Abstract
Neutralization of adenovirus (Ad) by anti-Ad neutralizing antibodies in serum involves formation of Ad-immune complexes that prevent the virus from interacting with target cells. We hypothesized that Ad-immune complexes likely contain viable Ad vectors which, although no longer capable of gaining access to receptors on target cells, may be able to express transgenes in cells bearing Fc receptors for immunoglobulins, i.e., that antibody-based “neutralization” of Ad vectors may be circumvented by the Fc receptor pathway. To test this hypothesis, we expressed the Fcγ receptor IIA (FcγR) in A549 lung epithelial cells or human dermal fibroblasts and evaluated gene transfer in the presence of human neutralizing anti-Ad serum. FcγR-expressing cells bound and internalized copious amounts of Ad, with a distinct population of internalized Ad trafficking to the nucleus. The dose-response curves for inhibition of gene transfer revealed that FcγR-expressing cells required a more-than-10-fold higher concentration of anti-Ad serum to achieve 50% inhibition of Ad-encoded β-galactosidase expression compared with non-FcγR-expressing cells. The discrepancy between neutralization of Ad during infection of FcγR-expressing cells and neutralization of Ad during infection of non-FcγR-expressing cells occurred with either heat-inactivated or non-heat-inactivated sera, was blocked by addition of purified Fc domain protein, and did not require the cytoplasmic domain of FcγR, suggesting that immune complex internalization proceeded via endocytosis rather than phagocytosis. FcγR-mediated infection by Ad-immune complexes did not require expression of the coxsackie virus-Ad receptor (CAR) since similar data were obtained when CAR-deficient human dermal fibroblasts were engineered to express FcγR. However, interaction of the Ad penton base with cell surface integrins contributed to the difference in neutralization between FcγR-expressing and non-FcγR-expressing cells. The data indicate that complexes formed from Ad and anti-Ad neutralizing antibodies, while compromised with respect to infection of non-FcγR-expressing target cells, maintain the potential to transfer genes to FcγR-expressing cells, with consequent expression of the transgene. The formation of Ad-immune complexes that can target viable virus to antigen-presenting cells may account for the success of Ad-based vaccines administered in the presence of low levels of neutralizing anti-Ad antibody.
One ubiquitous challenge regarding the use of viral vectors for gene transfer relates to the ability of immune-competent hosts to develop neutralizing humoral immunity against the viral capsid. In the case of adenovirus (Ad) gene transfer vectors based on subgroup C viruses, approximately one-half of the general patient population has a detectable neutralizing antibody titer (3, 10, 11, 19, 21, 21, 53, 61, 65, 68). The problem is exacerbated when one considers the impact of repeated administration of Ad vectors. Following each successive administration, the neutralizing antibody titer tends to increase and the efficacy of gene transfer decreases dramatically (10, 19, 33, 41, 42, 86).
Neutralization of Ad by antibodies has typically been described with one of two possible outcomes: intracellular neutralization or extracellular neutralization. Intracellular neutralization refers to an Ad-immune complex that enters the cell but fails to accomplish gene delivery to the nucleus. With purified antibodies against individual capsid proteins, several groups have demonstrated intracellular neutralization with accumulation of Ad inside organelles in the cytoplasm (12, 52, 55, 79-81). Extracellular neutralization, in which formation of Ad-immune complexes prevents Ad from interacting with target cells, is the predominant form of neutralization for unfractionated, anti-Ad sera (52, 74). However, it is not clear whether extracellularly neutralized Ad is necessarily neutralized with respect to intracellular trafficking. In other words, there may exist viable Ad capsids in extracellular Ad-immune complexes that could traffic to the nucleus and express viral genes if given the opportunity to interact with cells.
The relevance of this question lies in the fact that antigen-presenting cells express receptors for immune complexes and can internalize immune complexes via Fc receptors. The Fc receptor family includes both high-affinity and low-affinity receptors for the Fc portion of immunoglobulins (17). The low-affinity receptors (FcγRII and FcγRIII) clear immune complexes from tissue and serum and enhance the immune response to foreign antigens contained in the antibody-antigen complex (30). In the event that viable viral capsids gain entry to an antigen-presenting cell via Fc receptor interaction, there exists a potential viral gene expression with the antigen-presenting cell (14, 44), a mechanism that can lead to particularly strong immune responses to virus-encoded antigens.
With the knowledge that formation of Ad-immune complexes prevents Ad access to target cells, we hypothesized that Ad-immune complexes may contain viable virus that is capable of intracellular trafficking and infection if the Ad-immune complex is forced to interact with target cells via Fc receptors. To address this hypothesis, Fcγ receptor IIA (FcγR) was expressed in a nonhematopoietic target cell line. This strategy was based on the fact that this isoform of FcγR was previously shown to contain an immunoreceptor tyrosine-based activation domain and thus was capable of directing phagocytosis of immune complexes (46). In addition, the use of nonhematopoietic and nonmyeloid cell lines avoided potential confusion from endogenous expression of FcγR, a strategy previously validated in the literature (29, 30). Following formation of Ad-immune complexes with neutralizing anti-Ad serum, the intracellular trafficking of Ad capsids and Ad-mediated gene transfer were assessed over a range of ratios of Ad to neutralizing anti-Ad serum to determine the concentration of anti-Ad serum that gave 50% inhibition of gene transfer (IC50).
MATERIALS AND METHODS
Ad vectors.
All of the Ads used in this study were E1− E3− replication-deficient, recombinant Ad gene transfer vectors with an expression cassette inserted into the E1 position. The expression cassette included the cytomegalovirus promoter-enhancer, a simian virus 40 polyadenylation signal, and a transgene. The study included vectors encoding a β-galactosidase transgene (Adβgal) (22), an FcγR (CD32) transgene (AdFcγR) (8), a vector with a form of the Fc receptor that lacked the cytoplasmic domain (AdtaillessFcγR) (8), and a vector that was used as a control for vector infection into which no cDNA had been inserted (AdNull) (22). In addition to these vectors, one tropism-modified vector was employed. The vector was identical to Adβgal except that the nucleic acid sequence encoding the penton base protein was altered to modify the three amino acids (RGD) that confer integrin binding (AdΔRGDβgal) (76). All vectors were propagated, purified, and stored as described previously (56, 57). All vectors had particle-to-PFU ratios of less than 100. Virus concentration was determined on the basis of the extinction coefficient for Ad (9.09 × 10−13 M−1 cm−1) (47). Covalent conjugation of the red fluorophore Cy3 to the Ad capsid was accomplished by succinimidyl ester chemistry as previously described (39, 49).
Cell culture.
A549 lung epithelial carcinoma cells (CCL-185; American Type Culture Collection, Manassas, VA) express the high-affinity coxsackie virus-Ad receptor (CAR) (23) and were used as a model for CAR-sufficient cells. Human dermal fibroblasts are deficient in CAR expression (23). A549 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 50 U/ml penicillin, and 50 μg/ml streptomycin at 37°C in 5% CO2. Fibroblasts were maintained in RPMI 1640 cell culture medium supplemented with 10% fetal bovine serum, 50 U/ml penicillin, and 50 μg/ml streptomycin at 37°C in 5% CO2. All cell culture reagents were obtained from Life Technologies, Inc. (Gaithersburg, MD).
Anti-Ad neutralizing sera, titer determination, and heat inactivation.
Human neutralizing sera were obtained in the course of gene therapy clinical trials that have been previously described in detail (19). Sera were previously assayed for neutralizing anti-Ad titer by propagation of wild-type Ad5 infection in a monolayer culture of A549 cells and were stored at −80°C until use (19). Neutralizing titers of individual sera are described in the text according to the inverse of the lowest dilution that gave a 90% inhibition of adenovirus serotype 5 infection. Except where noted otherwise, the neutralizing titer of the anti-Ad serum was 2,560 (i.e., a 2,560-fold dilution was the lowest dilution to give 90% inhibition of adenovirus infection). Upon thawing, sera were heat inactivated at 55°C for 60 min prior to use, except where noted otherwise. This research was performed in compliance with the Health Information Privacy and Protection Act. This report does not contain information on the identities of patients from whom samples were collected.
Preinfection of A549 cells or human dermal fibroblasts with AdFcγR.
