Abstract
Transmissible spongiform encephalopathies (TSEs) can be ameliorated by prion protein (PrP)-specific antibodies, but active immunization is complicated by immune tolerance to the normal cellular host protein (PrPC). Here, we show that DNA immunization of wild-type mice can break immune tolerance against the prion protein, resulting in the induction of PrP-specific antibody and T-cell responses. PrP immunogenicity was increased by fusion to the lysosomal targeting signal from LIMPII (lysosomal integral membrane protein type II). Although mice immunized with a PrP-LIMPII DNA vaccine showed a dramatic delay in the onset of early disease signs after intracerebral challenge, immunization against PrP also had some deleterious effects. These results clearly confirm the feasibility of using active immunization to protect against TSEs and, in the absence of effective treatments, indicate a suitable alternative for combating the spread of these diseases.
Prion diseases belong to a class of conformational disorders including variant Creutzfeldt-Jacob disease, an increasing threat to human health (16). Transformation of the ubiquitous cellular prion protein (PrPC) into a pathological conformer (PrPSc) seems to be the key event in pathogenesis (5, 11). Emerging data indicate that this devastating group of diseases may be amenable to immunotherapy and immunoprophylaxis, raising the prospect that the spread of these diseases may be countered by vaccination (23). Several reports have shown that antibodies to the normal cellular protein, PrPC, when introduced by injection (27) or by transgenic means (9, 25), can arrest the accumulation of the pathological protein conformer PrPSc, thereby interfering with disease progression. However, active immunization strategies against this infectious disease are seriously compromised by host tolerance to PrPC (2); the feasibility of inducing humoral (7, 14, 24) and T-cell (22) responses to prion protein in wild-type (WT) mice has previously been demonstrated, but to our knowledge, no reports have described the concomitant induction of PrP-specific B-cell, CD4+ T-cell, and CD8+ T-cell responses in healthy wild-type mice after DNA immunization. Nucleic acid immunization has previously been shown to break tolerance to host proteins (6, 12), and we have exploited this phenomenon to confer protection against a murine melanoma (29). Herein we show that a DNA vaccine encoding PrPC can break tolerance to this host protein, inducing both humoral and PrP-specific T-cell responses and a degree of protection against PrPSc challenge. Several different plasmid constructs were evaluated, each encoding a different version of the mouse PrP. The first plasmid (pCMV-PrP) expressed an unmodified protein; the second (pCMV-UbPrP) encoded PrP fused to the cellular protein ubiquitin to enhance antigen presentation via major histocompatibility complex (MHC) class I, thereby improving the induction of antigen-specific CD8+ T cells (17, 21); and the third (pCMV-PrPLII) encoded PrP fused to the lysosomal integral membrane protein type II (LIMPII) lysosome-targeting signal, which enhances MHC class II antigen presentation and the induction of CD4+ T-cell responses (18). We find that the third construct was the most effective at breaking immune tolerance and enhanced both humoral and cellular responses in wild-type animals. Most importantly, this DNA vaccine conferred significant protection against intracerebral prion challenge, dramatically delaying the onset of disease. However, this benefit had an associated cost: once disease appeared, it was rapidly progressive. The advantages and disadvantages of vaccinating against prion diseases and the possible explanations for the clinical effects are discussed.
MATERIALS AND METHODS
Mice.
The 129/ola strain was purchased from Harlan, and 129/ola PrP knockout (PrPKO) mice were generously provided by Jean Manson, IAH, Edinburgh, United Kingdom.
Construction of recombinant plasmids expressing the mouse prion protein.
The mouse prion open reading frames with or without their termination codon was obtained by PCR using the forward (5′-AGATCTATGGCGAACCTTGGCTACTGGC-3′) and the stop reverse (5′-AGATCTCATCCCACGATCAGGAAGATG-3′) or the nonstop reverse (5′-AGATCTTCCCACGATCAGGAAGATGAGG) primers. The amplified fragments were cloned either in the pCMV-LII plasmid (18) or in the pCMV-F1/F2Ubiq plasmid (19) to obtain pCMV-PrP, pCMV-PrPLII, and pCMV-UbPrP plasmids. All the products were expressed under the control of the immediate early promoter of human cytomegalovirus using the pCMV expression vector (Clontech).
Protocol for DNA immunization.
DNA purification was carried out using endotoxin-free columns (QIAGEN). DNA was dissolved at 1 mg/ml in 1 N saline, and mice were immunized into each anterior tibial muscle with 100 μg of DNA per mouse and dose.
Inoculation of mice with prions.
The prion inoculum used in these experiments was derived by twofold serial passage of the BSE1 inoculum (4) in mice. Twenty microliters of a 10% brain homogenate was delivered by intracerebral injection into the right parietal lobes of mice. Mice were monitored weekly until the onset of terminal disease, at which time daily examination was performed. Early clinical signs included a waddling gait, ruffled coat, and kyphosis for at least three consecutive weeks. Severe clinical signs included tremors, a blank stare, lethargy, and immobility. Mice were sacrificed at the terminal stage of disease, with the exception of some mice, which were found dead.
