Abstract
We have previously established, using human immunodeficiency virus type 1 (HIV-1) strain LAI, that the HIV-1 central DNA Flap acts as a cis determinant of viral genome nuclear import. Although the impact of the DNA Flap on nuclear import has already found numerous independent confirmations in the context of lentivirus vectors, it has been claimed that it may be nonessential for infectious virus strains LAI, YU-2 (J. D. Dvorin et al., J. Virol. 76:12087-12096, 2002), HXB2, and NL4-3 (A. Limon et al., J. Virol. 76:12078-12086, 2002). We conducted a detailed analysis of virus infectivity using the provirus clones provided by the authors and analogous target cells. In contrast to published data, our results show that all cPPT mutant viruses exhibit reduced infectivity corresponding to a nuclear import defect irrespective of the viral genetic background or target cell.
The unique ability of human immunodeficiency virus type 1 (HIV-1) and other lentiviruses to replicate efficiently in nondividing cells implies the use of an active nuclear import strategy allowing the DNA genome to cross the nuclear membrane of an interphasic nucleus so as to gain access to, and integrate into, the cellular chromatin. The mitosis-independent replication of lentiviruses was the key feature for the generation of lentivirus-derived gene transfer vectors with promising therapeutic applications. Their capacity to transduce nonmitotic cells overcame one major limitation of the applicability of oncovirus-derived retrovirus vectors to gene therapy.
The search for the lentivirus determinants of mitosis-independent replication has constituted an active but strongly controversial field of investigation. An early premise, though unfounded, was that replication of a nuclear import-defective virus should be altered specifically in nondividing cells but not in dividing cells, where mitosis was expected to provide an alternative nuclear entry pathway for the HIV-1 preintegration complex (PIC). Based on the search for putative nuclear localization signals (NLS) within HIV-1 proteins that could account for active nuclear import of the PIC, a complex model involving several redundant factors has been drawn.
A first putative NLS was identified within the HIV-1 matrix protein (MA) and proposed as the critical determinant for viral genome nuclear import (8). However, the demonstration that replication was not affected in nondividing cells with identical MA mutants cast doubt on these first findings (14). It was then proposed that nuclear import was achieved by both MA and Vpr in a redundant manner (20) and that mutation of only one of the two determinants had no effect on HIV-1 genome nuclear import. Since myristoylated MA protein is expected to localize at the plasma membrane after fusion of the viral membrane with target cells, it was claimed that phosphorylation of the MA C-terminal tyrosine residue (Y132) acted to release from the plasma membrane a small fraction of the MA protein, which subsequently would interact, by virtue of a novel protein-protein interaction, with a central domain of the HIV-1 integrase (IN), thus allowing the nuclear import of the HIV-1 PIC (16, 17). However, the implication of the MA C-terminal tyrosine in the Vpr/MA model received no further confirmation, and the HIV-1 MA Y132F mutant demonstrated wild-type infection efficiency in nondividing primary macrophages (15). HIV-1 IN was then proposed as a third determinant of HIV-1 genome nuclear import (5, 18), and this time a replication defect was found in both dividing and nondividing cells (5), but there was no consensus about the location of the critical putative NLS. Again, these reports were subsequently reassessed (12, 23, 29).
In parallel, we have previously shown that a three-stranded DNA structure (called the central DNA Flap), present in the exact center of the HIV-1 genome and synthesized as a result of the presence of two central cis-acting sequences (the central polypurine tract [cPPT] and the central termination sequence [CTS]), acts as a cis determinant of HIV-1 nuclear import (35). Mutation of the cPPT sequence within the LAI full-length viral genome prevents central DNA Flap formation (10) and severely disrupts viral infectivity in both dividing and nondividing cells (35). The absence of the central DNA Flap results in a near-10-fold reduction in nuclear import of viral DNA, accounting entirely for the replication defect of cPPT mutant viruses (35).
