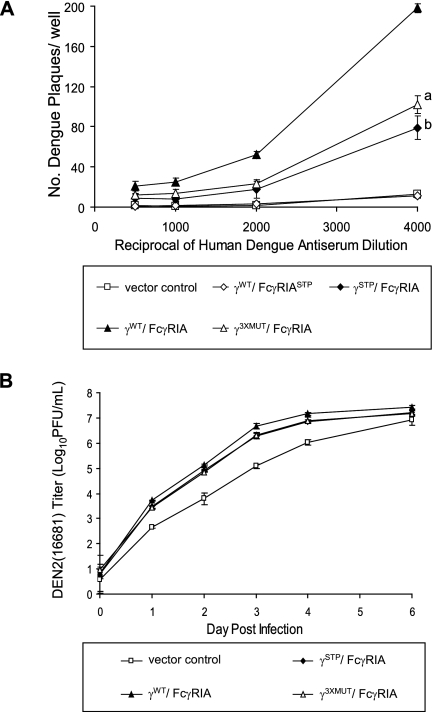
Second, we compared the relative infectivities of dengue virus immune complexes by direct plaque assay in COS cells expressing FcγRIA without γ-chain or FcγRIA associated with signaling-competent or signaling-incompetent γ-chain (Fig. 6A); a virus concentration (multiplicity of infection [MOI], 0.25) and antibody dilution range (1/500 to 1/4,000) that resulted in plaque amounts that approximated those observed with FcγRIIA were chosen (Fig. 4C and D). The infectivities of dengue virus immune complexes were up to ∼20-fold greater in signaling-competent, FcγRIA-expressing COS cells than in control COS transfectants. Immune complex infectivity was also significantly enhanced in signaling-incompetent COS cells that expressed FcγRIA without γ-chain or with a mutated γ-chain (P < 0.001). FcγRIA-mediated enhancement was greater when associated with signaling-incompetent γ-chain than without a γ-chain, but the difference was not statistically significant (P = 0.06).
FIG. 6.
FcγRIA bereft of γ-chain mediates dengue virus immune enhancement. Dengue 2 virus (16681) immune complexes prepared by incubating virus at a single MOI (0.25) and human dengue virus antiserum dilution (1/500 to 1/4,000 [A] and 1/4,000 [B]) were incubated in cluster plates overnight with trypsinized COS cells expressing FcγRIA alone (γSTP/FcγRIA) or in association with native (γWT/FcγRIA) or signaling-incompetent γ-chain (γ3XMUT/FcγRIA). After overnight incubation, an agarose overlay was added to the monolayered cells (A) or monolayers were washed to remove residual virus and replenished with fresh medium (B). In the experiment whose results are shown in panel A, agarose plugs were removed on the second postinfection day and plaques were detected by indirect immunostaining with a dengue 2 virus NS1-specific monoclonal antibody. Each condition was tested in quadruplicate. a, P = 0.06 (γ3XMUT/FcγRIA versus γSTP/FcγRIA); b, P < 0.001 (γSTP/FcγRIA versus vector control). In the experiment whose results are shown in panel B, supernatants were removed in their entirety from the respective wells daily and dengue virus was titrated by plaque assay in Vero cells. Virus concentrations are expressed as the log10 means ± standard deviations for an experiment performed in triplicate and titrated in duplicate. P < 0.003 (vector control versus γWT/FcγRIA, γ3XMUT/FcγRIA, and γSTP/FcγRIA); P < 0.015 (γWT/FcγRIA versus γ3XMUT/FcγRIA or γSTP/FcγRIA).