Production of recombinant PrPmu.
The mature form of the murine PrP (PrPmu) gene was cloned into the vector pET11a under the control of the T7lac promoter, and murine PrP protein expression was induced with IPTG (isopropyl-β-d-thiogalactopyranoside). PrPmu from inclusion bodies was solubilized in 8 M urea and fractionated by two rounds of cation-exchange chromatography using increasing concentrations of NaCl (0 to 1 M). The elution peak for PrP was achieved in the 0.5 to 1 M range. As a negative control for intracellular cytokine staining (ICCS), unrelated proteins were used by following the same protocol of semipurification.
Detection of epitope-specific T-cell responses by ICCS.
For the ICCS assay, splenocytes were incubated together with 1 μg/ml of the semipurified protein stimulus, as indicated in the figure legends. After a 36-h incubation at 37°C, brefeldin A was added at 5 μg/ml for the last 3 h to increase the accumulation of gamma interferon (IFN-γ) in the responding cells. Spleen cells were surface labeled with cytochrome-conjugated anti-CD8 antibody and with phycoerythrin-conjugated anti-CD4 antibody and intracellularly stained with a fluorescein-conjugated anti-IFN-γ antibody. Finally, the cells were fixed and analyzed by flow cytometry. Reagents were purchased from Pharmingen.
ELISA assay.
Enzyme-linked immunosorbent assay (ELISA) plates were coated overnight with 300 ng per well of semipurified PrP protein. Mice sera were applied, followed by peroxidase-conjugated goat anti-mouse polyvalent immunoglobulins (Sigma). Colorimetric detection was achieved by adding ABTS (2,2′-azino-di-[3-ethylbenzthiazoline sulfonate]-diammonium salt) substrate (Roche). Optical densities at 405 nm were calculated.
Western blot assay.
Brain proteins in phosphate-buffered saline homogenates were detergent extracted (5% Sarkosyl). Samples were precleared by centrifugation (2 min, 10,000 × g), and equivalent protein loads were adjusted by a bicinchoninic assay (Pierce) prior to treatment with 100 μg/ml of proteinase K (PK; Roche) for 45 min at 37°C when required. The reaction was stopped by the addition of 10 mM phenylmethylsulfonyl fluoride. Insoluble proteins were precipitated by centrifugation (25,000 × g, 45 min). Samples were finally loaded into 12% sodium dodecyl sulfate (SDS)-polyacrylamide gels or subjected to deglycosylation. For the latter, pellets were washed with 20 mM sodium phosphate, pH 7.8, and resuspended in deglycosylation buffer (1% SDS, 0.5% NP-40, dithiothreitol). Finally, samples were denatured by boiling them for 10 min and treated or mock treated with 250 units of peptide-N-glycosidase F (PNGase F) (NEB) in sodium phosphate buffer, pH 7.8. After overnight incubation, samples were kept for 2 h at −80°C after 4 volumes of ice-cold methanol were added. Proteins were finally precipitated by centrifugation (25,000 × g, 30 min), and pellets were resuspended in Laemmli's buffer before being loaded into SDS-polyacrylamide gels. Proteins were transferred to polyvinylidene difluoride (Millipore), and PK-resistant PrP (PrPres) proteins were detected by the specific monoclonal antibody 2A11, followed by incubation with peroxidase-labeled goat anti-mouse immunoglobulins (Sigma).
RESULTS
Targeting prion protein to the lysosomes improves the antibody responses induced after DNA immunization.
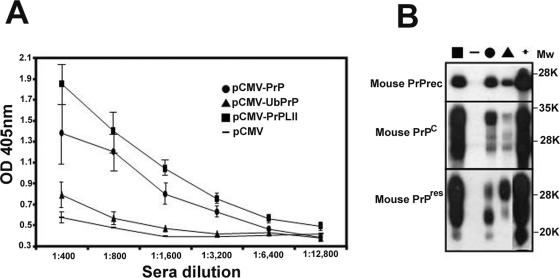
PrPKO mice lack PrPC and are, therefore, not tolerant of this normal host protein; these mice serve as a useful in vivo model with which to evaluate the potential immunogenicities of various PrP-encoding plasmid DNAs. Thus, we first immunized 129/ola PrPKO mice (13) with either pCMV-PrP (expressing the unmodified mouse prion protein), pCMV-UbPrP (encoding PrP fused to ubiquitin), pCMV-PrPLII (encoding PrP fused to the LIMPII lysosome-targeting signal), or pCMV (as a negative control). Each group contained four mice, and each mouse received three DNA injections at 14-day intervals. Mice were bled 6 weeks after the last immunization, and the presence of prion-specific antibodies in their sera was evaluated by ELISA (Fig. 1A), using semipurified recombinant murine PrP as the immobilized target. As shown previously (10), PrPKO mice immunized with a plasmid encoding an unmodified prion protein showed detectable PrP-specific antibody responses (Fig. 1A). In clear contrast, ubiquitin-tagged PrP induced substantially lower antibody responses (Fig. 1A), presumably because the protein was rapidly hydrolyzed by the proteasome, in agreement with what has been previously described for other antigens (21, 28). Most importantly, mice immunized with pCMV-PrPLII developed significantly higher titers of prion-specific antibodies (Fig. 1A); even at a dilution of 1:12,800, it was possible to detect specific responses above the background levels.
