Abstract
We recently found that human immunodeficiency virus (HIV)-specific CD4+ T cells express coreceptor CCR5 and activation antigen CD38 during early primary HIV-1 infection (PHI) but then rapidly disappear from the circulation. This cell loss may be due to susceptibility to infection with HIV-1 but could also be due to inappropriate apoptosis, an expansion of T regulatory cells, trafficking out of the circulation, or dysfunction. We purified CD38+++CD4+ T cells from peripheral blood mononuclear cells, measured their level of HIV-1 DNA by PCR, and found that about 10% of this population was infected. However, a small subset of HIV-specific CD4+ T cells also expressed CD127, a marker of long-term memory cells. Purified CD127+CD4+ lymphocytes contained fivefold more copies of HIV-1 DNA per cell than did CD127-negative CD4+ cells, suggesting preferential infection of long-term memory cells. We observed no apoptosis of antigen-specific CD4+ T cells in vitro and only a small increase in CD45RO+CD25+CD127dimCD4+ T regulatory cells during PHI. However, 40% of CCR5+CD38+++ CD4+ T cells expressed gut-homing integrins, suggesting trafficking through gut-associated lymphoid tissue (GALT). Furthermore, 80% of HIV-specific CD4+ T cells expressed high levels of the negative regulator CTLA-4 in response to antigen stimulation in vitro, which was probably contributing to their inability to produce interleukin-2 and proliferate. Taken together, the loss of HIV-specific CD4+ T cells is associated with a combination of an infection of CCR5+ CD127+ memory CD4+ T cells, possibly in GALT, and a high expression of the inhibitory receptor CTLA-4.
Preferential infection and loss of human immunodeficiency virus (HIV)-specific CD4+ T cells have been proposed as significant factors leading to the dysfunction of the immune response to HIV-1 infection (15, 31, 47). We recently found that HIV-specific CD4+ T cells from a long-term nonprogressor with unusually low viral replication expressed cell surface CCR5 (91), consistent with another report describing CCR5+ HIV-specific CD4+ T cells in subjects with chronic HIV-1 infection (90). We also observed that, very early in primary HIV-1 infection (PHI), HIV-specific CD4+ T cells expressed cell surface CCR5, together with a high expression of the activation antigen CD38 and the cell cycle marker Ki-67 but greatly reduced expression of CD127 (interleukin-7 receptor [IL-7R]) (94), a marker of long-term memory cells (63). During PHI and in most asymptomatic HIV-positive (HIV+) subjects, coreceptor usage by HIV-1 is largely directed towards CCR5 (10, 98), suggesting that CCR5+ HIV-specific CD4+ T cells will be targeted by the virus during the early stage of the infection. In vitro studies have shown that CD4+ T cells with activated memory phenotypes are preferentially susceptible to infection by HIV-1 (70, 72), while in vivo studies have shown that Ki-67+ CD4+ T cells are productively infected during PHI (96).
Therefore, we hypothesized that activated, proliferating HIV-specific CD4+ T cells coexpressing CCR5 and high levels of CD38 would be prime targets for HIV-1 infection in vivo during PHI. In our previous cross-sectional study, these cells appeared only transiently, exhibiting a rapid decline approximately 2 to 3 weeks following the onset of symptoms of the acute viral illness (94), consistent with cytopathic infection in vivo.
Dramatic losses of CCR5+ CD4+ T cells as a result of cytopathic infection, particularly in the gut, have been reported in primary simian immunodeficiency virus (SIV) infection (40, 45, 82), and a similar loss of gut CD4+ T cells occurs in primary HIV infection (7, 22, 48). If cytopathic infection of CCR5+ CD4+ T cells is particularly localized to gut-associated lymphoid tissue (GALT), then the trafficking of CCR5+ HIV-specific CD4+ T cells to GALT might also contribute to the rapid loss of these cells. The homing of memory CD4+ T cells to GALT is determined by the coexpression of the integrins α4 (CD49d) and β7, which specify binding to the mucosal vascular addressin MAdCAM-1 (42, 59, 87). A previous study of PHI reported a selective loss of CCR5+α4β7+ CD62L-negativeCD45RO+ CD4+ T cells from the circulation (34), suggesting that the expression of gut-homing integrins α4 (CD49d) and β7 on CCR5+ HIV-specific CD4+ T cells may determine their fates.
However, their transient appearance could be also be due to normal homeostatic processes, such as apoptosis or feedback regulation, since a similar peak of CD4+ T-cell responses has also been observed in other acute viral infections in both mice (80) and humans (3, 18, 55). HIV-specific CD4+ T cells during PHI were found to contain low levels of Bcl-2 (94), which has previously been associated with a propensity to undergo apoptosis in vitro and in vivo (74). Precursors of long-term memory CD4+ and CD8+ T cells in murine models selectively express IL-7Rα (CD127), which may play a role in sparing these cells from apoptosis by mediating the signaling leading to the reexpression of Bcl-2 (20, 30, 39, 63). However, 80 to 90% of HIV-specific CD4+ T cells lack CD127 during PHI (94), consistent with a predetermined apoptotic fate for most CCR5+CD38+++ CD4+ T cells.
Apart from apoptosis, the down-regulation of a CD4 T-cell response is also believed to be mediated by feedback inhibition exerted by CD25+ CD4+ T regulatory (T reg) cells, which have been shown to be important in the control of CD4-mediated inflammatory diseases (60). CD25+ T reg cells may also express CTLA-4, an important negative signaling molecule (56, 61, 76). The suppressive influence of CTLA-4 signaling has been inferred from the early fatal CD4+ lymphoproliferation observed in CTLA-4 gene knockout mice (78, 85). We previously observed that, compared to the expression of CTLA-4 by T cells from HIV-negative controls, intracellular CTLA-4 expression was greatly increased during PHI, especially in CD8+ T cells, when maximally stimulated with phorbol myristate acetate and ionomycin (J. Zaunders et al., unpublished results).
Therefore, in the current study we aimed to determine the extent to which all of these various factors may exert a negative influence on CCR5+CD38+++ HIV-specific CD4+ T cells during PHI. We measured their rate of infection with HIV-1 DNA, their rate of spontaneous apoptosis, as well as their expression of the gut-homing integrins α4 and β7, and also the possible generation of CTLA-4+ T regulatory cells during PHI. The results suggest that none of these factors alone leads to the loss of HIV-specific CD4+ T cells during the resolution of PHI but instead point to a complex multifactorial process that results in an impaired response.
MATERIALS AND METHODS
Subjects.
A total of 29 subjects, diagnosed with primary HIV-1 infection as previously described (92), were included in this study and then enrolled in the PHAEDRA/CORE 01 observational cohort. All subjects were males whose risk factor was sex with males. The median age was 34 years, the median CD4 count was 583 cells/μl, the median plasma HIV RNA was 111,450, the median number of days since the onset of symptoms was 21 (seven subjects were asymptomatic), and the median number of bands on HIV-1 Western blots was 1.
Six HIV+ subjects with established infections but undetectable plasma HIV RNA viral loads (<50 copies/ml) and without antiretroviral therapy at the time of study were also included and are referred to as “HIV+ controllers.”
Healthy HIV-negative university and hospital staff members were recruited as controls for this study. The PHAEDRA/CORE 01 study was approved by the St. Vincent's Hospital Ethics Committee, and all subjects gave written informed consent.
T-lymphocyte phenotyping of fresh whole blood.
The monoclonal antibodies used were CD3-PerCP-Cy5.5 and -Pacific Blue; CD4-phycoerythrin (PE)-Cy7, -Alexa Fluor 700, and -allophycocyanin (APC); CD8-Alexa Fluor 700 and -APC-Cy7; CD38-PE-Cy7, -APC, and -PE; CCR5-fluorescein isothiocyanate (FITC) gamma interferon (IFN-γ)-APC; CD45RO-FITC; CD25-APC; CD49d-PE; integrin β7-APC; activated caspase-3-PE; CTLA-4-PE; CD19-PE-Cy7; CD56-APC; CD16-APC-Cy7; HLA-DR-FITC; and CD123-PE (Becton Dickinson, San Jose, CA); CD4-energy-coupled dye (ECD), CD45RO-ECD, and IL-7R (CD127)-PE (Beckman Coulter, Hialeah, FL); and CD62L-APC-Cy7 (eBioscience, San Diego, CA). All antibodies were used according to the manufacturers' directions.