Third, we performed an experiment that compared virus replication in FcγRIA-expressing and control cells at multiple time points after immune complex inoculation by using a surrogate amplification plaque assay with Vero cells (Fig. 6B). Here, the respective COS transfectants were infected in triplicate with preformed dengue virus immune complexes in 12-well cluster plates (a single plate for each assay day) by using the same protocol as that for the direct plaque assay but omitting the agarose overlay. After overnight incubation, cell monolayers were washed to remove residual virus and were replenished with fresh medium before the first supernatant was collected (day 0) for virus titration. Supernatants were collected in their entirety daily thereafter and stored at −80°C for subsequent titration, in duplicate, in Vero cells. Figure 6B shows an experiment that measured virus replication over the course of 6 days. The results were in accord with those obtained by direct plaque assay with FcγRIA-expressing or control cells, with the highest levels of immune complex infectivity observed in cells expressing signaling-competent γWT/FcγRIA. Immune complex infectivity was up to ∼40-fold greater with the γWT/FcγRIA transfectant than with vector control cells. Significantly enhanced immune complex infectivity was also observed with FcγRIA alone or in association with mutated γ-chain, but at a lower (up to ∼15-fold) level. There was no significant difference between γSTP/FcγRIA and γ3XMUT/FcγRIA. These differential effects were observed up to 4 days after infection. Collectively, the results of these experiments indicated a hierarchy of immune complex infectivity in COS FcγRIA transfectants, in the following order: γWT/FcγRIA > γ3XMUT/FcγRIA ≥ γSTP/FcγRIA > vector control.
FcγRIA-mediated phagocytosis and immune complex infectivity are proportionately reduced by selective γ-chain mutation.
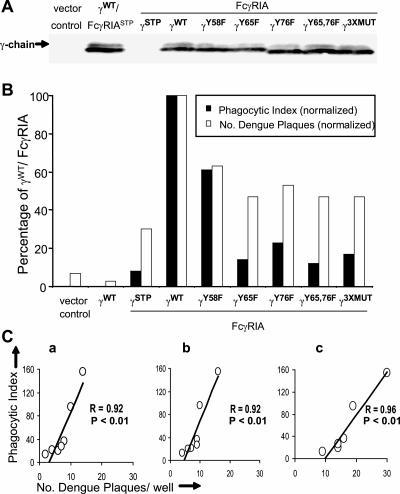
Previous molecular dissection of phagocytosis by COS cells that expressed FcγRIA-γ-chain chimeras revealed a hierarchy of effects of γ-chain Tyr-to-Phe residue changes on phagocytosis (18). To test the hypothesis that FcγRIA-mediated phagocytosis and dengue virus immune complex infectivity involve a common mechanism, we prepared a panel of bicistronic vectors consisting of FcγRIA and γ-chain versions with selected tyrosine residue mutations and measured phagocytosis and dengue virus immune complex infectivity in parallel within this COS transfectant panel. COS cells transfected with the empty vector or those expressing only γ-chain (γWT/FcγRIASTP) or FcγRIA (γSTP/FcγRIA) served as controls. Equivalent FcγRIA expression levels among the FcγRIA transfectants were verified by flow cytometry; in accord, the γ-chain abundances measured by Western blotting and densitometry were comparable among the respective COS transfectants (Fig. 7A). We observed equivalent bindings of opsonized yeast particles among the COS cells that expressed FcγRIA (data not shown). The quantitative phagocytoses of opsonized yeast particles and relative infectivities of dengue virus immune complexes among the COS transfectants are shown in Fig. 7B. COS transfectants that did not express FcγRIA exhibited no phagocytic activity. The highest phagocytic indices were observed in COS cells that expressed FcγRIA associated with γ-chain in native form. COS cells that expressed FcγRIA unassociated with γ-chain had the lowest receptor surface densities and exhibited only trivial phagocytosis. Single or double γ-chain ITAM tyrosine mutations were accompanied by up to ∼10-fold reduction in phagocytic activity. Mutation of the γ-chain non-ITAM tyrosine residue (Y58F) also led to a modest reduction in phagocytic activity. Dengue virus immune complex infectivity was essentially neutralized in control COS cells that did not express FcγRIA. Immune complex infectivity was increased more than 10-fold in COS cells that expressed FcγRIA associated with γ-chain in native form. Infectivity was also significantly (P < 0.05; two-tailed t test) increased in COS cells that expressed FcγRIA without a γ-chain, but at a much lower level. All γ-chain ITAM mutations and the γ-chain deletion (γSTP/FcγRIA) led to a modest (30 to 50%), but significant (P < 0.05), reduction in immune complex infectivity compared to that observed with the γWT/FcγRIA transfectant. Point mutation of upstream Tyr residue 58 was associated with reduced immune complex infectivity-enhancing activity that was not significant (P > 0.05) and that did not add to the effect of the ITAM mutations. These comparative findings were consistent over a range of virus and antibody concentrations (data not shown). Both single and double Tyr-to-Phe mutations of the γ-chain tail ITAM were accompanied by a parallel reduction in phagocytosis and immune complex infectivity. To discern whether phagocytosis and immune complex internalization might have similar mechanisms, we performed a linear regression analysis and found a highly significant correlation (P < 0.01) between phagocytic and immune enhancement capacities within the COS transfectant panel incubated with dengue virus immune complexes formed with serial MOIs of dengue virus (0.25, 0.5, and 1.0) and dengue virus antiserum (1/1,000) (Fig. 7C). Collectively, these data point to a shared pathway for phagocytosis and enhanced dengue virus immune complex infectivity mediated by signaling-competent FcγRIA and a second, somewhat less efficient entry mechanism that operates simply by FcγRIA engagement.
