Abstract
Alternative strategies for controlling the growing herpes simplex virus type 2 (HSV-2) epidemic are needed. A novel class of immunomodulatory microbicides has shown promise as antiherpetics, including intravaginally applied CpG-containing oligodeoxynucleotides that stimulate toll-like receptor 9 (TLR9). In the current study, we quantified protection against experimental genital HSV-2 infection provided by an alternative nucleic acid-based TLR agonist, polyinosine-poly(C) (PIC) (TLR3 agonist). Using a protection quantification paradigm, groups of mice were PIC treated and then subdivided into groups challenged with escalating doses of HSV-2. Using this paradigm, a temporal window of PIC efficacy for single applications was defined as 1 day prior to (prophylactic) through 4 h after (therapeutic) viral challenge. PIC treatment within this window protected against 10-fold-higher HSV-2 challenges, as indicated by increased 50% infectious dose values relative to those for vehicle-treated controls. Disease resolution and survival were significantly enhanced by repetitive PIC doses. Using optimal PIC regimens, cytokine induction was evaluated in murine vaginal lavages and in human vaginal epithelial cells. Similar induction patterns were observed, with kinetics that explained the limited durability of PIC-afforded protection. Daily PIC delivery courses did not generate sustained cytokine levels in murine vaginal fluids that would be indicative of local immunotoxicity. No evidence of immunotoxicity was observed in selected organs that were analyzed following repetitive vaginal PIC doses. Animal and in vitro data indicate that PIC may prove to be a valuable preventative microbicide and/or therapeutic agent against genital herpes by increasing resistance to HSV-2 and enhancing disease resolution following a failure of prevention.
Each year there are an estimated 500,000 new cases of symptomatic first-episode herpes simplex virus type 2 (HSV-2) infection (11, 45). Currently, there are no FDA-approved vaccines for this recurrent, lifelong infection. Chronic suppressive therapy is the “gold standard” for the treatment of genital herpes; however, such treatments are not curative. The growing epidemic of genital HSV-2 and lack of an approved vaccine have led to evaluation of approaches designed to protect the genital mucosa against infection (42).
One promising approach to prevention or reduction of transmission of sexually transmitted infections (STI) is the application of topical microbicides (14, 22). Microbicides can be applied locally to the vaginal or rectal mucosa prior to or just following a high-risk sexual encounter. The term microbicide implies that the compound kills microorganisms; this is somewhat misleading, because microbicides may work in a variety of ways, including disrupting the pathogen's membrane or envelope, blocking receptor interactions essential for infection, inhibiting intra- or extracellular pathogen replication, or enhancing local, mucosal immune responses, thereby reducing susceptibility (10, 22, 26, 30). A recent experimental microbicide strategy used CpG-containing oligonucleotides (CpG-ODN) delivered topically to the vaginal mucosa to induce a local immune response that protected against HSV-2 infection (21). CpG motifs are recognized by toll-like receptor 9 (TLR9) (25). When triggered by specific pathogen-produced agonists, TLR activation initiates innate immune mechanisms that are designed to limit the replication and/or spread of pathogens (1, 21).
Recently, as with CpG motifs, local treatment with another nucleic acid-based agonist recognized by TLR3 was shown to protect against experimental HSV-2 infection (6). Polyinosine-poly(C) (PIC) is a stabilized synthetic form of double-stranded RNA that triggers TLR3, leading to activation of at least two important transcription factors, NF-κB and interferon-regulatory factor 3 (37). These distinct pathways are potent stimulators of antiviral and innate immune responses important to limiting HSV-2 infection (2, 3, 5, 19, 27). Specifically, TLR3 activation elicits production of type I interferons, Th1-type cytokines, and chemokines, including MIP-1α and RANTES (4, 5, 15). Understanding the events following local PIC application and determining the optimal timing for delivery will aid in clinical trial development and will provide additional information for refinement of TLR agonist therapies.
To further evaluate PIC as a microbicide candidate, using the mouse genital HSV-2 model, we quantified the protection afforded by single and multiple delivery of PIC and defined a temporal window of efficacy. Because an ideal microbicide would be effective when delivered frequently without adverse side effects or toxicity, we evaluated consecutive daily PIC applications and found that this regimen provided enhanced disease resolution and survival relative to a single dose. In murine vaginal lavages, cytokines were induced transiently by PIC application with kinetics that correlated to the defined application window.
MATERIALS AND METHODS
HSV-2 propagation and cell culture.
Clarified stocks of HSV-2 strain 186 were used for all studies and were prepared from infected Vero cell monolayers (ATCC CCL81), with titers determined by standard plaque assay, and stored at −80°C as described previously (31). HEK-293 cells, constitutively expressing human TLR3 or TLR5, were purchased and cultured as recommended (InvivoGen, San Diego, CA). Primary human vaginal epithelial cells (EC), purchased from MatTek, were cultured in VEC-100 medium (MatTek, Ashland, MA). Vaginal EC, immortalized by human papillomavirus type 16 E6/E7 retroviral transduction using supernatant from PA317 LXSN 16E6E7 cells (CRL-2203; ATCC), were cultured in a 1:1 mixture of VEC-100 and keratinocyte serum-free medium (Invitrogen, Carlsbad, CA). For cell culture viral resistance studies, agonists were applied to triplicate cultures 24 h prior to viral challenge with HSV-2 strain 186 (multiplicity of infection [MOI] of 0.01). Forty-eight hours after challenge, the average titer of virus for untreated cultures served as a baseline (100%) for normalization of the viral titers in each culture. The percentage of the untreated titer was then averaged for each group.