The staining of CD4+ T-cell subsets in fresh peripheral blood was performed as previously described (91, 94) on whole blood within 1 h of venepuncture to minimize spontaneous loss of CCR5 expression. T regulatory CD4+ T-cell subsets were analyzed by five-color flow cytometry on an LSR II flow cytometer (Becton Dickinson) using CD3-PerCP-Cy5.5, CD4-PE-Cy7, CD25-APC, CD127-PE, and CD45RO-FITC as described elsewhere (65a). Foxp3 expression was studied in CD3-PerCP-Cy5.5+CD4-PE-Cy7+CD25-FITC+CD127-PElow cells using Foxp3-APC (eBioscience) according to the manufacturer's directions.
The immunophenotyping of gut-homing CD4+ T cells was analyzed by nine-color flow cytometry on the three-laser LSR II by staining with CD3-Pacific Blue, CD4-Alexa Fluor 700, CD8-APC-Cy7, CD45RO-ECD, CD38-PE-Cy7, HLA-DR-PerCP, CCR5-FITC, integrin β7-APC, and CD49d-PE. For analysis, a minimum of 100,000 events were collected. Spectral compensations were set using cells stained individually with the different fluorochrome conjugates and validated by staining peripheral blood mononuclear cells (PBMCs) with combinations of CD3-Pacific Blue, CD4-ECD, CD8-Alexa Fluor 700, CD19-PE-Cy7, CD56-APC, CD16-APC-Cy7, HLA-DR-PerCP, CD123-PE, and CD14-FITC monoclonal antibodies and obtaining expected patterns (data not shown).
Purification of CD4+ T-cell subsets by cell sorting of PBMCs.
Cryopreserved PBMCs from three PHAEDRA/CORE 01 subjects and a further three subjects, who had been included in a previous study (68), at presentation of PHI were used in cell sorting experiments. These PBMCs were obtained a median of 8 days after the onset of symptoms. Cryopreserved PBMCs (12 × 106 to 17 × 106) obtained from three subjects were thawed and stained with CD4-APC and CD38-PE. Cells were washed once with phosphate-buffered saline, fixed with 3% paraformaldehyde in phosphate-buffered saline for 30 min on ice, and then sorted using a FACSVantage by running FACSDiva software (version 4.1; Becton Dickinson). Four populations were obtained from PBMCs: CD38+++CD4+ and CD38dimCD4+ (Fig. 1A) as well as CD4-ve lymphocytes and monocytes defined as CD4dim and high side scatter cells. The purity of the cell subpopulations was >98% in all cases.
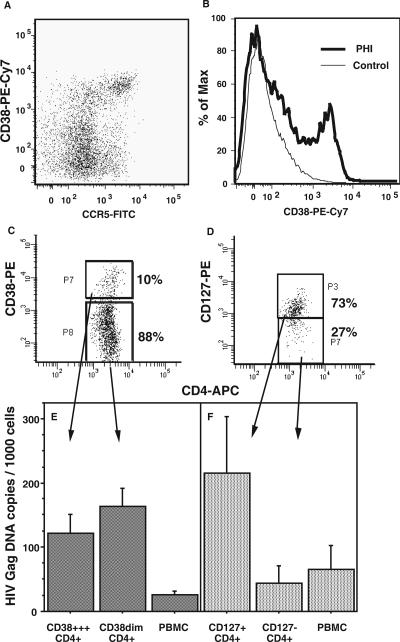
FIG. 1.
HIV DNA in CD38+++ and CD127+ subpopulations of CD4+ cells from PBMCs during PHI. A representative dot plot of CCR5+CD38+++ CD4+ T cells present in fresh whole blood during PHI is shown in panel A, and a representative comparison of CD38 expression on CD4+ T cells between a subject during PHI and a healthy adult control subject is shown in panel B. PBMCs were stained with CD4-APC and CD38-PE (C) or CD4-APC and CD127-PE (D) and sorted into different subpopulations. Representative histograms for each of the two sorting protocols are shown. DNA was extracted from purified cells, and the number of copies of HIV DNA per cell in each subpopulation was determined by quantitative PCR and normalized to beta-actin. Each column shows the mean (and standard error) from three independent experiments for each subpopulation of CD4+ cells defined by CD38 (E) or CD127 (F) as well as for the corresponding unsorted PBMC samples.
In further cell sorting experiments, cryopreserved PBMCs (15 × 106 to 23 × 106) from another three subjects with PHI were stained with CD4-APC and CD127-PE (Fig. 1B) and sorted into CD127+ CD4+ and CD127− CD4+ subpopulations as described above.
HIV-1 DNA quantification.
HIV-1 DNA was quantified by a real-time PCR assay specific for HIV-gag using the Rotor-Gene 3000 (Corbett Research, Sydney, Australia). HIV-1 DNA was compared with genomic DNA, determined by beta-actin detection as previously described (75). Both real-time assays used sequence-specific fluorogenic TaqMan probes. Standard curves were constructed by using pNL4-3 and purified human DNA (Sigma). The primers and probes used were the HIV-gag sense primer 5′-AGTGGGGGGACATCAAGCAGCCATGCAAAT-3′, antisense primer 5′-TACTAGTAGTTCCTGCTATGTCACTTCC-3′, detection probe 5′-6-carboxyfluorescein-ATCAATGAGGAAGCTGCAGAATGGGATAG-6-carboxytetramethylrhodamine-3′, beta-actin sense primer 5′-TCACCCACACTGTGCCCATCTACGA-3′, beta-actin antisense primer 5′-CAGCGGAACCGCTCATTGCCAATGG-3′, and detection probe 5′-6-carboxyfluorescein-ATGCCCTCCCCCATGCCATCCTGCG-6-carboxytetramethylrhodamine-3′. DNA extraction from sorted cells was performed using the High Pure viral nucleic acid reagents (Roche, Castle Hill, Australia) with an extended 16-h protease K digestion incubation at 56°C. To estimate the number of copies of HIV DNA per cell, it was assumed that the yield of DNA extracted was 1 ng per 150 cells (12).
Intracellular cytokine assay.
HIV Gag-specific CD4+ T cells were identified by using a 6-h whole-blood intracellular cytokine assay with six-color flow cytometry, as previously described (91, 94). Overlapping HIV-1 Gag 15-mer peptides, obtained from the NIH AIDS reference reagents program (catalog no. 11057), were used as a pool of 122 peptides at an individual concentration of 2 μg/ml each. Cytomegalovirus (CMV)-specific CD4+ T cells were identified as previously described (91, 94). For analysis, 300,000 events were collected. T lymphocytes were first gated on CD3-PerCP-Cy5.5 versus side scatter and then on CD4-PE-Cy7-positive/CD8-APC-Cy7-negative cells. Finally, IFN-γ-APC+ cells were analyzed for staining with either CTLA-4-PE or activated caspase-3-PE.
Statistical analyses.
All statistical tests were performed using StatView 5.0 for Macintosh (Abacus Concepts, Berkeley, CA). Results for replicate experiments are shown as means and standard errors. Results for each subject group are shown as medians and interquartile ranges. The Mann-Whitney U test was performed to compare continuous variables between subject groups. A two-sided P value of <0.05 was considered statistically significant.
RESULTS
HIV DNA in purified CD4+ T-cell subpopulations.
The aim of these experiments was to determine the level of infection of CCR5+CD38+++ CD4+ T cells during PHI (Fig. 1A) (94). However, CCR5 expression may be lost during PBMC isolation and cryopreservation (91). Therefore, elevated CD38 expression on CD4+ T cells during PHI (Fig. 1B), which survives cryopreservation and which on its own is a marker of antigen-specific CD4+ T cells from subjects with PHI (94), was used as a surrogate marker for the CCR5+CD38+++ HIV-specific CD4+ T cells in PBMCs. Purified CD38+++ CD4+ T cells and CD38dim subsets of CD4+ lymphocytes were obtained by cell sorting cryopreserved PBMC samples from three individuals with primary HIV-1 infection (Fig. 1C). The CD38+++ CD4+ T-cell subpopulation was exclusively CD45RO+ memory phenotype cells, while the CD38dim CD4+ T-cell subpopulation included both CD38-intermediate CD45RO-negative naïve phenotype cells and CD38-negative CD45RO+ resting memory CD4+ T cells (data not shown). We also purified CD4-negative lymphocytes and monocytes by cell sorting (data not shown).
DNA was extracted from the different subpopulations, and real-time PCR was used to measure copy numbers of HIV-1 DNA normalized to beta-actin (Fig. 1E). Very few copies (≤1 per 1,000 cells) of HIV-1 DNA were detected in CD4-negative lymphocyte or monocyte subpopulations (data not shown). However, both CD38+++ CD4+ T cells and CD38dim CD4+ T cells contained comparable levels of HIV-1 DNA on a per cell basis, and both purified subpopulations were enriched for HIV-1 DNA relative to the starting PBMCs (Fig. 1E).