FIG. 7.
FcγRIA-mediated phagocytosis and dengue virus immune complex infectivity are proportionately reduced by selective γ-chain mutation. COS cells were transfected with bicistronic vectors composed of γ-chain alone (γWT/FcγRIASTP), FcγRIA alone (γSTP/FcγRIA), or FcγRIA and γ-chain, in which its cytoplasmic tail residues (Y58, Y65, and Y76) were individually or multiply (γY65,76 and γ3XMUT) mutated by Tyr-to-Phe residue substitution. Results are from an experiment performed in triplicate. (A) γ-Chain abundance determined by Western blotting. Solubilized lysates prepared from 2.5 × 105 cells of each COS transfectant were electrophoresed and subjected to Western blotting using a monospecific rabbit serum against human γ-chain (22). (B) Phagocytoses of opsonized yeast particles and infectivities of dengue virus immune complexes. COS cells were transfected with γ-chain/FcγRIA versions or FcγRIA only. COS cells transfected with an empty vector or expressing γ-chain only (γWT/FcγRIASTP) served as controls. The phagocytic index was defined as the number of yeast particles internalized by 100 FcγRIA-expressing COS cells. In parallel, the respective COS transfectants were incubated with dengue 2 virus (16681) immune complexes formed with pooled human dengue virus antiserum (1/1,000) and serial concentrations of dengue virus; results for an experiment performed in triplicate with a virus MOI of 1.0 are shown. For both assays, the results were normalized against those for the γWT/FcγRIA transfectant. All γ-chain ITAM mutations and the γ-chain deletion (γSTP/FcγRIA) led to significant (P < 0.05, two-tailed t test) reductions in immune complex infectivity compared to that observed with the γWT/FcγRIA transfectant. (C) Correlation between phagocytosis and infectivity among dengue virus immune complexes formed with dengue virus antisera (1/1,000 dilution) and dengue 2 virus with MOIs of 0.25 (a), 0.50 (b), and 1.0 (c).
DISCUSSION
FcγR-mediated phagocytosis, internalization of relatively large opsonized particles, and endocytosis, internalization of soluble immune complexes, are biologically distinguishable processes. For both, ligand-clustered receptors are internalized, but only for phagocytosis does FcγR signaling competency appear essential for completion of the entry process. For example, cells expressing FcγRIA bereft of a γ-chain or FcγRIIA with tail ITAM alterations may internalize soluble IgG complexes but not opsonized particles (4, 25, 35, 48). Virus immune complexes are interesting ligands in this respect, since their sizes and infectivities depend on the nature and quantity of coating antibody (2, 37) and they may have access to other routes of internalization that utilize virus receptors which themselves may trigger signaling events (44).
In this study, we compared the influence of FcγRIA and FcγRIIA on the infectivities of dengue virus immune complexes prepared with human neutralizing dengue virus antibodies. Signaling-competent and signaling-incompetent versions of these receptors were expressed in dengue virus-permissive COS cells to discern whether dengue virus immune complex internalization, like that of opsonized large particles, depended on the receptors' activation properties.
