Abstract
Cells have evolved elaborate mechanisms to counteract the onslaught of viral infections. To activate these defenses, the viral threat must be recognized. Danger signals, or pathogen-associated molecular patterns, that are induced by pathogens include double-stranded RNA (dsRNA), viral single-stranded RNA, glycolipids, and CpG DNA. Understanding the signal transduction pathways activated and host gene expression induced by these danger signals is vital to understanding virus-host interactions. The vaccinia virus E3L protein is involved in blocking the host antiviral response and increasing pathogenesis, functions that map to separate C-terminal dsRNA- and N-terminal Z-DNA-binding domains. Viruses containing mutations in these domains allow modeling of the role of dsRNA and Z-form nucleic acid in the host response to virus infection. Deletions in the Z-DNA- or dsRNA-binding domains led to activation of signal transduction cascades and up-regulation of host gene expression, with many genes involved in the inflammatory response. These data suggest that poxviruses actively inhibit cellular recognition of viral danger signals and the subsequent cellular response to the viral threat.
The innate host defenses, including the interferon (IFN) response, play a key role in limiting the spread of virus soon after infection and in inducing an adaptive immune response (20, 25). Viruses have developed elaborate strategies to counteract the host antiviral response (20, 55). Vaccinia virus (VV), a type member of Orthopoxviridae, contains a large double-stranded DNA genome carrying approximately 190 genes. Replication of VV is divided into three temporally distinct phases: early, intermediate, and late. Immediately after infection, VV early genes are transcribed, but viral DNA replication must be initiated in order to transcribe the intermediate and late genes. VV replication occurs exclusively within the cytoplasm of the infected cell, thereby necessitating the requirement for many viral gene products to be utilized directly for transcription, genome replication, and viral assembly. Additionally, poxviruses have been shown to encode a multitude of gene products that interfere with or inhibit key components of the host inflammatory and antiviral responses. (26, 45, 51, 53, 54). The extent of these virus-encoded modulators of the cellular immune response suggests that the host cell recognizes the VV infection and responds through the induction of an array of antiviral defenses. The VV E3L gene is a necessary virulence gene providing IFN resistance and a broad host range to the virus in cells in culture (2, 31). The E3L gene is also a necessary factor for viral pathogenesis in a mouse model (6). The E3L gene encodes a 190-amino-acid protein with a highly conserved, carboxyl-terminal, double-stranded RNA (dsRNA)-binding domain (9, 41). This dsRNA-binding domain has been shown to be required for both the IFN resistance and broad-host-range phenotypes associated with VV (10). For VV, dsRNA is likely formed during virus replication due to convergent transcription at intermediate and late times postinfection (1, 34, 56).
The amino-terminal domain of E3L is also required for full pathogenesis in mice but is typically dispensable for replication in cells in culture (6). The N-terminal domain of E3L appears to be involved in binding to Z-form nucleic acid and is necessary for full inhibition of PKR activation (10, 27, 28, 32, 38, 58). The N-terminal domain of E3L involved in binding to Z-DNA is conserved among chordopoxviruses that contain an E3L gene (28). In a yeast one-hybrid system, the N-terminal domain of E3L has been shown to stimulate reporter gene expression from a potential Z-forming sequence and to shift potential Z-forming DNA sequences into a Z conformation in vitro (27). Thus, the N terminus of E3L can bind Z-DNA. Neurovirulence of VV deleted of the N-terminal Z-DNA-binding domain of E3L can be completely complemented by insertion of the distantly related Z-DNA-binding domains from ADAR1 or DLM-1, which share only approximately 30% sequence homology with the N terminus of E3L (28). Mutations which decrease the ability of the chimeric E3L protein to bind Z-DNA directly correlate with a decrease in pathogenesis, as do corresponding mutations in the wild-type (wt) E3L protein that are predicted to disrupt Z-DNA-binding of E3L, based on the published nuclear magnetic resonance structure of E3L (24) and based on modeling of E3L onto the structure of Zα ADAR1 bound to Z-DNA (28). Thus, binding of the E3L protein to Z-form nucleic acid appears to directly correlate with viral pathogenesis (28).
Host cells have evolved sophisticated systems to detect danger signals signifying the presence of viral infection. The goal of this research is to provide a comprehensive view and understanding of the role of dsRNA and possibly Z-form nucleic acid in the antiviral response and how viruses have developed elaborate mechanisms to evade or inhibit these cellular defenses. VV provides a key model system for understanding and deciphering these events. Recombinant VV constructs are available which are deleted of sequences coding for specific domains present in the E3L protein, including those that bind dsRNA and Z-DNA. Using these constructs, we have been able to begin to unravel cellular signal transduction responses and transcriptional up-regulation in response to virus infection in which free dsRNA was no longer sequestered and/or the Z-form nucleic binding domain was deleted. Our results demonstrate the transcriptional up-regulation of numerous host genes in response to deletion of the dsRNA-binding domain or Z-DNA-binding domain, many of which are involved in the host proinflammatory response. The up-regulation of cellular gene expression correlated well with the activation of several cellular signal transduction cascades and the activation of host transcription factors.
MATERIALS AND METHODS
Cells and viruses.
HeLa cells were maintained as previously described (32). The Copenhagen strain of VV, designated wtVV, was the parent strain used for all viruses in this study. wtVV constructs deleted of E3L (VVΔE3L), deleted of the first 83 N-terminal amino acids of E3L (VVE3LΔ83N), or deleted of the last 26 C-terminal amino acids of E3L (VVE3LΔ26C) were previously constructed (9, 10). For UV inactivation of virus, 1 ml of virus stock was exposed to a 253-nm-wavelength UV lamp (Philips G36Y6L) at a distance of 5 in. for 5 min. Inactivation of the virus was confirmed by titration.
Expression microarrays and statistical analysis.
HeLa cells (1 × 107 cells) were infected with the indicated viruses at a multiplicity of infection (MOI) of 5. At 2, 6, and 9 hours postinfection (hpi), total RNA was isolated as previously described (13, 14) and was purified using an RNeasy column (QIAGEN). One round of RNA amplification was performed using a RiboAmp kit (Arcturus KIT0201). Capillary gel electrophoresis (Hewlett-Packard Kayak XM600 Bioanalyzer) was used to check the purity of the RNA at each step and prior to target labeling. Briefly, fluorescent target cDNAs were prepared using 3 μl amplified RNA to generate Cy3/Cy5-labeled target cDNAs as previously described (13, 14). Human cDNA arrays (Human 1; Agilent Technologies) contained duplicate spots of 12,814 unique cDNA clones, and pretreatment and hybridization were performed as previously described (50). Raw data were combined and processed using the in-house programs Spot-on Image and Expression Array Manager. Briefly, a single experiment comparing two samples was performed using the dye label reverse technique hybridized to the cDNA microarrays, allowing for the calculation of mean ratios between expression levels of each gene in the analyzed sample pair, standard deviations, and P values for each experiment. All data were entered into a custom-designed Oracle 9i backed relational database, Expression Array Manager, and were then uploaded into Rosetta Resolver System 4.0 (Rosetta Biosoftware) and Spotfire Decision Site (Spotfire). Data normalization and the Resolver System error model specifically developed for our slide format are described on the website http://expression.microslu.washington.edu (7). The full microarray expression profile is available on ArrayExpress (http://www.ebi.ac.uk/arrayexpress/query/login).
