Abstract
One approach for a safer smallpox vaccine is to utilize recombinant subunits rather than live vaccinia virus (VACV). The products of the VACV envelope genes A27L, L1R, B5R, and A33R induce protective antibodies in animal models. We propose that proteins that elicit T-cell responses, as well as neutralizing antibodies, will be important to include in a molecular vaccine. To evaluate VACV-specific memory T-cell responses, peripheral blood mononuclear cells (PBMC) from four VACV vaccinees were tested against whole VACV and the individual envelope proteins A27, B5, L1, and A33, using gamma interferon enzyme-linked immunospot and cytokine flow cytometry assays. PBMC were stimulated with autologous dendritic cells infected with VACV or electroporated with individual VACV protein mRNAs. T-cell lines from all donors, vaccinated from 1 month to over 20 years ago, recognized all four VACV envelope proteins. Both CD4+ and CD8+ T-cell responses to each protein were detected. Further analysis focused on representative proteins B5 and A27. PBMC from a recent vaccinee exhibited high frequencies of CD4+ and CD8+ T-cell precursors to both B5 (19.8 and 20%, respectively) and A27 (6.8 and 3.7%). In comparison, B5- and A27-specific T-cell frequencies ranged from 0.4 to 1.3% in a donor vaccinated 3 years ago. Multiple CD4+ and CD8+ T-cell epitopes were identified from both A27 and B5, using overlapping 15-mer peptides. These data suggest that all four VACV envelope proteins may contribute to protective immunity, not only by inducing antibody responses, but also by eliciting T-cell responses.
It is important to develop safer alternatives to the live vaccinia virus (VACV) vaccine to immunize against smallpox (variola virus) infection. One approach is the use of modified vaccinia virus Ankara (MVA), a highly attenuated vaccinia virus that does not produce infectious progeny virions in human cells (24, 37). In comparison to VACV, however, MVA is less immunogenic and requires higher doses (8, 25). Since MVA is nonreplicating, it will also likely require more-frequent boosting. Additionally, there remain safety concerns about the use of a live, albeit attenuated, virus and the potential presence of adventitious pathogens. As an alternative live virus vaccine, one group has developed a mutant vaccinia virus, strain LC16m8 (expressing a truncated B5 envelope protein), that is less virulent in an animal model but appears to retain immunogenicity (26).
Another alternative is the use of a recombinant protein or DNA vaccine. Smallpox vaccine development is hampered, however, because little is known about the proteins that do or could play important roles in the generation of protective immune responses. Orthopoxviruses, including VACV and variola virus, are highly complex DNA viruses that encode over 180 proteins. There are also two different infectious forms of poxviruses, the intracellular mature virion (IMV) and the extracellular enveloped virion (EEV), that are associated with unique envelope proteins.
Animal studies suggest that VACV-specific neutralizing antibodies alone are sufficient to protect against challenge. For instance, mice were protected against lethal VACV infection after depletion of CD4+ and CD8+ T cells following vaccination or after passive transfer of immune sera (2, 20). In another study, rhesus macaques were protected against lethal monkeypox virus challenge after depletion of CD8+ T cells after vaccination but not by depletion of B cells before vaccination (9). In that study, passive transfer of human VACV-neutralizing antibodies also protected macaques against severe disease.
We propose that VACV envelope proteins that elicit both neutralizing antibodies and T-cell responses will be important to include in a potent and durable vaccine. There are several lines of evidence that support this hypothesis. CD4+ T-cell responses to lytic viruses help to generate and amplify B-cell, T-cell, and innate immune responses (34). Whereas antibodies may be sufficient to prevent infection, cytotoxic CD8+ T-cell responses are typically required to control and eradicate established viral infections (18, 19). Specifically, Belyakov et al. demonstrated that CD4+ and CD8+ T cells prevented mortality in vaccinated B-cell-deficient mice after VACV challenge (2). In another study, adoptive transfer of immune CD8+ T cells was protective in B-cell-depleted animals (45). Additionally, vaccination with an HLA A2-restricted epitope from the VACV host range protein HRP2 protected HLA A2 transgenic mice against lethal VACV infection (36).
Several VACV envelope proteins that induce protective antibodies have been identified, including the IMV proteins A27 and L1 and the EEV proteins A33 and B5 (10, 11, 14, 21, 32). Each of these protein sequences is highly conserved between VACV and variola virus. Recently, DNA vaccination with a combination of all four genes (A27L, L1R, A33R, and B5R) was documented to be completely protective against a lethal VACV challenge in both mouse and monkey models (15, 16). Protective antibody responses were identified in vaccinated animals, but T-cell responses were not studied.
The VACV proteins A27, B5, A33, and L1 represent promising smallpox vaccine candidates. The goal of this study was to determine whether any of the four envelope proteins A27, B5, A33, and L1 is recognized by memory T cells from vaccinated donors. VACV-specific T-cell lines (TCLs) were prepared from peripheral blood mononuclear cells (PBMC) from four donors. Dendritic cells (DC) were utilized to process and present input proteins via both major histocompatibility complex (MHC) class I and class II molecules in order to stimulate both CD4+ and CD8+ T-cell responses. VACV-specific T cells were amplified in vitro by stimulation with autologous DC treated with VACV after inactivation with psoralen and long-wave UV light (pUV). As targets, individual VACV proteins were expressed in DC via electroporation of mRNA. VACV-specific TCLs from each donor recognized each individual envelope protein expressed in autologous DC in a gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assay. Protein-specific TCLs, prepared from PBMC stimulated with autologous DC expressing each VACV protein, recognized VACV-treated targets. Both B5- and A27-specific CD4+ and CD8+ T cells were directly detected in PBMC by cytokine flow cytometry (CFC) assays. Additionally, both CD4+ and CD8+ T-cell epitopes were identified from B5- and A27-specific TCLs, using overlapping 15-mer synthetic peptides.
MATERIALS AND METHODS
Subjects.
Buffy coat collections were obtained from four donors previously vaccinated with VACV and from two unvaccinated healthy adult donors. Donors were vaccinated 1 month (donor 1), 3 years (donors 2 and 3), or >20 years ago (donor 4). This study was approved by the Institutional Review Board.
Viruses.
The Western Reserve strain of VACV was grown in 143 TK− cells, crude lysates were prepared, and titers were determined as previously described (44). VACV aliquots were inactivated with pUV, based on the method of Tsung et al. (42). Briefly, VACV was incubated with 1 μg/ml of psoralen (4′-aminomethyltrioxsalen hydrochloride; Sigma, St. Louis, Mo.) for 5 min at room temperature (1 ml/well in a 6-well plate) and exposed to long-wave UV light for 10 to 20 min, using the Stratalinker UV cross-linker (Stratagene, LaJolla, Calif.). This treatment led to a 102- to 104-fold reduction in titer. Adenovirus antigen was prepared from Ad2-infected cell lysates and inactivated with short-wave UV light, as previously described (28).
Cell lines and antibodies.
Epstein Barr virus-transformed lymphoblastoid cell lines (LCL) were prepared from donor PBMC by incubation with supernatant from the marmoset cell line B95-8 in the presence of 1 μg/ml cyclosporine, as previously described (28). The HLA homozygous LCL Boleth, Cox, and Mou were obtained from D. Eckels, Medical College of Wisconsin (46). CD40L-transfected 3T3 cells were obtained from J. L. Schultze, Harvard Medical School (35). Monoclonal antibodies (mAbs) specific for A27, A33, and L1 have previously been described (15). A B5-specific mAb (206C5-F12) was kindly provided by S. N. Isaacs, University of Pennsylvania. The HLA A-, B-, and C-specific mAb W632 and the anti-DR mAb L243 were purchased from Leinco Technologies (St. Louis, Mo.).