The number of HIV-1 DNA copies per cell indicate that if all infected cells contained only one copy of HIV-1 DNA each, then 10 to 15% of CD4+ T cells were infected. In a recent study of acute SIV infection, it was shown by single-cell analysis that infected cells contained an average of 1.5 copies per infected cell (45), consistent with previous studies of chronic HIV-1 infection (19, 29). Therefore, there is probably an upper limit of about 10% infected cells in both the activated CD38+++ and nonactivated CD38dim CD4+ T-cell subpopulations.
The results show that there was no preferential infection of the activated CD38+++ CD4+ T cells, despite our previous observation that they were predominantly CCR5+ in fresh whole blood. Instead, since a large majority of CD4+ T cells were CD38dim (Fig. 1A), then the majority of the copies of HIV-1 DNA in PBMCs were in the nonactivated CD38dim subpopulation.
Similarly, low CD127 expression was also used to enrich HIV-specific CD4+ T cells (94) in order to measure infection with HIV-1 DNA. Unexpectedly, purified CD127+ CD4+ T cells (Fig. 1D) were found to contain a disproportionately higher number of copies of HIV-1 DNA, fivefold more on a per cell basis than the more-activated CD127-negative CD4+ T cells (Fig. 1F). Similar to the CD38dim CD4+ T cells, the CD127+ subset makes up the greater part of CD4+ T cells within PBMCs, and therefore the vast majority of copies of HIV-1 DNA were in the CD127+ subset of CD4+ T cells. Conversely, during PHI, 80 to 90% of HIV-specific CD4+ T cells are CD127 negative (94), again suggesting that the majority of HIV-specific CD4+ T cells are not directly infected in vivo.
Combining the results of the two sets of sorting experiments suggests that the CD38+++ CD4+ T cells that are infected will be the 10 to 20% that are also CD127+ (94). However, in the current studies, there were insufficient cells available to directly confirm that CD38+++CD127+ CD4+ cells were highly infected; this will be addressed in future studies.
Apoptosis of HIV-specific CD4+ T cells.
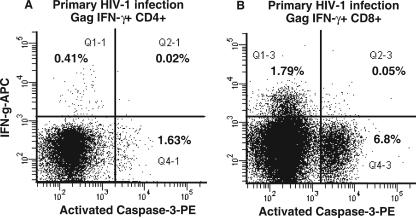
Since we estimated that only a minority of CCR5+CD38+++ CD4+ T cells were infected with HIV-1 DNA, the loss of these cells from the circulation (94) may not be due to direct cytopathic effect. We previously found that the rate of spontaneous apoptosis, in vitro, of CD4+ T cells from subjects with PHI was slightly elevated compared to that from healthy adult controls (93), and we expected to observe preferential apoptosis of IFN-γ+ gag-specific CD4+ T cells in early PHI, as was previously described for antigen-specific CD4+ T cells in chronic HIV-1 infection (89). Activated intracellular caspase-3 was used as a marker of apoptotic CD4+ T cells, in combination with the intracellular cytokine assay, by using fresh whole-blood samples from subjects in four independent experiments (Fig. 2A). The results in all cases showed that IFN-γ+ Gag-specific CD4+ T cells were clearly separate from apoptotic activated caspase-3+ CD4+ T cells. Therefore, we were unable to demonstrate directly a high rate of spontaneous apoptosis of Gag-specific CD4+ T cells that actively produced IFN-γ. Similarly, antigen-specific CD8+ T cells in the same samples were not positive for activated caspase-3, despite a very high level of apoptosis among CD8+ T cells (Fig. 2B).
FIG. 2.
Lack of apoptosis of Gag-specific CD4+ T cells in vitro during PHI. Following stimulation with Gag peptides in the intracellular cytokine assay, CD4+ (A) and CD8+ (B) T cells were simultaneously stained with monoclonal antibodies to IFN-γ and activated caspase-3. Representative histograms for one subject out of four consecutive subjects with PHI are shown. Also shown are percentages of cells in quadrants.
We also analyzed whether CD38+++ cells were caspase-3+ (data not shown). Apoptotic CD8+ T cells were mostly CD38+++, consistent with our previous results (93). In contrast, apoptotic CD4+ expressed only intermediate levels of CD38, again indicating that antigen-specific CD38+++ CD4+ T cells do not spontaneously undergo apoptosis in vitro.
Trafficking of CCR5+CD38+++ CD4+ T cells during primary HIV-1 infection.
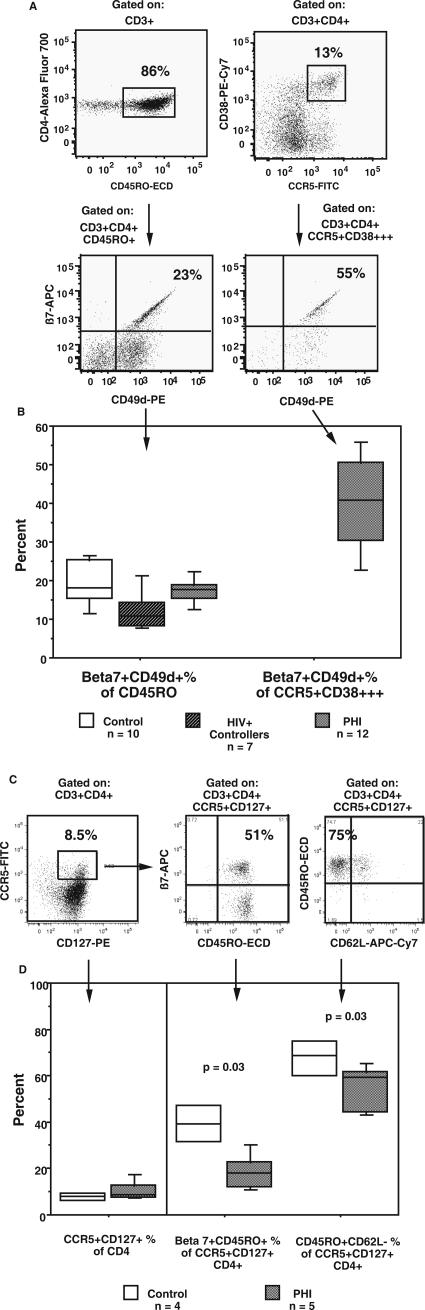
A third possible reason for the rapid loss of CCR5+CD38+++ CD4+ T cells from circulation could be the sequestration in tissues, as shown for CD8 effector cells in mice (44). Since it has recently been reported that gut lymphoid tissue is a major site of HIV-1 during primary infection (7, 22, 48), we examined the coexpression of the gut-homing markers CD49d and integrin β7 on CD4+ T cells during PHI, in particular on the CD45RO+ memory cell and CCR5+CD38+++ HIV-specific subsets of CD4+ T cells, respectively (Fig. 3A). The proportions of CD45RO+ memory CD4+ T cells expressing CD49d and integrin β7 were similar between controls and 12 consecutive subjects presenting during PHI (Fig. 3B), suggesting that there was no preferential loss of this circulating subset of memory CD4+ T cells. However, for the subjects with PHI, a median of 41% of CCR5+CD38+++ CD4+ T cells was found to be positive for the gut-homing markers CD49d and integrin β7, which was generally higher than that for CD45RO+ memory CD4+ T cells (Fig. 3B). These results suggest that a large fraction of the CCR5+CD38+++ CD4+ T cells present during PHI will preferentially traffic to gut-associated lymphoid tissue.
FIG. 3.
Gut-homing of CCR5+ CD4+ T cells during PHI. Fresh whole-blood samples were stained for CD3, CD4, CD45RO, CD62L, integrin β7, CD49d (integrin α4), CCR5, and CD38. Gut-homing CD4+ T cells were identified by coexpression of integrin β7 and CD49d. (A) The presence of integrin β7+CD49d+ cells within CD45RO+ and CCR5+CD38+++ CD4+ T-cell subsets is shown for 1 representative subject out of 12 subjects studied during PHI. The percentages of integrin β7+CD49d+ cells within their respective CD45RO+ and CCR5+CD38+++ CD4+ T-cell subsets are shown. (B) Box plots of integrin β7+CD49d+ cells as a percentage of CD45RO+CD4+ T cell for the three subject groups (left) and as a percentage of CCR5+CD38+++ CD4+ T cells for subjects with PHI only (right). Fresh whole-blood samples were also stained for CD3, CD4, CD45RO, CD62L, integrin β7, CCR5, and CD127. The CCR5+CD127+ subset present in a representative healthy adult control subject is shown in panel C, together with its expression of CD45RO, integrin β7, and CD62L. Box plots of CCR5+CD127+ cells as a percentage of CD4+ are shown at the left side of panel D, and β7+CD45RO+ and CD45RO+CD62L-negative cells as percentages of CCR5+CD127+ CD4+ T cells are also shown at the right side of the panel. Box plots depict the 90th, 75th, median, 25th, and 10th percentiles for each subject group, and the number of subjects in each group is shown. The P values shown are for subjects with PHI compared to those for healthy adult controls by a Mann-Whitney nonparametric test.