Accordingly, reinsertion of the cPPT and CTS cis-acting sequences within HIV-1-derived vectors significantly stimulates vector genome nuclear import and gene transfer efficiencies in all tissues and cell types, whether dividing or not, studied in our laboratory (19, 31, 36) and in independent studies (2-4, 6, 10, 11, 13, 25, 26, 28, 30, 32, 33). As a natural consequence, the central DNA Flap is today a common component of almost all lentivirus gene transfer vectors.
It has been suggested that the role of the central DNA Flap, while beneficial in the context of HIV-1-derived vectors, could be nonessential for viral replication in the context of full-length infectious viral genomes (12, 24). In order to verify these data, we conducted a detailed analysis of virus infectivity using the molecular proviral clones provided by the authors and the same target cell types. Here we show that the LAI, YU-2, HXB2 and NL4-3 viruses all have severe defects in infectivity and nuclear import in the absence of the central DNA Flap in all cell types studied, indicating that the role of the central DNA Flap within the context of replicative viruses is not strain or cell type dependent.
One-cycle titrations in P4-CCR5 indicator cells.
Viruses were produced by transient transfection of HeLa cells using calcium phosphate coprecipitation with the proviral plasmid. Wild-type and cPPT mutant proviral plasmids NL4-3 and HXB2 were provided by A. Engelman, YU-2 and LAI by M. H. Malim. The introduction of the cPPT mutations into the LAI backbone has been described previously (35). The same cPPT mutations were introduced into the YU-2 backbone by M. H. Malim (12) and into the NL4-3 and HXB2 backbones by A. Engelman (24). As a control for NL4-3, a wild-type strain provided by M.A. Martin was also included (1).
Virus infectivity was first assessed by titrations based on a single round of replication using our P4-CCR5 indicator cells, which are HeLa CD4+ CXCR4+ CCR5+ cells carrying the LacZ gene under the control of the HIV-1 long terminal repeat (LTR) promoter (9). Infection was carried out in triplicate in 96-well plates with 20, 10, 5, or 2.5 ng of p24 antigen/ml (three times more for HXB2) for 20,000 cells in 100 μl, and β-galactosidase activity was measured 48 h after infection by using a chemiluminescent β-galactosidase reporter gene assay (Roche Diagnostics). We found that cPPT mutants of strains LAI, YU-2, and HXB2 all had severe defects in infectivity relative to the wild-type controls (Fig. 1A, B, and C), as previously published by ourselves for LAI (35) and indeed by Limon et al. for HXB2 (24). In the case of the NL4-3 molecular clone provided by A. Engelman, we found, as did Limon et al. (24), that the wild-type virus exhibited an unaccountable replication defect, which resulted in replication levels consistently inferior to those of the corresponding cPPT mutant, a result of poor virological significance (Fig. 1D). It is very probable that this plasmid has an unexpected defect. Indeed, the use of the original NL4-3 wild-type proviral clone provided by M. A. Martin (1) indicated high infectivity, significantly above that of the corresponding cPPT mutant (Fig. 1D).
FIG. 1.
One-round titration assay of wild-type HIV-1 and cPPT mutants in dividing or nondividing P4-CCR5 indicator cells. (A to D) P4-CCR5 indicator cells were infected in triplicate with 20, 10, 5, or 2.5 ng of p24 antigen/ml (three times more for HXB2) from wild-type or cPPT mutant HIV-1 LAI (A), YU-2 (B), HXB2 (C), or NL4-3 (D). (E) Cell cycle arrest was induced by aphidicolin treatment (8 μM) from the day prior to infection, and nondividing P4-CCR5 cells were infected in triplicate with 20 ng of p24 antigen/ml from wild-type or cPPT mutant HIV-1 LAI, YU-2, HXB2, or NL4-3. β-Galactosidase activity was measured 48 h postinfection and was expressed as mean relative light units (RLU)/s (A to D) or RLU/s/ng of p24 (E) ± standard deviation. Data shown are representative of three independent experiments.
