Abstract
Filoviruses, represented by the genera Ebolavirus and Marburgvirus, cause a lethal hemorrhagic fever in humans and in nonhuman primates. Although filovirus can replicate in various tissues or cell types in these animals, the molecular mechanisms of its broad tropism remain poorly understood. Here we show the involvement of members of the Tyro3 receptor tyrosine kinase family—Axl, Dtk, and Mer—in cell entry of filoviruses. Ectopic expression of these family members in lymphoid cells, which otherwise are highly resistant to filovirus infection, enhanced infection by pseudotype viruses carrying filovirus glycoproteins on their envelopes. This enhancement was reduced by antibodies to Tyro3 family members, Gas6 ligand, or soluble ectodomains of the members. Live Ebola viruses infected both Axl- and Dtk-expressing cells more efficiently than control cells. Antibody to Axl inhibited infection of pseudotype viruses in a number of Axl-positive cell lines. These results implicate each Tyro3 family member as a cell entry factor in filovirus infection.
The family Filoviridae consists of two genera, Ebolavirus and Marburgvirus, which cause a severe hemorrhagic fever in humans and in nonhuman primates (15). Four species of Ebola virus—Zaire, Sudan, Ivory Coast, and Reston ebolaviruses—have been identified, with Zaire ebolavirus being the most pathogenic to humans (15). A recent outbreak of Marburg virus infection in Angola killed more than 300 people (fatality rate, ∼90%) (http://www.who.int/csr/don/2005_08_24/en/index.html), making this the most serious among several known outbreaks of Marburg virus.
In addition to primates, Ebola virus can infect rodents, such as mice (42) and guinea pigs, (7) and bats (52) under experimental conditions but is not lethal to these hosts unless adapted. In infected primates, the virus can be detected in virtually all organs (4, 6, 21, 43). Pseudotype viruses carrying filovirus glycoproteins (GPs)—the only surface proteins of the virus which mediate viral attachment and entry into cells—can infect avian and marsupial cells as well as many mammalian cell lines (8, 53, 62, 64). However, lymphoid cells do not appear to be susceptible to either authentic filovirus or pseudotype virus infection (8, 20, 21, 43, 47, 48, 62).
Numerous cellular factors contributing to filovirus entry into cells have been investigated in efforts to clarify the host/cell tropism and pathogenesis of these viruses. Chan et al. identified folate receptor alpha as a cofactor for filovirus cell entry (9), although a folate receptor alpha-independent pathway has been reported (9, 47, 50). Several groups, including our laboratory, have implicated calcium-dependent (C-type) lectins, dendritic cell-specific ICAM3-grabbing nonintegrin (DC-SIGN), DC-SIGN-related protein (DC-SIGNR), macrophage lectin specific for galactose/N-acetylgalactosamine (MGL), asialoglycoprotein receptor, and LSECtin, in this process (1, 5, 24, 32, 48, 55). The lectins are differentially expressed in dendritic cells, macrophages, sinusoidal endothelium, and hepatocytes, all of which are targeted by filoviruses (19-21, 33, 43, 65); however, at the terminal stage of infection, many types of cells become infected, regardless of lectin expression. We also showed that anti-β1 integrin antibodies or purified integrins only partially inhibited (∼50%) pseudotype virus infection (56). Thus, filovirus cell entry likely involves other, still unidentified molecules.
To gain a better understanding of the molecular mechanisms that drive the broad and distinctive infectivity of Ebola and Marburg viruses, we screened a cDNA library from cells (Vero E6) that are highly susceptible to filovirus infection, in order to identify molecules that participate in cell entry. The results indicate that three members of the Tyro3 family, Axl, Dtk, and Mer, which span the plasma membrane and contain intracellular tyrosine kinase domains (12, 13, 22, 36, 40), facilitate the cell entry of both Ebola and Marburg viruses. Most cells, except lymphocytes, express one or more of the Tyro3 family members (12, 22, 36, 39). Intriguingly, macrophages, one of the primary targets of filovirus infection (21, 43, 65), express all members of the family concomitantly (34). This distribution is consistent with filovirus tropism (8, 20, 21, 43, 47, 48, 62).
MATERIALS AND METHODS
Cells.
Vero E6, HT1080, 293, HeLa, COS-7, A549, and 293T cells were cultured in Dulbecco's modified Eagle's medium (Sigma). Jurkat (clone E6-1) cells were obtained from American Type Culture Collection and cultured in RPMI 1640 medium (Sigma). Both media were supplemented with 10% heat-inactivated fetal calf serum (FCS) and antibiotics. Plat-GP (murine leukemia virus [MLV]-based packaging) cells were kindly provided by T. Kitamura (University of Tokyo) and cultured in DMEM with 10% FCS and 10 μg/ml blasticidin (Invitrogen). Human umbilical vascular endothelial cells and hepatocytes were purchased from Cambrex Bio Science and cultured according to the manufacturer's protocols.
cDNA library.
A cDNA library was prepared from Vero E6 cells and cloned into the MLV retroviral vector pMX as described by Kitamura and Morikawa (29). The pMX plasmid was cotransfected with a vesicular stomatitis virus G (VSV-G) expression plasmid (pCAGGS) into Plat-GP cells by TransIT-293 reagent (Mirus Bio Corp). Two days later, culture supernatants were harvested, clarified through 0.45-μm MILLEX-HV filters (Millipore), and then used for infection of Jurkat cells at a multiplicity of infection of 0.3.
Pseudotype viruses.
GPs of Zaire ebolavirus (strain Zaire '76 Mayinga), Reston ebolavirus (Pennsylvania), Sudan ebolavirus (Boniface), Ivory Coast ebolavirus (Ivory Coast), and Lake Victoria marburgvirus (Musoke) were expressed from pCAGGS. These GPs, as well as the GP of Zaire Ebola virus lacking mucin-like domain (ZGPΔMuc) (49, 55), and VSV-G were used to pseudotype MLV and VSV. cDNA encoding feline CD2 (fCD2) (46) or green fluorescent protein (GFP) was cloned into pMXSL, which is a derivative of pMX lacking a 5′-primer binding site (29), and used as a reporter gene. MLV-based pseudotype virus was prepared by cotransfection of pMXSL and pCAGGS in Plat-GP cells. Two hundred milliliters of the transfected culture supernatants was clarified and concentrated to 13 ml by ultracentrifugation (50,000 × g, 2 h, 4°C) and then used as viral stocks. GFP expression in inoculated cells was assessed with a fluorescence microscope (TE300; Nikon) or by flow cytometry (FACSCalibur; Becton Dickinson) at 48 h postinoculation.
VSV pseudotyped with filovirus GP was prepared as described previously (53). It was treated with VSV-G-neutralizing antibody I1 before inoculation to reduce background infection mediated by residual virus possessing VSV-G, which was carried over during the preparation of the pseudotype virus.