Since the greatest burden of HIV-1 DNA was found in CD127+ CD4+ T cells (Fig. 1F), we also determined the proportion of CD4+ T cells which were CCR5+CD127+ and whether such cells expressed the gut-homing marker integrin β7 (Fig. 3C). In healthy adult controls, approximately 10% of CD4+ T cells were CCR5+CD127+, and furthermore, a large fraction of these cells were β7+ CD45RO+CD62L negative (Fig. 3C). We observed a similar subset of CCR5+CD127+ CD4+ T cells in subjects with PHI, but within this subset, there were significantly fewer β7+ CD45RO+CD62L-negative cells (Fig. 3D).
CD25+ CD4+ T regulatory cells during primary HIV-1 infection.
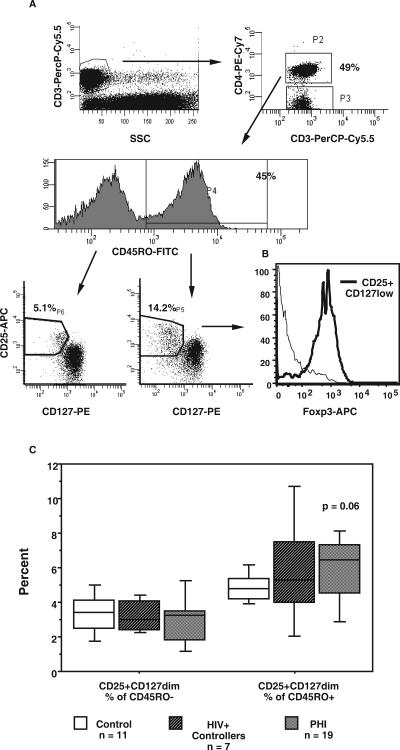
Another possible reason for the loss of antigen-specific CD4+ T cells during the resolution of PHI may be the development of CD25+ CD4+ T regulatory cells, which were recently reported in chronic HIV-1 infection (1, 33). We determined whether there was an elevation in the proportion or number of circulating CD25+ CD4+ T regulatory cells in subjects with PHI, compared to that in healthy adult controls. T regulatory cells were identified by an improved phenotypic method, combining increased expression of CD25 with reduced CD127 expression (Fig. 4A) (65a), and validated by the confirmation of the expression of Foxp3 selectively within these cells (Fig. 4B).
FIG. 4.
T regulatory cells during PHI. T reg CD3+CD4+ cells were identified within CD45RO+ and CD45RO− populations by high expression of CD25 and dim expression of CD127 (A). Representative histograms for a subject during PHI are shown. Identical gating was used for all cohorts. The selective expression of Foxp3 within CD25+CD127low CD4+ T cells, compared with that for the remaining CD4+ T cells, is shown in panel B. Overall results for all cohorts with the numbers of subjects in each cohort are shown in panel C. The results for each subpopulation are expressed as percentages of total CD4+ T cells. Box plots depict 10th, 25th, median, 75th, and 90th percentiles. The P value shown is for subjects with PHI compared to that for healthy adult controls, by a Mann-Whitney nonparametric test.
In subjects with PHI, there was a slight elevation in the proportion of CD45RO+ CD4+ T reg cells (Fig. 4C) compared to that in healthy adult controls. This phenotypic method also readily identifies CD45RO-negative CD4+ T reg cells, but these were not different between subjects with PHI and healthy adult controls (Fig. 4C). In some subjects, a small proportion, around 10%, of activated CCR5+CD38+++ CD4+ T cells during PHI also had the CD25+CD127low T reg phenotype (data not shown).
However, commensurate with the decrease in total CD4 T-cell counts in subjects with PHI, there was a decrease in the absolute numbers of T reg cells in subjects with PHI, compared to that in healthy adult controls (data not shown).
CTLA-4 expression by HIV-specific CD4+ T cells.
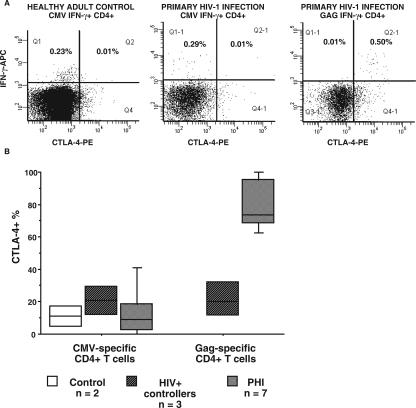
CTLA-4 may be involved in T reg activity (61), and we previously observed that there was increased expression of CTLA-4 by polyclonally stimulated T cells during PHI (J. Zaunders et al., unpublished). Therefore, we used the intracellular cytokine assay to assess intracellular expression of CTLA-4 by HIV-specific CD4+ T cells in response to antigen stimulation in vitro. The results show that a very large proportion of IFN-γ+ Gag-specific CD4+ T cells coexpressed CTLA-4 during PHI (Fig. 5A).
FIG. 5.
CTLA-4 expression by gag-specific CD4+ T cells during PHI. (A) Following the stimulation of whole blood from a healthy adult control with CMV lysate or from a subject during PHI with either CMV lysate or Gag peptides, in the intracellular cytokine assay, CD4+ T cells were simultaneously stained with monoclonal antibodies to IFN-γ and CTLA-4. Representative histograms are shown. (B) Overall results for all cohorts are shown with the number of subjects in each cohort. The results for CTLA-4+ cells are expressed as percentages of IFN-γ+ CD4+ T cells. Box plots depict the 10th, 25th, median, 75th, and 90th percentiles.
The expression of CTLA-4 by antigen-specific CD4+ T cells from the different subject groups is summarized in Fig. 5B. CMV-specific CD4+ T cells from healthy adult controls, HIV+ controllers, and subjects with PHI were all predominantly negative for CTLA-4 (Fig. 5B).
Importantly, Gag-specific CD4+ T cells from HIV+ controllers also expressed a very low level of CTLA-4 in response to antigen-specific restimulation in vitro in contrast to the high level of CTLA-4 expression by HIV-1 Gag-specific CD4+ T cells in subjects with PHI (Fig. 5B).
DISCUSSION
We embarked on the current studies with the hypothesis that the loss of HIV-specific CD4+ T cells during the resolution of primary HIV-1 infection was the result of preferential infection of highly activated CD38+++ CD127-negative CCR5+ effector CD4+ T cells, which we recently identified (94). In vitro and in vivo observations (14, 70, 72, 96) suggested that such activated CD38+++ CD127-negative antigen-specific CD4+ T cells carrying the coreceptor for transmitted strains of HIV-1 should have been a target for cytopathic HIV-1 infection during PHI.
While the initial results showed the presence of HIV-1 DNA in highly activated CD38+++ CD4+ T cells within the first weeks of symptomatic primary HIV-1 infection, the extent of infection of the CD38+++ CD4+ T cells in vivo appeared to be restricted. It was likely that only approximately 10% of these cells were infected, based on an average infection rate of 1.5 copies per infected cell (45). Therefore, the rapid decline in CCR5+CD38+++ HIV-specific CD4+ T cells in the circulation that we previously observed (94) was probably only partly due to cytopathic infection. This conclusion is supported by a parallel study of vaccinia virus-specific CD4+ T cells, where we observed a similar peak of antigen-specific CCR5+ CD38+++ CD4+ T cells, followed by a decline coincident with the resolution of the vaccinia virus infection (95).
Nevertheless, cytopathic infection probably remains a significant factor in the decline of CCR5+ HIV-specific CD4+ T cells. Previously, we found that proliferating CCR5+ Ki-67+ CD4+ T cells did not accumulate in the circulation during PHI, in contrast to a marked accumulation of CCR5+ Ki-67+ CD4+ T cells in subjects with acute Epstein-Barr virus infection (92). A dramatic loss of CCR5+ CD4+ T cells, particularly in the gut, has been reported in acute SIV infection (40, 45, 82) and a similar loss of gut CD4+ T cells may occur in primary HIV infection (7, 22, 48). It is therefore notable that during PHI, just under half of the circulating CCR5+CD38+++ CD4+ T cells expressed the gut-homing markers CD49d and integrin β7.