Dendritic cells.
PBMC were purified from buffy coat collections by Ficoll-Hypaque density gradient centrifugation. DC were prepared from CD14+ cells isolated from PBMC via positive immunomagnetic selection, as previously described (39). Briefly, CD14+ cells were incubated in RPMI 1640, supplemented with 1% human AB sera (Atlanta Biologicals, Atlanta, Ga.), 10 mM HEPES, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin, in the presence of granulocyte-macrophage colony-stimulating factor (800 U/ml) and interleukin-4 (IL-4; 1,000 U/ml; BD Pharmingen, San Diego, Calif.) for 5 days. Then DC maturation was induced by addition of 10 ng/ml IL-1β, 1,000 U/ml IL-6, 10 ng/ml tumor necrosis factor alpha, and 1 μg/ml prostaglandin E2 (Sigma, St. Louis, Mo.) for 48 h.
CD40-activated B-cell lines.
In some experiments, cells from CD40-activated B-cell lines (CD40-B) were used as autologous antigen-presenting cells in lieu of DC (to spare the large number of PBMC required to prepare DC). Similar to DC, CD40-B cells present input proteins via class I and class II antigens (43). CD40-B cells were generated from PBMC by repeated stimulation with irradiated CD40L-transfected NIH 3T3 cells, per the method of Schultze et al. (35).
Preparation of VACV protein mRNAs.
Cloned protein open reading frames (ORFs) (VACV Connaught strain) were amplified from the plasmids pWRG/A27L, pWRG/A33R, pWRG/B5R, and pWRG/L1R by PCR, using primers that added the restriction sites XhoI and HindIII (15). Each cDNA was gel purified, restricted with XhoI and HindIII, and inserted into the multicloning site of the transcription vector pBluescript II SK(+/−) (Invitrogen, Carlsbad, Calif.) behind a T7 polymerase promoter. As a control, the enhanced green fluorescent protein (GFP) ORF was cloned into the pBluescript vector. mRNA was prepared from each insert by in vitro transcription, using the mMESSAGE mMACHINE T7 kit (Ambion, Austin, Tex.), treated with DNase, and purified by LiCl precipitation, according to the manufacturer's instructions. mRNAs were analyzed by agarose-formaldehyde gel electrophoresis and stored at −80°C in small aliquots.
Electroporation of DC with mRNAs.
Mature DC were suspended at 1 × 106 to 2 × 106/100 μl in Nucleofector solution plus 5 μg of each mRNA and electroporated in an Amaxa electroporator using pulse program U08 (Amaxa, Gaithersburg, Md.) (3). Transfected DC were incubated overnight before being used. GFP expression was confirmed by direct examination for fluorescence by flow cytometry; more than 50% of cells expressed GFP (data not shown). The efficiency of electroporation of each VACV protein mRNA was measured by an indirect immunofluorescence assay using mAbs against each protein; expression ranged from 45% to 60% (data not shown).
Preparation of T-cell lines.
For whole VACV-specific TCLs, 3 × 106 aliquots of PBMC were stimulated with autologous mature DC treated overnight with pUV VACV (4 PFU/cell; PBMC to DC ratio, 10:1) in 24-well plates in RPMI 1640 supplemented with 10% human AB sera, 10 mM HEPES, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (T-cell media). On day 7, cells were restimulated with fresh pUV VACV-treated DC, and human recombinant IL-2 (5 U/ml; Becton Dickinson, Bedford, Mass.) was added starting at day 9. To prepare individual VACV protein-specific TCLs, PBMC were stimulated with autologous DC transfected with individual mRNAs (PBMC to DC ratio, 10:1) for 1 week.
CD4+ and CD8+ T-cell purification.
Purified populations of CD4+ and CD8+ T cells were separated from PBMC by negative immunomagnetic selection, as previously described (39).
Synthetic peptides.
Overlapping 15-mer peptides (11-amino-acid overlaps) from the VACV Connaught strain A27L and B5R protein sequences (GenBank accession numbers A4160184 and A4160185, respectively) were synthesized by Sigma Genosys (The Woodlands, Tex.). In a few cases where peptides containing the most hydrophobic sequences could not be synthesized, shorter peptides were synthesized. The purity of each crude peptide was confirmed by high-pressure liquid chromatography. Peptide stocks (10 mM) were prepared in dimethyl sulfoxide and frozen in small aliquots at −70°C.
IFN-γ ELISPOT assay.
T-cell responses were measured by IFN-γ ELISPOT assay, using mAbs from Mabtech (Stockholm, Sweden) and 96-well plates with Immobolin-P membrane (Millipore, Bedford, Mass.), as previously described (28). PBMC were incubated overnight with live VACV (1 PFU/cell) following a 90-min adsorption. TCLs were incubated overnight with peptides (10 μM each) or targets loaded with peptide, infected with live VACV, or treated with pUV VACV. Spot-forming cells (SFCs) were counted with an ImmunoSpot analyzer (Cleveland, Ohio).
Cytokine flow cytometry assay.
PBMC were incubated 6 h with mature autologous DC infected overnight with live VACV or treated with pUV VACV (4 PFU/cell). Uninfected DC were tested as a negative control. PBMC were also tested against autologous DC electroporated with mRNA for B5 and A27 VACV proteins and GFP (negative control). TCLs were incubated with or without peptide (10 μM) for 6 h. CD4+ and CD8+ T-cell responses were detected by measurements of intracellular IFN-γ and cell surface markers CD69, CD3, and CD8 by four-color staining, as previously described (38).
Cytotoxic T-cell assay.
Cytotoxicity was measured by a calcein-release assay, as previously described (27).
Statistical methods.
Data from the ELISPOT assays were analyzed using a paired t test to compare the mean number of spots in the triplicate control and experimental microwells. Standard deviations for all triplicate data points from both ELISPOT and CTL assays are shown in each figure.
RESULTS
Comparison of vaccinia-specific T-cell frequencies among vaccinees.
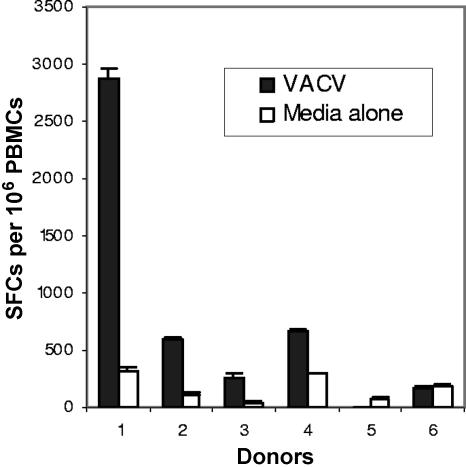
PBMC were collected from donors vaccinated with VACV 1 month (donor 1), 3 years (donors 2 and 3), or >20 years ago (donor 4). PBMC from each donor were directly assayed for T-cell responses to VACV (1 PFU/cell) by a short-term IFN-γ ELISPOT assay. PBMC were incubated with media alone as a negative control. Additionally, PBMC from two unvaccinated donors (5 and 6) were tested as negative controls. PBMC from all six donors responded to inactivated adenovirus antigen as a positive control (data not shown). All four vaccinees had detectable IFN-γ responses to VACV, whereas both nonimmune donors were negative (Fig. 1). VACV-specific T-cell frequencies from the donor vaccinated 1 month before (2,550 per 106 PBMC) were approximately sevenfold higher than those for the three other vaccinated donors (mean, 356 per 106 PBMC; range, 208 to 488). Titration of the VACV dose revealed nonlinear responses, and responses to a second antigen (adenovirus) were inhibited in comixing experiments with VACV, suggesting that VACV was also toxic to the T cells (data not shown). Thus, the levels of VACV-specific T cells were likely underestimated in this assay.