FIG. 1.
DNA immunization induces PrP antibody responses. (A) Serial dilutions of sera from PrPKO mice immunized three times with pCMV (dashes), pCMV-PrP (circles), pCMV-UbPrP (triangles), and pCMV-PrPLII (squares) were tested against ELISA plates coated with recombinant prion protein. The standard deviation for each group is shown. (B) Western blot using serum from a representative mouse from each immunization group (the data were identical for all mice in the same group). All sera were diluted 1:3,200 and were tested in a Western blot assay using as antigen (i) recombinant prion protein (PrPrec; top panel), (ii) brain extracts from a WT 129/ola mouse (PrPC; middle panel), or (iii) brain extracts from a PrPSc-infected mouse after proteinase K treatment (bottom panel). As a positive control, a hyperimmune mouse serum, raised in PrP null mice against E. coli recombinant PrP protein plus Freund's adjuvant, was used at a 1:2,000 dilution (lane marked “+”). Molecular weight (Mw) standards (in thousands [K]) were used as size markers.
To confirm the results described above, sera (1/3,200 dilution) from the same animals were tested in a Western blot assay (Fig. 1B). Antibodies induced after DNA immunization with pCMV-PrP (Fig. 1B) recognized the recombinant PrP protein expressed in Escherichia coli (which was also used in the ELISA). In correspondence with the ELISA results, antibodies from pCMV-PrPLII-immunized mice strongly recognized the recombinant PrP protein expressed in E. coli (Fig. 1B, upper panel). Furthermore, these antibodies recognized very strongly all PrP-specific bands from PrPC and PrPres (middle and lower panels). Sera from pCMV-UbPrP-immunized mice also reacted strongly with PrP in a Western blot assay (Fig. 1B). This was somewhat surprising because, at the dilution used in the Western blot (1/3,200), these sera were indistinguishable from control sera in the ELISA (Fig. 1A). We speculate that antibodies induced by pCMV-UbPrP might be generated against PrP degradation products and therefore will recognize linear sequences on a Western blot better than conformation-dependent native folding in an ELISA.
Targeting prion protein to the lysosomes improves both the T-helper and the CD8+ T-cell responses induced after DNA immunization.
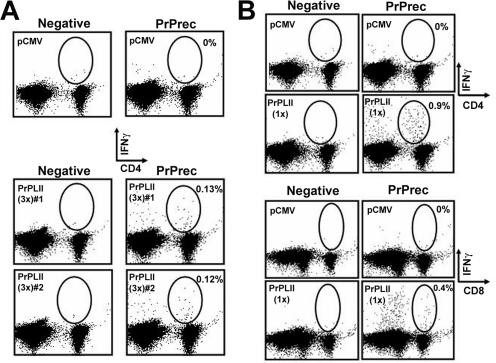
We have previously shown that lysosomal targeting can improve T-helper (CD4+ T-cell) responses after DNA immunization (18, 20). The enhancement in the antibody response observed by ELISA when prion protein is targeted for rapid hydrolysis in the lysosome (Fig. 1) might be due at least in part to its improved entry into the MHC class II processing pathway and the induction of a strong T-helper response. To evaluate the T-helper responses induced against the prion protein, PrPKO mice were immunized three times and sacrificed 6 weeks after the last immunization, and the purified splenocytes were incubated with, or without, recombinant PrP protein. Mice immunized with pCMV-PrPLII mounted a robust CD4+ T-cell response against PrP, as measured by the percentage of cells that expressed IFN-γ (indicator of T-cell activation), following incubation with recombinant PrP (Fig. 2A). Surprisingly, despite the clear antibody response induced by vaccination with pCMV-PrP, no specific IFN-γ response was detectable after stimulation with prion protein (data not shown).
FIG. 2.
DNA immunization induces PrP T-cell responses. PrPKO mice were immunized three times (A) or once (B) either with pCMV or with pCMV-PrPLII. Six weeks after the final immunization, splenocytes were harvested, incubated together with the indicated semipurified protein stimulus at 1 μg/ml for 36 h, and subjected to ICCS. Circles and percentages shown (values given after subtraction of the control value) indicate the PrP-specific CD4+ T-cells (A and upper panel in B) or the PrP-specific CD8+ T-cells (B, lower panel). PrPrec, recombinant PrP.