As previously shown for viral strain LAI (35), all cPPT mutants also demonstrated reduced infectivity in aphidicolin-treated nondividing P4 cells (Fig. 1E), indicating that central DNA Flap-defective viruses have a defect in infectivity in both dividing and nondividing cells, irrespective of the viral strain studied.
Viral replication kinetics in MT4-CCR5 cells and PBLs.
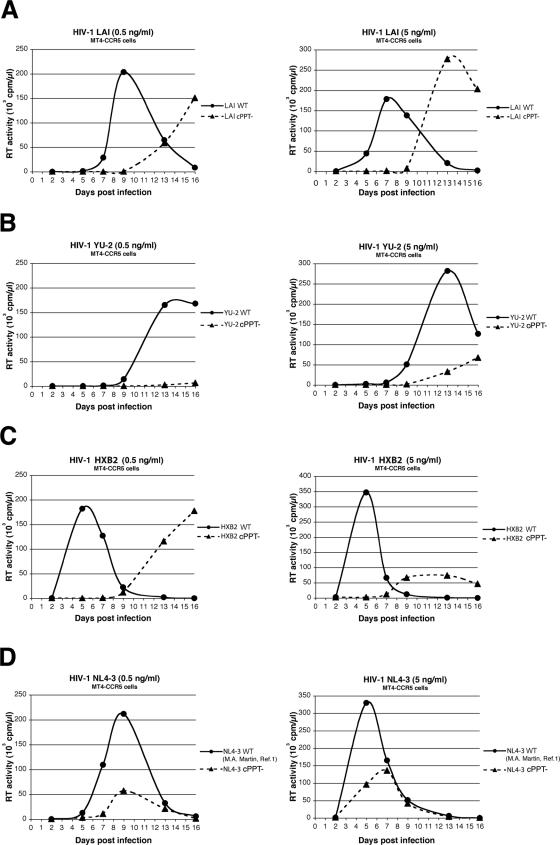
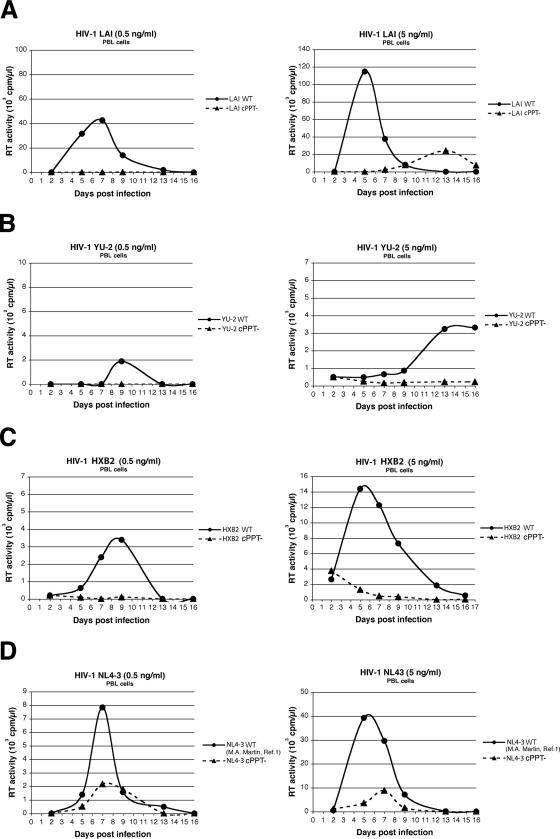
The infectivity of LAI, YU-2, HXB2, and NL4-3 was also evaluated in kinetic replication experiments in cell cultures as in the work of Dvorin et al. and Limon et al. (12, 24). MT4-CCR5 cells are human T-lymphotropic virus type 1-transformed human CD4+ T cells that stably express CCR5 and allow acute cytopathic HIV-1 infection. Peripheral blood lymphocytes (PBLs) were obtained from healthy donors, stimulated with phytohemagglutinin (5 μg/ml; Sigma), and maintained in the presence of interleukin-2 (20 U/ml; Sigma). MT4-CCR5 cells and PBLs were infected with 0.5 or 5 ng of p24 antigen/ml for 50,000 cells in 200 μl. Culture supernatants were collected at regular time points over 16 days and tested for reverse transcriptase activity. Results showed that while wild-type viruses of all four strains were able to establish productive infections, all cPPT mutant viruses exhibited a strong replication defect at both high and low multiplicities of infection in MT4-CCR5 cells, with a significant delay in peak replication compared with wild-type viruses and/or decreased viral production (Fig. 2). This viral-replication-defective phenotype was even starker for PBLs, where LAI, YU-2, and HXB2 cPPT mutants exhibited an almost complete loss of infectivity (Fig. 3).