Our approach using COS transfectants to measure dengue virus immune complex infectivity after Fc receptor engagement offered a number of advantages over studies that have employed macrophages or macrophage-like cell lines. First, FcγRIA and FcγRIIA were examined individually in isolation from other FcγR classes or unrelated macrophage receptors that may alter their function on such cells (3, 33, 36). Second, FcγRIA and FcγRIIA concentrations on the surfaces of COS transfectants were comparable (∼30,000 to 40,000 molecules per cell), which is generally not the case for monocytes/macrophages in which the abundance of these receptors is differentially regulated by inflammatory mediators and affected by culture conditions (15, 51), e.g., the surface concentrations of FcγRIIA on unstimulated THP-1 cells were ∼10-fold higher than those of FcγRIA (Table 1). Our determinations of FcγRIA and of FcγRIIA COS cell surface concentrations were within the range reported for FcγR on human peripheral blood monocytes/macrophages (15, 51). Interestingly, we found that FcγRIA surface concentrations were significantly higher when this receptor was associated with γ-chain than without it, in accord with the γ-chain requirement for efficient FcγRIA assembly and surface expression in vivo (45, 46, 52) and in contrast to the reduced FcγRIA expression in COS cells when separate vectors were used to deliver FcγRIA and γ-chain genes (30). That the pattern of FcγRIA expression in our transfectants appeared to more closely reflect the in vivo condition suggests that a precise stoichiometry between γ-chain and FcγRIA may be required for faithful receptor display and that our bicistronic vector design satisfied this requirement. Since FcγRIA and γ-chain are noncovalently linked at the transmembrane level (12, 17, 30), it is unlikely that mutations in the γ-chain cytoplasmic domain affected this association; that the FcγRIA surface concentration was the same when associated with a native or mutated γ-chain lent further support to this conclusion. The abrogation of Fc receptor signaling competency was verified by the significant reduction in phagocytic activity upon ITAM mutation. Serial Tyr-to-Phe mutations in the γ-chain cytoplasmic tail resulted in reduced phagocytic activities for yeast particles by FcγRIA/γ-chain complex-expressing COS cells, similar to those for SRBC by COS cells expressing the receptor complex in the form of a chimeric protein (18). Low-level yeast internalization observed among COS fibroblasts expressing signaling-incompetent FcγR transfectants was not too surprising, since many fibroblast cell types display “nonprofessional” phagocytic properties without Fc receptors (39) and COS cells that expressed FcγRIA without γ-chain have exhibited similar levels of opsonized SRBC internalization (25). It is also quite possible that yeast particles may be inherently more susceptible to internalization than SRBC when concentrated onto the fibroblast cell surface by FcγR engagement.
The extracellular portion of FcγRIA was earlier reported to be sufficient for increased dengue virus immune complex infectivity in COS cells, but a concurrent γ-chain modulating effect was not observed (42). This result was postulated due to reduced receptor density that attended cotransfection with γ-chain and FcγRIA in separate vectors. In the present experiments, we have revisited the question of a possible γ-chain role in dengue virus immune enhancement by using a significantly more efficient transfection method that resulted in two- to threefold-higher expression levels and bicistronic vectors designed to ensure uniform coexpression of FcγRIA and γ-chain versions among the cotransfectants which we have confirmed biochemically and by flow cytometry. This new methodology provided robust assays capable of more-precise discrimination of the relatively small differences (<3-fold) in dengue virus immune complex infectivity observed among the FcγRIA/γ-chain constructs compared to the much larger differences (>10-fold) that we observed between FcγRIA and FcγRIIA. Using three complementary methods to determine dengue virus immune complex infectivity in FcγRIA-expressing COS cells—direct plaque assay, surrogate virus replication assay, and flow cytometry—we found that enhanced immune complex infectivity mediated by FcγRIA was greatest when the receptor was associated with a γ-chain in its native form and that abrogation of γ-chain ITAM signaling capacity by Tyr-to-Phe mutation reduced but did not entirely eliminate this function. We also confirmed that FcγRIA bereft of a γ-chain is sufficient for enhancement. We interpret our results with FcγRIA as reflecting at least two virus immune complex internalization mechanisms at work. The first is a γ-chain signaling-dependent event wherein infectious virus immune complex aggregates of sufficient size triggered a classical phagocytosis entry pathway. This mechanism is suggested by the correlation between phagocytic capacity and immune complex infectivity among COS cells that expressed FcγRIA associated with γ-chain ITAM mutants. Indeed, antibody-virus complexes, including opsonized flaviviruses, can form lattice structures of considerable size (2, 7), so that for dengue virus (50-nm diameter), immune complexes composed of as few as 10 virions, i.e., a 500-nm “particle,” might be predicted to trigger phagocytosis (1). The second is a somewhat less efficient entry mechanism that did not require γ-chain activation (or, indeed, its presence) and relied simply on concentrating partially neutralized virions onto the cell for entry by a parallel endocytosis mechanism. Importantly, we observed no effect of isolated γ-chain expression on virus or virus immune complex infectivity, arguing against the enhanced replication explained by γ-chain association with a cell protein other than FcγRIA.