In order to study in more detail the immunogenicity of pCMV-PrPLII, 129/ola PrPKO mice were inoculated with a single dose of the DNA vaccine and, 6 weeks later, were sacrificed. Sera from these animals were used both in an ELISA and in a Western blot assay to determine the prion-specific antibody titers, which, as expected, were much lower after only one immunization with the pCMV-PrPLII vector than after two boosts (data not shown). In addition, splenocytes were analyzed by intracellular IFN-γ staining to identify PrP-specific T cells. Despite the low antibody response observed, a very robust CD4+ T-cell response was detectable after stimulation with PrP protein (Fig. 2B, upper panel). Strikingly, targeting prion protein to the lysosomes (pCMV-PrPLII-vaccinated mice) enhanced not only the specific T-helper responses but also the specific CD8+ T-cell responses; after ex vivo stimulation with PrP protein, specific CD8+ T cells expressed IFN-γ (Fig. 2B, lower panel). Other authors have previously demonstrated an improvement in CTL responses by targeting antigens to the lysosomes by using the lysosome-associated membrane protein 1 targeting signal (3). In clear contrast, and contrary to our expectations, CD8+ T-cell responses were not enhanced by fusion of ubiquitin to prion protein (data not shown). This result cannot be ascribed to the lack of degradation in the proteasomal complex, because ubiquitinated PrP was rapidly degraded after the transfection of BHK cells, and this degradation was reduced in the presence of proteasome inhibitors (not shown). Taken together, our results indicate that lysosomal targeting increases both humoral (Fig. 1) and cellular (Fig. 2) PrP-specific immune responses in PrPKO mice.
Breaking immune tolerance against PrP by DNA immunization.
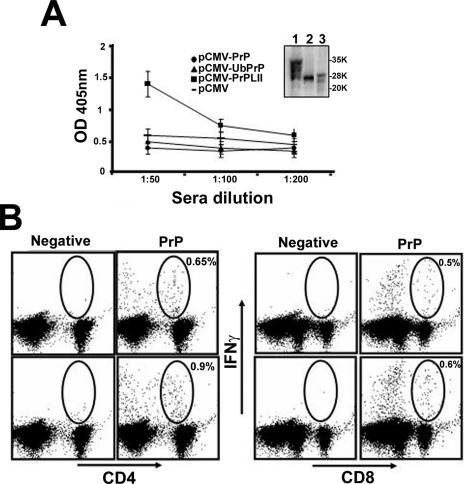
Next, we evaluated the capacity of our DNA vaccines to break tolerance in wild-type mice. Two WT 129/ola mice were immunized with each plasmid by following the protocol of immunization described above. Three doses of plasmid were administered, and mice were bled and sacrificed to evaluate their immune status 6 weeks after the last immunization. Prion-specific antibodies were detected in sera from mice immunized with pCMV-PrPLII by both ELISA (Fig. 3A) and Western blotting (Fig. 3A, inset), indicating that the DNA vaccine successfully broke tolerance to this host protein. As expected, the level of antibodies induced in wild-type mice was markedly lower than in PrPKO mice (compare Fig. 1 and 3A), presumably due to the tolerogenic effects of the endogenous host PrPC. Despite this constraint, the two WT mice immunized with pCMV-PrPLII also showed PrP-specific T-cell responses (Fig. 3B); both CD8+ and CD4+ (helper) T cells expressed IFN-γ in response to stimulation with recombinant PrP. Mice immunized with pCMV-PrP or with pCMV-UbPrP did not show PrP-specific T-cell responses distinguishable from those of the controls, and they had no detectable prion-specific antibodies in their sera, showing OD values even lower than those of the control sera (Fig. 3A).
FIG. 3.
Breaking tolerance against PrP by DNA immunization. (A) Serial dilutions of sera from WT 129/ola mice immunized three times with pCMV (dashes), pCMV-PrP (circles), pCMV-UbPrP (triangles), and pCMV-PrPLII (squares) were tested against ELISA plates coated with recombinant prion protein. The standard deviation for each group is shown. Serum from a WT 129/ola mouse immunized with pCMV-PrPLII was diluted 1:200 and tested in a Western blot assay (inset) using as the antigen (lane 1) brain extracts from a WT 129/ola mouse (PrPC), (lane 2) recombinant prion protein, or (lane 3) brain extracts from a PrPSc-infected mouse after proteinase K treatment. The data were identical for all mice in the same group. Molecular weight standards in thousands (K) were used as size markers. (B) Splenocytes from mice immunized with pCMV-PrPLII were harvested, incubated together with the indicated semipurified protein stimulus at 1 μg/ml for 36 h, and subjected to ICCS. Circles and percentages shown (values given after subtraction of the control value) indicate the PrP-specific CD4+ T cells (B, left panel) and CD8+ T cells (B, right panel) induced after DNA immunization with pCMV-PrPLII.