Genital mouse model of HSV-2 infection and viral dose-ranging paradigm.
Four- to five-week-old C57Bl/6 mice (Jackson Laboratories, Bar Harbor, ME) weighing 20 to 22 g were housed in Association for Assessment and Accreditation of Laboratory Animal Care-approved quarters and provided with unlimited food and water. All procedures were approved by the University of Texas Medical Branch IACUC and performed humanely to minimize pain and suffering. Animals were acclimated for 7 days before being randomly grouped (n = 9 to 15) prior to treatment or genital HSV-2 infection (31). Briefly, Depo-Provera (UpJohn, Kalamazoo, Mich.)-conditioned animals were challenged with a lethal dose of HSV-2 (104 PFU unless indicated otherwise) by vaginal instillation [31]). To quantify the protection provided by treatments, we employed a paradigm that utilized groups of animals treated similarly with the TLR agonist and then challenged with increasing 10-fold titers of HSV-2 by group. Vaginal swabs were collected from these animals on 2 days postinoculation (p.i.) and then tested for infectious virus using microscopic visualization of cytopathic effect 3 days after Vero cell inoculation. Animals were assessed daily for disease signs (hair loss and erythema) through 14 days p.i. Survival was followed for 21 days p.i. Resulting data were used to calculate the 50% infectious dose (ID50) from the infectious content of day 3 (d3) vaginal swabs and 50% lethal dose (LD50) values based upon survival at 21 days p.i. by end-point estimation (33). Vaginal lavages were collected by a positive displacement pipette (CP25 tips; Rainin, Oakland, CA), vaginally instilling 25 μl of sterile phosphate-buffered saline (PBS) five times. Recovered lavage fluid (∼100 μl) was stored at −80°C until cytokine quantification.
TLR agonist treatments.
PIC (Sigma, St. Louis, MO) was resuspended to 3.75 mg/ml in diethyl pyrocarbonate-treated 1× PBS (Mediatech, Herndon, VA). CpG-ODN 1018 ISS (31) was provided by Dynavax Technologies Corporation and also dissolved in PBS (5 mg/ml). Mice received a total of 100 μg of PIC or CpG-ODN 1018 ISS per dose intravaginally with a positive-displacement pipette. Cell cultures were treated with 100 μg/ml for each agonist or in the combination study received 100 μg/ml of both PIC and CpG-ODN 1018 ISS. Control groups of animals or wells of cells were treated with the PBS vehicle alone or for cell cultures with acyclovir (ACV) (20 μg/ml; GSK, Research Triangle Park, NC). For single dosing studies, control animals received a single intravaginal PBS application. Animals treated with multiple PIC doses were compared to PBS-treated mice that received seven daily applications to control for any potential effects of repetitive vaginal manipulations.
Cytokine quantification.
Vaginal cytokine levels were measured in lavages using a murine cytometric bead array (CBA) (Bio-Plex; Bio-Rad, Hercules, CA). Levels of murine interleukin 1α (IL-1α), IL-1β, IL-6, gamma interferon (IFN-γ), MIP-1α, and RANTES protein were established in duplicate for each lavage. For murine analyses, standards were diluted in PBS with 0.5% bovine serum albumin to reduce protein loss. For human culture studies, levels of IL-1β, IL-6, IFN-γ, IL-8, and MIP-1β were quantified similarly using a human CBA. These targets were selected to best overlap with the lavage targets to enhance comparisons. Overlap was limited by the expected cytokine expression profile from human EC cultures and the availability of the targets in the Bio-Plex system. Depending upon the analyte, a lower limit of detection of 1 to 10 pg/ml was established. Experimental samples were quantified by extrapolation from the standard curves, with samples below the lower limit being assigned a zero level. Murine vaginal alpha interferon (IFN-α) (A, 1, 4, 5, 6, and 9 IFN-α subspecies detected) and human beta interferon (IFN-β) were quantified by commercial enzyme-linked immunosorbent assay systems with detection ranges of 12.5 to 500 or 250 to 10,000 pg/ml, respectively (PBL Biomedical Laboratories, NJ).
Statistics.
Incidence and survival data were evaluated by log rank analyses or by two-tailed t tests with Welch's correction using Prism software (GraphPad, San Diego, CA). Fisher's exact test was performed for comparisons as indicated. A P value of <0.05 was considered significant.
RESULTS
Maximal efficacy of a single PIC application against genital HSV-2 was time dependent.
Prophylactic or therapeutic single-dose application regimens were evaluated to define a temporal window of PIC efficacy. Treatment groups (n = 9 to 15 mice) included 2-days-prior, 1-day-prior, 4-h-after, or 24-h-after viral challenge groups and a vehicle-treated (PBS) control group. A final group of mice received intravaginal CpG-ODN 1018 ISS delivered 2 days prior as a positive control for intervention (31). Prophylactic PIC treatment, either 2 days or 1 day prior to lethal HSV-2 challenge, significantly reduced the number of animals with disease signs (P < 0.005) and, in those animals that were infected, increased survival and delayed death relative to PBS vehicle-treated controls (P < 0.05) (Table 1). PIC applied 1 day prior to HSV-2 inoculation resulted, on average, in a ∼4 days' increase in survival (12.9 days) relative to application 2 days prior (9.1 days), indicating a limited durability of a single intravaginal application (P < 0.01) (Table 1).
TABLE 1.