FIG. 2.
Replication kinetics of wild-type HIV-1 and cPPT mutants in MT4-CCR5 lymphocytes. MT4-CCR5 cells were infected with 0.5 or 5 ng of p24 antigen/ml (left and right panels, respectively) from wild-type or cPPT mutant HIV-1 LAI (A), YU-2 (B), HXB2 (C), or NL4-3 (D). Reverse transcriptase (RT) activity was measured on culture supernatants collected at regular time points over 16 days postinfection and is expressed in counts per minute per microliter. Results are representative of two independent experiments.
FIG. 3.
Replication kinetics of wild-type HIV-1 and cPPT mutants in PBLs. PBLs were infected with 0.5 or 5 ng of p24 antigen/ml (left and right panels, respectively) from wild-type or cPPT mutant HIV-1 LAI (A), YU-2 (B), HXB2 (C), or NL4-3 (D). Reverse transcriptase (RT) activity was measured on culture supernatants collected at regular time points over 16 days postinfection and is expressed in counts per minute per microliter. Results are representative of two independent experiments.
Assessment of HIV-1 nuclear import by quantitative PCR on unintegrated 2-LTR circles.
We previously showed that the central DNA Flap is a cis-acting determinant of nuclear import in the context of the viral strain LAI (35). Having here determined that all four cPPT mutant viruses examined exhibit an important replication defect in three distinct cell types, we undertook to ascertain whether they also demonstrate a nuclear import defect. For this purpose, we used quantitative PCR amplification of unintegrated 2-LTR circles as an indicator of HIV-1 genome nuclear import.
MT4-CCR5 cells were infected with equal amounts of particles for wild-type and cPPT mutant viruses of each strain. Infection was carried out with 1,000 ng of p24 antigen/ml for strains LAI and HXB2, 300 ng/ml for NL4-3, and 100 ng/ml for YU-2, for 20 × 106 cells in 2 ml (LAI and HXB2) or 6 ml (NL4-3 and YU-2). Two hours after infection, cells were diluted in fresh medium to a final concentration of 1 × 106 cells/ml. To limit analysis to a single cycle of replication, the protease inhibitor saquinavir was added to a final concentration of 1 μM. For each viral strain, a control of infected cells cultured in the presence of 5 μM nevirapine, a nonnucleoside reverse transcriptase inhibitor, was included. Fixed-volume samples were collected at given time points postinfection (0, 6, 12, 24, and 48 h). In order to eliminate residual proviral plasmid from transfection, cell pellets were treated with 1,000 U of DNase I (Invitrogen) for 1 h at room temperature (7).
Total-cell DNA was extracted, and the copy numbers of 2-LTR circles and of total HIV-1 DNA (pol gene) were determined by real-time PCR using a LightCycler instrument (Roche Diagnostics) with the primers and probe sequences, as well as the PCR cycle conditions, previously described for 2-LTR junctions (7) and pol (27). Briefly, 1/50 (pol) or 1/10 (2-LTR) of total-cell DNA was used for real-time PCR carried out in triplicate for each reaction in a 20-μl mixture containing 1× LightCycler FastStart DNA master hybridization probes (Roche Diagnostics), 4 mM MgCl2, 300 nM each primer, and 200 nM each probe. Results were normalized to cell number based on the amplification of the CD3 gene as previously described (21).