Since the vast majority of HIV-1 DNA was found in CD127+ CD4+ T cells, we investigated the expression of CCR5 on these cells both in healthy adults and during PHI. Around 10% of CD4+ T cells in peripheral blood are CCR5+CD127+, but in healthy adults, nearly half also express integrin β7, suggesting trafficking to GALT. It is possible that the CCR5+CD127+β7+ CD4+ T cells subset is a significant target for HIV-1 infection due to direct infection of resting cells in GALT, as seen in the acute SIV infection model (40). In our current study, the overall proportion of gut-homing CD45RO+ memory cells in the circulation was not apparently reduced but theCCR5+CD127+β7+CD62L-negative subset of CD4+ T cells appeared to be selectively depleted during PHI.
Our findings are consistent with those of an earlier report of reduced circulating α4β7+ CCR5+ CD4+ T cells during PHI (34) as well as those of a previous study of acute SIV infection which reported a loss of the small subset of CD4+ T cells expressing CD103 (integrins αEβ7) (46), although this may direct localization around E-cadherin+ epithelial cells within GALT rather than direct trafficking to GALT (32). Detailed studies of cells from biopsies of GALT are therefore required to clarify the fate of HIV-specific CD4+ T cells.
The level of HIV-1 DNA within CD127+ CD4+ T cells was much higher than that in their CD127-negative counterparts. We had previously shown that the CD127-negative subpopulation of T cells contained most of the HIV-specific CD4+ T cells (94) but also contained highly activated CD38+, Ki-67+, and Bcl-2low cells prone to apoptosis during PHI (93). The observation that CD127+ CD4+ T cells are highly infected has two important implications for HIV-1 pathogenesis.
First, murine studies suggest that the small CD127+ subset of CCR5+CD38+++ HIV-specific effector CD4+ T cells that we previously observed (94) represents precursors of long-term memory CD4+ T cells (30, 39, 63). Therefore, HIV-1 infection may preferentially target these nascent HIV-specific memory CD4+ T cells. Future large-scale cell sorting experiments are required to purify sufficient CD127+CCR5+CD38+++ effector CD4+ T cells as well as CD127+β7+ CD4+ T cells to confirm that these cells are highly infected as implied by our current results.
Second, there is an essential role for frequent signaling by IL-7 in memory T-cell survival (38, 63, 66, 77). However, several studies have shown that the culture of PBMCs from subjects with chronic HIV-1 infection in the presence of IL-7 efficiently leads to the production of HIV-1 (9, 49, 69), specifically from latently infected resting cells (83), probably involving the induction of NF-kB (8, 17).
Other studies have shown that treatment of PBMCs with IL-7 makes resting CD4+ T cells permissive for productive HIV-1 infection (17, 64, 71, 81) without necessarily entering the cell cycle (81). Signaling by the IL-7 receptor leads to the up-regulation of NF-kB (reviewed in reference 28), which is a major transcription factor for the initiation of HIV-1 replication (reviewed in reference 57). We have found that plasma levels of IL-7 were elevated during PHI, and normal expression of the γ chain of the IL-7 receptor was perturbed (62). Taking these results together, it is therefore possible that increased IL-7 signaling in vivo can contribute significantly to promoting the infection of otherwise apparently resting CCR5+ CD127+ CD4+ T cells during PHI. High levels of infection of resting CD4+ T cells have been reported in lymphoid tissue during acute SIV infection (97), particularly in GALT (40), where IL-7 production is well described (28, 84). Subsequently, the homeostasis of resting CD4+ T cells, involving intermittent delivery of the IL-7 survival signal (38, 63, 66, 77), may lead to the depletion of CD127+ CD4+ T cells containing HIV-1 DNA.
Further studies are required to determine whether the HIV-1 DNA in resting CD4+ T cells was fully integrated or remained in a relatively labile unintegrated form (54) and to what extent infectious virus can be recovered from either activated or resting subsets, particularly when incubated with exogenous IL-7.
However, the major mechanism believed to mediate the decrease in effector T cells during the resolution of a primary immune response is apoptosis (43). Central to the apoptosis of CD8+ effector T cells is the balance between antiapoptotic Bcl-2 and proapoptotic Bim (52), while the reexpression of Bcl-2 mediated by IL-7R signaling is reportedly involved in the transition from effector to long-term memory cells (21, 63). We had directly observed a decrease in Bcl-2 expression in HIV-specific CD4+ T cells during PHI (94), so we expected to observe spontaneous apoptosis of these cells in vitro, as has been reported with chronic HIV-1 infection (89). However, this was not observed in our intracellular cytokine assays, even though elevated levels of apoptotic CD4+ and CD8+ T cells were clearly seen, consistent with our previous results (93).
This discrepancy may be due to a difference between chronic and acute HIV-1 infection. Cells committed to apoptosis in acute infection may be unable to produce cytokines in vitro. HLA class II tetramers (65, 67) would provide a more direct means to detect apoptotic antigen-specific CD4+ T cells.
Another possible mechanism of the down-regulation of the HIV-specific response is the suppression by CD25+ CD4+ T regulatory cells (60). While it has recently been reported that T reg cells can be infected by HIV-1 (50), most reports suggest that T reg cells (1, 4, 33, 86) are increased in chronic HIV-1 infection. We observed a slight increase in T reg cells, identified phenotypically in samples from subjects with PHI. It is possible that an effect of cytopathic infection simultaneously limited a potentially greater expansion of T reg cells, but our cell sorting experiments showed few copies of HIV-1 DNA in CD127-negative cells, which include T reg cells, suggesting that they were not preferentially infected.
A major finding is the high level of expression of CTLA-4 by HIV-specific CD4 T cells during PHI, and it is probable that this is a significant factor in their decline. Our results are consistent with reports of increased expression of CTLA-4 in CD4+ T cells in peripheral blood and lymphoid tissue in HIV-1 infection (4, 73), including CCR5+ and Ki-67+ CD4+ T cells (37). Importantly, murine genetic studies have shown that CTLA-4 expression is a potent constraint on CD4+ T-cell expansion in vivo (78, 85). However, it is not clear whether the expression of CTLA-4 by antigen-specific CD4+ T cells themselves is suppressive (16, 26) or whether there is an indirect suppressive effect mediated by other cells, such as T regulatory CD4+ T cells expressing CTLA-4 (5, 61, 79), and we had expected to find an increased expression of CTLA-4 associated with an increase in T reg cells.
The rapid expression of CTLA-4 in response to HIV-1 Gag antigen would be predicted to interfere with CD28 signaling (11, 36), reducing the synthesis and stability of IL-2 mRNA (2). Therefore, the overexpression of CTLA-4 may provide a mechanistic explanation for the lack of IL-2 production and in vitro proliferation by HIV-specific CD4+ effector T cells, which we and others have previously observed in PHI (24, 94) and which is a hallmark of untreated chronic HIV-1 infection (23, 27, 88). An exception may be those rare patients controlling viral replication without therapy who exhibit HIV-specific IL-2 production by CD4+ T cells and proliferation (6, 25, 58, 91). Therefore, it is significant that in the HIV-specific CD4+ T cells from HIV+ controllers included in this study, there was little CTLA-4 production in response to antigenic stimulation. Therefore, one avenue of investigation may be the possible beneficial effect of selectively interfering with the binding of CTLA-4 to B7 molecules on antigen-presenting cells (53).
In our parallel study of vaccinia virus-specific CD4+ T effector cells, less than half coexpressed CTLA-4 with IFN-γ (95), suggesting an important difference in cell fate decisions between early HIV- and vaccinia virus-specific CD4+ T cells. The cause of the altered differentiation of HIV-specific CD4+ T cells is currently unknown but could be due to differences in dendritic cells (35) between the two infections. HIV-1 infection reportedly leads to decreases in dendritic cell subsets in the circulation (13, 51) and alterations of the phenotype of these cells in lymphoid tissue (41). Therefore, there may be alterations in signaling by antigen-presenting cells in the very early stages of CD4+ memory T-cell differentiation following HIV-1 infection compared with that following vaccinia virus inoculation.
Altogether, the overall results of the current study argue against cytopathic infection and apoptosis of activated CCR5+ CD4+ effector T cells or an overabundance of T regulatory cells as major reasons for the loss of HIV-specific CD4+ T cells during the resolution of PHI. Instead our results indicate that the infection of CCR5+ CD127+ CD4+ T cells, including early HIV-specific memory CD4+ T cells that traffic through GALT, may be an important step in pathogenesis. Furthermore, the high level of CTLA-4 expression associated with limited IL-2 production probably plays a role in blocking the proliferative potential and differentiation of HIV-specific CD4+ T cells which escape infection.
Acknowledgments
We thank the trial participants, their physicians, and the trial nurses for their cooperation in providing timely samples, Jerome Darakdjian for cell sorting, and Ciara McGinley, Julie Yeung, and Michelle Bailey for PBMC cryopreservation as well as the NIH Reference Reagents Program for the provision of HIV-1 overlapping Gag peptides.