FcγRIIA was strikingly more efficient than FcγRIA in enhancing dengue virus immune complex infectivity. Abolishing FcγRIIA ITAM signaling competency led to impaired phagocytosis, but unlike with signaling-incompetent FcγRIA, immune enhancement appeared to be unaffected. Our experiments do not offer an immediate explanation for the divergent findings with these FcγR. FcγRIA and FcγRIIA exhibit differences in relative affinity for IgG subclasses, but it seems somewhat unlikely that skewed anti-dengue 2 virus isotype distribution accounted for the difference in Fc receptor behavior since dengue virus-specific IgG1 and IgG3, which have been found represented in the greatest abundances among IgG subclasses in dengue virus convalescent-phase sera (47), are bound by both receptor types with similar or identical affinities (45, 56). FcγRIIA preferentially binds immune complexes and exhibits a high dissociation rate constant (27), whereas FcγRIA preferentially binds monomeric IgG with notably high affinity. Ligand-clustered Fc receptors, including FcγRIIA, are known to concentrate in cell membrane regions, e.g., lipid rafts, rich in a variety of signaling molecules and potential virus receptor engagement sites (16, 21, 28, 43). It seems reasonable to speculate that FcγRIIA is better equipped than is FcγRIA to utilize alternative signaling pathways and entry mechanisms made available by relocation to such sites where weakly bound immune complexes might be more easily transferred to favorable entry pathways. Bispecific monoclonal antibodies that directed dengue virus to FcγRIIA or non-Fc receptor proteins on the surfaces of U937 human macrophage-like cells enhanced infection, arguably by such an alternate entry mechanism (26).
Our findings emphasize the conditional nature of virus neutralization or enhancement by antibody and suggest an approach for further investigation of an aspect of dengue virus-antibody interaction that is tied to both the protective and the pathological immune response to infection by this virus. Further studies are in progress to explain the apparent divergence in immune enhancement between FcγR and to discover to what extent epitope specificity and affinity in the IgG antibodies that comprise the dengue virus immune complex play a role in the outcome of Fc receptor engagement.
Acknowledgments
We thank Lihua Rong and Huiyuan Chen for expert technical assistance.
This work was supported by grants from the Pediatric Dengue Vaccine Initiative (TR03/04 to J.J.S. and TR16 to X.J.).
REFERENCES
- 1.Aderem, A., and D. M. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593-623. [DOI] [PubMed] [Google Scholar]
- 2.Almeida, J. D., and A. P. Waterson. 1969. The morphology of virus-antibody interaction. Adv. Virus Res. 15:307-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daeron, M. 1997. Fc receptor biology. Annu. Rev. Immunol. 15:203-234. [DOI] [PubMed] [Google Scholar]
- 4.Davis, W., P. T. Harrison, M. J. Hutchinson, and J. M. Allen. 1995. Two distinct regions of FC gamma RI initiate separate signalling pathways involved in endocytosis and phagocytosis. EMBO J. 14:432-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duchemin, A. M., L. K. Ernst, and C. L. Anderson. 1994. Clustering of the high affinity Fc receptor for immunoglobulin G (Fc gamma RI) results in phosphorylation of its associated gamma-chain. J. Biol. Chem. 269:12111-12117. [PubMed] [Google Scholar]
- 6.Fanger, N. A., K. Wardwell, L. Shen, T. F. Tedder, and P. M. Guyre. 1996. Type I (CD64) and type II (CD32) Fc gamma receptor-mediated phagocytosis by human blood dendritic cells. J. Immunol. 157:541-548. [PubMed] [Google Scholar]
- 7.Fauvel, M., H. Artsob, and L. Spence. 1977. Immune electron microscopy of arboviruses. Am. J. Trop. Med. Hyg. 26:798-807. [DOI] [PubMed] [Google Scholar]