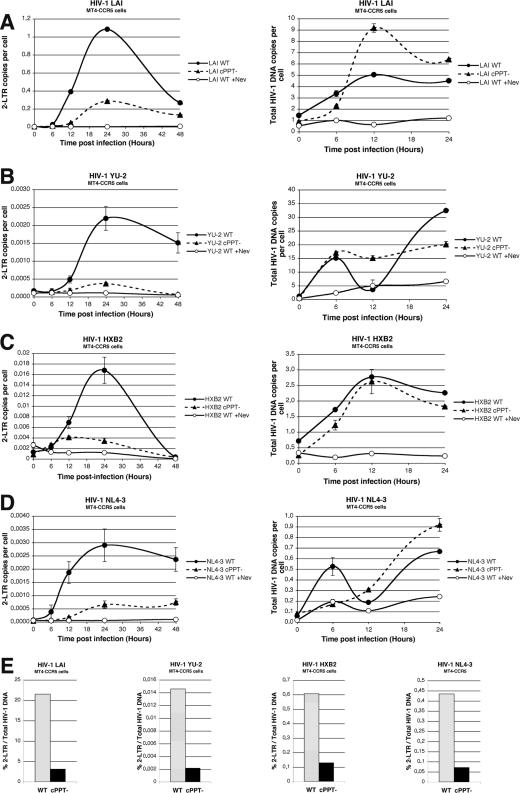
The detection of total HIV-1 DNA revealed a peak in total DNA synthesis at 6 to 12 h postinfection (Fig. 4A to D, right panels). Amplification of the 2-LTR junction indicated a peak in copy number at 24 h postinfection for all wild-type viruses (Fig. 4A to D, left panels). In the case of cPPT mutant viruses, however, only very small numbers of 2-LTR copies per cell were detected at any time point postinfection, irrespective of the viral strain, thus indicating a severe defect in the nuclear import of HIV-1 in the absence of the central DNA Flap. The 2-LTR/pol copy number ratios (Fig. 4E), calculated by dividing the peak of 2-LTR copy number (24 h postinfection) by the peak of total viral DNA (6 or 12 h postinfection), revealed 6.9-, 6.8-, 4.7-, and 6.1-fold decreases in 2-LTR formation for cPPT mutants of LAI, YU-2, HXB2, and NL4-3, respectively, compared to their wild-type counterparts. Taken together, these results demonstrate that the defect in viral replication exhibited by cPPT mutant LAI, YU-2, HXB2, and NL4-3 viruses is accounted for by a strong defect in nuclear import, as previously shown for LAI (35). The divergent results obtained previously (12, 24) cannot be explained by variations in experimental conditions, since we used the same viral molecular clones and the same cells (MT4, PBMC) as in these studies. We note that both reports (12, 24), although claiming a wild-type phenotype for all cPPT mutants, do paradoxically show defects in viral replication and nuclear import in the cases of HXB2, NL4-3 (24), and YU-2 (12), indicating that the conclusions drawn by these two reports do not match the primary data shown.
FIG. 4.
Quantitative PCR analysis of 2-LTR circle formation and total HIV-1 DNA copy number following infection of MT4-CCR5 cells with wild-type HIV-1 or cPPT mutants. (A to D) MT4-CCR5 cells were infected with wild-type or cPPT mutant LAI (A), YU-2 (B), HXB2 (C), or NL4-3 (D), and total DNA was extracted at 0, 6, 12, 24, and 48 h postinfection. Quantification of 2-LTR circles (A to D, left panels) and total HIV-1 DNA (A to D, right panels) was performed by real-time PCR at each time point. Cells infected with wild-type viruses in the presence of nevirapine (+Nev) served as controls. Results are means of triplicate (2-LTR) and duplicate (pol) determinations ± standard deviations. (E) Graphs show the ratio of the copy number of 2-LTR circles to that of total HIV-1 DNA for each virus. The ratio was calculated by dividing the peak of the 2-LTR copy number by the peak of total viral DNA.