The National Centre in HIV Epidemiology and Clinical Research is supported by the Commonwealth Department of Health and Ageing through the Australian National Council on AIDS, Hepatitis C, and Related Diseases. This project was funded by an AIEDRP grant through the NIH Division of AIDS and a program grant from the Australian National Health and Medical Research Council.
The authors have no conflicting financial interests.
REFERENCES
- 1.Aandahl, E. M., J. Michaelsson, W. J. Moretto, F. M. Hecht, and D. F. Nixon. 2004. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J. Virol. 78:2454-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acuto, O., and F. Michel. 2003. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat. Rev. Immunol. 3:939-951. [DOI] [PubMed] [Google Scholar]
- 3.Amyes, E., C. Hatton, D. Montamat-Sicotte, N. Gudgeon, A. B. Rickinson, A. J. McMichael, and M. F. Callan. 2003. Characterization of the CD4+ T cell response to Epstein-Barr virus during primary and persistent infection. J. Exp. Med. 198:903-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson, J., A. Boasso, J. Nilsson, R. Zhang, N. J. Shire, S. Lindback, G. M. Shearer, and C. A. Chougnet. 2005. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J. Immunol. 174:3143-3147. [DOI] [PubMed] [Google Scholar]
- 5.Bachmann, M. F., A. Gallimore, E. Jones, B. Ecabert, H. Acha-Orbea, and M. Kopf. 2001. Normal pathogen-specific immune responses mounted by CTLA-4-deficient T cells: a paradigm reconsidered. Eur. J. Immunol. 31:450-458. [DOI] [PubMed] [Google Scholar]
- 6.Boaz, M. J., A. Waters, S. Murad, P. J. Easterbrook, and A. Vyakarnam. 2002. Presence of HIV-1 Gag-specific IFN-gamma+IL-2+ and CD28+IL-2+ CD4 T cell responses is associated with nonprogression in HIV-1 infection. J. Immunol. 169:6376-6385. [DOI] [PubMed] [Google Scholar]
- 7.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chene, L., M. T. Nugeyre, F. Barre-Sinoussi, and N. Israel. 1999. High-level replication of human immunodeficiency virus in thymocytes requires NF-κB activation through interaction with thymic epithelial cells. J. Virol. 73:2064-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chene, L., M. T. Nugeyre, E. Guillemard, N. Moulian, F. Barre-Sinoussi, and N. Israel. 1999. Thymocyte-thymic epithelial cell interaction leads to high-level replication of human immunodeficiency virus exclusively in mature CD4+ CD8− CD3+ thymocytes: a critical role for tumor necrosis factor and interleukin-7. J. Virol. 73:7533-7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor, R. I., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 185:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis, S. J., S. Ikemizu, E. J. Evans, L. Fugger, T. R. Bakker, and P. A. van der Merwe. 2003. The nature of molecular recognition by T cells. Nat. Immunol. 4:217-224. [DOI] [PubMed] [Google Scholar]
- 12.Dib, C., S. Faure, C. Fizames, D. Samson, N. Drouot, A. Vignal, P. Millasseau, S. Marc, J. Hazan, E. Seboun, M. Lathrop, G. Gyapay, J. Morissette, and J. Weissenbach. 1996. A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380:152-154. [DOI] [PubMed] [Google Scholar]
- 13.Donaghy, H., A. Pozniak, B. Gazzard, N. Qazi, J. Gilmour, F. Gotch, and S. Patterson. 2001. Loss of blood CD11c+ myeloid and CD11c− plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood 98:2574-2576. [DOI] [PubMed] [Google Scholar]
- 14.Douek, D. C., J. M. Brenchley, M. R. Betts, D. R. Ambrozak, B. J. Hill, Y. Okamoto, J. P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, Z. Grossman, M. Dybul, A. Oxenius, D. A. Price, M. Connors, and R. A. Koup. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417:95-98. [DOI] [PubMed] [Google Scholar]
- 15.Douek, D. C., L. J. Picker, and R. A. Koup. 2003. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 21:265-304. [DOI] [PubMed] [Google Scholar]
- 16.Doyle, A. M., A. C. Mullen, A. V. Villarino, A. S. Hutchins, F. A. High, H. W. Lee, C. B. Thompson, and S. L. Reiner. 2001. Induction of cytotoxic T lymphocyte antigen 4 (CTLA-4) restricts clonal expansion of helper T cells. J. Exp. Med. 194:893-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ducrey-Rundquist, O., M. Guyader, and D. Trono. 2002. Modalities of interleukin-7-induced human immunodeficiency virus permissiveness in quiescent T lymphocytes. J. Virol. 76:9103-9111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gamadia, L. E., E. B. Remmerswaal, J. F. Weel, F. Bemelman, R. A. van Lier, and I. J. Ten Berge. 2003. Primary immune responses to human CMV: a critical role for IFN-γ-producing CD4+ T cells in protection against CMV disease. Blood 101:2686-2692. [DOI] [PubMed] [Google Scholar]
- 19.Gratton, S., R. Cheynier, M. J. Dumaurier, E. Oksenhendler, and S. Wain-Hobson. 2000. Highly restricted spread of HIV-1 and multiply infected cells within splenic germinal centers. Proc. Natl. Acad. Sci. USA 97:14566-14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grayson, J. M., K. Murali-Krishna, J. D. Altman, and R. Ahmed. 2001. Gene expression in antigen-specific CD8+ T cells during viral infection. J. Immunol. 166:795-799. [DOI] [PubMed] [Google Scholar]
- 21.Grayson, J. M., A. J. Zajac, J. D. Altman, and R. Ahmed. 2000. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J. Immunol. 164:3950-3954. [DOI] [PubMed] [Google Scholar]
- 22.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77:11708-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harari, A., S. Petitpierre, F. Vallelian, and G. Pantaleo. 2004. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood 103:966-972. [DOI] [PubMed] [Google Scholar]
- 24.Harari, A., F. Vallelian, P. Meylan, and G. Pantaleo. 2005. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J. Immunol. 174:1037-1045. [DOI] [PubMed] [Google Scholar]
- 25.Imami, N., A. Pires, G. Hardy, J. Wilson, B. Gazzard, and F. Gotch. 2002. A balanced type 1/type 2 response is associated with long-term nonprogressive human immunodeficiency virus type 1 infection. J. Virol. 76:9011-9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inobe, M., and R. H. Schwartz. 2004. CTLA-4 engagement acts as a brake on CD4+ T cell proliferation and cytokine production but is not required for tuning T cell reactivity in adaptive tolerance. J. Immunol. 173:7239-7248. [DOI] [PubMed] [Google Scholar]
- 27.Iyasere, C., J. C. Tilton, A. J. Johnson, S. Younes, B. Yassine-Diab, R. P. Sekaly, W. W. Kwok, S. A. Migueles, A. C. Laborico, W. L. Shupert, C. W. Hallahan, R. T. Davey, Jr., M. Dybul, S. Vogel, J. Metcalf, and M. Connors. 2003. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J. Virol. 77:10900-10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang, Q., W. Q. Li, F. B. Aiello, R. Mazzucchelli, B. Asefa, A. R. Khaled, and S. K. Durum. 2005. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 16:513-533. [DOI] [PubMed] [Google Scholar]
- 29.Jung, A., R. Maier, J. P. Vartanian, G. Bocharov, V. Jung, U. Fischer, E. Meese, S. Wain-Hobson, and A. Meyerhans. 2002. Multiply infected spleen cells in HIV patients. Nature 418:144. [DOI] [PubMed] [Google Scholar]
- 30.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191-1198. [DOI] [PubMed] [Google Scholar]
- 31.Kalams, S. A., and B. D. Walker. 1998. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 188:2199-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kilshaw, P. J., and J. M. Higgins. 2002. Alpha E: no more rejection? J. Exp. Med. 196:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinter, A. L., M. Hennessey, A. Bell, S. Kern, Y. Lin, M. Daucher, M. Planta, M. McGlaughlin, R. Jackson, S. F. Ziegler, and A. S. Fauci. 2004. CD25+CD4+ regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4+ and CD8+ HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J. Exp. Med. 200:331-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krzysiek, R., A. Rudent, L. Bouchet-Delbos, A. Foussat, C. Boutillon, A. Portier, D. Ingrand, D. Sereni, P. Galanaud, L. Grangeot-Keros, and D. Emilie. 2001. Preferential and persistent depletion of CCR5+ T-helper lymphocytes with nonlymphoid homing potential despite early treatment of primary HIV infection. Blood 98:3169-3171. [DOI] [PubMed] [Google Scholar]