Amplification was performed on the RNA samples. Amplification of RNA does not result in major changes in the populations of differentially expressed genes identified by cDNA microarrays (50).
Real-time PCR assays.
Quantitative real-time PCR was used to validate the gene expression changes. Briefly, total RNA samples were treated with DNase (Ambion) and reverse transcription was performed using TaqMan reverse transcription reagents (Applied Biosystems). Quantitative real-time PCR was performed on the ABI 7500 real-time PCR system, using TaqMan chemistry (Applied Biosystems). Each target was run in quadruplicate using TaqMan 2× PCR universal master mix (Applied Biosystems). GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and 18S were chosen as endogenous controls to normalize quantification of the target. Quantification of each gene was performed using Applied Biosystems Sequence Detection Software version 1.2.2. The probes (Applied Biosystems) used for analysis are as follows: eukaryotic 18s rRNA (Hs99999901_s1), CD14 (Hs00169122_g1), CD68 (Hs00154355_m1), IFI27 (Hs00271467_m1), IFIT2 (Hs00533665_m1), IFNB1 (Hs00277188_s1), IFNG (Hs00174143_m1), interleukin-6 (IL-6) (Hs00174131_m1), IL-8 (Hs00174103_m1), and PPIA (Hs99999904_m1).
mRNA isolation and quantification.
To determine total mRNA levels present during viral infection, HeLa cells (1 × 107 cells) were infected with the indicated viruses (purified) at an MOI of 5. At 6 and 9 hpi, the media were removed and the cells harvested and pelleted in phosphate-buffered saline solution. The pelleted cells were lysed, and mRNA was isolated per the manufacturer's instructions (FastTrack 2.0 mRNA isolation kit; Invitrogen). To confirm nonsaturation of the oligo(dT) cellulose resin, one sample contained two times the cell volume. RNA was quantified by optical densities at 260 and 280 nm.
Western blot analysis.
Western blot analysis was performed as previously described (32). Radioimmunoprecipitation assay cell lysates (32) were prepared for cytosolic proteins (eIF2α, ASK1, MKK3/MKK6, p38 mitogen-activated protein kinase [MAPK], IKKα/β, and IκBα), nuclear extracts were prepared for interferon regulatory factor 3 (IRF3) and NF-κB p65, and total cell lysates were prepared for STAT1 and ATF-2. Radioimmunoprecipitation assay lysates were prepared as described previously (32). For nuclear extracts, the cell pellet was lysed in NP-40 lysis buffer (0.1% Nonidet P-40, 10 mM HEPES, pH 7.5, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride; 100 μl per 4 × 106 cells). The lysate was centrifuged at 10,000 × g for 10 min, and the nuclear pellet was washed followed by lysis in nuclear lysis buffer (20 mM HEPES, pH 7.5, 25% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 5 μg/ml each of leupeptin, pepstatin A, aprotinin, spermine, and spermidine; 50 μl per 4 × 106 cells). The lysate was centrifuged at 10,000 × g for 10 min at 4°C and the supernatant collected. For total cell lysates, cells were lysed in the dish by the addition of 1× sodium dodecyl sulfate sample buffer containing 50 mM NaF. DNA was sheared by repeated pulling through a 26-gauge needle (15 times). The antibodies used were as follows: anti-eIF2α[pS52] (Biosource International); anti-IKKɛ[pT501] (Rockland); anti-p38 MAPK[pT180/pY182], anti-IκBα, anti-STAT1[pY701], anti-MKK3/MKK6[pS189/207], anti-ASK1[pS83], and anti-ATF-2[pT71] (Cell Signaling Technology); anti-IRF3 (Michael David, University of California, San Diego); and anti-NF-κB p65 (Oncogene Research). For PKR activation, measurements were based on levels of eIF2α phosphorylation, which we have previously shown to occur concomitantly in this system (32).
To measure viral regulation of STAT1 phosphorylation, HeLa cells were mock or virally infected at an MOI of 5. At 3 hpi, cells were treated with 100 IU/ml recombinant human alpha interferon A/D (Hoffman-LaRoche) for 15 min, followed by extract preparation and analysis as described above.
RESULTS
wtVV has previously been shown to be IFN resistant and to have a broad host range due in part to the expression of the viral E3L protein (2, 47). The E3L gene contains very distinct carboxyl- and amino-terminal domains important for phenotypic properties associated with VV. Deletion of the N terminus (VVE3LΔ83N) does not dramatically affect virus replication in cells in culture but reduces pathogenesis 500- to 5,000-fold (6). This reduction in pathogenesis directly correlates with the ability of a chimeric E3L protein to bind Z-DNA (28). With deletion of the C terminus of E3L (VVE3LΔ26C), the recombinant virus displays phenotypic characteristics very similar to those of a virus deleted of the entire E3L gene (data not shown). Since the C terminus of E3L contains the domain involved in binding to and sequestering dsRNA, these results demonstrate the key role of sequestering dsRNA during VV replication.
In order to assess the global cellular response to viral signals, microarray analysis of cDNA expression was performed on cells infected by wtVV and viruses containing mutations in the E3L protein. Of the more than 12,000 human genes analyzed on the microarray, approximately 2,500 genes were altered for expression by all of the VV constructs (data not shown). Of these, the majority of the host genes (60%) were repressed in levels of expression throughout the course of infection (data not shown). Previous metabolic studies suggest that cellular gene expression is virtually eliminated soon after VV infection (4, 36, 37). However, recent microarray analysis has reported that between 60.24% and 90% of differentially expressed genes are down-regulated during poxvirus infection and that cellular genes which are induced likely play key roles in viral replication (8, 19, 33). In our analysis, cells were infected at an MOI of 5, resulting in a 99.9% infection of cells as confirmed by viral expression of the lacZ gene (data not shown). The HeLa cells utilized in these studies have limited 2′5′-oligoadenylate synthetase (OAS) activity, thereby allowing RNA levels to be unaffected by activation of this pathway (data not shown). Total RNA and mRNA isolated from uninfected and infected cells did not vary significantly during the course of infection with any of our VV constructs [1.3-fold maximal difference in total RNA and 1.4-fold maximal difference in poly(A)+ RNA] (data not shown). Therefore, our results were likely not a result of gene induction in a significant population of uninfected cells, nor are our data skewed based on significant differences in target cDNA concentrations present during the microarray hybridization.
Since the microarray analysis performed in these assays was done over three time points and with four VV constructs, patterns and trends in VV regulation of cellular gene expression could be observed. Clusters of genes whose expression was likely regulated by the presence or absence of the C-terminal or N-terminal domains on E3L could be identified. For example, genes induced by the lack of the C-terminal domain on E3L could be clustered by identifying those genes regulated in common during infection with VVΔE3L and VVE3LΔ26C.