Our results show an important replication defect for LAI, YU-2, HXB2, and NL4-3 cPPT mutants in three different cell types, in both one-cycle titration assays and spreading infection in cell cultures, indicating that the nuclear import defect of HIV-1 DNA Flap mutants is not dependent on the viral strain or the target cell type. Of note, and as previously reported (35), mutations in the central DNA Flap do not lead to totally noninfectious viruses. Low infectivity is probably accounted for by a Flap-independent nuclear import mechanism that must be further investigated.
Most reports on HIV-1 nuclear import involving MA/Vpr or, more recently, CA (34) are based on the hypothesis that a nuclear import defect would manifest itself exclusively in nondividing cells, with a wild-type phenotype in dividing cells. However, experimental data directly addressing the notion that mitosis would provide an alternative pathway for lentivirus genome nuclear import are still lacking. Other reports indicate that an HIV-1 nuclear import defect phenotype can be seen in both dividing and nondividing cells (2, 5, 35) and that HIV-1 can undergo mitosis-independent nuclear import in cycling cells (22). Thus, a central question remaining to be addressed relates to the interplay between lentivirus genome nuclear import and the cell cycle.
Given the presence of DNA Flap sequences in all lentiviruses sequenced, we propose that the central DNA Flap-mediated nuclear import mechanism, like the capacity to replicate in nondividing cells, is a common feature of all lentiviruses.
Acknowledgments
This work was supported by grants from the Institut Pasteur, the Agence Nationale de Recherche sur le SIDA (ANRS), the Agence Nationale de la Recherche (ANR), and the Fondation pour la Recherche Médicale (FRM).
Wild-type and cPPT mutant provirus clones were provided by M. H. Malim (YU-2 and LAI) and A. Engelman (NL4-3 and HXB2).
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ao, Z., X. Yao, and E. A. Cohen. 2004. Assessment of the role of the central DNA flap in human immunodeficiency virus type 1 replication by using a single-cycle replication system. J. Virol. 78:3170-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baekelandt, V., A. Claeys, K. Eggermont, E. Lauwers, B. De Strooper, B. Nuttin, and Z. Debyser. 2002. Characterization of lentiviral vector-mediated gene transfer in adult mouse brain. Hum. Gene Ther. 13:841-853. [DOI] [PubMed] [Google Scholar]
- 4.Barry, S. C., B. Harder, M. Brzezinski, L. Y. Flint, J. Seppen, and W. R. Osborne. 2001. Lentivirus vectors encoding both central polypurine tract and posttranscriptional regulatory element provide enhanced transduction and transgene expression. Hum. Gene Ther. 12:1103-1108. [DOI] [PubMed] [Google Scholar]
- 5.Bouyac-Bertoia, M., J. D. Dvorin, R. A. Fouchier, Y. Jenkins, B. E. Meyer, L. I. Wu, M. Emerman, and M. H. Malim. 2001. HIV-1 infection requires a functional integrase NLS. Mol. Cell 7:1025-1035. [DOI] [PubMed] [Google Scholar]
- 6.Breckpot, K., M. Dullaers, A. Bonehill, S. van Meirvenne, C. Heirman, C. de Greef, P. van der Bruggen, and K. Thielemans. 2003. Lentivirally transduced dendritic cells as a tool for cancer immunotherapy. J. Gene Med. 5:654-667. [DOI] [PubMed] [Google Scholar]