FcγR expression was obtained by preinfecting cells with AdFcγR. For A549 cells, the preinfection conditions were 1,000 particles per cell, 60 min, 37°C. For human dermal fibroblasts, the preinfection conditions were 10,000 particles per cell, 60 min, 37°C. The use of a higher concentration of Ad to infect human dermal fibroblasts was previously shown to be required because of the absence of CAR (19, 39). Preinfected cells were prepared in parallel with uninfected cells (naive), as well as cells preinfected with AdNull as a control for vector infection. Where noted, preinfection was performed in the same manner with AdtaillessFcγR. After 3 h, preinfected A549 cells or fibroblasts were replated at a density of 104 cells per well on a 96-well plate for analysis of gene transfer or at a density of 104 cells per well of a coverslip bottom dish for microscopic analysis (19, 39).
Evaluation of Ad binding and FcγR expression.
Ad-immune complexes were formed as previously described, by preparing serial dilutions of neutralizing sera (0.01, 0.03, 0.1, 0.3, 1, 3, and 10%) in binding buffer (modified Eagle's medium without phenol red [Life Technologies], 1% bovine serum albumin [BSA; Sigma Chemical Co., St. Louis, MO], and 10 mM HEPES, pH 7.3 [BioFluids, Rockville, MD]) (19, 39). After preparation of the serial dilutions of serum, sufficient Ad was added to the mixture to yield a concentration of 1011 Cy3Ad particles/ml. The mixture was then incubated at 37°C for 5 min. Prior to infection, the cells were washed three times with binding buffer. A 30-μl volume of the Ad-serum mixture was added to 104 cells in the well of a coverslip bottom dish. The infection proceeded for 10 min at 37°C. The unbound virus was then washed away (three times with binding buffer) and returned to complete cell culture medium. Cells were incubated for an additional 60 min, washed three times with phosphate-buffered saline (PBS), and fixed with paraformaldehyde (4% in PBS, 20 min, 22°C; Electron Microscopy Sciences, Inc., Fort Washington, PA). After fixation, cells were prepared for indirect immunofluorescence by blocking with 5% goat serum (Calbiochem, La Jolla, CA) and 1% BSA in PBS plus 0.1% Triton X-100 (Sigma Chemical Co., St. Louis, MO) for 20 min. Primary antibody (fluorescein isothiocyanate-conjugated anti-CD32; Pharmingen/BD Biosciences, San Diego, CA) or an irrelevant primary antibody (fluorescein isothiocyanate-conjugated anti-CD64; Pharmingen/BD Biosciences) was applied to the cells at a 1:100 dilution in block for 60 min. Cells were washed thoroughly with 1% BSA in PBS, and nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI; Molecular Probes, Eugene, OR) at a concentration of 1 μg/ml in PBS. Cells were imaged with a Nikon Microphot SA microscope with a 60× N.A. 1.4 PlanApo differential interference contrast objective and DAPI, fluorescein, and Cy3 filters; a Princeton Instruments cooled charge-coupled device camera; and MetaMorph image analysis software (Universal Imaging, Inc., Downingtown, PA) as previously described (19, 39).
Evaluation of Ad-mediated β-galactosidase gene expression.
Monolayers of naive cells, AdNull-infected cells, or AdFcγR-infected cells were infected with Adβgal in a manner identical to the method described for infection with Cy3Ad above, with the exceptions that Adβgal was substituted for Cy3Ad and that infection occurred in wells of a 96-well plate instead of a coverslip bottom dish. Each condition was replicated in at least six wells per condition. After infection, cells were incubated for 24 h and β-galactosidase transgene expression was evaluated in cell lysates by quantitative chemiluminescence detection (Tropix, Bedford, MA) and normalized to the protein concentration of the cell lysate with the bicinchoninic acid reagent (Bio-Rad, Hercules, CA). In A549 cells, the β-galactosidase activity measured in cells infected in the absence of neutralizing serum was, on average, approximately 3 × 105 relative light units/mg or 2,000-fold above the background. In fibroblasts, the β-galactosidase activity measured in the absence of neutralizing serum was 2 × 104 relative light units/mg or 100-fold above the background.
To determine the IC50s for each condition, data were normalized to the control value for the data set (infection in the absence of serum). Normalized data from independent experiments were averaged to generate a dose-response curve for inhibition of Ad-mediated gene transfer by the serum. The data were used to generate a summary dose-response curve and were fitted with a spline (SigmaPlot for Windows; SPSS, Inc., Chicago, IL). The point at which the curve intersected a normalized transgene expression level of 0.5 (corresponding to 50% inhibition relative to the control) was taken as the IC50 for the experiment.
Competition with purified Fc domain.
To test the dependence of Ad-immune complex-mediated gene transfer on interaction of Fc domains with Fc receptors, complexes of Adβgal with 0.1% neutralizing serum (titer of 2,560) were used to infect AdNull- or AdFcγR-infected A549 cells. Infections were perfumed in the absence or presence of the purified Fc domain at 1 mg/ml (Calbiochem). Infection conditions and analysis of β-galactosidase activity were performed as described above.
Viability.
Because of reports of cytotoxic effects of Ad-immune complexes in the literature (34-37), the viability of cells was checked 1 h after the 10-min treatment with Ad-immune complexes created with dilutions of the serum with a titer of 2,560. Cell viability was assessed with two fluorophores, one that stains live cells (calcein AM) and one that stains dead cells (ethidium homodimer 1; Live-Dead Cytotoxicity Kit; Molecular Probes). Counts of live and dead cells were performed on fluorescence micrographs with automated image analysis software (MetaMorph; Universal Imaging).
Statistics.
All data are reported as the mean ± the standard error. Student's t test was used to compare conditions, with P values of <0.05 indicative of a significant difference.
RESULTS
FcγR-dependent cell association of Ad-immune complexes.
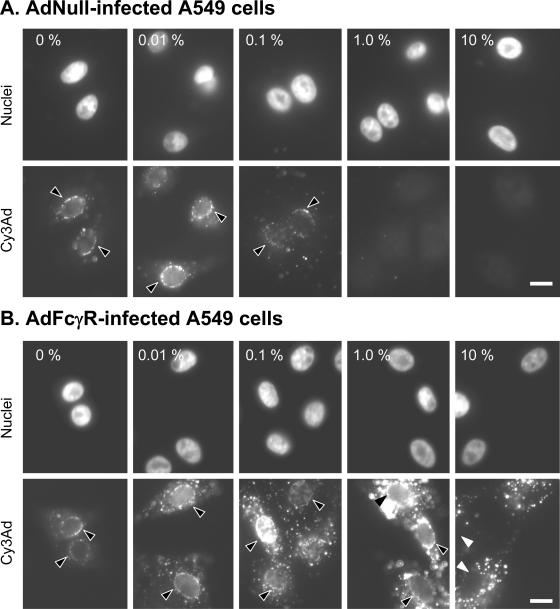
Immune complexes were formed by combining Cy3Ad with a range of concentrations of neutralizing serum (0.01% to 10%). The immune complexes were applied to A549 cells previously infected with either AdNull or AdFcγR. In AdNull-infected cells, cell-associated Ad decreased as the concentration of neutralizing serum increased, similar to data previously reported for naive A549 cells (74). At a concentration of 0.1% serum, cell-associated Cy3Ad was noticeably decreased and virtually all Cy3Ad was prevented from reaching the cells at a serum concentration of 1.0% (Fig. 1A). In contrast, A549 cells expressing FcγR maintained the ability to bind Ad-immune complexes at serum concentrations as high as 10% (Fig. 1B). Fluorescence intensity of cell-associated Cy3Ad indicated that the amount of viral capsid taken up by FcγR-expressing cells exceeded the amount of Cy3Ad internalized by a CAR-dependent mechanism in naive A549 cells. One striking observation concerned the ability of Cy3Ad to traffic to the nucleus. The nuclear perimeter in Fc receptor-bearing cells was demarcated by Cy3Ad even when Cy3Ad was mixed with as much as 1.0% neutralizing serum, indicating that some of the capsids internalized as Ad-immune complexes were capable of trafficking in a fashion similar to that of naive Cy3Ad. When AdNull-infected cells were compared to AdFcγR-infected cells, both in the presence of 1% neutralizing serum, it was clear that much of the capsid associated with FcγR-expressing cells was neutralized with respect to AdNull-infected cells. When FcγR-bearing A549 cells were exposed to Cy3Ad-immune complexes in the presence of neutralizing serum, the amount of Cy3Ad found in the cytoplasm rather than at the nucleus increased as the concentration of neutralizing serum increased (Fig. 1B). The intense, punctate concentrations of Cy3Ad in the cytoplasm indicated that some of virus was unable to traffic to the nucleus.
FIG. 1.