Library screening.
Library-transduced Jurkat cells were incubated with MLV carrying the ZGPΔMuc and fCD2 gene at a 1:5 dilution of viral stock. Two days later, the cells were transferred to an anti-fCD2 antibody-coated dish to capture fCD2-positive cells (45). After a further 8 days of culture, colony-forming cells were incubated with MLV carrying ZGPΔMuc and the GFP gene for 2 days. GFP-positive colonies were picked under a fluorescence microscope. From cellular genomes isolated with DNAzol reagent (Invitrogen), inserted cDNAs were amplified by PCR with LA Taq (Takara Bio Inc) and pMX primers (29) and then sequenced.
Antibodies and proteins.
Monoclonal antibodies to MGL (clone MLD-1) and the Zaire Ebola virus GP (133/3.16 and 226/8.1) were described previously (27, 54). Antibodies to the Tyro3 family and DC-SIGN/DC-SIGNR (DC28), recombinant mouse Gas6, and recombinant human chimeric proteins (Axl/Fc, Dtk/Fc, and Mer/Fc) were purchased from R&D Systems, Inc. Polyclonal and monoclonal antibodies were used for virus inhibition assays and flow cytometry, respectively, unless otherwise stated.
cDNA clones.
cDNAs encoding human Axl and Dtk were purchased from Invitrogen (clones 5205825 and 6095596). Mer cDNA was cloned from a placental cDNA library (Invitrogen) by PCR. The amino acid sequence deduced from cloned cDNA of Mer was identical to that under accession number AH010001 (18).
Inhibition assays.
To evaluate the effects of antibodies to cell molecules or ligands, we incubated cells with the reagents at room temperature for 30 min and then infected them with virus. The neutralizing activities of antibodies or chimeric proteins were assessed by incubation with virus at room temperature for 30 min before their use to infect cells.
Flow cytometry.
Adherent cells were detached in phosphate-buffered saline containing 0.02% EDTA and then washed once with cold phosphate-buffered saline supplemented with 2% FCS and 0.1% sodium azide (wash buffer). Cells were incubated with antibodies on ice for 20 min. After washing in buffer, the cells were further incubated with secondary antibodies labeled with fluorescent isothiocyanate (Zymed Laboratories). Cells were washed and then analyzed by FACSCalibur with Cell Quest software (Becton Dickinson).
Cell culture experiments with recombinant Ebola virus.
Recombinant Zaire ebolavirus (strain Zaire '76 Mayinga) expressing GFP was generated by inserting the GFP open reading frame with 3′- and 5′-untranslated regions of the VP24 gene containing transcriptional start and stop signals into the intergenic region between the VP30 and VP24 genes of the viral genome (unpublished data). The virus retained a functional GFP gene in the genome even after nine passages in Vero E6 cells (data not shown). Cells were inoculated with the GFP-expressing Ebola virus at a multiplicity of infection of 0.25 or 0.75 (determined with Vero E6 cells). Two days later, cells were fixed with 4% paraformaldehyde, and GFP-positive cells were counted by flow cytometry. All work with live Ebola viruses was performed in a BSL-4 laboratory at the National Microbiology Laboratory of the Public Health Agency of Canada.
Statistical analysis.
Welch's t test was used to measure differences between two group means.
Nucleotide sequences.
Sequences of simian Axl cDNAs isolated from Vero E6 cells are available from the DNA Data Bank of Japan (AB240639 and AB240640).
RESULTS
Infectivity of ZGPΔMuc-MLV.
Since the titers of pseudotype virus are higher with the GP of Zaire Ebola virus lacking a mucin-like domain (ZGPΔMuc) than with the intact GP (10, 49, 50), we first used ZGPΔMuc (with deletion of amino acids at positions 311 to 462) (55) for pseudotyping of MLV and assessing the specificity of viral infection. The infectivity of MLV pseudotyped with ZGPΔMuc possessing the GFP gene [ZGPΔMuc-MLV(GFP)] on Vero E6 cells was almost completely inhibited by a monoclonal antibody to GP (clone 133/3.16 or 226/8.1; 10 μg/ml) (data not shown) which neutralizes Ebola virus infection (54). When 1 × 105 Jurkat cells were inoculated with ZGPΔMuc-MLV(GFP) at a 1:5 dilution of stock, which would be expected to result in GFP expression in >7 × 104 of 1 × 105 Vero E6 cells, 15 to 30 cells expressed GFP. GFP expression was not observed when the virus was pretreated with GP-monoclonal antibody (100 μg/ml), indicating that Jurkat cells have limited but perceptible susceptibility to ZGPΔMuc-mediated infection.
cDNA library screening.
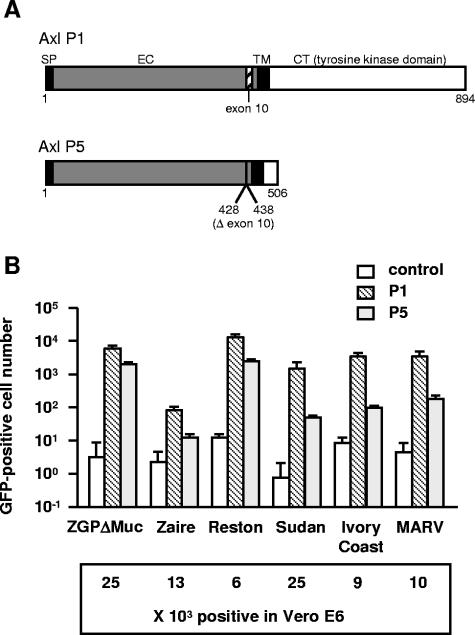
Expression of lectins MGL and DC-SIGN/DC-SIGNR in cells enhances Ebola virus infection (48, 55). Vero E6 cells are highly susceptible to filovirus infection, but we found that none of these lectins were expressed in these cells when tested by flow cytometry (data not shown), suggesting that Vero E6 cells have abundant, nonlectin receptor(s) for this virus. We therefore used a Vero E6 cDNA library as a resource for identification of Ebola virus cell entry factor(s). Infection of 2 × 107 library-transduced Jurkat cells with ZGPΔMuc-MLV(fCD2) led to the formation of approximately 1,200 colonies in anti-fCD2 antibody-coated dishes. After incubation with ZGPΔMuc-MLV(GFP), only five of the colonies expressed GFP. From each of these five (P1 to P5), we recovered Axl-coding cDNAs by PCR, although exon 10 (27 nucleotides) and most of the intracellular region were missing in P5 (illustrated in Fig. 1A). Axl cDNAs isolated from P1 (gene accession number AB240639) and P5 (AB240640) were cloned into the pMX vector. Axl cDNA from P5 lacked a stop codon, and therefore, translated Axl was fused at the C terminus (amino acid at position 506) to the PFQHSGRR sequence derived from the pMX vector sequence. After fresh Jurkat cells were transduced with the simian Axl cDNAs and the transduced cells were inoculated with ZGPΔMuc-MLV(GFP) (at a 1:25 viral stock dilution), we found that Axl expression enhanced viral infectivity (Fig. 1B). This effect was more pronounced with the Axl cDNA from P1, despite the higher level of Axl expression obtained with the cDNA from P5 (90% versus 33% positive and 99 mean fluorescence versus 28 mean fluorescence intensity in positive cells by flow cytometry). Enhanced viral infectivity upon Axl expression was also observed for the authentic Zaire GP, the GPs of Reston, Sudan, and Ivory Coast Ebola viruses, and the Marburg virus GP (Fig. 1B). To compare viral titers, we infected 1 × 105 Vero E6 cells with a 1:25 viral stock dilution and assessed GFP expression by flow cytometry (see bottom of Fig. 1B). The infectivities of MLV pseudotyped with VSV-G were similar in control and in simian Axl-expressing Jurkat cells (data not shown).