- 8.Fitzer-Attas, C. J., M. Lowry, M. T. Crowley, A. J. Finn, F. Meng, A. L. DeFranco, and C. A. Lowell. 2000. Fcgamma receptor-mediated phagocytosis in macrophages lacking the Src family tyrosine kinases Hck, Fgr, and Lyn. J. Exp. Med. 191:669-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleit, H. B., and C. D. Kobasiuk. 1991. The human monocyte-like cell line THP-1 expresses Fc gamma RI and Fc gamma RII. J. Leukoc. Biol. 49:556-565. [DOI] [PubMed] [Google Scholar]
- 10.Halstead, S. B. 1989. Antibody, macrophages, dengue virus infection, shock, and hemorrhage: a pathogenetic cascade. Rev. Infect. Dis. 11(Suppl. 4):S830-S839. [DOI] [PubMed] [Google Scholar]
- 11.Halstead, S. B., and P. Simasthien. 1970. Observations related to the pathogenesis of dengue hemorrhagic fever. II. Antigenic and biologic properties of dengue viruses and their association with disease response in the host. Yale J. Biol. Med. 42:276-292. [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison, P. T., L. Bjorkhaug, M. J. Hutchinson, and J. M. Allen. 1995. The interaction between human Fc gamma RI and the gamma-chain is mediated solely via the 21 amino acid transmembrane domain of Fc gamma RI. Mol. Membr. Biol. 12:309-312. [DOI] [PubMed] [Google Scholar]
- 13.Hennecke, M., M. Kwissa, K. Metzger, A. Oumard, A. Kroger, R. Schirmbeck, J. Reimann, and H. Hauser. 2001. Composition and arrangement of genes define the strength of IRES-driven translation in bicistronic mRNAs. Nucleic Acids Res. 29:3327-3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Indik, Z. K., J. G. Park, S. Hunter, and A. D. Schreiber. 1995. The molecular dissection of Fc gamma receptor mediated phagocytosis. Blood 86:4389-4399. [PubMed] [Google Scholar]
- 15.Jungi, T. W., and S. Hafner. 1986. Quantitative assessment of Fc receptor expression and function during in vitro differentiation of human monocytes to macrophages. Immunology 58:131-137. [PMC free article] [PubMed] [Google Scholar]
- 16.Katsumata, O., M. Hara-Yokoyama, C. Sautes-Fridman, Y. Nagatsuka, T. Katada, Y. Hirabayashi, K. Shimizu, J. Fujita-Yoshigaki, H. Sugiya, and S. Furuyama. 2001. Association of FcgammaRII with low-density detergent-resistant membranes is important for cross-linking-dependent initiation of the tyrosine phosphorylation pathway and superoxide generation. J. Immunol. 167:5814-5823. [DOI] [PubMed] [Google Scholar]
- 17.Kim, M. K., Z. Y. Huang, P. H. Hwang, B. A. Jones, N. Sato, S. Hunter, T. H. Kim-Han, R. G. Worth, Z. K. Indik, and A. D. Schreiber. 2003. Fcgamma receptor transmembrane domains: role in cell surface expression, gamma chain interaction, and phagocytosis. Blood 101:4479-4484. [DOI] [PubMed] [Google Scholar]
- 18.Kim, M. K., X. Q. Pan, Z. Y. Huang, S. Hunter, P. H. Hwang, Z. K. Indik, and A. D. Schreiber. 2001. Fc gamma receptors differ in their structural requirements for interaction with the tyrosine kinase Syk in the initial steps of signaling for phagocytosis. Clin. Immunol. 98:125-132. [DOI] [PubMed] [Google Scholar]
- 19.Kliks, S. C., A. Nisalak, W. E. Brandt, L. Wahl, and D. S. Burke. 1989. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 40:444-451. [DOI] [PubMed] [Google Scholar]
- 20.Kontny, U., I. Kurane, and F. A. Ennis. 1988. Gamma interferon augments Fc gamma receptor-mediated dengue virus infection of human monocytic cells. J. Virol. 62:3928-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwiatkowska, K., and A. Sobota. 2001. The clustered Fcgamma receptor II is recruited to Lyn-containing membrane domains and undergoes phosphorylation in a cholesterol-dependent manner. Eur. J. Immunol. 31:989-998. [DOI] [PubMed] [Google Scholar]