Association of Ad-immune complexes with A549 cells. A fluorochrome-conjugated Ad vector (Cy3Ad) was mixed with various concentrations of human serum (0.01% to 10%) containing anti-Ad neutralizing antibodies (undiluted titer, 2,560). After 5 min, the Cy3Ad-serum mixture was applied to monolayers of A549 cells previously infected with a control Ad (AdNull) or an Ad carrying the human FcγR gene (AdFcγR). After a 10-min infection period, unbound virus was removed by washing and Cy3Ad was allowed to traffic within cells for 60 min. The subcellular localization of Ad (Cy3, red channel) and nuclei (DAPI, blue channel) was evaluated by fluorescence microscopy. (A) AdNull-infected A549 cells treated with Cy3Ad in the presence of various concentrations of anti-Ad neutralizing antiserum. Note the association of Cy3Ad with the nucleus (black arrowheads) in the absence of serum and the disappearance of Cy3Ad from A549 cells with increasing serum concentrations. (B) AdFcγR-infected A549 cells treated with Cy3Ad in the presence of various concentrations of anti-Ad neutralizing antiserum. Note the sustained association of Cy3Ad with FcγR-bearing A549 cells despite the increasing concentration of serum. The association of Cy3Ad with the nuclear envelope in FcγR-expressing cells (black arrowheads) was maintained even at neutralizing serum concentrations that prevent association of Ad with AdNull-treated cells. At the highest serum concentration, no Ad was capable of trafficking to the nucleus (white arrowheads). Images were acquired and displayed under identical conditions. Bars = 10 μm.
To understand the significance of the subcellular localization of Ad following infection of FcγR-bearing cells with Ad-immune complexes, localization of the Ad capsid was compared to localization of the FcγRII protein. The amount of immune complexes associated with A549 cells corresponded to the level of FcγR expression in the cells (Fig. 2A). FcγRII-positive staining was found at the plasma membrane, as well as in a punctate pattern, typical of intracellular, endocytic vesicles that internalize and recycle the FcγR (Fig. 2B). The distribution of Cy3Ad included perinuclear accumulation, as well as a punctate pattern that colocalized with the punctate portion of FcγRII (Fig. 2B). Colocalization of Cy3Ad and FcγRII suggested that a portion of Cy3Ad failed to escape from FcγR-containing organelles. These data showed that anti-Ad neutralizing serum induced primarily extracellular neutralization at lower concentrations (e.g., 1% in the case of this serum) and induced complete intracellular neutralization only at higher concentrations (e.g., 10% in the case of this serum).
FIG. 2.
Intracellular accumulations of Ad-immune complexes in A549 cells colocalize with FcγR. FcγR-expressing A549 cells were infected with Cy3Ad in the presence of 0.1% anti-Ad neutralizing serum and processed as described in the legend to Fig. 1. After fixation, indirect immunofluorescence detection of FcγR was performed. The subcellular distribution of Cy3Ad (red), FcγR (green), and nuclei (blue) was evaluated by fluorescence microscopy. (A) FcγR-negative cells showed no interaction with Ad-immune complexes (white arrowheads), while FcγR-positive cells bound and internalized Ad-immune complexes, leading to successful trafficking of Cy3Ad to the nuclear envelope (black arrowheads). (B) High-magnification images of Cy3Ad and FcγR staining, showing colocalization. Note that colocalization of Cy3Ad and FcγR occurred in the cytoplasm (arrows), resulting in a yellow signal in the overlaid image. Bars = 10 μm.
FcγR-dependent Ad-mediated gene expression.
To confirm that trafficking of Cy3Ad corresponded to successful gene transfer, A549 cells were exposed to Ad-immune complexes that were formed with an Ad vector carrying the β-galactosidase transgene (Adβgal). In earlier in vitro studies using a different protocol for the formation of Ad-immune complexes, significant cytotoxicity was attributed to antibody-aggregated Ad (34-37). Differential cell viability could complicate the interpretation of the gene expression results. In the present studies, cell cultures were assessed for cell viability following infection with Ad-immune complexes. A549 cells maintained >90% viability regardless of the ratio of neutralizing serum to Ad particles (not shown).
The kinetics of neutralization were compared in naive A549 cells and A549 cells preinfected with either AdNull or Ad FcγR. Neutralization of Adβgal in AdNull-treated A549 cells was comparable to the kinetics of neutralization in naive A549 cells (Fig. 3). Ad-mediated gene expression was reduced by 50% when Ad was exposed to a low concentration of neutralizing serum (<0.1%). In contrast, AdFcγR-treated cells exhibited elevated levels of gene transfer to A549 cells and did not reach 50% neutralization until exposed to >1% serum (Fig. 3). Comparison of IC50s showed that cells expressing the Fc receptor required a 37-fold higher concentration of this neutralizing serum in order to achieve 50% inhibition of gene transfer compared with control AdNull-infected cells (Fig. 3). The precise shift in the IC50 is likely to be a unique property of a given serum and based on the precise combination of anti-Ad epitopes and anti-Ad isotypes in the serum.
FIG. 3.
FcγR-mediated gene transfer by neutralized Ad-immune complexes. An Ad vector expressing the β-galactosidase transgene (Adβgal) was mixed with various concentrations of human serum (0.01% to 10%) containing anti-Ad neutralizing antibodies (titer, 2,560). After 5 min, the Adβgal-serum mixture was applied to monolayers of naive A549 cells or A549 cells previously infected with either AdNull or AdFcγR. β-Galactosidase activity was assayed after 24 h and normalized to cellular protein content (relative light units per milligram of protein). Data were normalized to Adβgal infection in the absence of serum for each target cell type (dashed line at y = 1.0). The IC50 was determined as the concentration of serum that resulted in a 50% decrease in β-galactosidase gene expression (dotted line at y = 0.5). Comparable dose-response curves were obtained in the presence of neutralizing serum during infection of either naive or AdNull-infected A549 cells, In contrast, addition of neutralizing serum during infection of AdFcγR-infected A549 cells shifted the dose-response inhibition curve, resulting in a higher IC50.
To test whether the shift in IC50 was generally related to the neutralizing titer of the anti-Ad serum, two other sera with lower and higher neutralizing titers were evaluated. As predicted, the IC50 measured in naive A549 cells corresponded to the anti-Ad titer of the serum. With the same dilution series of sera with a low or high anti-Ad neutralizing titer (undiluted titer of either 20 or 49,000), the observed IC50 shifted correspondingly, indicating that the inhibition curve was governed by the anti-Ad antibodies rather than another serum component (Fig. 4). For each serum, the IC50 for FcγR-expressing cells was significantly higher than for AdNull-infected or naive cells. In fact, 50% inhibition did not occur in the serum with the lowest titer (undiluted titer, 20), even when the serum was present at the highest concentration (10%). The IC50s for one serum (undiluted titer, 2,560), either with or without prior heat inactivation, were similar, suggesting that complement was not involved in the interaction of Ad-immune complexes with FcγR-expressing cells (Fig. 4).
FIG. 4.
Effect of Fc receptor expression on IC50 of anti-Ad sera. Ad-immune complexes were formed and applied to naive, AdNull-infected, or AdFcγR-infected A549 cells with dilutions of sera with various titers, including titers of 20, 2,560, and 49,000. In addition, anti-Ad serum (titer, 2,560) was used to form Ad-immune complexes without prior heat inactivation of the serum. IC50s were determined as described in the legend to Fig. 3.
Characterization of the role of the Fc receptor in gene transfer following uptake of Ad-immune complexes.
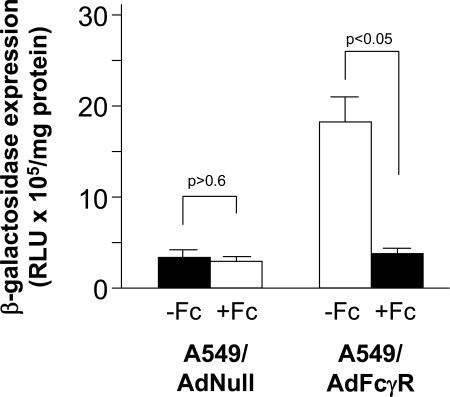
To confirm the importance of the Fc-Fc receptor interaction in the rescue of Ad-immune complexes, the uptake of Ad-immune complexes was assayed in the absence or presence of the purified Fc domain, a competitive inhibitor of antibody-Fc receptor binding. AdNull-infected A549 cells supported a minimal level of transgene expression in the presence of 0.1% serum, regardless of the presence or absence of the purified Fc domain (Fig. 5). In contrast, the high level of gene transfer in Fc receptor-bearing A549 cells was reduced by more than 75% in the presence of the purified Fc domain, indicating the importance of the Fc-Fc receptor interaction in uptake of Ad-immune complexes.