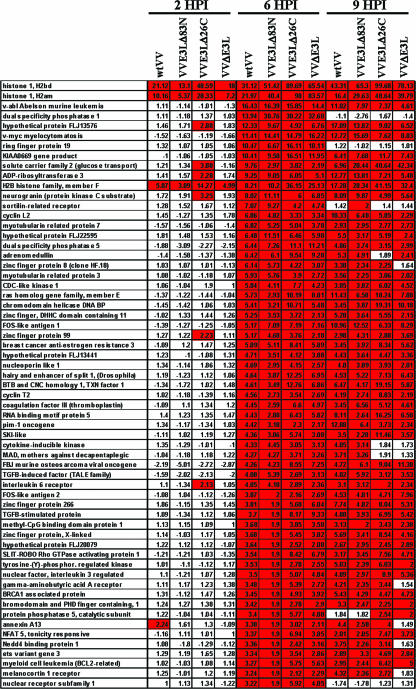
One subset of microarray data was the identification of host genes that were regulated by VV infection in general. These were genes that were differentially expressed commonly upon infection by all four VV constructs. Since host genes repressed by VV have a generalized, and likely, nonspecific decrease in mRNA levels, these genes will not be discussed in detail. However, VV infection led to a significant induction in mRNA levels of a number of host genes (Fig. 1). As previously shown (8, 18), the expression of histone RNA was greatly enhanced. As suggested by Brum et al., this is likely an artifact due to the virus-encoded poly(A) polymerase adding poly(A) to the histone mRNA which normally lacks poly(A) tails (8). Temporally, the majority of these genes were not induced at 2 hpi, but by 6 hpi and continuing through 9 hpi, high levels of induction were observed (Fig. 1). As a control, these results were compared to those obtained with microarray analysis performed using UV-inactivated wtVV. Infection with this virus led to only minor changes in gene expression, suggesting that changes in host gene expression with wtVV and the mutant E3L VV constructs were due to effects from active virus replication (data not shown).
FIG. 1.
Host gene expression regulated by VV. Host genes which were differentially induced during infection with all VV constructs are shown. Genes were sorted based on a threefold (P < 0.01) or greater level of induction for wtVV at 6 hpi and were also significantly induced by VVE3LΔ83N, VVE3LΔ26C, and VVΔE3L. Red boxes represent genes which were significantly induced. Changes (n-fold) in expression levels relative to those of mock-infected cells are shown within each box. TGFB, transforming growth factor β.
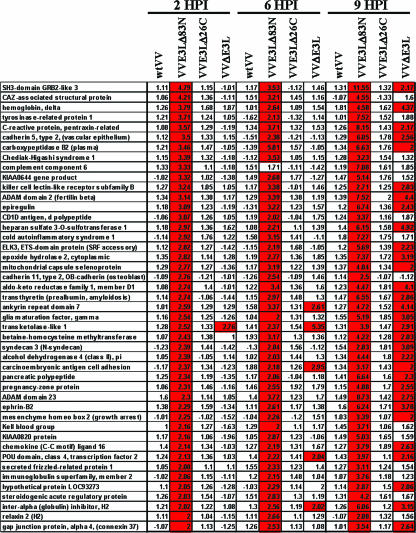
Two additional distinct populations of genes relating to host genes that were induced during infection with virus lacking the C-terminal domain of E3L (VVE3LΔ26C and VVΔE3L) (Fig. 2) and virus lacking the N-terminal domain (VVE3LΔ83N and VVΔE3L) (Fig. 3) were identified. Temporally, the induction of host genes by VVE3LΔ26C and VVΔE3L typically occurred at 6 hpi and continued through 9 hpi (Fig. 2). Several of the genes highly induced upon infection with VVE3LΔ26C and VVΔE3L were also induced upon infection with wtVV and VVE3LΔ83N, though to a much lower level (Fig. 2, right panel). These results may suggest that VV infection leads to the induction of these genes through accumulation of small amounts of free dsRNA or other inducing factors, which may be enhanced by the larger amounts of free dsRNA present during infection with VVE3LΔ26C and VVΔE3L.
FIG. 2.
Host gene expression regulated by the C-terminal dsRNA-binding domain of E3L. Host genes which were differentially expressed during infection with VV constructs lacking the C-terminal dsRNA-binding domain of E3L (VVE3LΔ26C and VVΔE3L) are shown. In the left panel, genes were sorted based on a threefold (P < 0.01) or greater level of induction for VVE3LΔ26C at 6 hpi and were unaltered during wtVV infection. The right panel represents genes which were induced during infection with all VV constructs (yellow boxes) but much more highly induced during infection with VVE3LΔ26C (red boxes). Changes (n-fold) in expression levels relative to those of mock-infected cells are shown within each box.
FIG. 3.
Host gene expression regulated by the N-terminal Z-DNA-binding domain of E3L. Host genes which were differentially expressed during infection with VV constructs lacking the N-terminal Z-DNA-binding domain of E3L (VVE3LΔ83N and VVΔE3L) are shown. Genes were sorted based on a twofold (P ≤ 0.01) or greater level of induction or repression for VVE3LΔ83N at 2 hpi and were unaltered during wtVV infection. Red boxes represent genes which were significantly induced. Changes (n-fold) in expression levels relative to those of mock-infected cells are shown within each box.
The induction of host genes by the loss of the N terminus of E3L appeared to be a very rapid event in the virus life cycle, with genes specifically induced by VVE3LΔ83N being detected by 2 hpi and continuing through the virus replication cycle (Fig. 3). The data in Fig. 3 were sorted based on cellular genes differentially regulated by VVE3LΔ83N which were unaltered for expression during infection with wtVV. In support of these genes being specifically regulated by the loss of the N terminus, infection with VVE3LΔ26C did not show any significant changes in the expression of these genes (Fig. 3). Upon infection with VVΔE3L, 69% of the host genes induced by VVE3LΔ83N were also induced by VVΔE3L, which also lacks the N-terminal domain of E3L (Fig. 3). However, the induction of these genes by VVΔE3L was delayed and not typically observed until 9 hpi. It is possible that this delay in induction may be related to additional effects due to VVΔE3L lacking the C-terminal domain of E3L as well. A significant group of genes induced commonly during infection with wtVV and VVE3LΔ26C (i.e., virus containing a functional N-terminal domain) was not observed (data not shown).
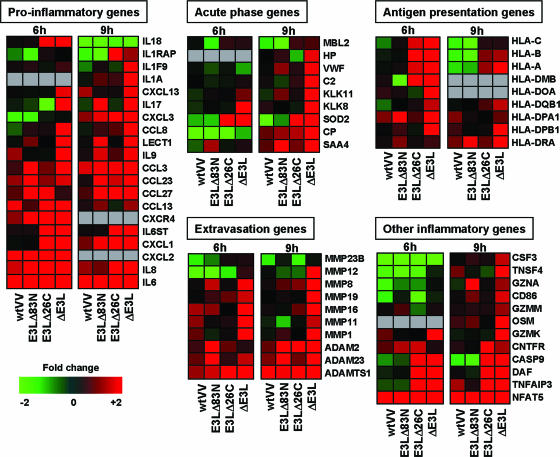
Our data suggest that the presence of the C-terminal domain and the presence of the N-terminal domain of E3L function independently to repress host gene expression. This may support the idea that free dsRNA and possibly Z-form nucleic acid may be key players in the host cell recognition of viral infection and the subsequent induction of a strong inflammatory response. Figure 4 shows results of regulation of proinflammatory gene expression during VV infection. Infection with VVΔE3L led to the induction of a large spectrum of proinflammatory genes, including interleukin genes, cytokine genes, cellular extravasation genes, antigen presentation genes, acute phase response genes, granzyme genes, and apoptotic genes (Fig. 4). Cells infected with virus deleted of just the C-terminal domain (VVE3LΔ26C) or just the N-terminal domain (VVE3LΔ83N) of E3L led to the induction of a smaller subset of inflammatory response genes (Fig. 4). During infection with wtVV, induction of the majority of inflammatory genes was inhibited, demonstrating the importance of the E3L gene in modulating host cell recognition of virus infection (Fig. 4).