FIG. 1.
Comparison of vaccinia virus-specific IFN-γ responses among donors. PBMC from VACV-immunized donors (1 to 4) and two nonimmune donors (5 and 6) were incubated overnight with live VACV (1 PFU/cell) or media alone (negative control). IFN-γ responses were measured by ELISPOT assay. SFCs, spot-forming cells.
Vaccinia virus-specific T cells recognize input virion proteins presented by dendritic cells.
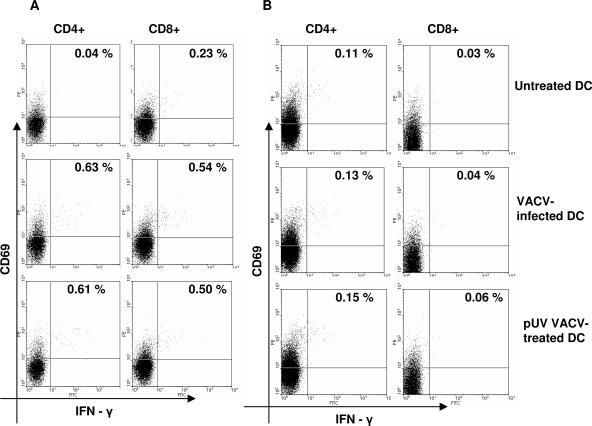
In order to identify T-cell responses to virion proteins, VACV was inactivated by pUV treatment to inhibit both expression of early viral proteins and viral replication (42). DC, professional antigen-presenting cells, were utilized to present input virion proteins from inactivated VACV via both MHC class I and class II antigens (12). Responses of PBMC from donor 4 to autologous DC infected with live VACV or treated with pUV-inactivated VACV (4 PFU/cell) were compared, using the short-term CFC assay. Uninfected DC were used to stimulate PBMC as a negative control. Additionally, PBMC from an unvaccinated donor (donor 5) were tested as a negative control. PBMC from both donors exhibited strong CD4+ and CD8+ T-cell responses to the mitogen phytohemagglutinin as a positive control (data not shown). As shown in Fig. 2, both CD4+ and CD8+ T-cell responses to live VACV-infected DC from donor 4 (0.59 and 0.31%, respectively) were detected. Similar levels of T-cell precursor frequencies to pUV VACV-treated DC from donor 4 (0.57 and 0.28%, respectively) were detected. No VACV-specific responses were detected from the unvaccinated donor. The fact that both CD4+ and CD8+ T-cell responses to DC treated with pUV-inactivated VACV were detected suggested that virus-specific T cells recognize input virion proteins.
FIG. 2.
CD4+ and CD8+ T-cell responses to vaccinia virus-infected DC. (A) PBMC from donor 4 were incubated 6 h with autologous DC infected overnight with live VACV or treated with pUV-inactivated VACV. (B) PBMC from a nonimmune donor (donor 5) were tested as a negative control. Activated IFN-γ- and CD69-positive cells were measured by cytokine flow cytometry using four-color staining. CD8+ T cells were gated on CD3+CD8+ lymphocytes; CD4+ T cells were gated on CD3+CD8− lymphocytes. VACV-specific precursor frequencies were calculated by subtracting the responses to uninfected DC (see text).
Virus-specific T-cell lines recognize individual vaccinia virus envelope proteins.
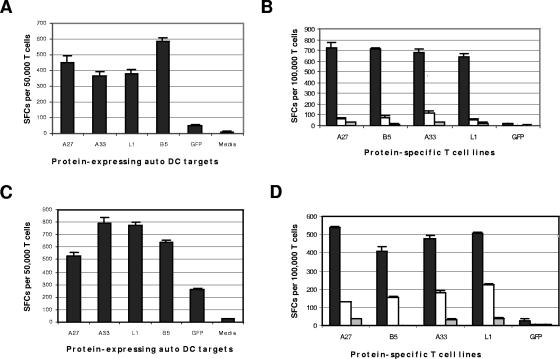
VACV-specific TCLs were prepared by stimulation of PBMC with pUV VACV-treated autologous DC for 2 weeks, as per Materials and Methods. TCLs were tested for responses to each VACV envelope protein expressed in autologous DC transfected with individual mRNAs via electroporation. As a negative control, TCLs were tested against autologous DC transfected with GFP mRNA. VACV-specific TCLs from all four donors demonstrated specific T-cell responses to each individual protein by IFN-γ ELISPOT assay, as illustrated with donor 3 (Fig. 3A) and donor 4 (Fig. 3C). Additionally, purified CD4+ and CD8+ T cells (donor 2) separately stimulated with pUV VACV-treated autologous DC exhibited IFN-γ responses to each protein (data not shown).
FIG. 3.
Vaccinia virus-specific T-cell lines recognize envelope proteins A27, L1, B5, and A33. VACV-specific TCLs prepared from PBMC from donor 3 (panels A and B) and donor 4 (panels C and D) were tested by IFN-γ ELISPOT assay. (A and C) Whole VACV-specific TCLs were stimulated for 2 weeks (donor 3) or 3 weeks (donor 4) with pUV VACV-treated autologous DC and tested against autologous DC electroporated with mRNA from A27, L1, B5, A33, or GFP (negative control). (B and D) Individual protein-specific TCLs (stimulated for 1 week with autologous DC electroporated with mRNA from either A27, L1, B5, A33, or GFP) were tested against pUV VACV-treated (4 PFU/cell) or untreated autologous CD40-activated B-cell targets (dark and open bars, respectively) or media alone (light gray bars). SFCs, spot-forming cells. Auto, autologous.
As an alternative approach, individual VACV protein-specific TCLs were prepared by stimulation of PBMC with autologous DC transfected with each protein mRNA for 1 week. PBMC stimulated with DC transfected with GFP mRNA were tested as a control. CD40-B cells were prepared from PBMC for use as autologous antigen-presenting cells, as per Materials and Methods, in order to spare the large number of PBMC required for DC preparation. CD40-B cells presented both live VACV and pUV VACV antigens to CD4+ and CD8+ T cells in the CFC assay, but not as efficiently as DC (data not shown). Each TCL was tested against pUV Vac-treated and untreated autologous CD40-B cell targets in the IFN-γ ELISPOT assay. From all four donors, TCLs against A27, B5, A33, and L1 each specifically recognized pUV Vac-treated autologous targets in the IFN-γ ELISPOT assay. The responses from donors 3 and 4 are illustrated in Fig. 3B and D, respectively. These data indicate that vaccinees exhibit T-cell responses to each envelope protein.
Quantitation of B5-specific CD4+ and CD8+ T-cell precursor frequencies.