FIG. 5.
Dependence of Ad-immune complex uptake on Fc-Fc receptor interaction. To demonstrate that Ad-immune complex uptake requires interaction of the Fc domain of immunoglobulins with the Fc receptor, A549 cells were infected with either AdNull or AdFcγR and then exposed to Ad-immune complexes prepared by mixing Adβgal with 0.1% anti-Ad serum (titer, 2,560) in the presence or absence of the purified Fc domain at 1 mg/ml. After 24 h, β-galactosidase activity was assessed and normalized to the protein content of the sample. RLU, relative light units.
Prior studies of FcγR have demonstrated that the cytoplasmic tail is required for phagocytosis of large ligands (29). In contrast, the cytoplasmic tail of endocytic receptors is normally required for optimal internalization but is not necessary to accomplish endocytosis of small ligands (32, 50). To test whether the cytoplasmic domain is required, A549 cells were preinfected with Ad vectors carrying either full-length FcγR or a truncated version of the FcγR gene that lacked the cytoplasmic domain (AdtaillessFcγR). When challenged with a range of doses of neutralizing serum, A549 cells expressing the full-length Fc receptor or the tailless Fc receptor had identical kinetics of inhibition, indicating that the cytoplasmic tail of the Fc receptor is not required for binding and entry of Ad-immune complexes into cells (Fig. 6).
FIG. 6.
Lack of dependence of Ad-immune complex uptake on the Fc receptor cytoplasmic domain. To evaluate the mechanism of internalization of Ad-immune complexes, gene transfer to Fc receptor-expressing cells was evaluated in cells expressing either full-length FcγR or a truncated version of FcγR that lacks the cytoplasmic tail. A549 cells were infected with Ad FcγR or AdtaillessFcγR and subsequently exposed to Ad-immune complexes prepared as described in the legend to Fig. 3. After 24 h, β-galactosidase activity was assessed and normalized to the protein content of the sample.
Receptor dependence of FcγR-mediated rescue of neutralized Ad.
A549 cells express both the Ad high-affinity receptor for the Ad fiber protein (CAR) and αV integrins, which serve as the lower-affinity receptor for the Ad penton base protein (23, 62). Since capsid-receptor interactions play a critical role during Ad escape to the cytosol (48, 49, 63, 77), the fiber-CAR interaction and the penton base-integrin interaction were investigated.
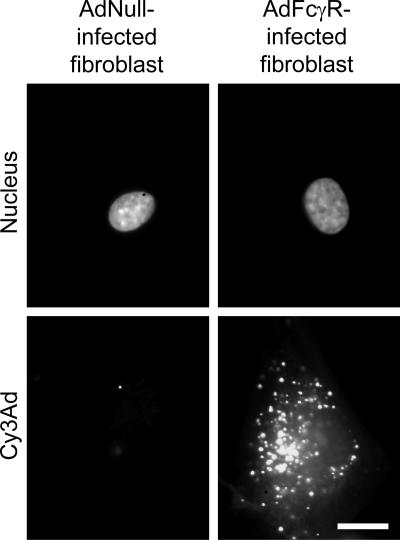
To evaluate the effect of loss of fiber-CAR interaction, CAR-deficient human dermal fibroblasts previously treated with AdNull or Ad FcγR were evaluated for uptake of Ad-immune complexes. Each condition was analyzed for viral trafficking with Cy3Ad-immune complexes and for gene expression with Adβgal-immune complexes. As expected, very little Cy3Ad bound to AdNull-infected fibroblasts, regardless of the presence of serum, since the fibroblasts lacked a high-affinity receptor for either the Ad capsid or immunoglobulins in the Ad-immune complexes. Ad FcγR-infected fibroblasts bound a small amount of Cy3Ad in the absence of serum, but in the presence of 0.1% neutralizing anti-Ad serum, copious amounts of Cy3Ad were associated with FcγR-bearing fibroblasts (Fig. 7). A smaller proportion of internalized Ad was observed at the nuclear envelope compared to A549 cells. This difference likely reflected the propensity of Ad to traffic to the microtubule organizing center, as well as the nucleus, in fibroblasts (5, 66).
FIG. 7.
Association of Ad-immune complexes with CAR-deficient human dermal fibroblasts modified to express FcγR. Cy3Ad-immune complexes were prepared and applied to human dermal fibroblasts as described in the legend to Fig. 1. The target cells used included human dermal fibroblasts previously infected with either AdNull or AdFcγR. After a 10-min infection, unbound virus was removed by washing. Cells were incubated for an additional 60 min to allow Cy3Ad trafficking within the cell. After fixation, cell nuclei were stained with a fluorophore (DAPI) and the position of virus within the cell was evaluated by fluorescence microscopy. Note that 0.1% neutralizing serum was sufficient to prevent Ad association with AdNull-infected fibroblasts. However, with the same concentration of neutralizing serum, a high level of cell-associated Cy3-Ad was observed. Bar = 10 μm.
To examine gene transfer via uptake of Ad-immune complexes in the absence of CAR, naive fibroblasts or fibroblasts previously infected with AdNull or AdFcγR were infected with immune complexes formed with an Ad vector that carried the β-galactosidase transgene (Adβgal) (Fig. 8A). The most striking aspect of the neutralization curve for Fc receptor-bearing fibroblasts was the dramatic increase in gene transfer at low concentrations of serum. The presence of 0.3% neutralizing serum led to an increase in gene transfer to Fc receptor-bearing fibroblasts of 64-fold compared to gene transfer in the absence of serum. The dramatic increase in gene transfer reflected the introduction of a high-affinity immunoglobulin G-FcγR interaction that enabled Ad-immune complexes to bind to cells. The high level of gene transfer to Fc receptor-bearing fibroblasts was accompanied by a 6.5-fold increase in the IC50 of the neutralizing serum (Fig. 8B). The IC50 for AdNull-infected fibroblasts was 0.2% serum, while the IC50 for AdFcγR-infected fibroblasts was 1.3% serum.
FIG. 8.
FcγR-mediated gene transfer by neutralized Ad-immune complexes in CAR-deficient fibroblasts. To determine the contribution of the native high-affinity interaction of Ad with CAR during immune complex uptake, immune complexes were exposed to naive (CAR-deficient) fibroblasts or fibroblasts infected with AdNull or AdFcγR. Immune complex formation and evaluation of gene transfer were performed as described in the legend to Fig. 3. (A) Ad-mediated gene transfer as a function of the anti-Ad serum concentration in naive, AdNull-infected, and AdFcγR-infected A549 cells. (B) IC50s for all of the conditions tested.
To evaluate the requirement for the penton base-integrin interaction during FcγR-dependent infection of A549 cells, Ad-immune complexes were created with either wild-type Ad capsid or an Ad capsid that had been genetically altered to remove the RGD integrin-binding motif in the penton base (AdΔRGDβgal) (76). A comparison of the kinetics of gene transfer following formation of Adβgal- or AdΔRGDβgal-immune complexes demonstrated striking differences in gene transfer in the presence or absence of integrin interaction (Fig. 9A). In A549 cells expressing FcγR, different IC50s were determined for the same serum, depending on the Ad vector that had been incorporated into the immune complex. Strikingly, when Ad-immune complexes were formed with AdΔRGDβgal, the IC50 of the serum for inhibiting gene transfer to FcγR-expressing A549 cells (0.09%) was comparable to the IC50 of immune complexes formed with Adβgal on non-FcγR expressing cells (0.03 to 0.06%) (Fig. 9B, black bars). These data differ from the result obtained when immune complexes were formed with Adβgal (Fig. 9B, white bars), suggesting that the penton base-integrin interaction is important for Ad-immune complexes to deliver viable Ad capsids to FcγR-expressing cells.
FIG. 9.
FcγR-mediated gene transfer by neutralized Ad-immune complexes in the absence of penton base-integrin interaction. To determine the contribution of the penton base-integrin interaction during immune complex uptake, Ad-immune complexes were formed from either Adβgal or an Ad vector that had been modified to remove the integrin-binding RGD sequence from the penton base protein (AdΔRGDβgal). The Ad-immune complexes were applied to A549 cells that had previously been infected with AdFcγR. Evaluation of gene transfer was performed as described in the legend to Fig. 3. (A) Ad-mediated gene transfer to FcγR as a function of anti-Ad serum concentration with either Adβgal or AdΔRGDβgal. (B) IC50s for all of the conditions tested.