- 7.Brussel, A., and P. Sonigo. 2004. Evidence for gene expression by unintegrated human immunodeficiency virus type1 DNA species. J. Virol. 78:11263-11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukrinsky, M. I., S. Haggerty, M. P. Dempsey, N. Sharova, A. Adzhubel, L. Spitz, P. Lewis, D. Goldfarb, M. Emerman, and M. Stevenson. 1993. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature 365:666-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charneau, P., G. Mirambeau, P. Roux, S. Paulous, H. Buc, and F. Clavel. 1994. HIV-1 reverse transcription. A termination step at the center of the genome. J. Mol. Biol. 241:651-662. [DOI] [PubMed] [Google Scholar]
- 10.Dardalhon, V., B. Herpers, N. Noraz, F. Pflumio, D. Guetard, C. Leveau, A. Dubart-Kupperschmitt, P. Charneau, and N. Taylor. 2001. Lentivirus-mediated gene transfer in primary T cells is enhanced by a central DNA flap. Gene Ther. 8:190-198. [DOI] [PubMed] [Google Scholar]
- 11.Demaison, C., K. Parsley, G. Brouns, M. Scherr, K. Battmer, C. Kinnon, M. Grez, and A. J. Thrasher. 2002. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum. Gene Ther. 13:803-813. [DOI] [PubMed] [Google Scholar]
- 12.Dvorin, J. D., P. Bell, G. G. Maul, M. Yamashita, M. Emerman, and M. H. Malim. 2002. Reassessment of the roles of integrase and the central DNA flap in human immunodeficiency virus type 1 nuclear import. J. Virol. 76:12087-12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Follenzi, A., L. E. Ailles, S. Bakovic, M. Geuna, and L. Naldini. 2000. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat. Genet. 25:217-222. [DOI] [PubMed] [Google Scholar]
- 14.Freed, E. O., and M. A. Martin. 1994. HIV-1 infection of non-dividing cells. Nature 369:107-108. [DOI] [PubMed] [Google Scholar]
- 15.Freed, E. O., G. Englund, F. Maldarelli, and M. A. Martin. 1997. Phosphorylation of residue 131 of HIV-1 matrix is not required for macrophage infection. Cell 88:171-173. [DOI] [PubMed] [Google Scholar]
- 16.Gallay, P., S. Swingler, C. Aiken, and D. Trono. 1995. HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is a key regulator. Cell 80:379-388. [DOI] [PubMed] [Google Scholar]
- 17.Gallay, P., S. Swingler, J. Song, F. Bushman, and D. Trono. 1995. HIV nuclear import is governed by the phosphotyrosine-mediated binding of matrix to the core domain of integrase. Cell 83:569-576. [DOI] [PubMed] [Google Scholar]
- 18.Gallay, P., T. Hope, D. Chin, and D. Trono. 1997. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc. Natl. Acad. Sci. USA 94:9825-9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giannini, C., S. Morosan, J. G. Tralhao, J. E. Guidotti, S. Battaglia, K. Mollier, L. Hannoun, D. Kremsdorf, H. Gilgenkrantz, and P. Charneau. 2003. A highly efficient, stable, and rapid approach for ex vivo human liver gene therapy via a FLAP lentiviral vector. Hepatology 38:114-122. [DOI] [PubMed] [Google Scholar]
- 20.Heinzinger, N. K., M. I. Bukrinsky, S. A. Haggerty, A. M. Ragland, V. Kewalramani, M. A. Lee, H. E. Gendelman, L. Ratner, M. Stevenson, and M. Emerman. 1994. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. USA 91:7311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iglesias, M. C., M. P. Frenkiel, K. Mollier, P. Souque, P. Despres, and P. Charneau. 2006. A single immunization with a minute dose of a lentiviral vector-based vaccine is highly effective at eliciting protective humoral immunity against West Nile virus. J. Gene Med. 8:265-274. [DOI] [PubMed] [Google Scholar]