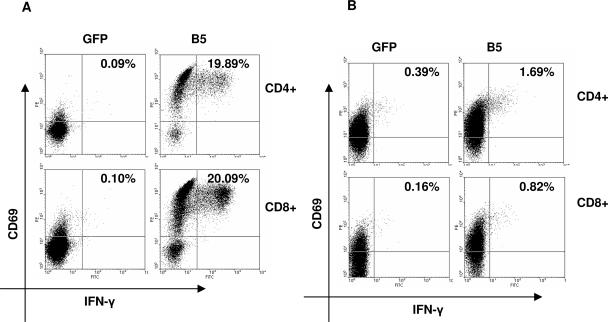
PBMC were directly assayed for B5-specific CD4+ and CD8+ T-cell precursor frequencies by CFC assay. PBMC were incubated for 6 h with autologous DC transfected with either B5 mRNA or GFP mRNA (negative control). B5-specific T-cell precursor frequencies correlated with the time interval postvaccination. As shown in Fig. 4A, the recent vaccinee (donor 1) exhibited very strong T-cell responses to B5: 19.8% for CD4 and 20% for CD8. In comparison, PBMC from donor 2, who was vaccinated 3 years ago, exhibited 20-fold-lower B5-specific CD4+ and CD8+ T-cell precursor frequencies (1.3 and 0.66%, respectively) (Fig. 4B). In contrast, PBMC from the donor vaccinated >20 years ago exhibited a detectable response to B5R only after amplification in vitro (Fig. 3C), consistent with the presence of lower precursor frequencies compared to the other donors. These data confirm the presence of both CD4+ and CD8+ T-cell responses to B5 in vaccinated donors.
FIG. 4.
Comparison of CD4+ and CD8+ T-cell responses to B5 of donors 1 and 2. PBMC were incubated 6 h with autologous DC electroporated with mRNA from B5 or GFP (negative control). (A) Donor 1 (vaccinated 1 month before). (B) Donor 2 (vaccinated 3 years before). Activated IFN-γ- and CD69-positive cells were measured by cytokine flow cytometry using four-color staining. CD8+ T cells were gated on CD3+CD8+ lymphocytes; CD4+ T cells were gated on CD3+CD8− lymphocytes. B5-specific precursor frequencies were calculated by subtracting the values of the responses to GFP-transfected DC (see text).
Identification of CD4+ and CD8+ T-cell epitopes from B5.
To identify both CD4+ and CD8+ T-cell epitopes, B5-specific TCLs from each donor were screened by ELISPOT assay for responses to eight pools of overlapping 15-mer peptides (8 to 10 peptides per pool) representing the entire 317-amino-acid (aa) B5 protein. The use of 15-mer synthetic peptides allows identification of both MHC class I- and class II-restricted T-cell epitopes, despite the fact that the optimal size of class I-restricted peptides is 9 to 10 aa (23). Then individual peptides from positive pools were tested. Lastly, B5-specific TCLs were stimulated with individual positive peptides for 2 weeks and analyzed by ELISPOT, CFC, and CTL assays when appropriate. B5-specific TCLs from all donors responded to at least one peptide pool.
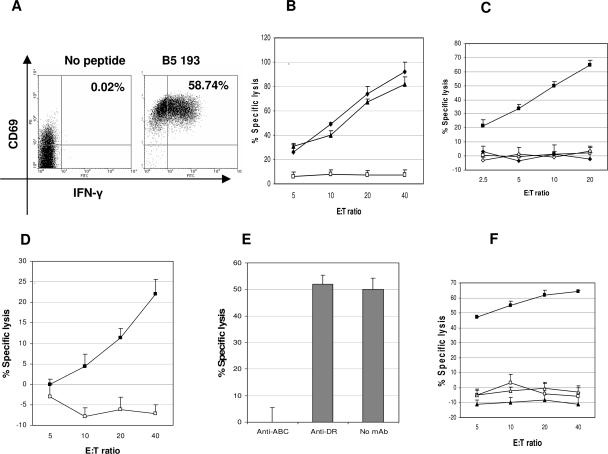
Major HLA B-restricted T-cell responses to the B5 peptides 193-07 and 225-239 (donor 1) and to the B5 peptide 105-119 (donor 3) were identified. Responses to each B5 peptide were documented to be mediated by CD8+ T cells by CFC assay, as illustrated for peptide 193-07 in Fig. 5A. Each peptide-specific line exhibited MHC-restricted cytotoxicity against both peptide-loaded and VACV-infected LCL, confirming that these epitopes are naturally processed in infected cells. The donor 1 (HLA A1, B35, B55) peptide 225-239-specific TCL responses are shown in Fig. 5B. The B5R 225-239-specific TCL was determined to be restricted by HLA B35, using a panel of HLA-matched and mismatched targets (Fig. 5C). The donor 1 B5 193-207-specific TCL was presumptively restricted by HLA B55; i.e., peptide-loaded autologous targets were killed, but neither HLA A1- nor B35-matched targets were recognized, and a B55-matched target was not available to test (data not shown). The donor 3 (A1, A23, B4, B44) peptide 105-119-specific TCL was documented to kill VACV-infected autologous targets (Fig. 5D and E) and was restricted by HLA B44 (Fig. 5F).
FIG. 5.
Identification of B5-specific epitopes restricted by HLA B alleles. A B5-specific TCL from donor 1 (HLA A1, B35, B55) was stimulated with individual B5 synthetic peptides for 2 weeks (panels A, B, and C). (A) A B5 193-207-specific TCL was incubated with or without peptide and analyzed by cytokine flow cytometry (gated on CD3+CD8+ lymphocytes). A B5 225-239-specific TCL was tested for cytotoxicity against autologous LCL infected with VACV (10 PFU/cell) (triangles), loaded with peptide (diamonds), or untreated as a negative control (open squares) (B) and HLA B35-matched LCL (HLA A3, B7, B35) only with or without peptide (filled and open squares, respectively) and HLA-mismatched Mou (HLA A29, B44) with or without peptide (filled and open diamonds, respectively) (C). A B5 105-119-specific TCL from donor 3 (HLA A1, A23, B8, B44) was tested for cytotoxicity against the following targets: autologous LCL infected with VACV (10 PFU/cell) (filled squares) or untreated (open squares) (D), VACV-infected autologous LCL preincubated with mAbs (40 μg/ml) against either HLA A, B, and C or HLA DR (effector to target ratio, 40:1) (E), HLA B44-matched Mou (HLA A29, B44) only with or without peptide (filled and opened squares, respectively), and HLA-mismatched Boleth (HLA A2, B75) with or without peptide (filled and open triangles, respectively) (F). Cytotoxicity was measured by a calcein-release assay. E:T, effector to target ratio.
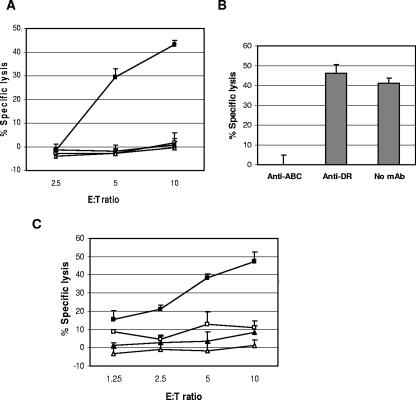
A major HLA A2-restricted T-cell response to the B5 peptide 5-19 from donor 2 was identified. The donor 2 (HLA A1, A2, B49, B57) B5 peptide-specific TCL response was mediated by CD8+ T cells by CFC assay (data not shown). The B5 5-19 peptide-specific TCL exhibited MHC-restricted cytotoxicity against VACV-infected autologous LCL, and cytotoxicity was blocked by a mAb against HLA A, B, and C antigens, not by anti-DR (Fig. 6A and B). This peptide response was documented to be restricted by HLA A2, using a panel of HLA-matched and mismatched targets (Fig. 6C). Donor 4 had detectable responses to peptide pool 2 which could not be amplified. Additional peptide responses were detected from donors 1 to 3 but were not further characterized (data not shown).