Optimal PIC-mediated efficacy was provided by local application at 1 day prior to or up to 4 h after HSV-2 challenge
| Treatmenta | Incidenceb (%) | Time to symptomsc (days) | Survival timec (days) | Survivald (%) |
|---|---|---|---|---|
| PIC, 2 d prior | 13/15 (87) | 6.3 ± 0.2e | 9.1 ± 0.3g | 1/15 (7) |
| PIC, 1 d prior | 13/15 (87) | 6.2 ± 0.2e | 12.9 ± 0.9f,h | 2/15 (13) |
| PIC, 4 h after | 6/9 (67) | 7.5 ± 0.7e | 11.2 ± 1.8e,i | 4/9 (44)g |
| PIC, 24 h after | 8/10 (80) | 5.9 ± 0.2 | 9.0 ± 0.4 | 2/10 (20) |
| CpG 1018, 2 d prior | 7/10 (70) | 7.7 ± 0.5f | 11.5 ± 1.2f | 2/10 (20) |
| PBS alone | 9/9 (100) | 5.7 ± 0.4 | 8.1 ± 0.5 | 0/9 (0) |
Intravaginal application of PIC was delivered prophylactically or therapeutically relative to viral challenge with 104 PFU. 2 d or 1 d prior, 2 or 1 day prior to challenge; 4 or 24 h after, 4 or 24 h after challenge.
Incidence of disease is measured by the development of hair loss and erythema and is expressed as the number of mice that develop symptoms/number of the animals in the group.
Mean for mice with disease signs within 15 days PI or mean survival time within 21 days PI. Log rank analysis was used to compare both the number of diseased/no. of live animals and the average time to disease/survival between groups.
Survival is presented as number of mice alive 21 days p.i./number of animals in the group.
P < 0.01 versus results with PBS.
P < 0.001 versus results with PBS.
P < 0.05 versus results with PBS.
P < 0.01 versus results with 2-days-prior PIC treatment.
P < 0.05 versus results with 2-days-prior PIC treatment by Fisher's exact test.
Therapeutically, PIC delivered 4 h after lethal viral challenge provided similar outcomes to treatment 1 day prior, with significant increases in survival (P < 0.04) and survival time and reductions in disease sign incidence relative to results with vehicle-treated controls (P < 0.01). Therapeutic efficacy for PIC waned by 24 h as indicated by the animals treated 4 or 24 h after viral challenge. Animals treated 24 h after HSV-2 inoculation had disease and survival similar to those of PBS-treated controls and were significantly different from animals treated 4 h after viral challenge (P < 0.01) (Table 1). These findings were confirmed in a second trial and in the quantification studies below.
Repetitive, daily PIC application significantly reduced disease and lethal outcomes compared to a single dose.
For clinical use, it is possible that PIC would be applied daily and as such could result in enhanced protection beyond a single application, tolerance, or immunotoxic outcomes. Therefore, daily PIC dosing regimens were evaluated. PIC administered once daily for three or seven consecutive doses followed by HSV-2 challenge 24 h later were compared to a single dose (1 day prior or 4 h after) or to PBS alone. Groups challenged 48 h after the third or seventh consecutive PIC dose also were evaluated to determine if repetitive delivery enhanced durability. Animals treated with the PBS vehicle alone controlled for the potential impact of local, repetitive manipulation and were found to be equivalent to animals that were not treated (data not shown).
Mice treated with either three or seven PIC doses and challenged 24 h later developed significantly less disease than PBS-treated animals (P < 0.002 and P < 0.008, respectively). These treatment regimens significantly enhanced survival time relative to results with PBS (P < 0.0001) (Table 2). Durability was not enhanced by three or seven doses as evidenced by outcomes of viral challenge 48 h after the last PIC dose, where animals experienced more disease and less survival than those treated 24 h prior (P < 0.01) (Table 2). Relative to PBS, however, repetitive dosing 48 h prior to challenge still reduced the incidence of disease signs and increased both survival and survival time (Table 2).
TABLE 2.
Repetitive delivery of PIC significantly enhanced disease resolution and survival beyond a single local application in a time-dependent manner
| Treatmenta | Incidenceb (%) | Time to symptomsc (days) | Survival timec (days) | Survivald (%) |
|---|---|---|---|---|
| 3 consecutive, 24 h prior | 7/15 (47)e | 8.7 ± 0.7f,g | 13.3 ± 0.8f,g,i | 7/15 (47)j |
| 3 consecutive, 48 h prior | 9/10 (90) | 6.9 ± 0.6e | 8.9 ± 0.3j | 2/10 (20) |
| 7 consecutive, 24 h prior | 4/10 (40)e | 8.5 ± 0.6f,h | 14.8 ± 1.7f,h | 5/10 (50)j |
| 7 consecutive, 48 h prior | 9/10 (90) | 7.9 ± 0.7f | 11.4 ± 1.1e | 2/10 (20) |
| PBS alone | 10/10 (100) | 5.4 ± 0.2 | 8.4 ± 0.5 | 0/10 (0) |
PIC was intravaginally applied prophylactically or therapeutically as indicated relative to viral challenge with 104 PFU.
Incidence of disease is measured by the development of hair loss and erythema and is expressed as the number of mice that develop signs/number of the animals in the group.
Mean for mice with disease signs within 14 days p.i. or mean survival time within 21 days p.i. (log rank analysis).
Survival is presented as number of mice alive 21 days p.i./number of animals in the group.
P < 0.01 versus PBS results.