Targeting prion protein to the lysosomes delays prion disease after intracerebral challenge.
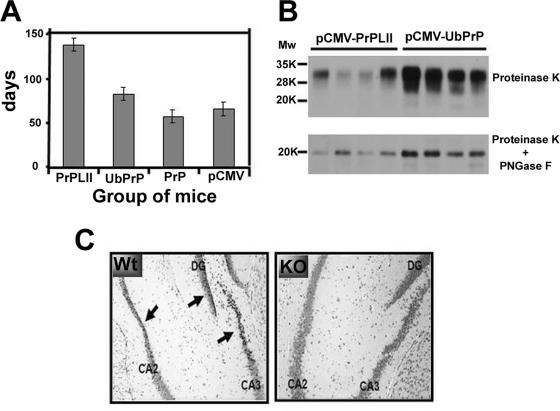
Finally, we evaluated the protective capacity of the three PrP DNA vaccines. WT 129/ola mice (four mice per group and one group per DNA vaccine) were immunized three times, as described above, and 6 weeks after the last boost were challenged by intracerebral inoculation of a lethal dose of an infectious mouse-adapted bovine spongiform encephalopathy PrP protein (4). A fourth group of mice received pCMV. Mice were evaluated weekly for clinical signs by observers blind to the vaccine groupings. The times at which signs of disease were first observed are shown in Fig. 4A. Negative-control mice (pCMV inoculated) first developed signs at ∼65 days (week 10) postchallenge, while mice immunized with pCMV-PrP had a time of disease onset similar to that of the control mice, developing signs at ∼57 days (week 9) postchallenge. All mice included in these two groups succumbed during week 20 after challenge. Mice immunized with pCMV-UbPrP had a statistically significant (P < 0.001, one-way analysis of variance) delay in disease onset, showing clear symptoms of the disease at ∼82 days (week 12) postchallenge. All mice within this group suffered a 2-week period of severe clinical symptoms of the disease before dying during week 22 after PrPSc challenge. Most strikingly, mice immunized with pCMV-PrPLII remained clear of clinical disease for almost twice as long as pCMV recipients, with an average of ∼138 days (week 20) before early clinical signs first appeared. However, these mice suddenly succumbed without clear signs of disease, very soon after the onset of early clinical signs and almost at the same time as pCMV-UbPrP-immunized mice (during week 22 after challenge). Postmortem Western blot and immunoblot analyses revealed no differences before PK treatment (data not shown) but did show much lower levels of PrPres accumulation in brains from all mice immunized with pCMV-PrPLII than was observed in pCMV-UbPrP-immunized animals (Fig. 4B), even though the mice in these groups died at almost exactly the same times postinoculation. This effect was very apparent not only after PK treatment (upper panel) but overall after PNGase digestion, which eliminates sugars from PrPres, permitting the reduction of all glycosylated forms to a sharper band of lower molecular weight easier to visualize (lower panel). pCMV-PrPLII-vaccinated mice showed less postmortem PrPres accumulation in their brains (data not shown), and they also succumbed 2 weeks later than pCMV- and pCMV-PrP-immunized mice and took longer to develop clinical disease. Thus, the accumulation of PrPres in the brain and severe clinical signs of prion disease seemed to correlate.
FIG. 4.
Vaccination confers protection against intracerebral prion challenge. (A) WT 129/ola mice were immunized three times with either pCMV, pCMV-PrP, pCMV-UbPrP, or pCMV-LII and 6 weeks later were intracerebrally challenged with PrPSc. Mice were examined weekly, and early signs of disease for each of the immunized groups were recorded and are shown. For each immunization group, all mice first developed the early symptoms within a few days of each other. (B) Mice immunized with pCMV-PrPLII or pCMV-UbPrP succumbed almost at the same time during week 22 (∼150 days) after PrPSc challenge. Equivalent amounts of brain protein extract from each dead mouse within these two vaccination groups were subjected to PK treatment (upper panel), followed by PNGase F digestion to eliminate N-linked glycans (lower panel). All four mice immunized with pCMV-PrPLII accumulated much smaller amounts of PrPres in their brains than the amounts observed in mice vaccinated with pCMV-UbPrP. (C) Twenty microliters of heat-inactivated serum from a PrPKO mouse previously immunized three times with pCMV-PrPLII (highly positive for prion-specific antibodies) was injected intracerebrally into three WT 129/ola mice or three PrPKO 129/ola mice. Twenty-four hours after intracerebral injection, mice were euthanized and brain tissues from WT mice (left panel) and PrPKO mice (right panel) were formalin fixed, cut in 5-μm-thick slices, and Nissl counterstained. Arrows indicate pyknotic neurons in the dentate gyrus (DG) and in cornu ammonis regions 2 and 3 (CA2 and CA3, respectively) of the hippocampus. Clear pyknosis can be observed in the hippocampi of WT mice (left panel), indicating neuronal death. In clear contrast, no significant neuronal damage was observed in PrPKO mice subjected to the same treatment (right panel).