DISCUSSION
The experiments described herein were designed to answer the following question: is neutralized Ad in Ad-immune complexes no longer infectious? This question was based on the observation that increasing concentrations of neutralizing serum led primarily to extracellular neutralization, i.e., abrogation of viral interaction with cells (74). This observation left open the possibility that viable Ad capsids, as well as nonviable Ad capsids, were caught in the Ad-immune complexes but that the viable Ad capsids no longer had access to the target cell surface. To answer this question, an experimental system was designed to force Ad-immune complexes to associate with target cells. Target cells were genetically modified to express FcγR, a receptor for the Fc domain of immunoglobulins. While it is a formal possibility that some nonspecific effect of the exogenous membrane of a membrane protein accounts for the observations described here, the fact that the enhanced infection of Fc receptor-expressing target cells was abrogated by an excess of the purified Fc domain argues that it was, in fact, the Fc-Fc receptor interaction that led to immune complex uptake. The Fc-Fc receptor interaction is physiologically relevant in that this receptor is expressed on tissue macrophages and dendritic cells and leads to uptake and antigen presentation of opsonized materials in vivo. Historically, viral neutralization has been characterized on the basis of quantitative and qualitative analyses of the neutralizing serum. This study demonstrates that viral neutralization is also a function of the characteristics of target cells, potentially leading to new interpretations of viral neutralization in the complexity of an in vivo setting.
The data clearly demonstrated that uptake of Ad-immune complexes via the Fc receptor not only led to elevated viral gene expression but also effectively changed the neutralization properties (IC50) of the serum. It is important to note that the extent to which a given serum will show a change in its IC50 is likely to be determined by a number of factors, including the isotypes of antibodies against the virus and the number and identity of epitopes contributing to the antiserum. Unlike prior studies of viral neutralization by serum in vitro which employed extended incubations of virus with neutralizing serum prior to infecting cells, the present study intended to model the interactions that might occur upon administration of a gene transfer vector to a patient with preexisting anti-Ad immunity. With the knowledge that viable Ad capsids are quickly cleared from serum (2, 70, 83), an acute in vitro model of immune complex formation and target cell infection was designed. To mimic acute exposure of Ad to immune sera, followed by rapid access to target cells, the experimental protocol called for mixing of Ad and anti-Ad serum for only 5 min prior to infection of a monolayer of target cells for 10 min. The concentration of virus used for the infections (1011 particles per ml) is comparable to the dose of virus administered in clinical trials for local or systemic administration (19).
In the present study, no immune complex-induced cytotoxicity was observed. Kjellen and Ankerst used a protocol in which 2 h was allowed for Ad-immune complex formation with wild-type Ad and 2 to 6 h was allowed for immune complex exposure to cells (34-37). These investigators reported cytotoxicities as high as 100%, depending on the ratio of serum to Ad and the time of exposure of complexes to target cells. In contrast, the protocol employed herein limited the time of formation of Ad-immune complexes and the duration of exposure of complexes to target cells as described above. As a result, cell viability was >90% for all conditions. The observation that gene transfer occurred independent of the presence of the cytoplasmic tail of the Fc receptor underscored the likelihood that endocytosis rather than phagocytosis was sufficient to internalize the small Ad-immune complexes formed by this protocol. There exists a potential for formation of cytotoxic Ad-immune complexes in vivo, and importantly, Ad-immune complexes formed with wild-type, replication-competent Ad have been proposed as causative agents in the canine eye disorder anterior uveitis and in canine and human nephrotoxicity (1, 84, 85, 87). However, cytotoxicity due to the formation of Ad-immune complexes from replication-deficient gene transfer vectors has not been reported, and under the conditions used in this study, significant cytotoxicity was not observed.
A number of avenues of research have focused on the ability of antibodies to modulate viral infection. The effect of antibodies reported here confirms previous reports of antibody-enhanced infection in a number of viruses, including human immunodeficiency virus (HIV), coxsackie virus B4, and rhinovirus (6, 24, 25, 69). Unlike the observations reported here, antibody-enhanced infection by viruses that are tropic for FcγR-bearing cells did not constitute a change in the tropism of the virus. The observations presented here show that binding of neutralizing antibodies to Ad may shift the tropism of Ad away from native target cells (CAR-expressing epithelial cells) toward a new target cell (FcγR-bearing antigen-presenting cells). This report is also distinct from studies in which nonneutralizing antibodies have been used to retarget Ad to FcγR-bearing cells (14, 44, 45), since modification of tropism was observed following treatment with whole, unfractionated neutralizing serum rather than selected monoclonal antibodies. Finally, this study is distinct from studies in which human sera have been analyzed to determine the relative contribution of neutralizing antibodies against each of the major capsid proteins (12, 52, 55, 59, 60, 67, 68, 73, 75, 79) since those studies examined the effects of subsets of antibodies rather than whole, unfractionated neutralizing serum on the infectivity of Ad. While this report does not test the hypothesis that Fc receptor-mediated uptake of Ad-immune complexes could be a means of propagating a wild-type infection in a host organism, it is interesting that persistent subgroup C Ad infections have been reported in lymphoid tissue of humans and mice (13, 15, 18, 31, 38, 51, 54, 58, 64, 72), where a high concentration of Fc receptor-bearing cells resides. Also of interest, follicular dendritic cells, T lymphocytes, and B lymphocytes are all capable of expressing Fc receptors (40, 71) and persistent Ad infections have been noted in patients with neutralizing anti-Ad antibodies (64). In contrast, these cell types have low or undetectable levels of CAR expression (26-28, 78, 82). Among the three lymphoid cell types, CD4+ CD8+ CD3+ T lymphocytes appear to be the major harbor for Ad in lymphoid tissue and support low levels of viral replication, although a small but detectable population of Ad was detected in a CD4+ CD8+ CD3− population that included dendritic cells (16, 43). Garnett et al. (16) noted that the level of Ad DNA decreased with age in the study population, correlating with the age-related loss of Fc receptor expression on follicular dendritic cells (4). The latent infection by Ad in lymphoid tissue may then result from a complex interaction of viruses, anti-Ad antibodies, Fc receptor-expressing cells, and low rates of viral replication.
The observation that a virus might be capable of gene expression in FcγR-bearing cells when it has been effectively neutralized with respect to infection in other cell types may have important implications for Ad-mediated therapeutic gene transfer and, in particular, Ad-based vaccines. The observations in the present report suggest a pathway for antigen presentation in which a host with preexisting anti-Ad immunity is exposed to Ad. After the formation of Ad-immune complexes, FcγR-bearing antigen-presenting cells may phagocytose the complexes, leading to presentation of viral capsid proteins via major histocompatibility complex (MHC) class II. However, some small fraction of the virus may escape from the phagosome and productively infect the antigen-presenting cell, leading to presentation of viral proteins via MHC class I. The implication of cross-priming both in pathogenic viral infection and in the clinical setting of viral gene transfer deserves additional study. The idea that the class I and class II pathways could be activated in a host with preexisting anti-Ad neutralizing antibody titers further predicts that an Ad-based vaccine could be effective in a patient with an anti-Ad neutralizing antibody titer. Some prior reports support this hypothesis. Although expression of marker genes cannot be detected following systemic administration of Ad vectors in the presence of a neutralizing anti-Ad antibody titer (9, 19, 33, 41, 42, 86), neutralizing antibodies and cytotoxic T-lymphocyte responses that can confer protective immunity have been developed following delivery of gene transfer vectors under similar conditions (7, 20, 60, 68). In fact, in a recent analysis of data relating to the use of Ad vectors to deliver an HIV vaccine in a human clinical trial, the trial sponsor chose to stratify the data, with one group of patients carrying preexisting anti-Ad antibody titers of <200 while a second tier had anti-Ad antibody titers of ≥200. The group with the detectable but low anti-Ad antibody titer developed an immune response to the Ad-encoded HIV antigen (N. Chirmule, personal communication). Even though neutralizing antibody titers are likely to prevent therapeutic gene transfer to a target cell, gene expression in antigen-presenting cells mediated by Ad-immune complexes might provide sufficient stimulation to boost an immune response. The observation that Ad-immune complexes can be used to transfer genes to dendritic cells in vitro (45) also supports the possibility that this phenomenon could contribute to immune recognition of Ad-encoded gene products in vivo.