FIG. 6.
Identification of an HLA A2-restricted B5 epitope. A B5-specific TCL from donor 2 (HLA A1, A2, B49, B57) was stimulated with B5 peptide 5-19 for 2 weeks. Cytotoxicity was measured by calcein-release assay against the following targets: VACV-infected (10 PFU/cell) and uninfected autologous LCL (filled and open squares, respectively) and VACV-infected and uninfected HLA-mismatched Mou (HLA A29, B44) (filled and open triangles, respectively) (A), VACV-infected autologous LCL preincubated with mAbs (40 μg/ml) against either HLA A, B, and C or HLA DR (effector to target ratio, 10:1) (B), and HLA A2-matched Boleth (HLA A2;B75) with or without peptide (filled and open squares, respectively) and HLA-mismatched Mou with or without peptide (filled and open triangles, respectively) (C). E:T, effector to target ratio.
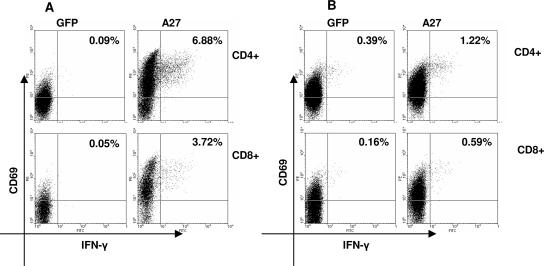
Quantitation of A27-specific CD4 and CD8+ T-cell precursor frequencies.
Using the same strategy as above, PBMC were directly tested for responses to autologous DC expressing A27 by CFC assay. Autologous DC expressing GFP were also tested as a negative control. As shown in Fig. 7A, the recent vaccinee exhibited strong responses to A27, although the precursor frequencies were two- to threefold lower than the B5-specific T-cell frequencies: 6.79% for CD4 and 3.67% for CD8. Donor 2, who was vaccinated 3 years ago, exhibited 10-fold lower CD4+ and CD8+ T-cell responses to A27 (0.83 and 0.43%, respectively) than the recent vaccinee (Fig. 7B). The A27-specific T-cell precursor frequencies were similar to the donor 2 responses to B5. PBMC from the donor vaccinated >20 years ago (donor 4) did not have a measurable direct response to A27-transfected DC but responded to A27 after in vitro amplification (Fig. 3C), consistent with lower precursor frequencies (data not shown).
FIG. 7.
Comparison of CD4+ and CD8+ T-cell responses to A27 of donors 1 and 2. PBMC were incubated 6 h with autologous DC electroporated with mRNA from A27 or GFP (negative control). (A) Donor 1 (vaccinated 1 month before). (B) Donor 2 (vaccinated 3 years before). Activated IFN-γ- and CD69-positive cells were measured by cytokine flow cytometry using four-color staining. CD8+ T cells were gated on CD3+CD8+ lymphocytes; CD4+ T cells were gated on CD3+CD8− lymphocytes. A27-specific precursor frequencies were calculated by subtracting the values of the responses to GFP-transfected DC (see text).
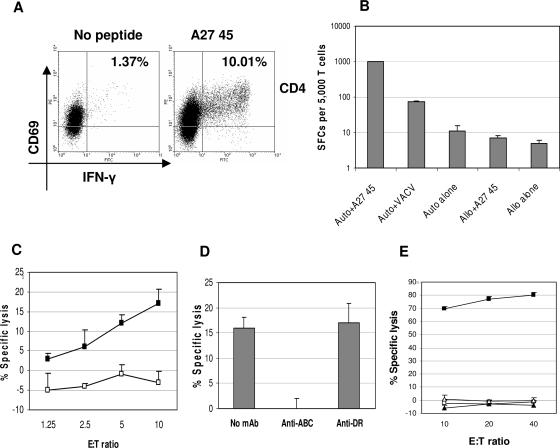
Identification of CD4+ and CD8+ T-cell epitopes from A27.
A27-specific TCLs were prepared by in vitro stimulation of PBMC with autologous DC transfected with A27 mRNA. A27-specific TCLs were screened for responses to four pools of overlapping 15-mer peptides representing the entire 110-aa A27 protein. All donors responded to at least one peptide pool and one or more individual peptides. TCLs were stimulated with individual positive peptides for 2 weeks and analyzed. Three CD4+ T-cell epitopes were identified: A27 peptides 24-39 (donor 3), 45-59 (donor 2), and 77-91 (donor 1). A representative CFC assay for the A27 45-59-specific TCL is illustrated in Fig. 8A. A27 peptide-specific TCLs exhibited MHC-restricted responses and recognized VACV-infected LCL in the IFN-γ ELISPOT assay, confirming that these epitopes are naturally processed, as illustrated in Fig. 8B. One CD8+ T-cell epitope, A27 89-103, was identified from donor 2. An A27 89-103-specific TCL exhibited MHC-restricted cytotoxicity against VACV-infected LCL that was blocked by mAb against class I HLA antigens and was documented to be restricted by HLA A1 (Fig. 8C, D, and E). Donor 4 exhibited a specific response to A27 17-31 which could not be further amplified. Additional peptide responses from donors 1 to 3 were detected but not further characterized (data not shown).
FIG. 8.
Identification of A27-specific T-cell epitopes. An A27-specific TCL from donor 2 (HLA A1,A2, B49, B57) was stimulated for 2 weeks with individual A27 peptides. An A27 45-59 peptide-specific TCL was analyzed by cytokine flow cytometry (gated on CD3+CD8− lymphocytes) (A) and tested for responses to VACV-infected or peptide-loaded autologous LCL and HLA-mismatched Mou (HLA A29, B44) by IFN-γ ELISPOT assay (B). An A27 89-103-specific TCL was tested for cytotoxicity against the following targets: VACV-infected and uninfected autologous LCL (filled and open squares, respectively) (C), VACV-infected autologous LCL preincubated with mAbs against either HLA A, B, and C or HLA DR (effector to target ratio, 10:1) (D), and HLA A1-matched Cox (HLA A1, B8) with and without peptide (filled and open squares, respectively) and HLA A2- and B49-matched LCL (HLA 2, 68, 38, 49) with and without peptide (filled and open triangles, respectively) (E). Cytotoxicity was measured by calcein-release assay. E:T, effector to target ratio. Auto, autologous. Allo, allogeneic.
The sequences of the identified B5 and A27 T-cell epitopes are displayed in Table 1. All peptide sequences share 100% sequence similarity with variola virus except for the B5 peptide 225-239, as noted.
TABLE 1.