FIG. 1.
(A) Schematic diagram of Axl obtained from Vero E6 cDNA library. Axl clone P1 is full length, whereas clone P5 lacks an exon 10-corresponding region (nine amino acids) and most of the cytoplasmic tyrosine kinase domain. SP, signal peptide; EC, extracellular domain; TM, transmembrane domain; CT, cytoplasmic domain. Left, N terminus; right, C terminus. Numbers indicate amino acid positions. (B) Jurkat cells were transduced with simian Axl (P1 and P5) by retroviral vectors. Control indicates cells transduced with empty vector. These cells (1 × 105 cells) were infected with MLV pseudotyped with filovirus GP from indicated species or virus (1:25 dilution), and 2 days later, GFP-expressing cells were counted. The data are means ± standard deviations (n = 3). Vero E6 cells were infected with pseudotyped viruses at the same dilution as that for Jurkat cells, and GFP expression was determined by flow cytometry (GFP-positive cell numbers are shown beneath the graph).
Effect of Tyro3 family expression in lymphoid cells.
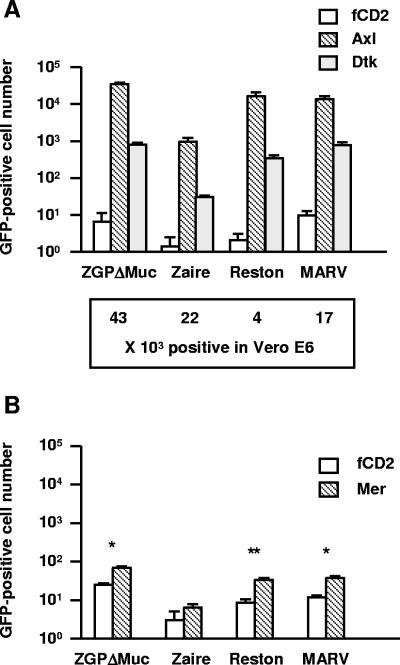
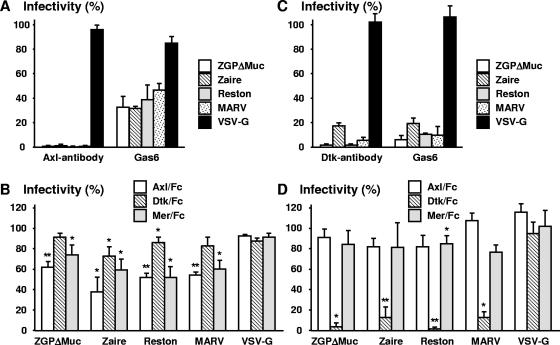
Axl (also called Ark, Tyro7, or Ufo) (40) is a receptor-type tyrosine kinase and, together with Dtk (also called Brt, Etk2, Rse, Sky, Tif, or Tyro3) (12, 13, 36) and Mer (also called Nyk or Tyro12) (22), constitutes the Tyro3 family. In a preliminary experiment, Mer/Fc, comprising an ectodomain of Mer fused with the immunoglobulin Fc, and Axl/Fc partially inhibited filovirus GP-mediated infection in simian Axl-expressing cells (P1) (data not shown), suggesting that expression of other members of the human Tyro3 family in Jurkat cells might promote GP-mediated infection with pseudotype MLV. Jurkat cells were stably transduced with the cDNA of human Axl, Dtk, or Mer by a retroviral vector and then examined in the following experiments. As with simian Axl, human Axl and Dtk enhanced GP-mediated infection (Fig. 2A) by comparison with fCD2 (negative control). Infectivity of the pseudotype viruses in Vero E6 cells is shown at the bottom of Fig. 2A. Mer expression also enhanced GP-mediated infection but to a lesser extent than Axl or Dtk expression (Fig. 2B). Flow cytometric analysis revealed a low level of Mer expression that could not be improved with use of alternative expression vectors (data not shown). Hence, Mer-expressing cells were not studied further, although a higher level of Mer expression may enhance infection to a level similar to that obtained with Axl. GP-mediated infection in Axl-expressing cells was reduced in a dose-dependent manner by pretreatment of cells with Axl polyclonal antibody (0.1 to 10 μg/ml) or Gas6 (a common ligand for the Tyro3 family) (0.05 to 5 μg/ml) (Fig. 3A). Pretreatment of viruses with Axl/Fc or Mer/Fc (20 μg/ml) also reduced their infectivity (Fig. 3B). Although Dtk/Fc significantly inhibited Zaire or Reston GP-mediated infection, its effects were generally less than those of Axl/Fc or Mer/Fc (Fig. 3B). In Dtk-expressing cells, infection was inhibited more than 80% by Dtk antibody, Gas6, and Dtk/Fc (Fig. 3C and D). Mer/Fc also had a statistically significant inhibitory effect on Reston GP-mediated infection, but it did not exceed 20% (Fig. 3D). Neutralizing monoclonal antibodies to GP (10 μg/ml) abolished ZGPΔMuc- and Zaire GP-mediated infection of Axl- and Dtk-expressing cells (>98% inhibition). None of the inhibitory reagents showed any effect on VSV-G-mediated infection (Fig. 3).
FIG. 2.
Jurkat cells were stably transduced with a human Tyro3 family member or fCD2 (negative control). These cells (1 × 105 cells) were infected with MLV pseudotyped with filovirus GP at a 1:25 (A) or 1:5 (B) stock dilution. GFP-positive cells were counted 2 days later. The data shown are means ± standard deviations (n = 3). For panel A, Vero E6 cells (1 × 105) were also inoculated with the pseudotypes at 1:25 viral dilution. GFP-positive cell numbers in the cells are indicated beneath the graph. The statistical analysis performed for panel B tested the means of fCD2- and Mer-expressing cells. *, P < 0.05; **, P < 0.01 (Welch's t test).