The analysis of Ad receptors that contribute to viral gene expression from Ad-immune complexes yielded important insights with implications for Ad-mediated gene therapy. FcγR-mediated infection by Ad-immune complexes formed with the native Ad capsid versus an AdΔRGD capsid in A549 cells demonstrated that the RGD sequence in the penton base was essential to observe the shift in IC50. If uptake of Ad-immune complexes by antigen-presenting cells in vivo leads to cross-priming through viral gene expression, then use of an AdΔRGD capsid may limit MHC class I presentation in antigen-presenting cells and therefore might blunt the cell-mediated immune response against vector-encoded genes in patients with preexisting anti-Ad humoral immunity.
Acknowledgments
We thank N. Mohamed for help in preparing the manuscript, B. G. Harvey (Weill Medical College) for providing characterized human anti-Ad neutralizing antiserum, S. Schreiber (University of Pennsylvania) for FcγR constructs, and R. McKinney for technical assistance.
These studies were supported, in part, by P01 HL59312; the Will Rogers Foundation, Los Angeles, CA; and the Cystic Fibrosis Foundation.
REFERENCES
- 1.Aguirre, G., L. Carmichael, and S. Bistner. 1975. Corneal endothelium in viral induced anterior uveitis. Ultrastructural changes following canine adenovirus type 1 infection. Arch. Ophthalmol. 93:219-224. [DOI] [PubMed] [Google Scholar]
- 2.Alemany, R., and D. T. Curiel. 2001. CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Ther. 8:1347-1353. [DOI] [PubMed] [Google Scholar]
- 3.Aste-Amezaga, M., A. J. Bett, F. Wang, D. R. Casimiro, J. M. Antonello, D. K. Patel, E. C. Dell, L. L. Franlin, N. M. Dougherty, P. S. Bennett, H. C. Perry, M. E. Davies, J. W. Shiver, P. M. Keller, and M. D. Yeager. 2004. Quantitative adenovirus neutralization assays based on the secreted alkaline phosphatase reporter gene: application in epidemiologic studies and in the design of adenovector vaccines. Hum. Gene Ther. 15:293-304. [DOI] [PubMed] [Google Scholar]
- 4.Aydar, Y., J. Wu, J. Song, A. K. Szakal, and J. G. Tew. 2004. FcγRII expression on follicular dendritic cells and immunoreceptor tyrosine-based inhibition motif signaling in B cells. Eur. J. Immunol. 34:98-107. [DOI] [PubMed] [Google Scholar]
- 5.Bailey, C. J., R. G. Crystal, and P. L. Leopold. 2002. Adenovirus-microtubule organizing center interaction and delayed kinetics of adenovirus association with the nucleus following infection of endothelial cells. Mol. Ther. 5:S54. [Google Scholar]
- 6.Baravalle, G., M. Brabec, L. Snyers, D. Blaas, and R. Fuchs. 2004. Human rhinovirus type 2-antibody complexes enter and infect cells via Fc-γ receptor IIB1. J. Virol. 78:2729-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barouch, D. H., M. G. Pau, J. H. Custers, W. Koudstaal, M. J. Havenga, D. M. Truitt, S. M. Sumida, M. G. Kishko, J. C. Arthur, B. Korioth-Schmitz, M. H. Newberg, D. A. Gorgone, M. A. Lifton, D. L. Panicali, G. J. Nabel, N. L. Letvin, and J. Goudsmit. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 172:6290-6297. [DOI] [PubMed] [Google Scholar]
- 8.Bezdicek, P., S. Worgall, I. Kovesdi, M. K. Kim, J. G. Park, T. Vincent, P. L. Leopold, A. D. Schreiber, and R. G. Crystal. 1999. Enhanced liver uptake of opsonized red blood cells after in vivo transfer of FcγRIIA cDNA to the liver. Blood 94:3448-3455. [PubMed] [Google Scholar]
- 9.Chen, P., I. Kovesdi, and J. T. Bruder. 2000. Effective repeat administration with adenovirus vectors to the muscle. Gene Ther. 7:587-595. [DOI] [PubMed] [Google Scholar]
- 10.Chen, Y., D. C. Yu, D. Charlton, and D. R. Henderson. 2000. Pre-existent adenovirus antibody inhibits systemic toxicity and antitumor activity of CN706 in the nude mouse LNCaP xenograft model: implications and proposals for human therapy. Hum. Gene Ther. 11:1553-1567. [DOI] [PubMed] [Google Scholar]
- 11.Chirmule, N., K. Propert, S. Magosin, Y. Qian, R. Qian, and J. Wilson. 1999. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 6:1574-1583. [DOI] [PubMed] [Google Scholar]
- 12.Couch, R. B., J. A. Kasel, H. G. Perreira, A. T. Haase, and V. Knight. 1973. Induction of immunity in man by crystalline adenovirus type 5 capsid antigens. Proc. Soc. Exp. Biol. Med. 143:905-910. [DOI] [PubMed] [Google Scholar]
- 13.Devi, K. I. 1968. Adenovirus infections in Delhi: isolation from tonsils and a serological survey. Indian J. Med. Res. 56:20-26. [PubMed] [Google Scholar]
- 14.Ebbinghaus, C., A. Al-Jaibaji, E. Operschall, A. Schoffel, I. Peter, U. F. Greber, and S. Hemmi. 2001. Functional and selective targeting of adenovirus to high-affinity Fcγ receptor I-positive cells by using a bispecific hybrid adapter. J. Virol. 75:480-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans, A. S. 1958. Latent adenovirus infections of the human respiratory tract. Am. J. Hyg. 67:256-266. [DOI] [PubMed] [Google Scholar]
- 16.Garnett, C. T., D. Erdman, W. Xu, and L. R. Gooding. 2002. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J. Virol. 76:10608-10616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gergely, J., and G. Sarmay. 1990. The two binding-site models of human IgG binding Fc γ receptors. FASEB J. 4:3275-3283. [DOI] [PubMed] [Google Scholar]
- 18.Green, M., W. S. Wold, J. K. Mackey, and P. Rigden. 1979. Analysis of human tonsil and cancer DNAs and RNAs for DNA sequences of group C (serotypes 1, 2, 5, and 6) human adenoviruses. Proc. Natl. Acad. Sci. USA 76:6606-6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvey, B. G., P. L. Leopold, N. R. Hackett, T. M. Grasso, P. M. Williams, A. L. Tucker, R. J. Kaner, B. Ferris, I. Gonda, T. D. Sweeney, R. Ramalingam, I. Kovesdi, S. Shak, and R. G. Crystal. 1999. Airway epithelial CFTR mRNA expression in cystic fibrosis patients after repetitive administration of a recombinant adenovirus. J. Clin. Investig. 104:1245-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto, M., J. L. Boyer, N. R. Hackett, J. M. Wilson, and R. G. Crystal. 2005. Induction of protective immunity to anthrax lethal toxin with a nonhuman primate adenovirus-based vaccine in the presence of preexisting anti-human adenovirus immunity. Infect. Immun. 73:6885-6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemminki, A., M. Wang, R. A. Desmond, T. V. Strong, R. D. Alvarez, and D. T. Curiel. 2002. Serum and ascites neutralizing antibodies in ovarian cancer patients treated with intraperitoneal adenoviral gene therapy. Hum. Gene Ther. 13:1505-1514. [DOI] [PubMed] [Google Scholar]
- 22.Hersh, J., R. G. Crystal, and B. Bewig. 1995. Modulation of gene expression after replication-deficient, recombinant adenovirus-mediated gene transfer by the product of a second adenovirus vector. Gene Ther. 2:124-131. [PubMed] [Google Scholar]
- 23.Hidaka, C., E. Milano, P. L. Leopold, J. M. Bergelson, N. R. Hackett, R. W. Finberg, T. J. Wickham, I. Kovesdi, P. Roelvink, and R. G. Crystal. 1999. CAR-dependent and CAR-independent pathways of adenovirus vector-mediated gene transfer and expression in human fibroblasts. J. Clin. Investig. 103:579-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hober, D., W. Chehadeh, A. Bouzidi, and P. Wattre. 2001. Antibody-dependent enhancement of coxsackievirus B4 infectivity of human peripheral blood mononuclear cells results in increased interferon-alpha synthesis. J. Infect. Dis. 184:1098-1108. [DOI] [PubMed] [Google Scholar]