Vaccinia virus B5 and A27 protein CD4+ and CD8+ T-cell epitopes and their HLA-restricting elements
| Peptidea | Sequenceb | HLA restriction |
|---|---|---|
| B5 5-19 | SVVTLLCVLPAVVYS | A2 |
| B5 105-119 | TKYFRCEEKNGNTSW | B44 |
| B5 193-207 | NGLISGSTFSIGGVI | B55c |
| B5 225-239 | CIDGKWNPILPTCVR | B35 |
| A27 24-39 | AKKPEAKREAIVKAD | Class IId |
| A27 45-59 | ETLKQRLTNLEKKIT | Class IId |
| A27 77-91 | EVLFRLENHAETLRA | Class IId |
| A27 89-103 | LRAAMISLAKKIDVQ | A1 |
Peptides are denoted by amino acid numbers.
All sequences share 100% sequence similarity with variola virus except for the variola virus B5 225-239 peptide (CIDGKWNPVLPICIR contains two conserved [bold] amino acid differences and one nonconserved [underlined] amino acid difference).
B55 restriction is presumptive only (see text).
Peptide-specific responses were mediated by CD4+ T cells, as measured by cytokine flow cytometry. The specific class II restricting elements were not identified.
DISCUSSION
DNA vaccination with a combination of four VACV envelope protein genes, A27L, B5R, A33R, and L1R, is effective against lethal poxvirus infection in both mouse and monkey models, suggesting that these proteins may be suitable smallpox vaccine candidates (15, 16). Protective antibody responses to each protein were documented in vaccinated animals, but T-cell responses were not studied. In this report, PBMC from donors vaccinated with VACV from 1 month to over 20 years ago were documented to exhibit both CD4+ and CD8+ T-cell responses to each of these four VACV envelope proteins. Additionally, specific CD4+ and CD8+ T-cell epitopes were identified from the representative EEV protein B5 and the IMV protein A27.
VACV-specific TCLs from four of four vaccinated donors exhibited specific responses to the individual VACV proteins A27, B5, A33, and L1 in the IFN-γ ELISPOT assay. VACV-specific TCLs were prepared by stimulation of PBMC with pUV VACV-treated autologous DC. DC are professional antigen-presenting cells that efficiently process and present input proteins to both CD4+ and CD8+ T cells. The use of pUV-inactivated VACV allowed presentation of input virion proteins by DC and avoided the toxicity associated with live VACV in both the DC and TCL cultures. For use as targets, autologous DC were transfected with mRNA for each protein via electroporation, and expression of each VACV protein was confirmed by immunofluorescence assay. Additionally, individually purified CD4+ and CD8+ VACV-specific TCLs were documented to recognize each VACV protein, indicating the presence of both CD4+ and CD8+ T-cell epitopes.
As another approach, individual VACV protein-specific TCLs were successfully prepared by stimulation of PBMC with autologous DC expressing each VACV protein. TCLs specific for A27, B5, A33, and L1 each recognized pUV VACV-treated autologous targets in the IFN-γ ELISPOT assay.
A27 and B5 were selected as representative IMV and EEV proteins, respectively, for more detailed analysis. Precursor frequencies of VACV protein-specific T cells were compared among PBMC from different donors by CFC assay. The recent vaccinee exhibited high precursor frequencies of both CD4+ and CD8+ T cells specific for B5 (19 and 20%, respectively) and for A27 (7 and 4%, respectively). A donor vaccinated 3 years previously exhibited 10- to 20-fold-lower frequencies of B5- and A27-specific T cells. In contrast, the donor vaccinated >20 years ago exhibited T-cell responses to B5 and A27 after in vitro amplification only. These data confirm that memory T-cell responses to B5 and A27 are long lasting but that precursor frequencies decline over time.
Representative CD4+ and CD8+ T-cell epitopes were identified from B5 and A27. B5 and A27-specific TCLs were screened with overlapping 15-mer peptides that are predicted to detect both HLA class I- and class II-restricted epitopes (Table 1). Four epitopes each from B5 and A27 were characterized by ELISPOT, CFC, and, in some cases, CTL assays. Interestingly, three of four peptides identified from A27 were CD4+ T-cell epitopes, whereas all four B5-specific peptides were CD8+ T-cell epitopes. All peptide-specific TCLs recognized live VACV-infected cells, confirming that each epitope was naturally processed in infected cells. Additionally, each peptide-specific CD8+ TCL exhibited MHC-restricted killing of VACV-infected cells. Additional B5- and A27-specific peptide responses were identified but have not yet been characterized.
This study documents the presence of long-lasting VACV protein-specific T-cell responses in vaccinated donors, consistent with prior data on whole VACV-specific T-cell responses. Ennis and coworkers were the first to document long-lasting whole-VACV-specific T-cell memory (6). Hammarlund et al. reported high frequencies of whole-VACV-specific T-cell responses after vaccination that declined slowly over time with a half-life of 8 to 15 years (13).
This is the first study to detect specific human T-cell responses to the individual VACV envelope proteins A27, B5, A33, and L1. These data are consistent with the limited information regarding VACV-specific T-cell responses from other animal studies. In an early study, Demkowicz et al. demonstrated cell proliferation when spleen cells from VACV-vaccinated mice were incubated with purified A27 protein (7). More recently, murine CD4+ T-cell responses to an A27L DNA vaccine were documented (30). In another study, IFN-γ responses to a B5R DNA vaccine in a mouse model were detected (32).
Human T-cell epitopes have been identified from other VACV proteins. Ennis and coworkers identified two HLA A2-restricted epitopes from the VACV early/late proteins 189R and 018L (unknown functions) in recent vaccinees (40). Oseroff et al. recently identified 48 class I-restricted T-cell epitopes from 35 different VACV proteins by screening PBMC from vaccinated donors with a large number of synthetic peptides (29). Additionally, human T-cell epitopes have been identified utilizing VACV-infected HLA transgenic mice. Snyder et al. identified an HLA A2-restricted epitope in HRP2 that induced protective immune responses in HLA A2 transgenic mice (36). In another study using HLA A2, A11, and B7 transgenic mice, multiple CD8+ T-cell epitopes were identified from both regulatory and structural VACV proteins, including the envelope proteins A14, A17, and H3 (31).
The present study was limited to the analysis of T-cell responses to four VACV envelope proteins. The VACV proteins B5, A27, A33, and L1 were selected for analysis based on the strength of the vaccine data with these immunogens in animal models. Vaccination with each individual protein induces protective antibody responses in animal models (11, 15, 33). Neutralizing antibodies were documented to B5, A27, and L1 in vaccinated animals. Protective antibodies against A33 were also detected but were not neutralizing, possibly because they mediate complement-dependent killing (22; Alan Schmaljohn, unpublished data). Previous studies of antibody responses from human vaccinees have also identified neutralizing antibodies to B5 (1). Human VACV-specific immune globulin also recognizes A27 and A33 proteins, but antibodies against L1 have not been specifically identified (5, 17).
The fact that four randomly selected donors (with diverse HLA types) exhibited T-cell responses to each protein suggests that these proteins are highly immunogenic and may be recognized by most individuals. However, it will also be important to extend these analyses to additional vaccinees. Several representative B5- and A27-specific T-cell epitopes were identified. Using the same strategies described herein, further studies need to be performed to characterize additional B5- and A27-specific T-cell epitopes, as well as to identify T-cell epitopes from A33 and L1.