- 22.Letourneur, O., I. C. Kennedy, A. T. Brini, J. R. Ortaldo, J. J. O'Shea, and J. P. Kinet. 1991. Characterization of the family of dimers associated with Fc receptors (Fc epsilon RI and Fc gamma RIII). J. Immunol. 147:2652-2656. [PubMed] [Google Scholar]
- 23.Libraty, D. H., S. Pichyangkul, C. Ajariyakhajorn, T. P. Endy, and F. A. Ennis. 2001. Human dendritic cells are activated by dengue virus infection: enhancement by gamma interferon and implications for disease pathogenesis. J. Virol. 75:3501-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Littaua, R., I. Kurane, and F. A. Ennis. 1990. Human IgG Fc receptor II mediates antibody-dependent enhancement of dengue virus infection. J. Immunol. 144:3183-3186. [PubMed] [Google Scholar]
- 25.Lowry, M. B., A. M. Duchemin, J. M. Robinson, and C. L. Anderson. 1998. Functional separation of pseudopod extension and particle internalization during Fc gamma receptor-mediated phagocytosis. J. Exp. Med. 187:161-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mady, B. J., D. V. Erbe, I. Kurane, M. W. Fanger, and F. A. Ennis. 1991. Antibody-dependent enhancement of dengue virus infection mediated by bispecific antibodies against cell surface molecules other than Fc gamma receptors. J. Immunol. 147:3139-3144. [PubMed] [Google Scholar]
- 27.Maenaka, K., P. A. van der Merwe, D. I. Stuart, E. Y. Jones, and P. Sondermann. 2001. The human low affinity Fcgamma receptors IIa, IIb, and III bind IgG with fast kinetics and distinct thermodynamic properties. J. Biol. Chem. 276:44898-44904. [DOI] [PubMed] [Google Scholar]
- 28.Manes, S., G. del Real, and A. C. Martinez. 2003. Pathogens: raft hijackers. Nat. Rev. Immunol. 3:557-568. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Salas, E. 1999. Internal ribosome entry site biology and its use in expression vectors. Curr. Opin. Biotechnol. 10:458-464. [DOI] [PubMed] [Google Scholar]
- 30.Miller, K. L., A. M. Duchemin, and C. L. Anderson. 1996. A novel role for the Fc receptor gamma subunit: enhancement of Fc gamma R ligand affinity. J. Exp. Med. 183:2227-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell, M. A., M. M. Huang, P. Chien, Z. K. Indik, X. Q. Pan, and A. D. Schreiber. 1994. Substitutions and deletions in the cytoplasmic domain of the phagocytic receptor Fc gamma RIIA: effect on receptor tyrosine phosphorylation and phagocytosis. Blood 84:1753-1759. [PubMed] [Google Scholar]
- 32.Morens, D. M., S. B. Halstead, P. M. Repik, R. Putvatana, and N. Raybourne. 1985. Simplified plaque reduction neutralization assay for dengue viruses by semimicro methods in BHK-21 cells: comparison of the BHK suspension test with standard plaque reduction neutralization. J. Clin. Microbiol. 22:250-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukhopadhyay, S., J. Herre, G. D. Brown, and S. Gordon. 2004. The potential for Toll-like receptors to collaborate with other innate immune receptors. Immunology 112:521-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nimmerjahn, F., and J. V. Ravetch. 2006. Fcgamma receptors: old friends and new family members. Immunity 24:19-28. [DOI] [PubMed] [Google Scholar]
- 35.Odin, J. A., J. C. Edberg, C. J. Painter, R. P. Kimberly, and J. C. Unkeless. 1991. Regulation of phagocytosis and [Ca2+]i flux by distinct regions of an Fc receptor. Science 254:1785-1788. [DOI] [PubMed] [Google Scholar]
- 36.Ortiz-Stern, A., and C. Rosales. 2003. Cross-talk between Fc receptors and integrins. Immunol. Lett. 90:137-143. [DOI] [PubMed] [Google Scholar]
- 37.Parren, P. W., and D. R. Burton. 2001. The antiviral activity of antibodies in vitro and in vivo. Adv. Immunol. 77:195-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peiris, J. S., and J. S. Porterfield. 1981. Antibody-dependent enhancement of plaque formation on cell lines of macrophage origin—a sensitive assay for antiviral antibody. J. Gen. Virol. 57:119-125. [DOI] [PubMed] [Google Scholar]