- 35.Langenkamp, A., K. Nagata, K. Murphy, L. Wu, A. Lanzavecchia, and F. Sallusto. 2003. Kinetics and expression patterns of chemokine receptors in human CD4+ T lymphocytes primed by myeloid or plasmacytoid dendritic cells. Eur. J. Immunol. 33:474-482. [DOI] [PubMed] [Google Scholar]
- 36.Leibson, P. J. 2004. The regulation of lymphocyte activation by inhibitory receptors. Curr. Opin. Immunol. 16:328-336. [DOI] [PubMed] [Google Scholar]
- 37.Leng, Q., Z. Bentwich, E. Magen, A. Kalinkovich, and G. Borkow. 2002. CTLA-4 upregulation during HIV infection: association with anergy and possible target for therapeutic intervention. AIDS 16:519-529. [DOI] [PubMed] [Google Scholar]
- 38.Lenz, D. C., S. K. Kurz, E. Lemmens, S. P. Schoenberger, J. Sprent, M. B. Oldstone, and D. Homann. 2004. IL-7 regulates basal homeostatic proliferation of antiviral CD4+T cell memory. Proc. Natl. Acad. Sci. USA 101:9357-9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li, J., G. Huston, and S. L. Swain. 2003. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J. Exp. Med. 198:1807-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148-1152. [DOI] [PubMed] [Google Scholar]
- 41.Lore, K., A. Sonnerborg, C. Brostrom, L. E. Goh, L. Perrin, H. McDade, H. J. Stellbrink, B. Gazzard, R. Weber, L. A. Napolitano, Y. van Kooyk, and J. Andersson. 2002. Accumulation of DC-SIGN+CD40+ dendritic cells with reduced CD80 and CD86 expression in lymphoid tissue during acute HIV-1 infection. AIDS 16:683-692. [DOI] [PubMed] [Google Scholar]
- 42.Mackay, C. R., D. P. Andrew, M. Briskin, D. J. Ringler, and E. C. Butcher. 1996. Phenotype, and migration properties of three major subsets of tissue homing T cells in sheep. Eur. J. Immunol. 26:2433-2439. [DOI] [PubMed] [Google Scholar]
- 43.Marrack, P., and J. Kappler. 2004. Control of T cell viability. Annu. Rev. Immunol. 22:765-787. [DOI] [PubMed] [Google Scholar]
- 44.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 45.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 46.Mattapallil, J. J., N. L. Letvin, and M. Roederer. 2004. T-cell dynamics during acute SIV infection. AIDS 18:13-23. [DOI] [PubMed] [Google Scholar]
- 47.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune responses to HIV. Nature 410:980-987. [DOI] [PubMed] [Google Scholar]
- 48.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moran, P. A., M. L. Diegel, J. C. Sias, J. A. Ledbetter, and J. M. Zarling. 1993. Regulation of HIV production by blood mononuclear cells from HIV-infected donors: I. Lack of correlation between HIV-1 production and T cell activation. AIDS Res. Hum. Retrovir. 9:455-464. [DOI] [PubMed] [Google Scholar]
- 50.Oswald-Richter, K., S. M. Grill, N. Shariat, M. Leelawong, M. S. Sundrud, D. W. Haas, and D. Unutmaz. 2004. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2:E198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pacanowski, J., S. Kahi, M. Baillet, P. Lebon, C. Deveau, C. Goujard, L. Meyer, E. Oksenhendler, M. Sinet, and A. Hosmalin. 2001. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood 98:3016-3021. [DOI] [PubMed] [Google Scholar]
- 52.Pellegrini, M., G. Belz, P. Bouillet, and A. Strasser. 2003. Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim. Proc. Natl. Acad. Sci. USA 100:14175-14180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phan, G. Q., J. C. Yang, R. M. Sherry, P. Hwu, S. L. Topalian, D. J. Schwartzentruber, N. P. Restifo, L. R. Haworth, C. A. Seipp, L. J. Freezer, K. E. Morton, S. A. Mavroukakis, P. H. Duray, S. M. Steinberg, J. P. Allison, T. A. Davis, and S. A. Rosenberg. 2003. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl. Acad. Sci. USA 100:8372-8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pierson, T., J. McArthur, and R. F. Siliciano. 2000. Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annu. Rev. Immunol. 18:665-708. [DOI] [PubMed] [Google Scholar]
- 55.Precopio, M. L., J. L. Sullivan, C. Willard, M. Somasundaran, and K. Luzuriaga. 2003. Differential kinetics and specificity of EBV-specific CD4+ and CD8+ T cells during primary infection. J. Immunol. 170:2590-2598. [DOI] [PubMed] [Google Scholar]
- 56.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rohr, O., C. Marban, D. Aunis, and E. Schaeffer. 2003. Regulation of HIV-1 gene transcription: from lymphocytes to microglial cells. J. Leukoc. Biol. 74:736-749. [DOI] [PubMed] [Google Scholar]
- 58.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 59.Rott, L. S., J. R. Rose, D. Bass, M. B. Williams, H. B. Greenberg, and E. C. Butcher. 1997. Expression of mucosal homing receptor α4β7 by circulating CD4+ cells with memory for intestinal rotavirus. J. Clin. Investig. 100:1204-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sakaguchi, S. 2004. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22:531-562. [DOI] [PubMed] [Google Scholar]
- 61.Sakaguchi, S. 2005. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 6:345-352. [DOI] [PubMed] [Google Scholar]
- 62.Sasson, S. C., J. J. Zaunders, G. Zanetti, E. M. King, K. M. Merlin, D. E. Smith, K. K. Stanley, D. A. Cooper, and A. D. Kelleher. 2006. Increased plasma interleukin-7 level correlates with decreased CD127 and increased CD132 extracellular expression on T cell subsets in patients with HIV-1 infection. J. Infect. Dis. 193:505-514. [DOI] [PubMed] [Google Scholar]
- 63.Schluns, K. S., and L. Lefrancois. 2003. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 3:269-279. [DOI] [PubMed] [Google Scholar]
- 64.Schmitt, N., L. Chene, D. Boutolleau, M. T. Nugeyre, E. Guillemard, P. Versmisse, C. Jacquemot, F. Barre-Sinoussi, and N. Israel. 2003. Positive regulation of CXCR4 expression and signaling by interleukin-7 in CD4+ mature thymocytes correlates with their capacity to favor human immunodeficiency X4 virus replication. J. Virol. 77:5784-5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scriba, T. J., H. T. Zhang, H. L. Brown, A. Oxenius, N. Tamm, S. Fidler, J. Fox, J. N. Weber, P. Klenerman, C. L. Day, M. Lucas, and R. E. Phillips. 2005. HIV-1-specific CD4+ T lymphocyte turnover and activation increase upon viral rebound. J. Clin. Investig. 115:443-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65a.Seddiki, N., B. Santner-Nanan, J. Martinson, J. Zaunders, S. Sasson, A. Landay, M. Solomon, W. Selby, S. I. Alexander, R. Nanan, A. Kelleher, and B. Fazekas de St. Groth. 2006. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 203:1693-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seddon, B., and R. Zamoyska. 2003. Regulation of peripheral T-cell homeostasis by receptor signalling. Curr. Opin. Immunol. 15:321-324. [DOI] [PubMed] [Google Scholar]
- 67.Seth, N., D. Kaufmann, T. Lahey, E. S. Rosenberg, and K. W. Wucherpfennig. 2005. Expansion and contraction of HIV-specific CD4 T cells with short bursts of viremia, but physical loss of the majority of these cells with sustained viral replication. J. Immunol. 175:6948-6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith, D. E., G. R. Kaufmann, J. O. Kahn, F. M. Hecht, P. A. Grey, J. J. Zaunders, P. H. Cunningham, A. Carr, C. Duncombe, D. C. Quan, A. Petersen, and D. A. Cooper. 2003. Greater reversal of CD4+ cell abnormalities and viral load reduction after initiation of antiretroviral therapy with zidovudine, lamivudine, and nelfinavir before complete HIV type 1 seroconversion. AIDS Res. Hum. Retrovir. 19:189-199. [DOI] [PubMed] [Google Scholar]