A few other VACV proteins have also been demonstrated to induce protective immune responses, including T-cell responses. In particular, the IMV protein H3, which may function as a virus attachment protein, appears to play a role. Davies et al. identified neutralizing antibodies to H3 from VACV-immunized donors (4). Additionally, this group demonstrated that vaccination with a VACV H3 recombinant protein or passive transfer with anti-H3 serum provided partial protection from VACV infection in a mouse model (5). Drexler et al. also identified an HLA A2-restricted epitope from H3 in PBMC from human vaccinees (8). Another group identified murine T-cell responses to the VACV early protein B8 (a soluble IFN-γ receptor) and found that immunization with a VACV B8 CD8+ T-cell peptide provided partial protection against mousepox (ectromelia virus) infection in a mouse model (41). Other VACV proteins that represent potential vaccine candidates may be evaluated for human T-cell epitopes using the same strategy as outlined in this report.
In conclusion, CD4+ and CD8+ T-cell responses to the VACV proteins A27, B5, A33, and L1 were identified from four of four vaccinated donors. Vaccines (DNA or protein) comprised of combinations of these immunogens induce protective antibodies and protect against lethal VACV infection in animal models. Antibody responses to these proteins are likely to play important roles in protective immunity. Nevertheless, VACV-specific T cells will boost B-cell responses, eradicate infected cells, and help maintain virus-specific immunologic memory. Therefore, the presence of human T-cell epitopes strengthens the potential for use of each of these immunogens in a safe and effective molecular smallpox vaccine.
Acknowledgments
This work was supported by National Institutes of Health grant AI059246.
We thank Christina Coughlin (University of Pennsylvania) for advice regarding the electroporation of DC and William Koch for use of an Amaxa electroporator. We especially thank all of the donors who participated in this study.
REFERENCES
- 1.Bell, E., M. Shamim, J. C. Whitbeck, G. Sfyroera, J. D. Lambris, and S. N. Isaacs. 2004. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology 325:425-431. [DOI] [PubMed] [Google Scholar]
- 2.Belyakov, I. M., P. Earl, A. Dzutsev, V. A. Kuznetsov, M. Lemon, L. S. Wyatt, J. T. Snyder, J. D. Ahlers, G. Franchini, B. Moss, and J. A. Berzofsky. 2003. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. USA 100:9458-9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coughlin, C. M., B. A. Vance, S. A. Grupp, and R. H. Vonderheide. 2004. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood 103:2046-2054. [DOI] [PubMed] [Google Scholar]
- 4.Davies, D. H., X. Liang, J. E. Hernandez, A. Randall, S. Hirst, Y. Mu, K. M. Romero, T. T. Nguyen, M. Kalantari-Dehaghi, S. Crotty, P. Baldi, L. P. Villarreal, and P. L. Felgner. 2005. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc. Natl. Acad. Sci. USA 102:547-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies, D. H., M. M. McCausland, C. Valdez, D. Huynh, J. E. Hernandez, Y. Mu, S. Hirst, L. Villarreal, P. L. Felgner, and S. Crotty. 2005. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J. Virol. 79:11724-11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demkowicz, W. E., Jr., R. A. Littaua, J. Wang, and F. A. Ennis. 1996. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J. Virol. 70:2627-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demkowicz, W. E., J. S. Maa, and M. Esteban. 1992. Identification and characterization of vaccinia virus genes encoding proteins that are highly antigenic in animals and are immunodominant in vaccinated humans. J. Virol. 66:386-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drexler, I., C. Staib, W. Kastenmuller, S. Stevanovic, B. Schmidt, F. A. Lemonnier, H. G. Rammensee, D. H. Busch, H. Bernhard, V. Erfle, and G. Sutter. 2003. Identification of vaccinia virus epitope-specific HLA-A*0201-restricted T cells and comparative analysis of smallpox vaccines. Proc. Natl. Acad. Sci. USA 100:217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edghill-Smith, Y., H. Golding, J. Manischewitz, L. R. King, D. Scott, M. Bray, A. Nalca, J. W. Hooper, C. A. Whitehouse, J. E. Schmitz, K. A. Reimann, and G. Franchini. 2005. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 11:740-747. [DOI] [PubMed] [Google Scholar]
- 10.Fang, M., H. Cheng, Z. Dai, Z. Bu, and L. J. Sigal. 2006. Immunization with a single extracellular enveloped virus protein produced in bacteria provides partial protection from a lethal orthopoxvirus infection in a natural host. Virology 345:231-243. [DOI] [PubMed] [Google Scholar]
- 11.Fogg, C., S. Lustig, J. C. Whitbeck, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2004. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J. Virol. 78:10230-10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonteneau, J. F., M. Gilliet, M. Larsson, I. Dasilva, C. Munz, Y. J. Liu, and N. Bhardwaj. 2003. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood 101:3520-3526. [DOI] [PubMed] [Google Scholar]
- 13.Hammarlund, E., M. W. Lewis, S. G. Hansen, L. I. Strelow, J. A. Nelson, G. J. Sexton, J. M. Hanifin, and M. K. Slifka. 2003. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9:1131-1137. [DOI] [PubMed] [Google Scholar]
- 14.Hooper, J. W., D. M. Custer, C. S. Schmaljohn, and A. L. Schmaljohn. 2000. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology 266:329-339. [DOI] [PubMed] [Google Scholar]
- 15.Hooper, J. W., D. M. Custer, and E. Thompson. 2003. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology 306:181-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hooper, J. W., E. Thompson, C. Wilhelmsen, M. Zimmerman, M. A. Ichou, S. E. Steffen, C. S. Schmaljohn, A. L. Schmaljohn, and P. B. Jahrling. 2004. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 78:4433-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones-Trower, A., A. Garcia, C. A. Meseda, Y. He, C. Weiss, A. Kumar, J. P. Weir, and M. Merchlinsky. 2005. Identification and preliminary characterization of vaccinia virus (Dryvax) antigens recognized by vaccinia immune globulin. Virology 343:128-140. [DOI] [PubMed] [Google Scholar]
- 18.Jonjic, S., M. del Val, G. M. Keil, M. J. Reddehase, and U. H. Koszinowski. 1988. A nonstructural viral protein expressed by a recombinant vaccinia virus protects against lethal cytomegalovirus infection. J. Virol. 62:1653-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klavinskis, L. S., J. L. Whitton, and M. B. Oldstone. 1989. Molecularly engineered vaccine which expresses an immunodominant T-cell epitope induces cytotoxic T lymphocytes that confer protection from lethal virus infection. J. Virol. 63:4311-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Law, M., M. M. Putz, and G. L. Smith. 2005. An investigation of the therapeutic value of vaccinia-immune IgG in a mouse pneumonia model. J. Gen. Virol. 86:991-1000. [DOI] [PubMed] [Google Scholar]