- 25.Homsy, J., M. Meyer, and J. A. Levy. 1990. Serum enhancement of human immunodeficiency virus (HIV) infection correlates with disease in HIV-infected individuals. J. Virol. 64:1437-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horvath, J., and J. M. Weber. 1988. Nonpermissivity of human peripheral blood lymphocytes to adenovirus type 2 infection. J. Virol. 62:341-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang, S., R. I. Endo, and G. R. Nemerow. 1995. Upregulation of integrins αvβ3 and αvβ5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J. Virol. 69:2257-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang, S., T. Kamata, Y. Takada, Z. M. Ruggeri, and G. R. Nemerow. 1996. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J. Virol. 70:4502-4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Indik, Z., C. Kelly, P. Chien, A. I. Levinson, and A. D. Schreiber. 1991. Human FcγRII, in the absence of other Fc γ receptors, mediates a phagocytic signal. J. Clin. Investig. 88:1766-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Indik, Z. K., J. G. Park, S. Hunter, and A. D. Schreiber. 1995. Structure/function relationships of Fcγ receptors in phagocytosis. Semin. Immunol. 7:45-54. [DOI] [PubMed] [Google Scholar]
- 31.Israel, M. 1962. The viral flora of enlarged tonsils and adenoids. J. Pathol. Bacteriol. 84:169-176. [Google Scholar]
- 32.Johnson, L. S., K. W. Dunn, B. Pytowski, and T. E. McGraw. 1993. Endosome acidification and receptor trafficking: bafilomycin A1 slows receptor externalization by a mechanism involving the receptor's internalization motif. Mol. Biol. Cell 4:1251-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kass-Eisler, A., E. Falck-Pedersen, D. H. Elfenbein, M. Alvira, P. M. Buttrick, and L. A. Leinwand. 1994. The impact of developmental stage, route of administration and the immune system on adenovirus-mediated gene transfer. Gene Ther. 1:395-402. [PubMed] [Google Scholar]
- 34.Kjellen, L. 1972. Cytotoxic virus-antibody complexes. J. Infect. Dis. 126:682-683. [DOI] [PubMed] [Google Scholar]
- 35.Kjellen, L. 1972. Cytotoxicity by antigen aggregation. Adenovirus neutralization assays as a model. Arch. Gesamte Virusforsch. 39:1-12. [DOI] [PubMed] [Google Scholar]
- 36.Kjellen, L., and J. Ankerst. 1973. Cytotoxicity of adenovirus-antibody aggregates: sensitivity to different cell strains, and inhibition by hexon antiserum and by complement. J. Virol. 12:25-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kjellen, L., and J. Ankerst. 1973. Cytotoxicity of adenovirus-antibody complexes. Specificity of antibody and virus-antibody ratio at which cytotoxicity is induced. Eur. J. Immunol. 3:78-84. [DOI] [PubMed] [Google Scholar]
- 38.Lambriex, M., and J. van der Veen. 1976. Comparison of replication of adenovirus type 2 and type 4 in human lymphocyte cultures. Infect. Immun. 14:618-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leopold, P. L., B. Ferris, I. Grinberg, S. Worgall, N. R. Hackett, and R. G. Crystal. 1998. Fluorescent virions: dynamic tracking of the pathway of adenoviral gene transfer vectors in living cells. Hum. Gene Ther. 9:367-378. [DOI] [PubMed] [Google Scholar]
- 40.Lynch, R. G. 2000. Regulatory roles for FcγRIII (CD16) and FcγRII (CD32) in the development of T- and B-lineage lymphoid cells. J. Leukoc. Biol. 67:279-284. [DOI] [PubMed] [Google Scholar]
- 41.Mack, C. A., W. R. Song, H. Carpenter, T. J. Wickham, I. Kovesdi, B. G. Harvey, C. J. Magovern, O. W. Isom, T. Rosengart, E. Falck-Pedersen, N. R. Hackett, R. G. Crystal, and A. Mastrangeli. 1997. Circumvention of anti-adenovirus neutralizing immunity by administration of an adenoviral vector of an alternate serotype. Hum. Gene Ther. 8:99-109. [DOI] [PubMed] [Google Scholar]
- 42.Mastrangeli, A., B. G. Harvey, J. Yao, G. Wolff, I. Kovesdi, R. G. Crystal, and E. Falck-Pedersen. 1996. “Sero-switch” adenovirus-mediated in vivo gene transfer: circumvention of anti-adenovirus humoral immune defenses against repeat adenovirus vector administration by changing the adenovirus serotype. Hum. Gene Ther. 7:79-87. [DOI] [PubMed] [Google Scholar]
- 43.McNees, A. L., J. A. Mahr, D. Ornelles, and L. R. Gooding. 2004. Postinternalization inhibition of adenovirus gene expression and infectious virus production in human T-cell lines. J. Virol. 78:6955-6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meier, O., M. Gastaldelli, K. Boucke, S. Hemmi, and U. F. Greber. 2005. Early steps of clathrin-mediated endocytosis involved in phagosomal escape of Fcγ receptor-targeted adenovirus. J. Virol. 79:2604-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mercier, S., H. Rouard, M. H. fau-Larue, and M. Eloit. 2004. Specific antibodies modulate the interactions of adenovirus type 5 with dendritic cells. Virology 322:308-317. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell, M. A., M. M. Huang, P. Chien, Z. K. Indik, X. Q. Pan, and A. D. Schreiber. 1994. Substitutions and deletions in the cytoplasmic domain of the phagocytic receptor FcγRIIA: effect on receptor tyrosine phosphorylation and phagocytosis. Blood 84:1753-1759. [PubMed] [Google Scholar]
- 47.Mittereder, N., K. L. March, and B. C. Trapnell. 1996. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 70:7498-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyazawa, N., R. G. Crystal, and P. L. Leopold. 2001. Adenovirus serotype 7 retention in a late endosomal compartment prior to cytosol escape is modulated by fiber protein. J. Virol. 75:1387-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyazawa, N., P. L. Leopold, N. R. Hackett, B. Ferris, S. Worgall, E. Falck-Pedersen, and R. G. Crystal. 1999. Fiber swap between adenovirus subgroups B and C alters intracellular trafficking of adenovirus gene transfer vectors. J. Virol. 73:6056-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mukherjee, S., R. N. Ghosh, and F. R. Maxfield. 1997. Endocytosis. Physiol. Rev. 77:759-803. [DOI] [PubMed] [Google Scholar]
- 51.Neumann, R., E. Genersch, and H. J. Eggers. 1987. Detection of adenovirus nucleic acid sequences in human tonsils in the absence of infectious virus. Virus Res. 7:93-97. [DOI] [PubMed] [Google Scholar]
- 52.Norrby, E. 1969. The structural and functional diversity of adenovirus capsid components. J. Gen. Virol. 5:221-236. [DOI] [PubMed] [Google Scholar]
- 53.Nwanegbo, E., E. Vardas, W. Gao, H. Whittle, H. Sun, D. Rowe, P. D. Robbins, and A. Gambotto. 2004. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin. Diagn. Lab. Immunol. 11:351-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pereira, H. G. 1972. Persistent infection by adenoviruses. J. Clin. Pathol. Suppl. (R. Coll. Pathol.) 6:39-42. [PMC free article] [PubMed] [Google Scholar]
- 55.Pettersson, U., L. Philipson, and S. Hoglund. 1968. Structural proteins of adenoviruses. II. Purification and characterization of the adenovirus type 2 fiber antigen. Virology 35:204-215. [DOI] [PubMed] [Google Scholar]
- 56.Rosenfeld, M. A., W. Siegfried, K. Yoshimura, K. Yoneyama, M. Fukayama, L. E. Stier, P. K. Paakko, P. Gilardi, L. D. Stratford-Perricaudet, M. Perricaudet, S. Jallat, A. Pavirani, J.-P. Lecocq, and R. G. Crystal. 1991. Adenovirus-mediated transfer of a recombinant α1-antitrypsin gene to the lung epithelium in vivo. Science 252:431-434. [DOI] [PubMed] [Google Scholar]
- 57.Rosenfeld, M. A., K. Yoshimura, B. C. Trapnell, K. Yoneyama, E. R. Rosenthal, W. Dalemans, M. Fukayama, J. Bargon, L. E. Stier, L. Stratford-Perricaudet, M. Perricaudet, W. B. Guggino, A. Pavirani, J.-P. Lecocq, and R. G. Crystal. 1992. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell 68:143-155. [DOI] [PubMed] [Google Scholar]
- 58.Rowe, W. P., R. J. Huebner, L. K. Gilmore, R. H. Parrott, and T. G. Ward. 1953. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc. Soc. Exp. Biol. Med. 84:570-573. [DOI] [PubMed] [Google Scholar]