P < 0.0001 versus PBS results.
P < 0.01 versus results with three consecutive PIC treatments 48 h prior.
P < 0.05 versus results with seven consecutive PIC treatments 48 h prior.
P < 0.01 versus results with seven consecutive treatments 48 h prior.
P < 0.05 versus PBS results (Fisher's exact test).
Local PIC application protected against 10-fold-higher challenge titers of HSV-2.
To quantify the amount of protection afforded by a single-dose therapeutic regimen, as well as prophylactic single- and multidosing regimens, an escalating HSV-2 dose-ranging paradigm was utilized. Treatment groups were inoculated with 10-fold serial dilutions of HSV-2, ranging from 2 × 101 to 2 × 105 PFU (10 to 15 mice per dilution), to establish the ID50 relative to that for PBS controls. Active infection was indicated by infectious viral content in vaginal swabs collected 3 days p.i. LD50 (50% lethal dose) values were established to show outcomes in cases of prevention failure and served to confirm previous results. PIC increased both the ID50 and LD50 by 10-fold relative to PBS when delivered as a single dose 1 day prior to challenge (Table 3). A single therapeutic (4 h after) PIC dose was not as effective at preventing infection, as indicated by the ID50 (1.8 × 102), but was extremely effective at resolving disease and preventing lethal outcomes, as evidenced by the 100-fold LD50 shift relative to results with PBS (>2.3 × 104) (Table 3).
TABLE 3.
Quantification of protection against experimental genital HSV-2 afforded by single or repetitive intravaginal PIC application
| HSV-2 dose (PFU) | Result with treatmenta
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PIC, 1 day prior
|
PIC, 4 h after
|
1d prior and 4 h after PIC
|
3 consec. PIC 24 h prior
|
PBS control
|
|||||||||||
| % Infected | % Survival | % Infected | % Survival | % Infected | % Survival | % Infected | % Survival | % Infected | % Survival | ||||||
| 2 × 101 | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 20 | 90 | |||||
| 2 × 102 | 10c | 90d | 60 | 60 | 13c | 100c | 0d | 100c | 70 | 40 | |||||
| 2 × 103 | 90 | 20 | 100 | 80c | 73d | 73c,e | 80 | 80c | 100 | 0 | |||||
| 2 × 104 | 90 | 10 | 100 | 80c,f | 93 | 13 | 100 | 80c,f | NA | NA | |||||
| 2 × 105 | 100 | 0 | NAg | NA | 90 | 20 | NA | NA | NA | NA | |||||
| ID50b | 1.4 × 103
|
1.8 × 102
|
1.8 × 103
|
1.6 × 103
|
1.4 × 102
|
||||||||||
| LD50b | 1.4 × 103 | >2.3 × 104 | 1.1 × 104 | >2.3 × 104 | 2.8 × 102 | ||||||||||
% infected, percentage of animals with vaginal viral replication as detected in swabs collected on day 2 p.i. Fisher's-exact-test comparisons were made between the same viral doses. % survival, percentage of animals that survived HSV-2 challenge 21 days p.i. Data were analyzed using Fisher's-exact-test comparisons between the same viral doses. Consec., consecutive.
The dose of virus required to infect or cause lethal outcomes in 50% (ID50 or LD50, respectively) of the mice, calculated by Reed and Muench end-point estimation (33), is shown. ID50 was established by infectious content in vaginal swabs collected 3 days p.i. LD50 values were based upon survival to 21 days p.i.
P < 0.001 versus results with PBS.
P < 0.01 versus results with PBS.
P < 0.01 versus results with 1-day-prior treatment.
P < 0.01 versus results results with 1-day-prior and 4-h-after dual treatment.
NA, not analyzed.
Protection afforded by a dual PIC treatment (1 day prior and 4 h after) also was quantified to determine if combined prophylactic and therapeutic effects could be achieved. The dual treatment produced a 10-fold increase in ID50, similar to prophylactic treatment, and an ∼100-fold shift in LD50, consistent with the therapeutic 4-h application (Table 3). Disease resolution was not as pronounced in the dual-treatment group based upon the significant difference in survival following challenge with 2 × 104 PFU relative to a single treatment 4 h after challenge. Because three and seven consecutive PIC doses produced similar enhanced outcomes relative to single doses (Table 2), protection afforded by three daily doses of PIC also was quantified. Similar to the dual-treatment regimen, consecutive daily dosing resulted in an ∼11-fold increase in the ID50 and a 100-fold shift for the LD50 relative to results with PBS treatment (Table 3). Three PIC doses did increase survival in the 104 PFU challenge group (80%) (Table 3) relative to results with a similar viral challenge and 3-day course in the prior study (47%) (Table 2), but the difference was not significant.
PIC elicited cytokine and chemokine production in murine vaginal lavages.
To better understand the time frame associated with protection, cytokine kinetics following PIC application were evaluated in mouse vaginal lavages. Lavages were collected at selected times after treatment and analyzed for induction of cytokines (IFN-α, IL-1α, IL-1β, IL-6, and IFN-γ) and chemokines (MIP-1α and RANTES), shown to play a role in limiting HSV infection (12, 13, 19). In the absence of HSV-2 infection, individual vaginal lavages (n = 5/time point) were collected at 2, 4, 18, 24, and 48 h after PIC treatment and were compared to PBS-treated controls. Mice were sampled only once in a 24-h period to prevent an artificial “washout” of cytokine levels in an effort to ensure accurate outcomes.