FIG. 3.
Inhibition of MLV-based pseudotype virus infection by antibody, ligand, or soluble ectodomain of Tyro3 family. Axl (A,B)-expressing and Dtk (C,D)-expressing Jurkat cells were treated with polyclonal antibody to Axl or Dtk (0.1 to 10 μg/ml) or with Gas6 ligand (0.05 to 5 μg/ml) and then infected (A,C). Results with 10 μg/ml antibody and 5 μg/ml ligand are shown. Pseudotype viruses were treated with soluble ectodomains of Tyro3 family members (20 μg/ml) prior to inoculation (B,D). Percent infectivity was calculated as follows: (GFP-positive cell number in reagent-treated culture/GFP-positive cell number in mock-treated culture) × 100. The data shown are means ± standard deviations (n = 3). Statistical analysis was performed for panels B and D: Welch's t test. *, P < 0.05; **, P < 0.01 (all versus mock treated).
The infectivity of a VSV-based pseudotype virus carrying filovirus GPs was also enhanced by Axl or Dtk expression (Fig. 4). The data indicate that the enhancing effects of Axl and Dtk on filovirus GP-mediated infection do not depend on the backbones of the pseudotype viruses. The infectivities of pseudotyped VSVs in Vero E6 cells were assessed and are shown in Fig. 4.
FIG. 4.
Infectivity of VSV-based pseudotype virus in Jurkat cells. Cells (1 × 105 cells) were infected with viruses pseudotyped with the indicated filovirus GPs. GFP-positive cells were counted 1 day later. The data are means ± standard deviations (n = 3). The infectivities of VSV pseudotype viruses in Vero E6 cells are given beneath the graph.
Effects of Axl and Dtk expression on infection with live Ebola virus.
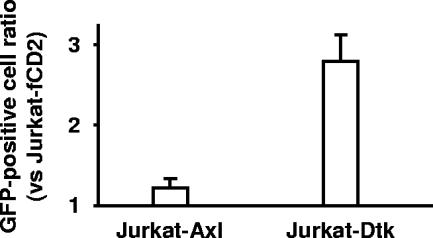
Jurkat cells expressing Axl, Dtk, or fCD2 (control) (5 × 104 cells) were inoculated with recombinant Zaire Ebola virus carrying a GFP gene, and 2 days later, GFP-positive cells were counted (this relatively short culture period permits only limited cycles of replication by Ebola virus). GFP-positive cells (45 to 373 cells) were observed in each cell culture. The ratios of positive cells in Axl- or Dtk-expressing cells to those in fCD2-expressing cells exceeded 1 (1.2 and 2.8) (Fig. 5), indicating that Axl and Dtk expression promoted cell entry of the recombinant Ebola virus. Together with the results of pseudotype virus assays, these findings demonstrate that Tyro3 family members participate in cell entry of Ebola virus.
FIG. 5.
Live Ebola virus infection in Axl- or Dtk-expressing Jurkat cells. fCD2-expressing cells were used as controls. Cells were infected with recombinant Zaire Ebola virus expressing GFP, and GFP-positive cells were counted 2 days later. Ratios of positive cells in Axl- or Dtk-expressing cells to those in fCD2-expressing cells are shown. Experiments were performed in quadruplicate and the data reported as means ± standard deviations.
Enhancement of infection for pseudotype virus was higher with Axl than with Dtk (Fig. 2A and 4), contrary to results with live Ebola virus (Fig. 5). This discrepancy may originate from differences in virion morphology, or possibly the viral internal proteins (e.g., Gag, M, and VP40) affect the degree of enhancement.
Tyro3 family expression and GP-mediated infection.
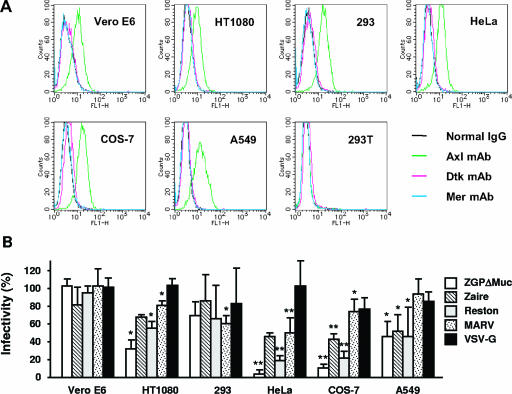
By flow cytometry, Axl expression was detected in Vero E6, HT1080, 293, HeLa, COS-7, and A549 but not 293T cells (Fig. 6A), all of which are susceptible to pseudotype viruses carrying filovirus GPs or to wild-type Ebola viruses (8, 35, 50, 53, 62). Neither Dtk nor Mer expression was detected in the cell lines (Fig. 6A). Using the Axl-positive cell lines, we examined the effects of antibody to Axl on infection with an MLV-based pseudotype virus. The Axl antibody significantly inhibited infection in five of the cell lines but not in Vero E6 cells (Fig. 6B). The inhibitory effects of Axl antibody were especially striking in HeLa and COS-7 cells infected with ZGPΔMuc- or Reston GP-pseudotyped virus (90 to 95% and 80%, respectively), indicating that infection of these cell lines by the pseudotyped viruses was mediated primarily by Axl. Filovirus GP-mediated infection in Jurkat cells expressing simian Axl (P1 from Vero E6) was >90% inhibited by the Axl antibody (data not shown); therefore, the failure of the antibody to inhibit infection in Vero E6 cells indicates the existence of Axl-independent entry. Antibody to Axl failed to inhibit VSV-G-mediated infection in all cell lines tested (Fig. 6B). Nor did the addition of antibodies to Dtk and Mer enhance Axl antibody-mediated inhibition of viral infection (data not shown).
FIG. 6.
(A) Expression of the Tyro3 family members in cell lines. Cells were detached and incubated with monoclonal antibodies (mAb) to the Tyro3 family members or normal immunoglobulin G. The cells were then incubated with secondary antibodies labeled with fluorescein isothiocyanate and assessed by flow cytometry. (B) Inhibition of infection of viruses pseudotyped with GPs by polyclonal antibody to Axl. Cell lines indicated were treated with antibody to Axl (10 μg/ml) and then infected with MLV-based pseudotype virus. Percent infectivity was calculated as follows: (GFP-positive cell number in antibody-treated culture/GFP-positive cell number in normal IgG-treated culture) × 100. The data are means ± standard deviations (n = 3). *, P < 0.05; **, P < 0.01 (all versus normal immunoglobulin G treated) by Welch's t test.