FIG. 4.
VV regulation of the host inflammatory response. Cells were infected with wtVV, VVE3LΔ83N, VVE3LΔ26C, or VVΔE3L, and the differential expression levels of host genes involved in several proinflammatory responses are shown. Shades of red indicate host gene induction, and shades of green indicate host gene repression. Gray represents cDNA sequences that were not present on all arrays used.
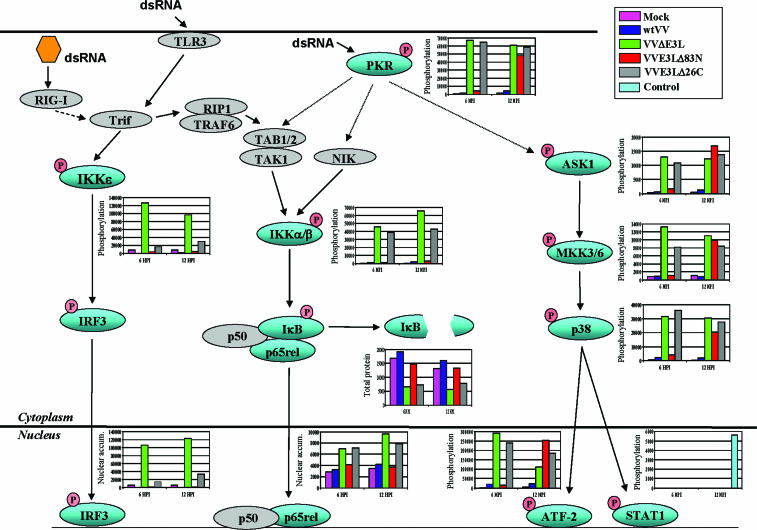
Our microarray data suggest that the VV E3L gene product may be directly involved in inhibiting the cellular response to dsRNA and possibly Z-form nucleic acid. These data would suggest that the E3L protein modulates cellular signal transduction cascades and activation of subsequent transcription factors involved in the recognition of and response to viral danger signals. To test this hypothesis, the activations of key players in various signal transduction cascades in response to VV infection were determined. These assays were performed (in duplicate) using Western blot analysis to observe established events in the activation process, including phosphorylation state, nuclear translocation, and protein degradation. Reliability and confidence of the Western blot data are supported by similar activations of multiple components in each of the signal transduction cascades (Fig. 5).
FIG. 5.
VV regulation of signal transduction. Cells were mock infected or infected with wtVV, VVE3LΔ83N, VVE3LΔ26C, or VVΔE3L. Extracts were prepared at 6 hpi and 12 hpi and analyzed for the presence of phosphorylation, nuclear translocation, or degradation of key components of several host signal transduction cascades by Western blot analysis. During gel electrophoresis, equal protein amounts were loaded into each lane. Chemiluminescent bands corresponding to each protein were quantified using ImageQuant software and graphed. Extracts were assayed in duplicate, and graphs illustrate representative results. Nuclear accum., nuclear accumulation.
None of the pathways involved in IRF3, NF-κB, ATF-2, and STAT1 activation were activated during wtVV infection (Fig. 5). During infection with VVΔE3L, phosphorylation of IKKɛ leading to translocation of IRF3 to the nucleus was observed; phosphorylation of IKKα/β and subsequent IκB degradation and translocation of the p65rel subunit of NF-κB to the nucleus were observed, and activation of ASK1, MKK3/6, and p38 MAPK leading to the subsequent phosphorylation and nuclear translocation of ATF-2 was observed (Fig. 5). During infection with VVE3LΔ26C, activations of the NF-κB and ATF-2 cascades were similar to that of VVΔE3L (Fig. 5). However, VVE3LΔ26C led to only minor levels of IKKɛ phosphorylation and IRF3 nuclear translocation, suggesting that the N-terminal domain of E3L is sufficient to inhibit activation of this cascade (Fig. 5). During infection with VVE3LΔ83N, only activation of the p38 MAPK/ATF-2 cascade was observed (Fig. 5). This activation was observed only at very late times postinfection (12 hpi), which is consistent with previous reports demonstrating PKR activation at very late times during the replication of VVE3LΔ83N (32) and which suggests that signaling through the p38 MAPK pathway is mediated by PKR.
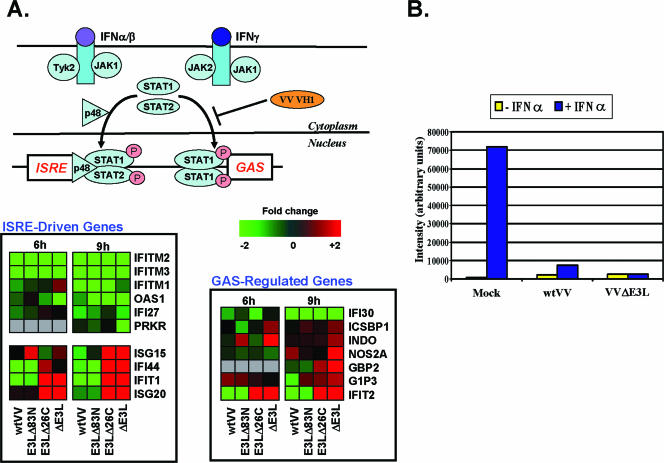
During infection with any of the VV constructs, activation of STAT1 was not observed (Fig. 5). Given that STAT1 is a well-characterized substrate for p38 MAPK and given the role of STAT1 in the IFN and antiviral response, this result was somewhat unexpected. Consistent with the lack of phosphorylation of STAT1, microarray analysis demonstrated that, especially for the type I IFN response, the majority of IFN-regulated genes were either down-regulated or unchanged (Fig. 6A). A few genes, including interferon-stimulated gene 15 (ISG15) and ISG20, were up-regulated during infection with VV constructs deleted of the C-terminal dsRNA-binding domain (Fig. 6A), but this may be due to activation of other transcription factors, such as IRF3 and NF-κB, respectively, which can also regulate some STAT1-responsive genes (12, 43). It was previously reported that the VV-encoded VH1 phosphatase can bind to and dephosphorylate STAT1, blocking type II IFN signal transduction (35). The inhibition of STAT1 activation and transcriptional response observed in Fig. 5 and 6A may be due to activity associated with the VH1 phosphatase. In support of this, preinfection with wtVV or VVΔE3L blocked STAT1 phosphorylation mediated by type I IFN treatment (Fig. 6B). STAT1 phosphorylation was readily detectable in uninfected cells treated with type I IFN (Fig. 5 and 6B). These results suggest that VV encodes a general inhibitor of STAT1 phosphorylation that can inhibit phosphorylation of STAT1 in response to type I and type II IFN signaling and in response to activation of the p38 MAPK pathway.
FIG. 6.