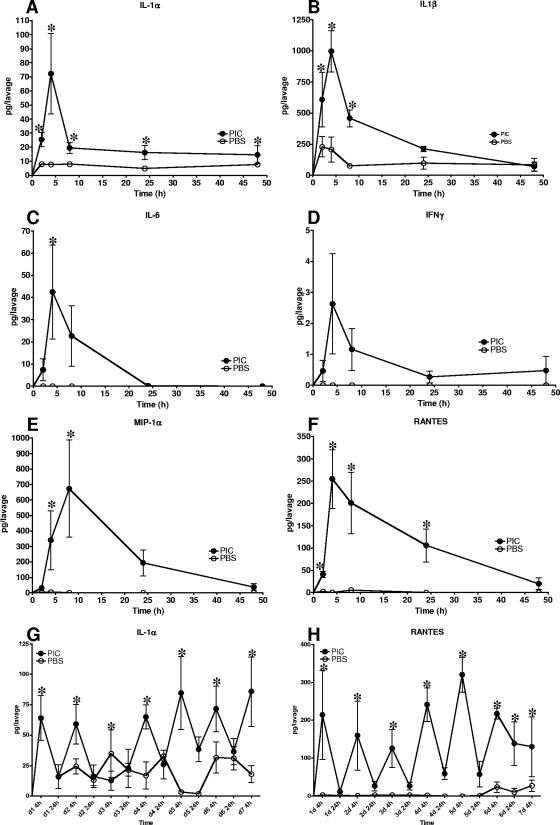
Most cytokine (IL-1α, IL-1β, and IL-6) and chemokine (RANTES) production peaked at or after 4 h and decreased to lower or baseline levels by 24 to 48 h following PIC application relative to results with PBS controls (Fig. 1A to F). IFN-α was not significantly induced (data not shown). IFN-γ production was not significant in vaginal lavages following PIC application (Fig. 1C). MIP-1α production peaked at 18 h and returned to low, baseline levels by 24 to 48 h following PIC application (Fig. 1E).
FIG. 1.
Kinetic analysis of cytokine and chemokine induction by intravaginal PIC application in vaginal lavages. Lavages (n = 5 mice per time point) collected at 2, 4, 18, 24, and 48 h following PIC or application of the PBS vehicle were quantified by CBA. Induction of the cytokine IL-1α, IL-1β, IL-6, or IFN-γ (A to D) or the chemokine MIP-1α or RANTES (E and F) is expressed as pg/lavage. Following 7 days of PIC delivery, the daily cyclic pattern of induction was observed (G and H). Statistical significance was determined by t test with Welch's correction. *, P < 0.01.
Repetitive PIC application did not result in sustained cytokine production in vaginal lavages or enlargement of local or peripheral organs.
Cytokine levels in vaginal lavages collected 4 or 24 h after each consecutive, daily PIC application also were established. The cyclic cytokine induction pattern observed for single PIC applications was repeated after subsequent PIC doses. Only IL-1α and RANTES levels were found to be statistically elevated 24 h after PIC application, but they returned to basal levels by 48 h (Fig. 1A and F, respectively). Repetitive dosing did not alter the cyclic nature of IL-1α or RANTES induction even after a seventh PIC application, where baseline levels were found 24 to 48 h later (Fig. 1G and H).
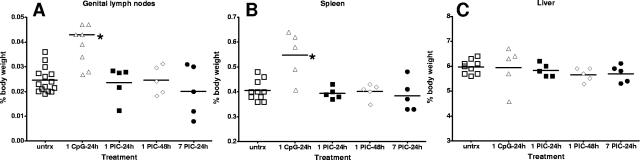
To determine if repetitive delivery induced gross changes to local or peripheral organs, mice again were treated intravaginally with PIC or with CpG-ODN 1018 ISS. Mice were euthanized humanely following the treatments, and genital lymph nodes (gLN), spleen, and liver were examined for gross changes in weight reflecting possibly increased cellularity associated with inflammation. A single intravaginal treatment with CpG-ODN 1018 ISS produced significant enlargement of the gLN and spleen within 24 h of application (P < 0.05) (Fig. 2A and B). Liver weight was not effected by CpG-ODN 1018 ISS application (Fig. 2C) (20). Following single or multiple PIC dosing regimens, enlargement of the local gLN, spleen, or liver was not observed in any of the animals relative to multiply dosed PBS controls (Fig. 2A to C). In a limited parallel study, 14 consecutive daily applications of PIC also produced no significant changes in organ weights (data not shown).
FIG. 2.
Single or repetitive intravaginal PIC applications did not result in local or peripheral organ enlargement. gLN (A), spleen (B), and liver (C) were removed and weighed following a single intravaginal dose of CpG-ODN 1018 ISS or PIC or consecutive PIC applications. Data are presented as percentages of total body weight, with each point representing an animal in the group (n = 5). Groups were compared for statistical significance by analysis of variance. untrx, untreated.
PIC-afforded protection was TLR3 mediated in human epithelial cells.