DISCUSSION
In the present study, we identified three members of the Tyro3 family (Axl, Dtk, and Mer) as novel cell entry factors for Ebola and Marburg viruses. These molecules are widely distributed in many types of cells throughout the body (11-13, 16, 18, 22, 26, 31, 34, 36, 39-41, 61), except on lymphocytes and granulocytes (12, 22, 36, 39), and are highly conserved among different animal species (23, 28), consistent with the cell and host tropisms of filovirus. While we also found a Tyro3 family-independent pathway(s) susceptible to pseudotype virus infection (Fig. 6B, e.g., Vero E6 cells), filovirus likely infects some cells in vivo via Tyro3 family members, since Axl antibody significantly inhibited GP-mediated infection in a number of cell lines (Fig. 6B). Knockout mice may provide a model for studying the significance of the Tyro3 family members in vivo.
The exact roles of Tyro3 family proteins during cell entry of filoviruses remain unclear. Gas6 binding results in movement of Tyro3 family members toward lysosomes through endocytosis (57) or engulfment of apoptotic cells (38, 44, 63). Moreover, given that the cytoplasmic tails of receptor tyrosine kinases are essential for ligand-induced internalization (44, 57), we find it intriguing that a simian Axl clone from P5, lacking most of the tyrosine kinase domain, showed a reduced ability to enhance infection compared with that of full-length Axl from P1 (Fig. 1). The Axl clone from P5 also lacked exon 10, but we found that Axl lacking only the exon 10-corresponding region showed enhancing effects on filovirus GP-mediated infection, similar to those of full-length Axl (data not shown). Considered together, these observations suggest a mechanism in which Tyro3 family members promote filovirus cell entry by facilitating transfer of viral particles to endosomes, thus allowing invasion into the cytoplasm (8, 10, 53, 62).
Interaction between the filovirus GP and Tyro3 family members may also contribute to viral pathogenesis. Filovirus infection activates macrophages/monocytes, resulting in the release of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) (25, 51). Elevated levels of TNF-α in blood, together with other factors, increase vascular permeability (3, 14, 58-60). Indeed, coagulation defects are features often observed in persons infected with filovirus (4, 17, 19). Gas6 binding to Axl or other members of the Tyro3 family induce cell type-specific effects, such as deactivation of stimulated macrophages (34). In the vasculature, Gas6-Tyro3 binding prevents endothelial cells from TNF-α-induced cytotoxicity (37, 41) and stabilizes platelet aggregation (2, 11). Thus, by interfering with this binding, filovirus GPs could alter the physical functions of the Tyro3 family, resulting in dysregulation of the immune and vascular systems, a hallmark of filovirus infection (15).
A Dtk/Fc chimeric protein efficiently inhibited GP-mediated infection in Dtk-expressing cells (Fig. 3D) but not in those expressing Axl (Fig. 3B). Axl/Fc and Mer/Fc behaved in a similar fashion (Fig. 3B and D). These results suggest that Axl and Mer may interact with the same or overlapping regions of GP, while Dtk may bind to a region distinct from those recognized by Axl and Mer. Although a more detailed analysis will be required to test this possibility, the results agree with comparisons showing a greater similarity of Axl and Mer within the Tyro3 family (23). Recently, the amino acids/regions of GPs critical for filovirus cell entry/binding were identified with use of 293T, HeLa, and/or Vero E6 cells (30, 35). However, Dtk was not detected in either of these cell lines in flow cytometry, although Axl was detected in HeLa and Vero E6 cells (data not shown). Thus, it is possible that GP amino acids/regions different from those identified with these cell lines would be found if Dtk-positive cells are used as targets.
Expression of Tyro3 family members enhanced the infection of pseudotype viruses carrying any of the filovirus GPs tested, and inhibition patterns by reagents such as antibodies and chimeric proteins did not differ substantially among Ebola virus GPs (Fig. 3). However, there appeared to be an inverse correlation between infectivity in Vero E6 cells and that in Jurkat cells. That is, the infectivity of viruses pseudotyped with Zaire GP was always high in Vero E6 cells but low in Axl-expressing Jurkat cells, by comparison with viruses carrying Reston GP (Fig. 1B, 2A, and 4). The extent of enhancement of viral infectivity by Axl expression was also lower with Zaire GP than with Reston GP (39- versus 1,061-fold in Fig. 1B; 726- versus 7,546-fold in Fig. 2A; 21- versus 67-fold in Fig. 4). In HeLa and COS-7 cells, antibody to Axl inhibited infection with Zaire GP-pseudotyped MLV less efficiently than did MLV pseudotyped with Reston GP (Fig. 6B). These results indicate that Zaire GP-mediated infection by pseudotype virus does not depend as much on Axl as does infection mediated by Reston GP. Such preferential usage of cellular receptors may account for the virulence of Ebola virus (15).
That ZGPΔMuc-mediated infection was enhanced more profoundly by Axl (roughly 10-fold) than was Zaire GP-mediated infection (Fig. 1B, 2A, and 4) and that the former was more efficiently inhibited than the latter by Axl antibody in HeLa and COS-7 cells (Fig. 6B) likely indicate that the mucin-like domain broadens the tropism of Zaire GP-mediated infection. While this domain contributes to MGL-mediated Ebola virus infection (55), we found no expression of the lectin in HeLa and COS-7 cells (data not shown). Hence, the mucin-like domain of Zaire GP appears to play a role in the cell entry of filoviruses, whether mediated by MGL or by other molecules.
The limited, but measurable, susceptibility of parental Jurkat cells to filovirus GP-mediated infection we observed is inconsistent with previous reports by others (1, 8, 47, 64). None of 17 Jurkat clones established by limiting dilution were completely resistant to infection by pseudotype virus carrying a GFP gene (data not shown). We found that detection of pseudotype virus infection is more sensitive when we use GFP, rather than luciferase, as a reporter (data not shown), which may explain the inconsistency between our results and those of others, who used pseudotype virus with the latter reporter protein. Consistent with our finding, Chan et al. reported that a small population of Jurkat cells was susceptible to infection with pseudotype virus carrying Marburg virus GP and a drug-resistant gene (9), suggesting that Jurkat cells, in fact, have a low but appreciable level of susceptibility to filovirus GP-mediated infection.
The effects of Axl or Dtk expression on the enhancement of live Ebola virus infection were small (less than 10-fold) (Fig. 5) compared with those of pseudotype virus infection (10- to 1,000-fold or more) (Fig. 1B, 2A, and 4). Our assay with live virus reflects both viral cell entry and many following steps, such as viral genomic replication and transcription, while the assays with pseudotype virus measure only cell entry of the viruses. The limited enhancement for live Ebola virus compared with pseudotype virus, therefore, may be due to inefficient replication of the former virus in lymphoid cells. Alternatively, the differences observed between the pseudotype virus and Ebola virus could reflect differences in their cell entry processes.
Our findings highlight the complexity of the filovirus entry mechanism, in which many cellular molecules, including Tyro3 family members, integrins, and lectins, can be involved. Comprehensive analyses with these molecules will be important to elucidate the filovirus entry mechanism. Such studies ultimately not only will lead to a better understanding of the host/cellular range of filoviruses but might also support development of prophylactic/therapeutic strategies against these highly pathogenic viruses.