Thus, we are faced with an apparent contradiction: mice immunized with pCMV-PrPLII show a dramatic prolongation of good health and a reduction in the accumulation of PrPres but then die suddenly. How might this paradox be resolved? It has recently been shown that PrP-specific antibodies can cross-link cellular PrP, leading to neuronal apoptosis in vivo (26). Therefore, we hypothesized that the repertoire of antibodies induced by pCMV-PrPLII might have pathogenic, as well as beneficial, effects. To investigate this issue, WT 129/ola mice were inoculated intracerebrally with 20 μl of inactivated serum highly enriched with PrP-specific antibodies (obtained from PrP null mice immunized with pCMV-PrPLII) and 24 h later were sacrificed. Histological analyses revealed neuronal death (Fig. 4C, left panel). Neuronal damage was not observed in PrPKO 129/ola mice that received the same sera (Fig. 4C, right panel), suggesting that this effect was dependent upon the expression of cellular PrP on the surfaces of the affected neurons.
DISCUSSION
Transmissible spongiform encephalopathies (TSEs) form a group of neurodegenerative disorders whose frequency is increasing and for which no effective prophylactic or preventive strategies exist, other than attempting to control exposure to the infectious agent. However, there is an increasing amount of evidence that points toward the effective role of the immune system in combating these diseases (8, 3). Recent reports clearly demonstrate that antibodies specific to prion protein can delay the onset of the disease (15). However, the relevance of T-cell responses to protection has not been fully addressed. In order to enhance the CD8+ T-cell responses induced after DNA immunization of wild-type mice, ubiquitin was fused to the PrP protein, driving it for rapid degradation in the proteasome. Although sera from PrPKO mice immunized with pCMV-UbPrP recognized PrP on a Western blot (Fig. 1B), no specific T-cell responses were detected, perhaps due to the low sensitivity of our assay. Our results suggest the possibility that immune responses induced by immunization of wild-type mice with pCMV-UbPrP, even below the in vitro detection threshold, might have been sufficient to cause the slight, but statistically significant, in vivo delay in the onset of prion disease following intracerebral challenge (Fig. 4A). However, the effects of linking PrPC to the LIMPII tail were more striking and enhanced not only PrP-specific CD4+ T-cell responses but also PrP-specific antibody and CD8+ T-cell responses. Interestingly, while strong T-cell responses were obtained in PrPKO mice with only one dose of the vaccine (Fig. 2B), at least two doses of pCMV-PrPLII were required to induce PrP-specific antibodies. This may have implications for vaccine protocol if, for example, one wishes to induce strong cellular responses but weaker antibody responses. We are currently studying all these processes in more detail. To our knowledge, this is the first report showing the induction of PrP-specific CD8+ T-cell responses upon vaccination. We are currently studying the role that these cells might play in protection against prion diseases.
Following PrPSc challenge, mice immunized with pCMV-PrP died at the same time as control mice immunized with the empty pCMV plasmid (during week 20 after challenge). In correspondence with the significant delay observed in the disease onset, mice immunized with pCMV-UbPrP succumbed during week 22 after challenge, 9 weeks after the onset of early clinical signs and before suffering severe clinical signs for almost 2 weeks. Intriguingly, pCMV-PrPLII-immunized mice remained clinically healthy for almost twice as long as any other group (Fig. 4A). However, these mice suddenly succumbed at ∼148 days postchallenge (during week 22 after challenge), very soon after the onset of early clinical signs and almost at the same time as pCMV-UbPrP-immunized mice, perhaps as a result of immunopathology mediated by PrP-specific antibodies induced by pCMV-PrPLII (Fig. 4C). We hypothesized that PrPSc inoculation might somehow affect the blood-brain barrier, facilitating the transfer of antibodies from the bloodstream to the central nervous system.
These results open new ethical concerns for TSE immunization, similar to those that have been voiced regarding vaccination against Alzheimer's disease (1). The cost-to-benefit ratio of immunizing against TSEs also might be different depending on the route of challenge. Here, the pathogen was delivered by intracranial injection, and it is possible that the deleterious effects might have been fewer if the PrPSc had been administered by more physiologically relevant routes of infection (oral or parenteral). We are currently performing experiments not only to follow PK-resistant PrP accumulation during the infection period but also to study histologically and immunohistochemically the kinetics of the neuropathological changes taking place in the brains of immunized mice. We are also evaluating the efficacy of new plasmid constructs and alternative routes of DNA administration that might avoid the adverse effects of vaccination, without reducing the protective efficacy.