- 59.Roy, S., D. S. Clawson, R. Calcedo, C. Lebherz, J. Sanmiguel, D. Wu, and J. M. Wilson. 2005. Use of chimeric adenoviral vectors to assess capsid neutralization determinants. Virology 333:207-214. [DOI] [PubMed] [Google Scholar]
- 60.Santra, S., M. S. Seaman, L. Xu, D. H. Barouch, C. I. Lord, M. A. Lifton, D. A. Gorgone, K. R. Beaudry, K. Svehla, B. Welcher, B. K. Chakrabarti, Y. Huang, Z. Y. Yang, J. R. Mascola, G. J. Nabel, and N. L. Letvin. 2005. Replication-defective adenovirus serotype 5 vectors elicit durable cellular and humoral immune responses in nonhuman primates. J. Virol. 79:6516-6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schulick, A. H., G. Vassalli, P. F. Dunn, G. Dong, J. J. Rade, C. Zamarron, and D. A. Dichek. 1997. Established immunity precludes adenovirus-mediated gene transfer in rat carotid arteries. Potential for immunosuppression and vector engineering to overcome barriers of immunity. J. Clin. Investig. 99:209-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seidman, M. A., S. M. Hogan, R. L. Wendland, S. Worgall, R. G. Crystal, and P. L. Leopold. 2001. Variation in adenovirus receptor expression and adenovirus vector-mediated transgene expression at defined stages of the cell cycle. Mol. Ther. 4:13-21. [DOI] [PubMed] [Google Scholar]
- 63.Seth, P., M. Rosenfeld, J. Higginbotham, and R. G. Crystal. 1994. Mechanism of enhancement of DNA expression consequent to cointernalization of a replication-deficient adenovirus and unmodified plasmid DNA. J. Virol. 68:933-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Snejdarova, V., V. Vonka, L. Kutinova, D. Rezacova, and V. Chladek. 1975. The nature of adenovirus persistence in human adenoid vegetations. Arch. Virol. 48:347-357. [DOI] [PubMed] [Google Scholar]
- 65.Stewart, A. K., N. J. Lassam, I. C. Quirt, D. J. Bailey, L. E. Rotstein, M. Krajden, S. Dessureault, S. Gallinger, D. Cappe, Y. Wan, C. L. Addison, R. C. Moen, J. Gauldie, and F. L. Graham. 2000. Adenovector-mediated gene delivery of interleukin-2 in metastatic breast cancer and melanoma: results of a phase 1 clinical trail. Gene Ther. 6:350-363. [DOI] [PubMed] [Google Scholar]
- 66.Strunze, S., L. C. Trotman, K. Boucke, and U. F. Greber. 2005. Nuclear targeting of adenovirus type 2 requires CRM1-mediated nuclear export. Mol. Biol. Cell 16:2999-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sumida, S. M., D. M. Truitt, M. G. Kishko, J. C. Arthur, S. S. Jackson, D. A. Gorgone, M. A. Lifton, W. Koudstaal, M. G. Pau, S. Kostense, M. J. Havenga, J. Goudsmit, N. L. Letvin, and D. H. Barouch. 2004. Neutralizing antibodies and CD8+ T lymphocytes both contribute to immunity to adenovirus serotype 5 vaccine vectors. J. Virol. 78:2666-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sumida, S. M., D. M. Truitt, A. A. Lemckert, R. Vogels, J. H. Custers, M. M. Addo, S. Lockman, T. Peter, F. W. Peyerl, M. G. Kishko, S. S. Jackson, D. A. Gorgone, M. A. Lifton, M. Essex, B. D. Walker, J. Goudsmit, M. J. Havenga, and D. H. Barouch. 2005. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J. Immunol. 174:7179-7185. [DOI] [PubMed] [Google Scholar]
- 69.Takada, A., and Y. Kawaoka. 2003. Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications. Rev. Med. Virol. 13:387-398. [DOI] [PubMed] [Google Scholar]
- 70.Tao, N., G. P. Gao, M. Parr, J. Johnston, T. Baradet, J. M. Wilson, J. Barsoum, and S. E. Fawell. 2001. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol. Ther. 3:28-35. [DOI] [PubMed] [Google Scholar]
- 71.Tew, J. G., J. Wu, M. Fakher, A. K. Szakal, and D. Qin. 2001. Follicular dendritic cells: beyond the necessity of T-cell help. Trends Immunol. 22:361-367. [DOI] [PubMed] [Google Scholar]
- 72.van der Veen, J., and M. Lambriex. 1973. Relationship of adenovirus to lymphocytes in naturally infected human tonsils and adenoids. Infect. Immun. 7:604-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Varghese, R., Y. Mikyas, P. L. Stewart, and R. Ralston. 2004. Postentry neutralization of adenovirus type 5 by an antihexon antibody. J. Virol. 78:12320-12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vincent, T., B. G. Harvey, S. M. Hogan, C. J. Bailey, R. G. Crystal, and P. L. Leopold. 2001. Rapid assessment of adenovirus serum neutralizing antibody titer based on quantitative, morphometric evaluation of capsid binding and intracellular trafficking: population analysis of adenovirus capsid association with cells is predictive of adenovirus infectivity. J. Virol. 75:1516-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang, M., A. Hemminki, G. P. Siegal, M. N. Barnes, I. Dmitriev, V. Krasnykh, B. Liu, D. T. Curiel, and R. D. Alvarez. 2005. Adenoviruses with an RGD-4C modification of the fiber knob elicit a neutralizing antibody response but continue to allow enhanced gene delivery. Gynecol. Oncol. 96:341-348. [DOI] [PubMed] [Google Scholar]
- 76.Wickham, T. J., M. E. Carrion, and I. Kovesdi. 1995. Targeting of adenovirus penton base to new receptors through replacement of its RGD motif with other receptor-specific peptide motifs. Gene Ther. 2:750-756. [PubMed] [Google Scholar]
- 77.Wickham, T. J., E. J. Filardo, D. A. Cheresh, and G. R. Nemerow. 1994. Integrin αvβ5 selectively promotes adenovirus mediated cell membrane permeabilization. J. Cell Biol. 127:257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wickham, T. J., G. M. Lee, J. A. Titus, G. Sconocchia, T. Bakacs, I. Kovesdi, and D. M. Segal. 1997. Targeted adenovirus-mediated gene delivery to T cells via CD3. J. Virol. 71:7663-7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilcox, W. C., and H. S. Ginsberg. 1963. Production of specific neutralizing antibody with soluble antigens of type 5 adenovirus. Proc. Soc. Exp. Biol. Med. 114:37-42. [DOI] [PubMed] [Google Scholar]
- 80.Wohlfart, C. 1988. Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J. Virol. 62:2321-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wohlfart, C., and E. Everitt. 1985. Human adenovirus 2 as immunogen in rabbits yields antisera with high titers of antibodies against the nonstructural 72K DNA-binding protein. Virus Res. 3:77-85. [DOI] [PubMed] [Google Scholar]
- 82.Worgall, S., A. Busch, M. Rivara, D. Bonnyay, P. L. Leopold, R. Merritt, N. R. Hackett, P. W. Rovelink, J. T. Bruder, T. J. Wickham, I. Kovesdi, and R. G. Crystal. 2004. Modification to the capsid of the adenovirus vector that enhances dendritic cell infection and transgene-specific cellular immune responses. J. Virol. 78:2572-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Worgall, S., G. Wolff, E. Falck-Pedersen, and R. G. Crystal. 1997. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum. Gene Ther. 8:37-44. [DOI] [PubMed] [Google Scholar]
- 84.Wright, N. G., W. I. Morrison, H. Thompson, and H. J. Cornwell. 1973. Experimental adenovirus immune complex glomerulonephritis. Br. J. Exp. Pathol. 54:628-633. [PMC free article] [PubMed] [Google Scholar]
- 85.Wright, N. G., W. I. Morrison, H. Thompson, and H. J. Cornwell. 1974. Mesangial localization of immune complexes in experimental canine adenovirus glomerulonephritis. Br. J. Exp. Pathol. 55:458-465. [PMC free article] [PubMed] [Google Scholar]
- 86.Yang, Y., Q. Li, H. C. Ertl, and J. M. Wilson. 1995. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J. Virol. 69:2004-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yuzawa, Y., N. Aoi, A. Fukatsu, S. Ichida, F. Yoshida, Y. Akatsuka, S. Minami, Y. Kodera, and S. Matsuo. 1993. Acute renal failure and degenerative tubular lesions associated with in situ formation of adenovirus immune complexes in a patient with allogeneic bone marrow transplantation. Transplantation 55:67-72. [DOI] [PubMed] [Google Scholar]