Acknowledgments
We thank Michael Whitt for VSVΔG*, Douglas Lyles for VSV-G antibody I1, and John Gilbert for editing the manuscript.
This work was supported by grants for the Core Research for Evolutional Science and Technology Agency from the Japan Science and Technology Agency and by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor and Welfare, Japan, by the National Institute of Allergy and Infectious Diseases Public Health Service research grants, and by grants from the Public Health Agency of Canada and the Canadian Institute of Health Research (MOP-43921).
REFERENCES
- 1.Alvarez, C., F. Lasala, J. Carrillo, O. Muniz, A. L. Corbí, and R. Delgado. 2002. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 76:6841-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelillo-Scherrer, A., L. Burnier, N. Flores, P. Savi, M. DeMol, P. Schaeffer, J.-M. Herbert, G. Lemke, S. P. Goff, G. K. Matsushima, H. S. Earp, C. Vesin, M. F. Hoylaerts, S. Plaosance, D. Collen, E. M. Conway, B. Wehrle-Haller, and P. Carmeliet. 2005. Role of Gas6 receptors in platelet signaling during thrombus stabilization and implications for antithrombotic therapy. J. Clin. Investig. 115:237-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baize, S., E. M. Leroy, A. J. Georges, M.-C. Georges-Courbot, M. Capron, I. Bedjabaga, J. Lansoud-Soukate, and E. Mavoungou. 2002. Inflammatory responses in Ebola virus-infected patients. Clin. Exp. Immunol. 128:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baskerville, A., S. P. Fisher-Hoch, G. H. Neild, and A. B. Dowsett. 1985. Ultrastructural pathology of experimental Ebola haemorrhagic fever virus infection. J. Pathol. 147:199-209. [DOI] [PubMed] [Google Scholar]
- 5.Becker, S., M. Spiess, and H.-D. Klenk. 1995. The asialoglycoprotein receptor is a potential liver-specific receptor for Marburg virus. J. Gen. Virol. 76:393-399. [DOI] [PubMed] [Google Scholar]
- 6.Bowen, E. T., G. S. Platt, D. I. Simpson, L. B. McArdell, and R. T. Raymond. 1978. Ebola haemorrhagic fever: experimental infection of monkeys. Trans. R. Soc. Trop. Med. Hyg. 72:188-191. [DOI] [PubMed] [Google Scholar]
- 7.Bowen, E. T. W., G. S. Platt, G. Lloyd, A. Baskerville, W. J. Harris, and E. E. Vella. 1977. Viral haemorrhagic fever in southern Sudan and northern Zaire. Preliminary studies on the aetiological agent. Lancet 12:571-573. [DOI] [PubMed] [Google Scholar]
- 8.Chan, S., R. F. Speck, M. C. Ma, and M. A. Goldsmith. 2000. Distinct mechanisms of entry by envelope glycoproteins of Marburg and Ebola (Zaire) viruses. J. Virol. 74:4933-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan, S. Y., C. J. Empig, F. J. Welte, R. F. Speck, A. Schmaljohn, J. F. Kreisberg, and M. A. Goldsmith. 2001. Folate receptor-alpha is a cofactor for cellular entry by Marburg and Ebola viruses. Cell 106:117-126. [DOI] [PubMed] [Google Scholar]
- 10.Chandran, K., N. J. Sullivan, U. Felbor, S. P. Whelan, and J. M. Cunningham. 2005. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 308:1643-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, C., Q. Li, A. L. Darrow, Y. Wang, C. K. Derian, J. Yang, L. de Garavilla, P. Andrade-Gordon, and B. P. Damiano. 2004. Mer receptor tyrosine kinase signaling participates in platelet function. Arterioscler. Thromb. Vasc. Biol. 24:1118-11123. [DOI] [PubMed] [Google Scholar]
- 12.Crosier, K. E., L. R. Hall, P. M. Lewis, C. M. Morris, C. R. Wood, J. C. Morris, and P. S. Crosier. 1994. Isolation and characterization of the human DTK receptor tyrosine kinase. Growth Factors 11:137-144. [DOI] [PubMed] [Google Scholar]
- 13.Dai, W., H. Pan, H. Hassanain, S. L. Gupta, and M. J. Murphy, Jr. 1994. Molecular cloning of a novel receptor tyrosine kinase, tif, highly expressed in human ovary and testis. Oncogene 9:975-979. [PubMed] [Google Scholar]
- 14.Feldmann, H., H. Bugany, F. Mahner, H.-D. Klenk, D. Drenckhahn, and H.-J. Schnittler. 1996. Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J. Virol. 70:2208-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldmann, H., T. W. Geisbert, P. B. Jahrling, H.-D. Klenk, S. V. Netesov, C. J. Peters, A. Sanchez, R. Swanepoel, and V. E. Volchkov. 2004. Family Filoviridae, p645-653. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy, 8th ed. Elsevier/Academic Press, London, United Kingdom.