In conclusion, these data show that DNA immunization with pCMV-PrPLII breaks tolerance to PrP in wild-type mice, leading to the induction of PrP-specific antibodies and T-cell responses and to a substantial degree of protection. These findings demonstrate clearly the feasibility of prophylactic immunization against prion-related diseases and provide strong validation for continuing the search for vaccines against these and other neurological diseases, such as Alzheimer's disease.
Acknowledgments
This work has been entirely supported by research project grants EET2002-4217-C02-01 and AGL2004-07857-C03-01 and also by the Ramón y Cajal Program from the Spanish Ministerio de Educación y Ciencia (MEC). J.L.W. was supported by NIH award AI-27028.
We thank Jean Manson from the Institute for Animal Health, Neuropathogenesis Unit, Edinburgh, United Kingdom, for providing us with the PrPKO mice. We thank Joaquin Castilla and Esteban Domingo from CISA-INIA Valdeolmos for their scientific support. Finally, we thank Stephanie Harkins from The Scripps Research Institute and all technical and administrative staff from Valdeolmos and CReSA for their invaluable assistance.
REFERENCES
- 1.Abbott, A. 2004. Doctors seek lost data on Alzheimer's vaccine. Nature 430:715. [DOI] [PubMed] [Google Scholar]
- 2.Aguzzi, A., and C. J. Sigurdson. 2004. Antiprion immunotherapy: to suppress or to stimulate? Nat. Rev. Immunol. 4:725-736. [DOI] [PubMed] [Google Scholar]
- 3.Bonini, C., S. P. Lee, S. R. Riddell, and P. D. Greenberg. 2001. Targeting antigen in mature dendritic cells for simultaneous stimulation of CD4+ and CD8+ T cells. J. Immunol. 166:5250-5257. [DOI] [PubMed] [Google Scholar]
- 4.Castilla, J., A. Gutierrez Adan, A. Brun, B. Pintado, M. A. Ramirez, B. Parra, D. Doyle, M. Rogers, F. J. Salguero, C. Sanchez, J. M. Sanchez-Vizcaino, and J. M. Torres. 2003. Early detection of PrPres in BSE infected bovine PrP transgenic mice. Arch. Virol. 148:677-691. [DOI] [PubMed] [Google Scholar]
- 5.Castilla, J., P. Saa, C. Hetz, and C. Soto. 2005. In vitro generation of infectious scrapie prions. Cell 121:195-206. [DOI] [PubMed] [Google Scholar]
- 6.Djilali-Saiah, I., P. Lapierre, S. Vittozi, and F. Alvarez. 2002. DNA vaccination breaks tolerance for a neo-self antigen in liver: a transgenic murine model of autoimmune hepatitis. J. Immunol. 169:4889-4896. [DOI] [PubMed] [Google Scholar]
- 7.Goni, F., E. Knudsen, F. Schreiber, H. Scholtzova, J. Pankiewicz, R. Carp, H. C. Meeker, R. Rubenstein, D. R. Brown, M. S. Sy, J. A. Chabalgoity, E. M. Sigurdsson, and T. Wisniewski. 2005. Mucosal vaccination delays or prevents prion infection via an oral route. Neuroscience 133:413-421. [DOI] [PubMed] [Google Scholar]
- 8.Heppner, F. L., and A. Aguzzi. 2004. Recent developments in prion immunotherapy. Curr. Opin. Immunol. 16:594-598. [DOI] [PubMed] [Google Scholar]
- 9.Heppner, F. L., C. Musahl., I. Arrighi, M. A. Klein, T. Rulicke, B. Oesch, R. M. Zinkernagel, U. Kalinke, and A. Aguzzi. 2001. Prevention of scrapie pathogenesis by transgenic expression of anti-prion protein antibodies. Science 294:178-182. [DOI] [PubMed] [Google Scholar]
- 10.Krasemann, S., M. H. Groschup, S. Harmeyer, G. Hunsmann, and W. Bodemer. 1996. Generation of monoclonal antibodies against human prion proteins in PrP0/0 mice. Mol. Med. 2:725-734. [PMC free article] [PubMed] [Google Scholar]
- 11.Legname, G., I. V. Baskakov, H. O. Nguyen, D. Riesner, F. E. Cohen, S. J. DeArmond, and S. B. Prusiner. 2004. Synthetic mammalian prions. Science 305:673-676. [DOI] [PubMed] [Google Scholar]
- 12.Leitner, W., L. N. Wang, M. J. deVeer, A. Zhou, R. H. Silverman, B. R. Williams, T. W. Dubensky, H. Ying, and N. P. Restifo. 2003. Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways. Nat. Med. 9:33-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manson, J. C., A. R. Clarke, M. L. Hooper, L. Aitchison, I. McConnell, and J. Hope. 1994. 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol. Neurobiol. 8:121-127. [DOI] [PubMed] [Google Scholar]