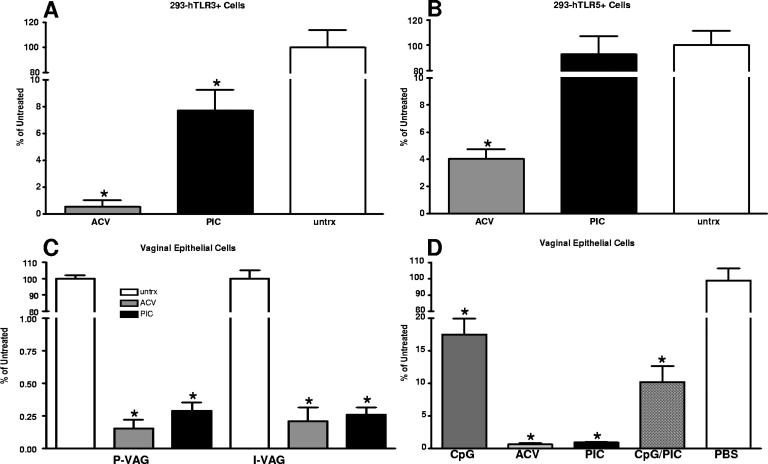
To determine if the PIC protection was TLR3 mediated or rather was due to direct antiviral inhibition, HEK293 cells constitutively expressing human TLR3 or TLR5 were pretreated with PIC for 1 day prior to HSV-2 challenge (MOI of 0.01). Cell supernatants were harvested 48 h later, and titers were determined (Fig. 3). The average titer for the untreated wells was set at 100%, and each culture's titer was expressed as a percentage of the average value, averaged by group, and then compared. PIC treatment of 293-hTLR3+ cells significantly reduced HSV-2 replication relative to that for untreated cells (18-fold, P < 0.01; Fig. 3A). This reduction was statistically similar to results with ACV treatment of parallel cultures. In contrast, PIC-treated 293-hTLR5+ cells exhibited HSV-2 titers similar to those of PBS-treated cultures (Fig. 3B). In addition, primary (P-VAG) or immortalized (I-VAG) human vaginal EC cultures were pretreated with PIC for 1 day prior to HSV-2 challenge. Human vaginal EC are the first cells to encounter intravaginal PIC and express high levels of TLR3 (M. Herbst-Kralovetz and R. Pyles, unpublished observations). In PIC-treated vaginal EC cultures, viral replication was significantly reduced in both P-VAG (346-fold) and I-VAG (271-fold) EC relative to results for untreated parallel cultures (P < 0.01) (Fig. 3C). Again these reductions were comparable to those provided by ACV.
FIG. 3.
PIC-afforded protection against HSV-2 was TLR3 mediated. Parallel cultures of HEK-293 cells that selectively express human TLR3 (A) or TLR5 (B) were treated with PIC (100 μg/ml) or acyclovir (ACV) (20 μg/ml; positive control) or left untreated (untrx) for 24 h prior to HSV-2 challenge (MOI, 0.01). The average titer in untreated cells was set at 100%, and the titers of each culture were expressed as percentages of the average untreated. Cells expressing human TLR3 or those treated with ACV had significantly decreased HSV-2 replication relative to that for untreated controls (P < 0.01). Human primary (P-VAG) or immortalized (I-VAG) vaginal EC were treated for 24 h with PIC or ACV as described above prior to HSV-2 challenge (MOI, 0.01) and normalized to untreated parallel cultures. Cells were collected by scraping them into the culture supernatant 48 h after viral challenge, and titers were determined on Vero monolayers. P-VAG and I-VAG cells treated with PIC exhibited significantly (P < 0.01) less HSV-2 replication than untreated controls. PIC HSV-2 titers were equivalent to those of ACV-treated cultures (C). Codelivered PIC and CpG-ODN 1018 ISS significantly (P < 0.01) reduced viral replication relative to untreated controls; however, no significant synergy or antagonism was observed (P > 0.05 PIC or CpG-ODN 1018 ISS alone compared to the combination) (D). Statistical comparisons were made by analysis of variance. *, P < 0.01.
To evaluate the potential for synergy or antagonism, P-VAG EC were treated with PIC, CpG-ODN 1018 ISS, or both PIC and CpG-ODN 1018 ISS (100 μg/ml of each) and again compared to untreated and ACV-treated wells. Results with single treatments were similar to those of the first study and confirmed the observed reductions in viral replication. Treatment with both agonists simultaneously significantly reduced viral replication relative to that for untreated controls (P < 0.001) (Fig. 3D), indicating a lack of antagonism. The data indicated that the two treatments were not synergistic either, since there was no significant difference between results with a single treatment with PIC or CpG-ODN 1018 ISS and those with the dual-treated cultures (P > 0.05) (Fig. 3D).
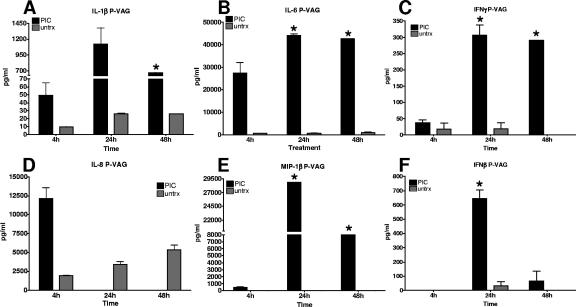
Utilizing the vaginal EC cultures, a set of cytokines implicated in HSV clearance were selected from the available human targets to overlap with the evaluated murine cytokines (7, 13, 23, 28). Levels of human IFN-β, IL-1β, IL-6, IFN-γ, IL-8, and MIP-1β were measured at 4, 24, and 48 h following PIC treatment in P-VAG EC. PIC treatment of P-VAG EC induced a robust cytokine response beginning at 4 h, peaking at 24 h, and decreasing by 48 h (Fig. 4A to F). In contrast to lavage levels, significant IL-6, IFN-γ, and MIP-1β levels were detected 48 h after PIC treatment. PBS-treated cultures were analyzed for the same time points and used for comparisons. A subset of these control samples did not have detectable cytokines; these were designated zero for the analyses and are included on the graphs.