- 69.Smithgall, M. D., J. G. Wong, K. E. Critchett, and O. K. Haffar. 1996. IL-7 up-regulates HIV-1 replication in naturally infected peripheral blood mononuclear cells. J. Immunol. 156:2324-2330. [PubMed] [Google Scholar]
- 70.Spina, C. A., H. E. Prince, and D. D. Richman. 1997. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J. Clin. Investig. 99:1774-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steffens, C. M., E. Z. Managlia, A. Landay, and L. Al-Harthi. 2002. Interleukin-7-treated naive T cells can be productively infected by T-cell-adapted and primary isolates of human immunodeficiency virus 1. Blood 99:3310-3318. [DOI] [PubMed] [Google Scholar]
- 72.Stevenson, M., T. L. Stanwick, M. P. Dempsey, and C. A. Lamonica. 1990. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 9:1551-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stone, S. F., P. Price, and M. A. French. 2005. Dysregulation of CD28 and CTLA-4 expression by CD4 T cells from previously immunodeficient HIV-infected patients with sustained virological responses to highly active antiretroviral therapy. HIV Med. 6:278-283. [DOI] [PubMed] [Google Scholar]
- 74.Strasser, A., L. O'Connor, and V. M. Dixit. 2000. Apoptosis signaling. Annu. Rev. Biochem. 69:217-245. [DOI] [PubMed] [Google Scholar]
- 75.Suzuki, K., T. Shijuuku, T. Fukamachi, J. Zaunders, G. Guillemin, D. Cooper, and A. Kelleher. 2005. Prolonged transcriptional silencing and CpG methylation induced by dsRNAs targeted to the HIV-1 promoter region. J. RNAi and Gene Silencing 1:66-78. [PMC free article] [PubMed] [Google Scholar]
- 76.Takahashi, T., T. Tagami, S. Yamazaki, T. Uede, J. Shimizu, N. Sakaguchi, T. W. Mak, and S. Sakaguchi. 2000. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 192:303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tan, J. T., E. Dudl, E. LeRoy, R. Murray, J. Sprent, K. I. Weinberg, and C. D. Surh. 2001. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl. Acad. Sci. USA 98:8732-8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tivol, E. A., F. Borriello, A. N. Schweitzer, W. P. Lynch, J. A. Bluestone, and A. H. Sharpe. 1995. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3:541-547. [DOI] [PubMed] [Google Scholar]
- 79.Tivol, E. A., and J. Gorski. 2002. Re-establishing peripheral tolerance in the absence of CTLA-4: complementation by wild-type T cells points to an indirect role for CTLA-4. J. Immunol. 169:1852-1858. [DOI] [PubMed] [Google Scholar]
- 80.Topham, D. J., and P. C. Doherty. 1998. Longitudinal analysis of the acute Sendai virus-specific CD4+ T cell response and memory. J. Immunol. 161:4530-4535. [PubMed] [Google Scholar]
- 81.Unutmaz, D., V. N. KewalRamani, S. Marmon, and D. R. Littman. 1999. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J. Exp. Med. 189:1735-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Veazey, R. S., K. G. Mansfield, I. C. Tham, A. C. Carville, D. E. Shvetz, A. E. Forand, and A. A. Lackner. 2000. Dynamics of CCR5 expression by CD4+ T cells in lymphoid tissues during simian immunodeficiency virus infection. J. Virol. 74:11001-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang, F. X., Y. Xu, J. Sullivan, E. Souder, E. G. Argyris, E. A. Acheampong, J. Fisher, M. Sierra, M. M. Thomson, R. Najera, I. Frank, J. Kulkosky, R. J. Pomerantz, and G. Nunnari. 2005. IL-7 is a potent and proviral strain-specific inducer of latent HIV-1 cellular reservoirs of infected individuals on virally suppressive HAART. J. Clin. Investig. 115:128-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watanabe, M., Y. Ueno, T. Yajima, Y. Iwao, M. Tsuchiya, H. Ishikawa, S. Aiso, T. Hibi, and H. Ishii. 1995. Interleukin 7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J. Clin. Investig. 95:2945-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Waterhouse, P., J. M. Penninger, E. Timms, A. Wakeham, A. Shahinian, K. P. Lee, C. B. Thompson, H. Griesser, and T. W. Mak. 1995. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270:985-988. [DOI] [PubMed] [Google Scholar]
- 86.Weiss, L., V. Donkova-Petrini, L. Caccavelli, M. Balbo, C. Carbonneil, and Y. Levy. 2004. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood 104:3249-3256. [DOI] [PubMed] [Google Scholar]
- 87.Williams, M. B., and E. C. Butcher. 1997. Homing of naive and memory T lymphocyte subsets to Peyer's patches, lymph nodes, and spleen. J. Immunol. 159:1746-1752. [PubMed] [Google Scholar]
- 88.Younes, S. A., B. Yassine-Diab, A. R. Dumont, M. R. Boulassel, Z. Grossman, J. P. Routy, and R. P. Sekaly. 2003. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 198:1909-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yue, F. Y., C. M. Kovacs, R. C. Dimayuga, X. X. Gu, P. Parks, R. Kaul, and M. A. Ostrowski. 2005. Preferential apoptosis of HIV-1-specific CD4+ T cells. J. Immunol. 174:2196-2204. [DOI] [PubMed] [Google Scholar]
- 90.Yue, F. Y., C. M. Kovacs, R. C. Dimayuga, P. Parks, and M. A. Ostrowski. 2004. HIV-1-specific memory CD4+ T cells are phenotypically less mature than cytomegalovirus-specific memory CD4+ T cells. J. Immunol. 172:2476-2486. [DOI] [PubMed] [Google Scholar]
- 91.Zaunders, J. J., W. B. Dyer, B. Wang, M. L. Munier, M. Miranda-Saksena, R. Newton, J. Moore, C. R. Mackay, D. A. Cooper, N. K. Saksena, and A. D. Kelleher. 2004. Identification of circulating antigen-specific CD4+ T lymphocytes with a CCR5+, cytotoxic phenotype in an HIV-1 long-term nonprogressor and in CMV infection. Blood 103:2238-2247. [DOI] [PubMed] [Google Scholar]
- 92.Zaunders, J. J., G. R. Kaufmann, P. H. Cunningham, D. Smith, P. Grey, K. Suzuki, A. Carr, L. E. Goh, and D. A. Cooper. 2001. Increased turnover of CCR5+ and redistribution of CCR5- CD4 T lymphocytes during primary human immunodeficiency virus type 1 infection. J. Infect. Dis. 183:736-743. [DOI] [PubMed] [Google Scholar]
- 93.Zaunders, J. J., L. Moutouh-de Parseval, S. Kitada, J. C. Reed, S. Rought, D. Genini, L. Leoni, A. Kelleher, D. A. Cooper, D. E. Smith, P. Grey, J. Estaquier, S. Little, D. D. Richman, and J. Corbeil. 2003. Polyclonal proliferation and apoptosis of CCR5+ T lymphocytes during primary human immunodeficiency virus type 1 infection: regulation by interleukin (IL)-2, IL-15, and Bcl-2. J. Infect. Dis. 187:1735-1747. [DOI] [PubMed] [Google Scholar]
- 94.Zaunders, J. J., M. L. Munier, D. E. Kaufmann, S. Ip, P. Grey, D. Smith, T. Ramacciotti, D. Quan, R. Finlayson, J. Kaldor, E. S. Rosenberg, B. D. Walker, D. A. Cooper, and A. D. Kelleher. 2005. Early proliferation of CCR5+ CD38+++ antigen-specific CD4+ Th1 effector cells during primary HIV-1 infection. Blood 106:1660-1667. [DOI] [PubMed] [Google Scholar]
- 95.Zaunders, J. J., W. B. Dyer, M. L. Munier, S. Ip, J. Liu, E. Amyes, W. Rawlinson, R. De Rose, S. J. Kent, J. S. Sullivan, D. A. Cooper, and A. D. Kelleher. 2006. CD127+ CCR5+ CD38+++ CD4+ Th1 effector cells are an early component of the primary immune response to vaccinia virus and precede development interleukin-2+ memory CD4+ T cells. J. Virol. 80:10151-10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang, Z., T. Schuler, M. Zupancic, S. Wietgrefe, K. A. Staskus, K. A. Reimann, T. A. Reinhart, M. Rogan, W. Cavert, C. J. Miller, R. S. Veazey, D. Notermans, S. Little, S. A. Danner, D. D. Richman, D. Havlir, J. Wong, H. L. Jordan, T. W. Schacker, P. Racz, K. Tenner-Racz, N. L. Letvin, S. Wolinsky, and A. T. Haase. 1999. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286:1353-1357. [DOI] [PubMed] [Google Scholar]
- 97.Zhang, Z. Q., S. W. Wietgrefe, Q. Li, M. D. Shore, L. Duan, C. Reilly, J. D. Lifson, and A. T. Haase. 2004. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc. Natl. Acad. Sci. USA 101:5640-5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu, T., H. Mo, N. Wang, D. S. Nam, Y. Cao, R. A. Koup, and D. D. Ho. 1993. Genotypic and phenotypic characterization of HIV-1 in patients with primary infection. Science 261:1179-1181. [DOI] [PubMed] [Google Scholar]