- 14.Nikles, D., P. Bach, K. Boller, C. A. Merten, F. Montrasio, F. L. Heppner, A. Aguzzi, K. Cichutek, U. Kalinke, and C. J. Buchholz. 2005. Circumventing tolerance to the prion protein (PrP): vaccination with PrP-displaying retrovirus particles induces humoral immune responses against the native form of cellular PrP. J. Virol. 79:4033-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polymenidou, M., F. L. Heppner, E. C. Pellicioli, E. Urich, G. Miele, N. Braun, F. Wopfner, H. M. Schatzl, B. Becher, and A. Aguzzi. 2004. Humoral immune response to native eukaryotic prion protein correlates with anti-prion protection. Proc. Natl. Acad. Sci. USA 101:14670-14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricketts, M. N. 2004. Public health and the BSE epidemic. Curr. Top. Microbiol. Immunol. 284:99-119. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez, F., L. L. An, S. Harkins, J. Zhang, M. Yokoyama, G. Widera, J. T. Fuller, C. Kincaid, I. L. Campbell, and J. L. Whitton. 1998. DNA immunization with minigenes: low frequency of memory CTL and inefficient antiviral protection are rectified by ubiquitination. J. Virol. 72:5174-5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez, F., S. Harkins, J. M. Redwine, J. M. de Pereda, and J. L. Whitton. 2001. CD4+ T cells induced by a DNA vaccine: immunological consequences of epitope-specific lysosomal targeting. J. Virol. 75:10421-10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez, F., M. K. Slifka, S. Harkins, and J. L. Whitton. 2001. Two overlapping subdominant epitopes identified by DNA immunization induce protective CD8+ T-cell populations with differing cytolytic activities. J. Virol. 75:7399-7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez, F., and J. L. Whitton. 2000. Enhancing DNA immunization. Virology 268:233-238. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez, F., J. Zhang, and J. L. Whitton. 1997. DNA immunization: ubiquitination of a viral protein enhances CTL induction, and antiviral protection, but abrogates antibody induction. J. Virol. 71:8497-8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosset, M. B., C. Ballerini, S. Gregoire, P. Metharom, C. Carnaud, and P. Aucouturier. 2004. Breaking immune tolerance to the prion protein using prion protein peptides plus oligodeoxynucleotide-CpG in mice. J. Immunol. 172:5168-5174. [DOI] [PubMed] [Google Scholar]
- 23.Sadowski, M., and T. Wisniewski. 2004. Vaccines for conformational disorders. Expert Rev. Vaccines 3:279-290. [DOI] [PubMed] [Google Scholar]
- 24.Sigurdsson, E. M., D. R. Brown, M. Daniels, R. J. Kascsak, R. Kascsak, R. Carp, H. C. Meeker, B. Frangione, and T. Wisniewski. 2002. Immunization delays the onset of prion disease in mice. Am. J. Pathol. 161:13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sigurdsson, E. M., M. S. Sy, R. Li, H. Scholtzova, R. J. Kascsak, R. Kascsak, R. Carp, H. C. Meeker, B. Frangione, and T. Wisniewski. 2003. Antiprion antibodies for prophylaxis following prion exposure in mice. Neurosci. Lett. 336:185-187. [DOI] [PubMed] [Google Scholar]
- 26.Solforosi, L., J. R. Criado, D. B. McGavern, S. Wirz, M. Sanchez-Alavez, S. Sugama, L. A DeGiorgio, B. T. Volpe, E. Wiseman, G. Abalos, E. Masliah, D. Gilden, M. B. Oldstone, B. Conti, and R. A. Williamson. 2004. Cross-linking cellular prion protein triggers neuronal apoptosis in vivo. Science 303:1514-1516. [DOI] [PubMed] [Google Scholar]
- 27.White, A. R., and S. H. Hawke. 2003. Immunotherapy as a therapeutic treatment for neurodegenerative disorders. J. Neurochem. 87:801-808. [DOI] [PubMed] [Google Scholar]
- 28.Wu, Y., and T. J. Kipps. 1997. Deoxyribonucleic acid vaccines encoding antigens with rapid proteasome-dependent degradation are highly efficient inducers of cytolytic T lymphocytes. J. Immunol. 159:6037-6043. [PubMed] [Google Scholar]
- 29.Xiang, R., H. N. Lode, T. H. Chao, J. M. Ruehlmann, C. S. Dolman, F. Rodriguez, J. L. Whitton, W. W. Overwijk, N. P. Restifo, and R. A. Reisfeld. 2000. An autologous oral DNA vaccine protects against murine melanoma. Proc. Natl. Acad. Sci. USA 97:5492-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]