- 39.Rabinovitch, M. 1995. Professional and non-professional phagocytes: an introduction. Trends Cell Biol. 5:85-87. [DOI] [PubMed] [Google Scholar]
- 40.Ravetch, J. V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19:275-290. [DOI] [PubMed] [Google Scholar]
- 41.Sabin, A. 1952. Research on dengue during World War II. Am. Trop. Med. Hyg. 1:30-50. [DOI] [PubMed] [Google Scholar]
- 42.Schlesinger, J. J., and S. E. Chapman. 1999. Influence of the human high-affinity IgG receptor FcgammaRI (CD64) on residual infectivity of neutralized dengue virus. Virology 260:84-88. [DOI] [PubMed] [Google Scholar]
- 43.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 44.Smith, A. E., and A. Helenius. 2004. How viruses enter animal cells. Science 304:237-242. [DOI] [PubMed] [Google Scholar]
- 45.Takai, T. 2002. Roles of Fc receptors in autoimmunity. Nat. Rev. Immunol. 2:580-592. [DOI] [PubMed] [Google Scholar]
- 46.Takai, T., M. Li, D. Sylvestre, R. Clynes, and J. V. Ravetch. 1994. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell 76:519-529. [DOI] [PubMed] [Google Scholar]
- 47.Thein, S., J. Aaskov, T. T. Myint, T. N. Shwe, T. T. Saw, and A. Zaw. 1993. Changes in levels of anti-dengue virus IgG subclasses in patients with disease of varying severity. J. Med. Virol. 40:102-106. [DOI] [PubMed] [Google Scholar]
- 48.Van den Herik-Oudijk, I. E., P. J. Capel, T. van der Bruggen, and J. G. Van de Winkel. 1995. Identification of signaling motifs within human Fc gamma RIIa and Fc gamma RIIb isoforms. Blood 85:2202-2211. [PubMed] [Google Scholar]
- 49.Van den Herik-Oudijk, I. E., M. W. Ter Bekke, M. J. Tempelman, P. J. Capel, and J. G. Van de Winkel. 1995. Functional differences between two Fc receptor ITAM signaling motifs. Blood 86:3302-3307. [PubMed] [Google Scholar]
- 50.Van den Herik-Oudijk, I. E., N. A. Westerdaal, N. V. Henriquez, P. J. Capel, and J. G. Van De Winkel. 1994. Functional analysis of human Fc gamma RII (CD32) isoforms expressed in B lymphocytes. J. Immunol. 152:574-585. [PubMed] [Google Scholar]
- 51.van de Winkel, J. G., and C. L. Anderson. 1991. Biology of human immunoglobulin G Fc receptors. J. Leukoc. Biol. 49:511-524. [DOI] [PubMed] [Google Scholar]
- 52.van Vugt, M. J., A. F. Heijnen, P. J. Capel, S. Y. Park, C. Ra, T. Saito, J. S. Verbeek, and J. G. van de Winkel. 1996. FcR gamma-chain is essential for both surface expression and function of human Fc gamma RI (CD64) in vivo. Blood 87:3593-3599. [PubMed] [Google Scholar]
- 53.Vaughn, D. W., S. Green, S. Kalayanarooj, B. L. Innis, S. Nimmannitya, S. Suntayakorn, T. P. Endy, B. Raengsakulrach, A. L. Rothman, F. A. Ennis, and A. Nisalak. 2000. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 181:2-9. [DOI] [PubMed] [Google Scholar]
- 54.Warmerdam, P. A., P. W. Parren, A. Vlug, L. A. Aarden, J. G. van de Winkel, and P. J. Capel. 1992. Polymorphism of the human Fc gamma receptor II (CD32): molecular basis and functional aspects. Immunobiology 185:175-182. [DOI] [PubMed] [Google Scholar]
- 55.Wellington, M., J. M. Bliss, and C. G. Haidaris. 2003. Enhanced phagocytosis of Candida species mediated by opsonization with a recombinant human antibody single-chain variable fragment. Infect. Immun. 71:7228-7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woof, J. M., and D. R. Burton. 2004. Human antibody-Fc receptor interactions illuminated by crystal structures. Nat. Rev. Immunol. 4:89-99. [DOI] [PubMed] [Google Scholar]
- 57.Wu, S. J., G. Grouard-Vogel, W. Sun, J. R. Mascola, E. Brachtel, R. Putvatana, M. K. Louder, L. Filgueira, M. A. Marovich, H. K. Wong, A. Blauvelt, G. S. Murphy, M. L. Robb, B. L. Innes, D. L. Birx, C. G. Hayes, and S. S. Frankel. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 6:816-820. [DOI] [PubMed] [Google Scholar]