- 16.Fiebeler, A., J.-K. Park, D. N. Muller, C. Lindschau, M. Mengel, S. Merkel, B. Banas, F. C. Luft, and H. Haller. 2004. Growth arrest specific protein 6/Axl signaling in human inflammatory renal diseases. Am. J. Kidney Dis. 43:286-295. [DOI] [PubMed] [Google Scholar]
- 17.Fisher-Hoch, S. P., G. S. Platt, G. H. Neild, T. Southee, A. Baskerville, R. T. Raymond, G. Lloyd, and D. I. H. Simpson. 1985. Pathophysiology of shock and hemorrhage in a fulminating viral infection (Ebola). J. Infect. Dis. 152:887-894. [DOI] [PubMed] [Google Scholar]
- 18.Gal, A., Y. Li, D. A. Thompson, J. Weir, U. Orth, S. G. Jacobson, E. Apfelstedt-Sylla, and D. Vollrath. 2000. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat. Genet. 26:270-271. [DOI] [PubMed] [Google Scholar]
- 19.Geisbert, T. W., H. A. Young, P. B. Jahrling, K. J. Davis, T. Larsen, E. Kagan, and L. E. Hensley. 2003. Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am. J. Pathol. 163:2371-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geisbert, T. W., L. E. Hensley, T. R. Gibb, K. E. Steele, N. K. Jaax, and P. B. Jahrling. 2000. Apoptosis induced in vitro and in vivo during infection by Ebola and Marburg viruses. Lab. Investig. 80:171-186. [DOI] [PubMed] [Google Scholar]
- 21.Geisbert, T. W., P. B. Jahrling, M. A. Hanes, and P. M. Zack. 1992. Association of Ebola-related Reston virus particles and antigen with tissue lesions of monkeys imported to the United States. J. Comp. Pathol. 106:137-152. [DOI] [PubMed] [Google Scholar]
- 22.Graham, D., T. L. Dawson, D. L. Mullaney, H. R. Snodgrass, and H. R. Earp. 1994. Cloning and mRNA expression analysis of a novel human protooncogene, c-mer. Cell Growth Differ. 5:647-657. [PubMed] [Google Scholar]
- 23.Graham, D. K., G. W. Bowman, T. L. Dawson, W. L. Stanford, H. S. Earp, and H. R. Snodgrass. 1995. Cloning and developmental expression analysis of the murine c-mer tyrosine kinase. Oncogene 10:2349-2359. [PubMed] [Google Scholar]
- 24.Gramberg, T., H. Hofmann, P. Möller, P. F. Lalor, A. Marzi, M. Geier, M. Krumbiegel, T. Winkler, F. Kirchhoff, D. H. Adams, S. Becker, J. Münch, and S. Pöhlmann. 2005. LSECtin interacts with filovirus glycoproteins and the spike protein of SARS coronavirus. Virology 340:224-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta, M., S. Mahanty, R. Ahmed, and P. E. Rollin. 2001. Monocyte-derived human macrophages and peripheral blood mononuclear cells infected with ebola virus secrete MIP-1α and TNF-α and inhibit poly-IC-induced IFN-α in vitro. Virology 284:20-25. [DOI] [PubMed] [Google Scholar]
- 26.Healy, A. M., J. J. Schwartz, X. Zhu, B. E. Herrick, B. Varnum, and H. W. Farber. 2001. Gas 6 promotes Axl-mediated survival in pulmonary endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L1273-L1281. [DOI] [PubMed] [Google Scholar]
- 27.Higashi, N., A. Morikawa, K. Fujioka, Y. Fujita, Y. Sano, M. Miyata-Takeuchi, N. Suzuki, and T. Irimura. 2002. Human macrophage lectin specific for galactose/N-acetylgalactosamine is a marker for cells at an intermediate stage in their differentiation from monocytes into macrophages. Int. Immunol. 14:545-554. [DOI] [PubMed] [Google Scholar]
- 28.Janssen, J. W. G., A. S. Schulz, A. C. M. Steenvoorden, M. Schmidberger, S. Strehl, P. F. Ambros, and C. R. Bartram. 1991. A novel putative tyrosine kinase receptor with oncogenic potential. Oncogene 6:2113-2120. [PubMed] [Google Scholar]
- 29.Kitamura, T., and Y. Morikawa. 2000. Isolation of T-cell antigens by retrovirus-mediated expression cloning. Methods Mol. Biol. 134:143-152. [DOI] [PubMed] [Google Scholar]
- 30.Kuhn, J. H., S. R. Radoshitzky, A. C. Guth, K. L. Warfield, W. Li, M. J. Vincent, J. S. Towner, S. T. Nichol, S. Bavari, H. Choe, M. J. Aman, and M. Farzan. 2006. Conserved receptor-binding domains of Lake Victoria marburgvirus and Zaire ebolavirus bind a common receptor. J. Biol. Chem. 281:15951-15958. [DOI] [PubMed] [Google Scholar]
- 31.Li, R., J. Chen, G. Hammonds, H. Phillips, M. Armanini, P. Wood, R. Bunge, P. J. Godowski, M. X. Sliwkowski, and J. P. Mather. 1996. Identification of Gas6 as a growth factor for human Schwann cells. J. Neurosci. 16:2012-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin, G., G. Simmons, S. Pöhlmann, F. Baribaud, H. Ni, G. J. Leslie, B. S. Haggarty, P. Bates, D. Weissman, J. A. Hoxie, and W. Doms. 2003. Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC-SIGN and DC-SIGNR. J. Virol. 77:1337-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu, W., L. Tang, G. Zhang, H. Wei, Y. Cui, L. Guo, Z. Gou, X. Chen, D. Jiang, Y. Zhu, G. Kang, and F. He. 2004. Characterization of a novel C-type lectin-like gene, LSECtin: demonstration of carbohydrate binding and expression in sinusoidal endothelial cells of liver and lymph node. J. Biol. Chem. 279:18748-18758. [DOI] [PubMed] [Google Scholar]
- 34.Lu, Q., and G. Lemke. 2001. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science 293:306-311. [DOI] [PubMed] [Google Scholar]
- 35.Manicassamy, B., J. Wang, H. Jiang, and L. Rong. 2005. Comprehensive analysis of ebola virus GP1 in viral entry. J. Virol. 79:4793-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mark, M. R., D. T. Scadden, Z. Wang, Q. Gu, A. Goddard, and P. J. Godowski. 1994. rse, a novel receptor-type tyrosine kinase with homology to Axl/Ufo, is expressed at high levels in the brain. J. Biol. Chem. 269:10720-10728. [PubMed] [Google Scholar]
- 37.Melaragno, M. G., Y.-W. C. Fridell, and B. C. Berk. 1999. The Gas6/Axl system: a novel regulator of vascular cell function. Trends Cardiovasc. Med. 9:250-253. [DOI] [PubMed] [Google Scholar]
- 38.Nakano, T., Y. Ishimoto, J. Kishino, M. Umeda, K. Inoue, K. Nagata, K. Ohashi, K. Mizuno, and H. Arita. 1997. Cell adhesion to phosphatidylserine mediated by a product of growth arrest-specific gene 6. J. Biol. Chem. 272:29411-29414. [DOI] [PubMed] [Google Scholar]
- 39.Neubauer, A., A. Fiebeler, D. K. Graham, J. P. O'Bryan, C. A. Schmidt, P. Barckow, S. Serke, W. Siegert, H. R. Snodgrass, D. Huhn, and E. T. Liu. 1994. Expression of axl, a transforming receptor tyrosine kinase, in normal and malignant hematopoiesis. Blood 84:1931-1941. [PubMed] [Google Scholar]