- 21.Lustig, S., C. Fogg, J. C. Whitbeck, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2005. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J. Virol. 79:13454-13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lustig, S., C. Fogg, J. C. Whitbeck, and B. Moss. 2004. Synergistic neutralizing activities of antibodies to outer membrane proteins of the two infectious forms of vaccinia virus in the presence of complement. Virology 328:30-35. [DOI] [PubMed] [Google Scholar]
- 23.Maecker, H. T., H. S. Dunn, M. A. Suni, E. Khatamzas, C. J. Pitcher, T. Bunde, N. Persaud, W. Trigona, T. M. Fu, E. Sinclair, B. M. Bredt, J. M. McCune, V. C. Maino, F. Kern, and L. J. Picker. 2001. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J. Immunol. Methods 255:27-40. [DOI] [PubMed] [Google Scholar]
- 24.McCurdy, L. H., B. D. Larkin, J. E. Martin, and B. S. Graham. 2004. Modified vaccinia Ankara: potential as an alternative smallpox vaccine. Clin. Infect. Dis. 38:1749-1753. [DOI] [PubMed] [Google Scholar]
- 25.Meseda, C. A., A. D. Garcia, A. Kumar, A. E. Mayer, J. Manischewitz, L. R. King, H. Golding, M. Merchlinsky, and J. P. Weir. 2005. Enhanced immunogenicity and protective effect conferred by vaccination with combinations of modified vaccinia virus Ankara and licensed smallpox vaccine Dryvax in a mouse model. Virology 339:164-175. [DOI] [PubMed] [Google Scholar]
- 26.Morikawa, S., T. Sakiyama, H. Hasegawa, M. Saijo, A. Maeda, I. Kurane, G. Maeno, J. Kimura, C. Hirama, T. Yoshida, Y. Asahi-Ozaki, T. Sata, T. Kurata, and A. Kojima. 2005. An attenuated LC16m8 smallpox vaccine: analysis of full-genome sequence and induction of immune protection. J. Virol. 79:11873-11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olive, M., L. Eisenlohr, N. Flomenberg, S. Hsu, and P. Flomenberg. 2002. The adenovirus capsid protein hexon contains a highly conserved human CD4+ T-cell epitope. Hum. Gene Ther. 13:1167-1178. [DOI] [PubMed] [Google Scholar]
- 28.Olive, M., L. C. Eisenlohr, and P. Flomenberg. 2001. Quantitative analysis of adenovirus-specific CD4+ T cell responses from healthy adults. Viral Immunol. 14:403-413. [DOI] [PubMed] [Google Scholar]
- 29.Oseroff, C., F. Kos, H. H. Bui, B. Peters, V. Pasquetto, J. Glenn, T. Palmore, J. Sidney, D. C. Tscharke, J. R. Bennink, S. Southwood, H. M. Grey, J. W. Yewdell, and A. Sette. 2005. HLA class I-restricted responses to vaccinia recognize a broad array of proteins mainly involved in virulence and viral gene regulation. Proc. Natl. Acad. Sci. USA 102:13980-13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otero, M., S. A. Calarota, A. Dai, A. S. De Groot, J. D. Boyer, and D. B. Weiner. 2006. Efficacy of novel plasmid DNA encoding vaccinia antigens in improving current smallpox vaccination strategy. Vaccine 24:4461-4470. [DOI] [PubMed] [Google Scholar]
- 31.Pasquetto, V., H. H. Bui, R. Giannino, F. Mirza, J. Sidney, C. Oseroff, D. C. Tscharke, K. Irvine, J. R. Bennink, B. Peters, S. Southwood, V. Cerundolo, H. Grey, J. W. Yewdell, and A. Sette. 2005. HLA-A*0201, HLA-A*1101, and HLA-B*0702 transgenic mice recognize numerous poxvirus determinants from a wide variety of viral gene products. J. Immunol. 175:5504-5515. [DOI] [PubMed] [Google Scholar]
- 32.Pulford, D. J., A. Gates, S. H. Bridge, J. H. Robinson, and D. Ulaeto. 2004. Differential efficacy of vaccinia virus envelope proteins administered by DNA immunisation in protection of BALB/c mice from a lethal intranasal poxvirus challenge. Vaccine 22:3358-3366. [DOI] [PubMed] [Google Scholar]
- 33.Ramirez, J. C., E. Tapia, and M. Esteban. 2002. Administration to mice of a monoclonal antibody that neutralizes the intracellular mature virus form of vaccinia virus limits virus replication efficiently under prophylactic and therapeutic conditions. J. Gen. Virol. 83:1059-1067. [DOI] [PubMed] [Google Scholar]
- 34.Schmid, D. S., and B. T. Rouse. 1992. The role of T cell immunity in control of herpes simplex virus. Curr. Top. Microbiol. Immunol. 179:57-74. [DOI] [PubMed] [Google Scholar]
- 35.Schultze, J. L., S. Michalak, M. J. Seamon, G. Dranoff, K. Jung, J. Daley, J. C. Delgado, J. G. Gribben, and L. M. Nadler. 1997. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J. Clin. Investig. 100:2757-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snyder, J. T., I. M. Belyakov, A. Dzutsev, F. Lemonnier, and J. A. Berzofsky. 2004. Protection against lethal vaccinia virus challenge in HLA-A2 transgenic mice by immunization with a single CD8+ T-cell peptide epitope of vaccinia and variola viruses. J. Virol. 78:7052-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutter, G., and B. Moss. 1992. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl. Acad. Sci. USA 89:10847-10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang, J., P. Flomenberg, L. Harshyne, L. Kenyon, and D. W. Andrews. 2005. Glioblastoma patients exhibit circulating tumor-specific CD8+ T cells. Clin. Cancer Res. 11:5292-5299. [DOI] [PubMed] [Google Scholar]
- 39.Tang, J., M. Olive, R. Pulmanausahakul, M. Schnell, N. Flomenberg, L. Eisenlohr, and P. Flomenberg. 2006. Human CD8+ cytotoxic T cell responses to adenovirus capsid proteins. Virology 350:312-322. [DOI] [PubMed] [Google Scholar]
- 40.Terajima, M., J. Cruz, G. Raines, E. D. Kilpatrick, J. S. Kennedy, A. L. Rothman, and F. A. Ennis. 2003. Quantitation of CD8+ T cell responses to newly identified HLA-A*0201-restricted T cell epitopes conserved among vaccinia and variola (smallpox) viruses. J. Exp. Med. 197:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tscharke, D. C., G. Karupiah, J. Zhou, T. Palmore, K. R. Irvine, S. M. Haeryfar, S. Williams, J. Sidney, A. Sette, J. R. Bennink, and J. W. Yewdell. 2005. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J. Exp. Med. 201:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsung, K., J. H. Yim, W. Marti, R. M. Buller, and J. A. Norton. 1996. Gene expression and cytopathic effect of vaccinia virus inactivated by psoralen and long-wave UV light. J. Virol. 70:165-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Bergwelt-Baildon, M. S., R. H. Vonderheide, B. Maecker, N. Hirano, K. S. Anderson, M. O. Butler, Z. Xia, W. Y. Zeng, K. W. Wucherpfennig, L. M. Nadler, and J. L. Schultze. 2002. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood 99:3319-3325. [DOI] [PubMed] [Google Scholar]
- 44.Wysocka, M., L. C. Eisenlohr, L. Otvos, Jr., D. Horowitz, J. W. Yewdell, J. R. Bennink, and C. J. Hackett. 1994. Identification of overlapping class I and class II H-2d-restricted T cell determinants of influenza virus N1 neuraminidase that require infectious virus for presentation. Virology 201:86-94. [DOI] [PubMed] [Google Scholar]
- 45.Xu, R., A. J. Johnson, D. Liggitt, and M. J. Bevan. 2004. Cellular and humoral immunity against vaccinia virus infection of mice. J. Immunology. 172:6265-6271. [DOI] [PubMed] [Google Scholar]
- 46.Yang, S. Y., E. Milford, U. Hammerling, and B. Dupont. 1989. Description of the reference panel of B-lymphoblastoid cell lines for factors of the HLA system: the B-cell line panel designed for the Tenth International Histocompatibility Workshop, p. 11-19. In B. Dupont (ed.), Immunobiology of HLA, vol. I. Histocompatibility testing 1987. Springer-Verlag, New York, N.Y. [Google Scholar]