- 22.Katz, A. R., J. G. Greger, P. Boimel, and A. M. Shalka. 2003. Human immunodeficiency virus type 1 DNA nuclear import and integration are mitosis independent in cycling cells. J. Virol. 77:13412-13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Limon, A., E. Devroe, R. Lu, H. Z. Ghory, P. A. Silver, and A. Engelman. 2002. Nuclear localization of human immunodeficiency virus type 1 preintegration complexes (PICs): V165A and R166A are pleiotropic integrase mutants primarily defective for integration, not PIC nuclear import. J. Virol. 76:10598-10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Limon, A., N. Nakajima, R. Lu, H. Z. Ghory, and A. Engelman. 2002. Wild-type levels of nuclear localization and human immunodeficiency virus type 1 replication in the absence of the central DNA flap. J. Virol. 76:12078-12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manganini, M., M. Serafini, F. Bambacioni, C. Casati, E. Erba, A. Follenzi, L. Naldini, S. Bernasconi, G. Gaipa, A. Rambaldi, A. Biondi, J. Golay, and M. Introna. 2002. A human immunodeficiency virus type 1 pol gene-derived sequence (cPPT/CTS) increases the efficiency of transduction of human nondividing monocytes and T lymphocytes by lentiviral vectors. Hum. Gene Ther. 13:1793-1807. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen, T. H., J. Oberholzer, J. Birraux, P. Majno, P. Morel, and D. Trono. 2002. Highly efficient lentiviral vector-mediated transduction of nondividing, fully reimplantable primary hepatocytes. Mol. Ther. 6:199-209. [DOI] [PubMed] [Google Scholar]
- 27.Nobile, C., C. Petit, A. Moris, K. Skrabal, J. P. Abastado, F. Mammano, and O. Schwartz. 2005. Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN-expressing cells promotes long-term transmission to lymphocytes. J. Virol. 79:5386-5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park, F., and M. A. Kay. 2001. Modified HIV-1 based lentiviral vectors have an effect on viral transduction efficiency and gene expression in vitro and in vivo. Mol. Ther. 4:164-173. [DOI] [PubMed] [Google Scholar]
- 29.Petit, C., O. Schwartz, and F. Mammano. 2000. The karyophilic properties of human immunodeficiency virus type 1 integrase are not required for nuclear import of proviral DNA. J. Virol. 74:7119-7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seppen, J., M. Rijnberg, M. P. Cooreman, and R. P. Oude Elferink. 2002. Lentiviral vectors for efficient transduction of isolated primary quiescent hepatocytes. J. Hepatol. 36:459-465. [DOI] [PubMed] [Google Scholar]
- 31.Sirven, A., F. Pflumio, V. Zennou, M. Titeux, W. Vainchenker, L. Coulombel, A. Dubart-Kupperschmitt, and P. Charneau. 2000. The human immunodeficiency virus type-1 central DNA flap is a crucial determinant for lentiviral vector nuclear import and gene transduction of human hematopoietic stem cells. Blood 96:4103-4110. [PubMed] [Google Scholar]
- 32.Van Maele, B., J. De Rijck, E. De Clercq, and Z. Debyser. 2003. Impact of the central polypurine tract on the kinetics of human immunodeficiency virus type 1 vector transduction. J. Virol. 77:4685-4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitwam, T., M. Peretz, and E. Poeschla. 2001. Identification of a central DNA flap in feline immunodeficiency virus. J. Virol. 75:9407-9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamashita, M., and M. Emerman. 2004. Capsid is a dominant determinant of retrovirus infectivity in nondividing cells. J. Virol. 78:5670-5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zennou, V., C. Petit, D. Guetard, U. Nerhbass, L. Montagnier, and P. Charneau. 2000. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell 101:173-185. [DOI] [PubMed] [Google Scholar]
- 36.Zennou, V., C. Serguera, C. Sarkis, P. Colin, E. Perret, J. Mallet, and P. Charneau. 2001. The HIV-1 DNA flap stimulates HIV vector-mediated cell transduction in the brain. Nat. Biotechnol. 19:446-450. [DOI] [PubMed] [Google Scholar]