VV regulation of the host IFN response. (A) Cells were infected with wtVV, VVE3LΔ83N, VVE3LΔ26C, or VVΔE3L, and the differential expression levels of host genes involved in the IFN response are shown. Shades of red indicate host gene induction, and shades of green indicate host gene repression. Gray represents samples which were eliminated due to chip errors. (B) Cells were mock infected or infected with wtVV or VVΔE3L. At 3 hpi, cells were treated with 100 IU/ml IFN-α for 15 min. Extracts were prepared and analyzed for activation (phosphorylation) of STAT1 by Western blot analysis. During gel electrophoresis, equal protein amounts were loaded into each lane. Chemiluminescent bands corresponding to STAT1 were quantified using ImageQuant software and graphed. ISRE, interferon-stimulated response element; GAS, gamma interferon-activated site.
DISCUSSION
The data presented suggest an important role of the VV E3L gene product in regulating host gene expression. Poxviruses have evolved elaborate mechanisms to counteract the activity associated with immune and antiviral functions (45, 51, 54). Similar mechanisms have been described as being employed by a wide variety of viruses (55). Though many of these viral proteins, including E3L, have been shown to function as inhibitors of PKR or OAS activity or as modulators of the IFN response, an alternative role, either direct or indirect, may be to regulate the host immune response by interfering with cellular gene expression.
The work presented in the manuscript utilizes microarray and Western blot analyses to determine the role of the E3L protein in regulating host gene expression. Of significance for validation of our results, compared to viral infections with our other VV constructs, VVE3LΔ83N had a gene expression profile very similar to that of wtVV and the results obtained for VVΔE3L were very similar to those for VVE3LΔ26C (Fig. 2). These data, along with similarities in temporal responses, levels of change (n-fold), and validation of select genes based on real-time PCR, provide confidence in the interpretation of the microarray data.
The up-regulation of gene expression induced by virus constructs lacking the C-terminal dsRNA-binding domain of E3L was not observed until 6 hpi. This is in agreement with previous data showing that PKR, OAS, and IRF3 activation during infection with VVΔE3L occurs around 4 hpi (31, 49; unpublished observations). The activation of these antiviral pathways is likely due to the presence of RNase A-resistant dsRNA formed during intermediate and late transcription of viral genes (31, 34, 56). This supports the concept that dsRNA formed during a vaccinia virus infection may act as a broad pathogen-associated molecular pattern involved in the activation of antiviral enzymes and the induction of specific host gene expression.
A significant cluster of induced genes was not observed during infection with wtVV and VVE3LΔ83N and was not altered during infection with VVE3LΔ26C and VVΔE3L (data not shown). This supports the concept that the dsRNA-binding domain of E3L is not directly involved in the induction of cellular transcription but instead acts to block host gene expression by sequestering the dsRNA activator molecule, which, when recognized by the host as a danger signal, leads to the induction of cellular transcription. In general, the classic enzymes involved in the cellular antiviral state, including PKR and OAS, were not induced during infection with VVE3LΔ26C and VVΔE3L. Instead, these infected cells appear to induce factors which may be involved in warning surrounding cells or immune cells of the viral threat. A comprehensive study on the regulation of gene expression by exogenous dsRNA using microarray technology was previously done by Geiss et al. (13). Based on their results, 47% of the genes they observed to be up-regulated by dsRNA were similarly altered in our arrays during infection with viruses lacking the C terminus of E3L. Those authors utilized cells treated with exogenous dsRNA, thereby performing these assays in a distinctly different environment compared to dsRNA formed intracellularly during a viral infection. Our VV mutants provide the opportunity to observe the global regulation of host gene expression specifically by dsRNA formed during a virus infection. Ludwig et al. (33) have performed microarray analysis on cells infected with modified vaccinia virus Ankara (MVA) deleted of E3L in comparison to wtMVA cells. In comparison to our data, 55% of the host genes those authors observed to be up-regulated were also altered during our infection with VVΔE3L. This difference is not unexpected since MVA lacks numerous other immunomodulatory genes, including the K1L gene, which is required for the inhibition of NF-κB activation (3, 46).
dsRNA and/or PKR has been shown to have a role in the activation of the IRF3, NF-κB, and ATF-2 signal transduction cascades (16, 22, 48, 49, 52, 57). Our data are consistent with activation of the ATF-2 cascade through a PKR-dependent mechanism. However, our data also suggest that activation of the IRF3 and NF-κB cascades occurred through a PKR-independent mechanism, since infection with VVE3LΔ83N did not activate either of these pathways and VVE3LΔ26C did not activate the IRF3 cascade, but both viruses led to PKR activation. These data also support previous data showing that IRF3 and NF-κB activation could occur in cells treated with dsRNA but devoid of PKR (22, 49).
The function of the N-terminal domain of E3L has been more controversial. This domain is required for viral pathogenesis and inhibition of PKR activation (28, 32). Biochemically, the N-terminal domain can bind to Z-DNA both in vitro and in a yeast one-hybrid system (29). Viral pathogenesis appears to correlate with Z-DNA-binding activity, since mutations in E3L which disrupt conserved amino acids of the Z-DNA-binding motif lead to decreased pathogenesis in mice (28). The N terminus of E3L has also been reported to be involved in nuclear localization of the E3L protein. Transfection of cells with a plasmid expressing E3L led to cytoplasmic and nuclear localization of the E3L protein, whereas expression of E3L deleted of the N-terminal 83 amino acids led to solely cytoplasmic expression (10, 29). However, during infection of cells with wtVV or VVE3LΔ83N, immunofluorescent localizations of E3L appear identical between the two viruses, with the protein present in both the cytoplasm and the nucleus (unpublished observations). This suggests that the role of the N terminus of E3L in nuclear localization may be a phenomenon observed only during transfection. Therefore, the only well-characterized role of the N terminus of E3L in an infected cell is interaction with Z-form nucleic acid.
The microarray clustering data suggest that the N terminus of E3L is involved in regulating host gene expression. Vaccinia virus deleted of E3L or expressing E3L deleted of the N-terminal 83 amino acids led to the induction of a significant cluster of cellular genes. These data may suggest that the N terminus of E3L synthesized during infection with VVE3LΔ26C or wtVV may be blocking the induction of these cellular genes. Since both of these viruses express the N-terminal, Z-DNA-binding motif of E3L, the data may suggest that the presence of the Z-DNA-binding domain of E3L is not acting to induce host gene expression but instead may support a role of this domain in blocking the induction of specific cellular genes. Like the dsRNA-binding domain of E3L, the Z-DNA-binding domain may be acting to sequester Z-form nucleic acids which otherwise would be recognized by the host as a virus-induced/exposed danger signal. Kwon and Rich (29) reported that transfection of HeLa cells with an E3L expression plasmid led to increased gene expression from IL-6, NF-AT, and p53 promoter elements as well as increased mRNA levels of IL-6, NF-AT, and p53. This increased expression was not observed when cells were transfected with an expression plasmid expressing E3LΔ83N. These data suggested that the presence of the Z-DNA-binding domain of E3L may act to function to increase gene expression. These results are contradictory to our data showing that the N-terminal domain of E3L appeared to repress host gene expression. Notably, p53 and NF-AT expression is unaltered during infection with any of our viruses, and IL-6 expression was highly induced during infection with viruses deleted of the dsRNA-binding domain and induced to a lesser extent during infection with wtVV virus or VV deleted of the Z-DNA-binding domain of E3L (data not shown). Again, Kwon and Rich performed these assays under transfection conditions which may yield results different from those for a viral infection (29).