FIG. 4.
Human primary vaginal EC responded robustly to PIC treatment. Duplicate human primary vaginal (P-VAG) EC cultures were stimulated with PIC. Selected cytokine and chemokine secretion into the medium was evaluated at 4, 24, and 48 h following PIC treatment (A to F). In several cases, lavages from a group yielded protein levels below the detection limit of the assay and are indicated on the graphs as a zero value. The plots represent the averages for three independent studies. Data were compared for statistical significance using a t test with Welch's correction (*, P < 0.01; **, P < 0.001). untrx, untreated.
DISCUSSION
The ideal HSV-2 microbicide would completely protect against infection, because HSV-2 establishes a lifelong latent infection that can periodically reactivate, resulting in a recurrent lesion or asymptomatic viral shedding at the epithelial surface (42). To evaluate PIC as a candidate HSV-2 microbicide, protection against experimental infection was quantified for selected dosing regimens by comparing the ID50 for treated animals relative to that for vehicle-treated controls. The data indicated that maximal PIC efficacy was of shorter duration than that reported for CpG-ODN (6, 19, 31). Prophylactic application within 24 h before viral challenge provided optimal outcomes relative to that 48 h prior to HSV-2 inoculation (increased survival by ∼4 days). As with PIC, we and others showed that CpG-ODN vaginal application enhanced disease resolution in those mice that became infected (6, 19, 31). In the guinea pig model, enhanced disease resolution translated into fewer recurrences and reduced the magnitude of HSV-2 shedding (31). Interventions that reduce viral shedding can substantially reduce transmission rates (9, 16, 34). Studies to examine the impact of PIC on recurrence and shedding must be completed to establish if in cases of prevention failure, PIC will alter pathogenesis and thereby reduce the HSV-2 epidemic (9).
In a recent study, Ashkar et al. reported that prophylactically delivered PIC could completely protect against HSV-2 infection (6). Our data at a similar challenge level did not show this level of protection, but there were several differences between the studies. Ashkar and colleagues employed strain 333 rather than the 186 strain utilized in this study and evaluated PIC protection with older animals (6 to 8 weeks old) purchased from an alternate supplier. Our results and the positive findings reported by Ashkar et al. support our conclusion that PIC is a promising candidate HSV-2 microbicide that should be studied further.
The HSV-2 dose-ranging paradigm revealed that enhanced protection is provided by multiple PIC doses. Prophylactic PIC application protected against a 10-fold-higher challenge (increased ID50), completely protected mice at lower challenge doses, and enhanced disease resolution and survival (10-fold LD50 shift) (Table 3). Therapeutically, PIC did not prevent infection; however, the LD50 was increased by 100-fold, signifying a profound enhancement of disease resolution. Dual PIC application 1 day prior to and 4 h after challenge generally provided both the prophylactic and therapeutic benefits (Table 3), protecting against infection with ∼10 times more HSV-2 and against lethal outcomes with ∼100 times more virus than PBS alone. At higher viral challenge doses, dual treatment did not enhance survival as effectively as the 4-h single therapeutic treatment, suggesting that PIC delivery 24 h prior may not be the ideal time interval for maximal benefits observed for single-dose regimens.
The results also showed that daily PIC application enhanced survival but did not increase the duration of protection beyond 24 h (Table 2). Enhancement of survival was more pronounced in the second study (Table 3) for the group that received a 3 days PIC and was challenged 24 h after with 104 PFU, but the result was not significantly different from that for the comparable group in the first study (Table 2). Although the translation of mouse data to humans must be supported by further research and clinical trials, as a microbicide, the animal data suggest PIC would be most effective when delivered prior to and soon after a high-risk sexual encounter. The data also suggest that if prevention failed, PIC likely would profoundly impact the disease course.
Mechanistically, PIC-afforded protection against HSV-2 was TLR3 mediated in human EC in the absence of other cell types (Fig. 3). Additional studies are under way to establish the role of TLR3 in PIC protection in vivo. Recently, EC have been shown to play a large part in innate and early immune responses to both TLR agonists and pathogens and thereby play a crucial role in the development of protective Th1 responses against HSV-2 (32, 36, 41). Human vaginal EC cultures responded robustly to PIC by producing cytokines similar to those induced in murine lavages, as well as IFN-β (Fig. 4). Intracellular levels of IFN-β or other cytokines were not measured in the EC cultures and may be equally or wholly responsible for the resistance to viral replication that was observed. In vitro, the prolonged cytokine secretion observed relative to that in the lavages may be due to an extended half-life in cell culture medium and/or prolonged activity of PIC in the sterile culture environment. Studies to establish the fate and/or stability of PIC delivered in vitro and in vivo are in progress.
In vivo, the lavage cytokine and chemokine kinetics suggest that either intracellular levels of antiviral cytokines, not measured in the lavages, or active cellular recruitment and activation play an important role in the extended period of protection observed for PIC. The secreted levels of cytokines and chemokines supported local recruitment and/or activation of professional immune cells but for PIC did not translate to the loading of gLN or other tissues based on crude tissue weights (Fig. 2). CpG-ODN 1018 ISS application, however, led to a significant increase in gLN and spleen weights within 24 h of application and may help to explain the extended protection afforded by CpG-ODN relative to that with PIC (19, 21, 31, 41). The lavage data also suggest that IFN-β produced by EC lining the genital tract may play more of a role in PIC-mediated protection than IFN-α, which was not secreted at detectable levels into murine vaginal lavages. Further animal studies will be required to quantify intracellular or secreted forms of IFN-β in lavages, but the data from human vaginal EC cultures support this supposition. IFN-β directly inhibits HSV-2 gene expression and activates other immune system components, including antigen-presenting cells and lymphocytes (23, 24).