- 40.O'Bryan, J. P., R. A. Frye, P. C. Cogswell, A. Neubauer, B. Kitch, C. Prokop, R. Espinosa III, M. M. Le Beau, H. S. Earp, and E. T. Liu. 1991. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol. Cell. Biol. 11:5016-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Donnell, K., I. C. Harkes, L. Dougherty, and I. P. Wicks. 1999. Expression of receptor tyrosine kinase Axl and its ligand Gas6 in rheumatoid arthritis: evidence for a novel endothelial cell survival pathway. Am. J. Pathol. 154:1171-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pattyn, S., W. Jacob, G. van der Groen, P. Piot, and G. Courteille. 1977. Isolation of Marburg-like virus from a case of haemorrhagic fever in Zaire. Lancet 12:573-574. [DOI] [PubMed] [Google Scholar]
- 43.Ryabchikova, E. I., L. V. Kolesnikova, and S. V. Luchko. 1999. An analysis of features of pathogenesis in two animal models of Ebola virus infection. J. Infect. Dis. 179:S199-S202. [DOI] [PubMed] [Google Scholar]
- 44.Scott, R., E. J. McMahon, S. M. Pop, E. A. Reap, R. Caricchio, P. L. Cohen, H. S. Earp, and G. K. Matsushima. 2001. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411:207-211. [DOI] [PubMed] [Google Scholar]
- 45.Shimojima, M., T. Miyazawa, Y. Sakurai, Y. Nishimura, Y. Tohya, Y. Matsuura, and H. Akashi. 2003. Usage of myeloma and panning in retrovirus-mediated expression cloning. Anal. Biochem. 315:138-140. [DOI] [PubMed] [Google Scholar]
- 46.Shimojima, M., Y. Nishimura, T. Miyazawa, K. Kato, K. Nakamura, Y. Izumiya, H. Akashi, and Y. Tohya. 2002. A feline CD2 homologue interacts with human red blood cells. Immunology 105:360-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simmons, G., A. J. Rennekamp, N. Chai, L. H. Vandenberghe, J. L. Riley, and P. Bates. 2003. Folate receptor alpha and caveolae are not required for Ebola virus glycoprotein-mediated viral infection. J. Virol. 77:13433-13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simmons, G., J. D. Reeves, C. C. Grogan, L. H. Vandenberghe, F. Baribaud, J. C. Whitbeck, E. Burke, M. J. Buchmeier, E. J. Soilleux, J. L. Riley, R. W. Doms, P. Bates, and S. Pöhlmann. 2003. DC-SIGN and DC-SIGNR bind ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology 305:115-123. [DOI] [PubMed] [Google Scholar]
- 49.Simmons, G., R. J. Wool-Lewis, F. Baribaud, R. C. Netter, and P. Bates. 2002. Ebola virus glycoproteins induce global surface protein down-modulation and loss of cell adherence. J. Virol. 76:2518-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sinn, P., M. A. Hickey, P. D. Staber, D. E. Dylla, S. A. Jeffers, B. L. Davidson, D. A. Sanders, and P. B. McCray, Jr. 2003. Lentivirus vectors pseudotyped with filoviral envelope glycoproteins transduce airway epithelia from the apical surface independently of folate receptor alpha. J. Virol. 77:5902-5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ströher, U., E. West, H. Bugany, H.-D. Klenk, H.-J. Schnittler, and H. Feldmann. 2001. Infection and activation of monocytes by Marburg and Ebola viruses. J. Virol. 75:11025-11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swanepoel, R., P. A. Leman, F. J. Burt, N. A. Zachariades, L. E. Braack, T. G. Ksiazek, P. E. Rollin, S. R. Zaki, and C. J. Peters. 1996. Experimental inoculation of plants and animals with Ebola virus. Emerg. Infect. Dis. 2:321-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takada, A., C. Robison, H. Goto, A. Sanchez, K. G. Murti, M. A. Whitt, and Y. Kawaoka. 1997. A system for functional analysis of Ebola virus glycoprotein. Proc. Natl. Acad. Sci. USA 94:14764-14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takada, A., H. Feldmann, U. Stroeher, M. Bray, S. Watanabe, H. Ito, M. McGregor, and Y. Kawaoka. 2003. Identification of protective epitopes on ebola virus glycoprotein at the single amino acid level by using recombinant vesicular stomatitis viruses. J. Virol. 77:1069-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takada, A., K. Fujioka, M. Tsuiji, A. Morikawa, N. Higashi, H. Ebihara, D. Kobasa, H. Feldmann, T. Irimura, and Y. Kawaoka. 2004. Human macrophage C-type lectin specific for galactose and N-acetylgalactosamine promotes filovirus entry. J. Virol. 78:2943-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takada, A., S. Watanabe, H. Ito, K. Okazaki, H. Kida, and Y. Kawaoka. 2000. Downregulation of β1 integrins by Ebola virus glycoprotein: implication for virus entry. Virology 278:20-26. [DOI] [PubMed] [Google Scholar]
- 57.Valverde, P. 2005. Effects of Gas6 and hydrogen peroxide in Axl ubiquitination and downregulation. Biochem. Biophys. Res. Commun. 333:180-185. [DOI] [PubMed] [Google Scholar]
- 58.Villinger, F., P. E. Rollin, S. S. Brar, N. F. Chikkala, J. Winter, J. B. Sundstrom, S. R. Zaki, R. Swanepoel, A. A. Ansari, and C. J. Peters. 1999. Markedly elevated levels of interferon (IFN)-γ, IFN-α, interleukin (IL)-2, IL-10, and tumor necrosis factor-α associated with fatal Ebola virus infection. J. Infect. Dis. 179:S188-S191. [DOI] [PubMed] [Google Scholar]
- 59.Wahl-Jensen, V. M., T. A. Afanasieva, J. Seebach, U. Ströher, H. Feldmann, and H.-J. Schnittler. 2005. Effects of Ebola virus glycoproteins on endothelial cell activation and barrier function. J. Virol. 79:10442-10450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wahl-Jensen, V., S. K. Kurz, P. R. Hazelton, H.-J. Schnittler, U. Ströher, D. R. Burton, and H. Feldmann. 2005. Role of Ebola virus secreted glycoproteins and virus-like particles in activation of human macrophages. J. Virol. 79:2413-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wimmel, A., D. Glitz, A. Kraus, J. Roeder, and M. Schuermann. 2001. Axl receptor tyrosine kinase expression in human lung cancer cell lines correlates with cellular adhesion. Eur. J. Cancer 37:2264-2274. [DOI] [PubMed] [Google Scholar]
- 62.Wool-Lewis, R. J., and P. Bates. 1998. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J. Virol. 72:3155-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu, Y., S. Singh, M.-M. Georgescu, and R. B. Birge. 2005. A role for Mer tyrosine kinase in αvβ5 integrin-mediated phagocytosis of apoptotic cells. J. Cell Sci. 118:539-553. [DOI] [PubMed] [Google Scholar]
- 64.Yang, Z., R. Delgado, L. Xu, R. F. Todd, E. G. Nabel, A. Sanchez, and G. J. Nabel. 1998. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science 279:1034-1037. [DOI] [PubMed] [Google Scholar]
- 65.Zaki, S. R., W.-J. Shieh, P. W. Greer, C. S. Goldsmith, T. Ferebee, J. Katshitshi, F. K. Tshioko, M. A. Khan, E. Lloyd, P. E. Rollin, T. G. Ksiazek, C. J. Peters, et al. 1999. A novel immunohistochemical assay for the detection of Ebola virus in skin: implications for diagnosis, spread, and surveillance of Ebola hemorrhagic fever. J. Infect. Dis. 179:S36-S47. [DOI] [PubMed] [Google Scholar]