The correlation between Z-DNA-binding proteins and the antiviral IFN system is expanding. Two mammalian IFN-inducible Z-DNA-binding proteins have been described. The RNA-specific adenosine deaminase (ADAR1) catalyzes the deamination of adenosine to inosine in viral and cellular RNAs. Two forms of ADAR1 which initiate from different promoters, one constitutively active and the other IFN inducible, have been identified (15). Only the IFN-inducible form is capable of binding Z-DNA (42). A second cellular protein, DLM-1, has also been shown to bind Z-DNA and is IFN inducible (39). Recently, a PKR-like kinase (PKZ) was isolated from zebrafish (40). The kinase domain of this protein is more similar to PKR than to any other known eIF-2α kinases; however, the two dsRNA-binding domains present on PKR have been replaced with two Z-DNA-binding domains. PKZ expression is induced upon treatment with dsRNA, suggesting a role of this enzyme in the fish host antiviral response (40). The presence of all of these proteins suggests a role of Z-DNA-binding proteins in the IFN response and/or antiviral activity.
The presence of potential Z-DNA-forming sequences in the upstream regulatory region of several host genes has suggested a role for the transient formation of Z-DNA in regulation of transcription (21, 44). E3L, which is synthesized very early during vaccinia virus replication, may be translocating to the nucleus and binding to Z-DNA structures transiently induced on host genes that are actively being transcribed during infection with vaccinia virus. Binding of E3L to these sites might block transcriptional activation of these genes and dampen the host response to virus infection. Alternatively, Z-form nucleic acid (either RNA or DNA) may be a product of viral infection, and the role of the N terminus of E3L may be to sequester these Z-form nucleic acids, which, if left unsequestered, might initiate a cascade of antiviral defenses.
Previous work by our lab has illustrated the importance of both the N and C termini of E3L related to vaccinia virus replication and pathogenesis. Furthermore, the ability of VV-containing mutations in the E3L gene to act as potential vaccines has been determined. Scarification of mice with wtVV, VVE3LΔ83N, or VVΔE3L provided full protection from a subsequent wtVV challenge (data not shown). For wtVV and VVE3LΔ83N, high levels of viral replication are required for this protective response (109 PFU/g) compared to VVΔE3L, which required much lower levels of viral replication (106 PFU/g) to induce a similar protective immune response. Previous data have shown that VVE3LΔ83N replicates to high titers in the nasal tissue upon intranasal inoculation, but subsequent spread to the lungs and brain is limited (5). For VVΔE3L, replication was much more reduced in all tissues, including the nasal turbinates (5). Therefore, VVΔE3L was able to induce a protective immune response against wtVV at much lower tissue antigen doses than wtVV or VVE3LΔ83N.
It is likely that the array of host inflammatory responses is directly related to the virulence and vaccine efficacy of these viruses after infection of mice. Despite replicating poorly at the site of infection in mice, VVΔE3L and VVE3LΔ26C induce a very strong protective immune response as measured by subsequent wtVV challenge (6; unpublished observations). The induction of antigen presentation genes and cellular extravasation genes permitting leukocyte influx into the site of infection along with the heightened cytokine response would support the high level of vaccine efficacy associated with these viruses.
The results from our signal transduction experiments agree well with the gradient of host inflammatory genes induced during infection with the VV constructs. Loss of E3L resulted in activation of IRF3, NF-κB, and ATF-2 and resulted in an increase in expression for the majority of inflammatory genes. VVE3LΔ26C activated NF-κB and ATF-2 and induced the expression of fewer proinflammatory genes than VVΔE3L. VVE3LΔ83N activated only the ATF-2 cascade and induced fewer proinflammatory genes than either VVΔE3L or VVE3LΔ26C. Finally, wtVV did not activate any of the transcription cascades investigated and led to the induction of few inflammatory response genes. Together, these data not only suggest which pathways and genes may be regulated by dsRNA and possibly Z-form nucleic acid but also suggest which transcription factors may be involved in the up-regulation of specific inflammatory genes. These data also fit well with the virulence associated with these viruses, where wtVV > VVE3LΔ83N > VVE3LΔ26C ≥ VVΔE3L. It is likely that these pathways and inflammatory genes are responsible for limiting the replication of some of these constructs as well as inducing the protective immune response.
The signal transduction data regarding VVΔE3L and VVE3LΔ26C suggest that either the N or C terminus of E3L can function independently to inhibit activation of the IRF3 cascade. Presumably, the C terminus of E3L may act to sequester the dsRNA activator of the IRF3 cascade, whereas the N terminus blocks an additional step in the cascade. Some points clearly remain to be investigated. Both VVΔE3L and VVE3LΔ26C led to up-regulation of ISG15 expression. ISG15 expression is known to be regulated by IRF3 (17). This up-regulation of ISG15 expression may be due to minor levels of IRF3 nuclear translocation observed during infection with VVE3LΔ26C (Fig. 5). It is also of note that many other cellular genes demonstrated to be induced by a constitutively active form of IRF3 are not induced by either VVΔE3L or VVE3LΔ26C (17). For example, IFN-β levels remained unchanged for all viruses. However, Dave et al. (11) reported that IFN-β expression could not be induced in HeLa cells. These results may also emphasize the complexity of cross talk between signal transduction cascades, promoter regulation by multiple transcription factors, or additional viral components, making the interpretation of key players in gene regulation difficult.
It is clear that dsRNA and, quite possibly, Z-form nucleic acid are associated with activation of the IFN system. Since dsRNA is very often produced in virus-infected cells (20, 23, 30), the presence of dsRNA is a key signal in cellular recognition of viral invasion. With Z-DNA or Z-RNA, a possible source of this nucleic acid during viral infection is unknown. With the VV E3L protein, sequestration of dsRNA and possibly Z-form nucleic acid is necessary for viral evasion of host defenses. Given the global nature of these viral danger signals, this information not only is important in understanding poxvirus pathogenesis but also can likely be applied to many viral systems.
Acknowledgments
This work was supported by NIH grants AI066501, AI066326, and AI052347 to the Jacobs laboratory. The Katze laboratory is supported by NIH grants DA015625-03, AI022646-20A, and AI056214-02.
REFERENCES
- 1.Baylis, C. D., and R. C. Condit. 1993. Temperature-sensitive mutants in the vaccinia virus A18R gene increase double-stranded RNA synthesis as a result of aberrant viral transcription. Virology 194:254-262. [DOI] [PubMed] [Google Scholar]
- 2.Beattie, E., E. B. Kaufman, H. Marinez, M. E. Perus, B. L. Jacobs, E. Paoletti, and J. Tartaglia. 1996. Host-range restriction of vaccinia virus E3L-specific deletion mutants. Virus Genes 12:89-94. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard, T. J., P. Alcami, and G. L. Smith. 1998. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J. Gen. Virol. 79:1159-1167. [DOI] [PubMed] [Google Scholar]
- 4.Boone, R., and B. Moss. 1978. Sequence complexity and relative abundance of vaccinia virus mRNAs synthesized in vivo and in vitro. J. Virol. 26:554-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandt, T., M. Heck, A. Vijaysri, G. Jentarra, J. Cameron, and B. Jacobs. 2005. The N-terminal domain of the vaccinia virus E3L-protein is required for neurovirulence, but not for induction of a protective immune response. Virology 333:263-270. [DOI] [PubMed] [Google Scholar]
- 6.Brandt, T. A., and B. L. Jacobs. 2001. Both the carboxyl- and amino-terminal domains of the vaccinia virus interferon resistance gene, E3L, are required for pathogenesis in a mouse model. J. Virol. 75:850-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brazma, A., P. Hingamp, J. Quackenbush, G. Sherlock, P. Spellman, C. Stoeckert, J. Aach, W. Ansorge, C. A. Ball, H. C. Causton, T. Gaasterland, P. Glenisson, F. C. Holstege, I. F. Kim, V. Markowitz, J. C. Matese, H. Parkinson, A. Robinson, U. Sarkans, S. Schulze-Kremer, J. Stewart, R. Taylor, J. Vilo, and M. Vingron. 2001. Minimum information about a microarray experiment (MIAME)—toward standards for microarray data. Nat. Genet. 29:365-371. [DOI] [PubMed] [Google Scholar]
- 8.Brum, L. M., M. C. Lopez, J. C. Varela, H. V. Baker, and R. W. Moyer. 2003. Microarray analysis of A549 cells infected with rabbitpox virus (RPV): a comparison of wild-type RPV and RPV deleted for the host gene, SPI-1. Virology 315:322-334. [DOI] [PubMed] [Google Scholar]
- 9.Chang, H. W., and B. L. Jacobs. 1993. Identification of a conserved motif that is necessary for binding of the vaccinia virus E3L gene product to double-stranded RNA. Virology 194:537-547. [DOI] [PubMed] [Google Scholar]
- 10.Chang, H. W., L. H. Uribe, and B. L. Jacobs. 1995. Rescue of vaccinia virus lacking the E3L gene by mutants of E3L. J. Virol. 69:6605-6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dave, R. S., J. P. McGettigan, T. Qureshi, M. J. Schnell, G. Nunnari, and R. J. Pomerantz. 2006. siRNA targeting vaccinia virus double-stranded RNA binding protein [E3L] exerts potent antiviral effects. Virology 348:489-497. [DOI] [PubMed] [Google Scholar]
- 12.Espert, L., C. Rey, L. Gonzalez, G. Degols, M. K. Chelbi-Alix, N. Mechti, and C. Gongora. 2004. The exonuclease ISG20 is directly induced by synthetic dsRNA via NF-kappaB and IRF1 activation. Oncogene 23:4636-4640. [DOI] [PubMed] [Google Scholar]
- 13.Geiss, G., G. Jin, J. Guo, R. Bumgarner, M. G. Katze, and G. C. Sen. 2001. A comprehensive view of regulation of gene expression by double-stranded RNA-mediated cell signaling. J. Biol. Chem. 276:30178-30182. [DOI] [PubMed] [Google Scholar]
- 14.Geiss, G. K., R. E. Bumgarner, M. C. An, M. B. Agy, A. B. van 't Wout, E. Hammersmark, V. S. Carter, D. Upchurch, J. I. Mullins, and M. G. Katze. 2000. Large-scale monitoring of host cell gene expression during HIV-1 infection using cDNA microarrays. Virology 266:8-16. [DOI] [PubMed] [Google Scholar]
- 15.George, C., and C. Samuel. 1999. Human RNA-specific adenosine deaminase ADAR1 transcripts possess alternative exon 1 structures that initiate from different promoters, one constitutively and the other interferon inducible. Proc. Natl. Acad. Sci. USA 96:4621-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goh, K., M. deVeer, and B. Williams. 2000. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. 19:4292-4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grandvaux, N., M. J. Servant, B. tenOever, G. C. Sen, S. Balachandran, G. N. Barber, R. Lin, and J. Hiscott. 2002. Transcriptional profiling of interferon regulatory factor 3 target genes: direct involvement in the regulation of interferon-stimulated genes. J. Virol. 76:5532-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerra, S., L. A. Lopez-Fernandez, A. Pascual-Montano, M. Munoz, K. Harshman, and M. Esteban. 2003. Cellular gene expression survey of vaccinia virus infection of human HeLa cells. J. Virol. 77:6493-6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerra, S., L. Lopez-Fernandex, R. Conde, A. Pascual-Montano, K. Harshman, and M. Esteban. 2004. Microarray analysis reveals characteristic changes of host gene expression in response to attenuated modified vaccinia virus Ankara infection of human HeLa cells. J. Virol. 78:5820-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haller, O., G. Kochs, and F. Weber. 2006. The interferon response circuit: induction and suppression by pathogenic viruses. Virology 344:119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herbert, A., and A. Rich. 1999. Left-handed Z-DNA: structure and function. Genetica 106:37-47. [DOI] [PubMed] [Google Scholar]
- 22.Iordanov, M., J. Wong, J. Bell, and B. Magun. 2001. Activation of NF-κB by double-stranded RNA (dsRNA) in the absence of protein kinase R and RNase L demonstrates the existence of two separate dsRNA-triggered antiviral programs. Mol. Cell. Biol. 21:61-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs, B. L., and J. O. Langland. 1996. When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology 219:339-349. [DOI] [PubMed] [Google Scholar]
- 24.Kahmann, J. D., D. A. Wecking, V. Putter, K. Lowenhaupt, Y. G. Kim, P. Schmieder, H. Oschkinat, A. Rich, and M. Schade. 2004. The solution structure of the N-terminal domain of E3L shows a tyrosine conformation that may explain its reduced affinity to Z-DNA in vitro. Proc. Natl. Acad. Sci. USA 101:2712-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawai, T., and S. Akira. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7:131-137. [DOI] [PubMed] [Google Scholar]
- 26.Kibler, K. V., T. Shors, K. B. Perkins, C. C. Zeman, M. P. Banaszak, J. Biesterfeldt, J. O. Langland, and B. L. Jacobs. 1997. Double-stranded RNA is a trigger for apoptosis in vaccinia virus-infected cells. J. Virol. 71:1992-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim, Y., K. Lowenhaupt, D. Oh, K. K. Kim, and A. Rich. 2004. Evidence that vaccinia virulence factor E3L binds to Z-DNA in vivo: implications for development of a therapy for poxvirus infection. Proc. Natl. Acad. Sci. USA 101:1514-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, Y., M. Muralinath, T. Brandt, M. Pearcy, K. Hauns, K. Lowenhaupt, B. L. Jacobs, and A. Rich. 2003. A role for Z-DNA binding in vaccinia virus pathogenesis. Proc. Natl. Acad. Sci. USA 100:6974-6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon, J., and A. Rich. 2005. Biological function of the vaccinia virus Z-DNA-binding protein E3L: gene transactivation and antiapoptotic activity in HeLa cells. Proc. Natl. Acad. Sci. USA 102:12759-12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langland, J. O., J. M. Cameron, M. C. Heck, J. K. Jancovich, and B. L. Jacobs. 2006. Inhibition of PKR by RNA and DNA viruses. Virus Res. 119:100-110. [DOI] [PubMed] [Google Scholar]
- 31.Langland, J. O., and B. L. Jacobs. 2002. The role of the PKR-inhibitory genes, E3L and K3L, in determining vaccinia virus host range. Virology 299:133-141. [DOI] [PubMed] [Google Scholar]
- 32.Langland, J. O., and B. L. Jacobs. 2004. Inhibition of PKR by vaccinia virus: role of the N- and C-terminal domains of E3L. Virology 324:419-429. [DOI] [PubMed] [Google Scholar]
- 33.Ludwig, H., J. Magas, C. Staib, M. Lehmann, R. Lang, and G. Sutter. 2005. Role of viral factor E3L in modified vaccinia virus Ankara infection of human HeLa cells: regulation of the virus life cycle and identification of differentially expressed host genes. J. Virol. 79:2584-2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lugwig, H., Y. Suezer, Z. Waibler, U. Kalinke, B. S. Schnierle, and G. Sutter. 2006. Double-stranded RNA-binding protein E3 controls translation of viral intermediate RNA, marking an essential step in the life cycle of modified vaccinia virus Ankara. J. Gen. Virol. 87:1145-1155. [DOI] [PubMed] [Google Scholar]
- 35.Najarro, P., P. Traktman, and J. A. Lewis. 2001. Vaccinia virus blocks gamma interferon signal transduction: viral VH1 phosphatase reverses Stat1 activation. J. Virol. 75:3185-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedley, S., and R. J. Cooper. 1984. The inhibition of HeLa cell RNA synthesis following infection with vaccinia virus. J. Gen. Virol. 65:1687-1697. [DOI] [PubMed] [Google Scholar]
- 37.Rice, A. P., and B. E. Roberts. 1983. Vaccinia virus induces cellular mRNA degradation. J. Virol. 47:529-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romano, P. R., F. Zhang, S. Tan, M. T. Garcia-Barrio, M. G. Katze, T. E. Dever, and A. G. Hinnebusch. 1998. Inhibition of double-stranded RNA-dependent protein kinase PKR by vaccinia virus E3: role of complex formation and the E3 N-terminal domain. Mol. Cell. Biol. 18:7304-7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothenburg, S., T. Schwartz, F. Koch-Nolte, and F. Haag. 2002. Complex regulation of the human gene for the Z-DNA binding protein DLM-1. Nucleic Acids Res. 30:993-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rothenburg, S., N. Deigendesch, K. Dittmar, F. Koch-Nolte, F. Haag, K. Lowenhaupt, and A. Rich. 2005. A PKR-like eukaryotic initiation factor 2 alpha kinase from zebrafish contains Z-DNA binding domains instead of dsRNA binding domains. Proc. Natl. Acad. Sci. USA 102:1602-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saunders, L. R., and G. N. Barber. 2003. The dsRNA binding protein family: critical roles, diverse cellular functions. FASEB J. 17:961-983. [DOI] [PubMed] [Google Scholar]
- 42.Schade, M., C. Turner, R. Kuhne, P. Schmieder, K. Lowenhaupt, A. Herbert, A. Rich, and H. Oschkinat. 1999. The solution structure of the Zalpha domain of the human RNA editing enzyme ADAR1 reveals a prepositioned binding surface for Z-DNA. Proc. Natl. Acad. Sci. USA 96:12465-12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schafer, S. L., R. Lin, P. A. Moore, J. Hiscott, and P. M. Pitha. 1998. Regulation of type I interferon gene expression by interferon regulatory factor-3. J. Biol. Chem. 273:2714-2720. [DOI] [PubMed] [Google Scholar]
- 44.Schroth, G. P., P. J. Chou, and P. S. Ho. 1992. Mapping Z-DNA in the humane genome. Computer-aided mapping reveals a nonrandom distribution of potential Z-DNA-forming sequences in human genes. J. Biol. Chem. 267:11846-11855. [PubMed] [Google Scholar]
- 45.Seet, B. T., J. B. Johnston, C. R. Brunetti, J. W. Barrett, H. Everett, C. Cameron, J. Sypula, S. H. Nazarian, A. Lucas, and G. McFadden. 2003. Poxvirus and immune evasion. Annu. Rev. Immunol. 21:377-423. [DOI] [PubMed] [Google Scholar]
- 46.Shisler, J. L., and X.-L. Jin. 2004. The vaccinia virus K1L gene product inhibits host NF-κB activation by preventing IκBα degradation. J. Virol. 78:3553-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shors, T., K. V. Kibler, K. B. Perkins, R. Seidler-Wulff, M. P. Banaszak, and B. L. Jacobs. 1997. Complementation of vaccinia virus deleted of the E3L gene by mutants of E3L. Virology 239:269-276. [DOI] [PubMed] [Google Scholar]
- 48.Silva, A., M. Whitmore, Z. Xu, Z. Jiang, X. Li, and B. R. Williams. 2004. Protein kinase R (PKR) interacts with and activates mitogen activated protein kinase 6 (MKK6) in response to double stranded RNA stimulation. J. Biol. Chem. 279:37670-37676. [DOI] [PubMed] [Google Scholar]
- 49.Smith, E. J., I. Marie, A. Prakash, A. Garcia-Sastre, and D. E. Levy. 2001. IRF3 and IRF7 phosphorylation in virus-infected cells does not require double-stranded RNA-dependent protein kinase R or IkB kinase but is blocked by vaccinia virus E3L protein. J. Biol. Chem. 276:8951-8957. [DOI] [PubMed] [Google Scholar]
- 50.Smith, M. W., Z. N. Yue, M. J. Korth, H. A. Do, L. Boix, N. Fausto, J. Bruix, R. L. Carithers, Jr., and M. G. Katze. 2003. Hepatitis C virus and liver disease: global transcriptional profiling and identification of potential markers. Hepatology 38:1458-1467. [DOI] [PubMed] [Google Scholar]
- 51.Smith, S. A., and G. J. Kotwal. 2002. Immune response to poxvirus infections in various animals. Crit. Rev. Microbiol. 28:149-185. [DOI] [PubMed] [Google Scholar]
- 52.Takenori, T., C. Tatematsu, and Y. Nakanishi. 2002. Double-stranded RNA activated protein kinase interacts with apoptosis signal-regulating kinase 1. Eur. J. Biochem. 269:6126-6132. [DOI] [PubMed] [Google Scholar]
- 53.Trapani, J. A., V. R. Sutton, and M. J. Smyth. 1999. CTL granules: evolution of vesicles essential for combating virus infections. Immunol. Today 20:351-356. [DOI] [PubMed] [Google Scholar]
- 54.Turner, P. C., and R. W. Moyer. 2002. Poxvirus immune modulators: functional insights from animal models. Virus Res. 88:35-53. [DOI] [PubMed] [Google Scholar]
- 55.Weber, F., G. Kochs, and O. Haller. 2004. Inverse interference: how viruses fight the interferon system. Viral Immunol. 17:498-515. [DOI] [PubMed] [Google Scholar]
- 56.Xiang, Y., D. A. Simpson, J. Spiegel, A. Zhou, R. H. Silverman, and R. C. Condit. 1998. The vaccinia virus A18R DNA helicase is a postreplicative negative transcription elongation factor. J. Virol. 72:7012-7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 58.Yuwen, H., J. H. Cox, J. W. Yewdell, J. R. Bennink, and R. Moss. 1993. Nuclear localization of a double-stranded RNA-binding protein encoded by the vaccinia virus E3L gene. Virology 195:732-744. [DOI] [PubMed] [Google Scholar]