The kinetics of cytokine induction following PIC application also provided an explanation for the observed in vivo prophylactic and therapeutic protection. Most measured cytokines reached significant levels in vaginal lavages by 2 to 4 h after treatment, decreasing by 18 to 24 h and returning to baseline by 48 h (Fig. 1). Ashkar et al. did not observe IFN-γ in vaginal washes at 24 or 48 h (6), times when we found IFN-γ had returned to baseline levels near the lower limit of the assay (Fig. 1). Prolonged, elevated release of cytokines, indicative of toxicity, was not observed in lavages even after repetitive delivery. The observed, transient upregulation of acute-phase cytokines (IL-1 and IL-6) likely contributed to PIC-afforded protection by recruiting and/or activating T cells, dendritic cells, and macrophages (35, 39, 43). The observed PIC upregulation of RANTES, a chemokine that enhances Th1 CD4+ T-cell-mediated immunity against HSV-2 (19, 27, 29, 38), supports T-cell involvement. Cellular recruitment may explain the observed protection >1 day prior to challenge, a time when cytokine levels were near baseline. Alternatively, the rapid induction of cytokines by 4 h may be the basis for reduced disease and delayed lethality associated with therapeutic PIC application. Such inductions would follow initial infection but would peak prior to completion of the first round of HSV-2 replication, ∼12 h after inoculation (13).
With regard to potential toxicity, repetitive systemic CpG-ODN but not systemic PIC administration was reported to elicit substantial and possibly toxic alterations in lymph nodes, spleen, and liver (20, 35). Unlike the case with CpG-ODN 1018 ISS, no gross pathological changes in the gLN, spleen, or liver resulted from up to 14 doses of PIC applied locally. As noted, this lack of immune induction, in part, explains the short-lived PIC durability. Conversely, the extended durability of protection provided by CpG-ODN (19, 31) may be explained by the substantial recruitment and/or retention of lymphocytes in the gLN (35, 41).
Due to PIC's short durability and the observed lack of toxicity associated with this microbicide candidate, repetitive delivery may be recommended clinically. The simultaneous presence of PIC and other STI pathogens and their natural TLR agonists potentially could alter outcomes and induce toxicity, a possibility that must be addressed with further studies with animal models. Although the safety of administering TLR agonists in humans is not clearly defined, there have been several clinical trials evaluating administration of TLR3, -7, and -9 agonists with promising results and no severe adverse reactions (8, 18, 17, 44). In fact, a TLR7 agonist (Aldara) has been licensed as a treatment for genital warts, utilizing an every-other-day application regimen (40).
Our data support continued study of PIC as a microbicide with prophylactic potential to protect against HSV-2. The enhanced disease resolution and survival afforded by PIC also support its continued evaluation as a therapeutic. Epidemiological studies and the data presented in this report suggest that if successfully introduced to the clinic, PIC could profoundly impact the HSV-2 epidemic (9, 16, 34). Finally, ideal microbicides should be potent and broad spectrum. Further testing will be required to determine if PIC will prevent infection or limit replication and pathology induced by other STI.
Acknowledgments
This work was supported by the Gulf South Sexually Transmitted Infections and Topical Microbicide Cooperative Research Center, NIAID U19 AI61972. M.M.H.-K. was supported by an NIH T-32 training grant in mucosal immunology, AI07626-04, the Zelda Zinn Casper Endowed Scholarship, and the James W. McLaughlin Fellowship Fund.
We thank Dynavax Technologies for providing the CpG-ODN 1018 ISS oligonucleotide. We also thank Marguerite Maurer and Bhagavathi Ramasubramanian for their technical support.
The authors do not have a commercial affiliation that might pose a conflict of interest.
REFERENCES
- 1.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S. 2003. Toll-like receptor signaling. J. Biol. Chem. 278:38105-38108. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S., and H. Hemmi. 2003. Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 85:85-95. [DOI] [PubMed] [Google Scholar]
- 4.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 5.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 6.Ashkar, A. A., X. D. Yao, N. Gill, D. Sajic, A. J. Patrick, and K. L. Rosenthal. 2004. Toll-like receptor (TLR)-3, but not TLR4, agonist protects against genital herpes infection in the absence of inflammation seen with CpG DNA. J. Infect. Dis. 190:1841-1849. [DOI] [PubMed] [Google Scholar]
- 7.Baron, S., S. K. Tyring, W. R. Fleischmann, Jr., D. H. Coppenhaver, D. W. Niesel, G. R. Klimpel, G. J. Stanton, and T. K. Hughes. 1991. The interferons. Mechanisms of action and clinical applications. JAMA 266:1375-1383. [DOI] [PubMed] [Google Scholar]
- 8.Beutner, K. R., S. K. Tyring, K. F. Trofatter, Jr., J. M. Douglas, Jr., S. Spruance, M. L. Owens, T. L. Fox, A. J. Hougham, and K. A. Schmitt. 1998. Imiquimod, a patient-applied immune-response modifier for treatment of external genital warts. Antimicrob. Agents Chemother. 42:789-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blower, S., A. Wald, H. Gershengorn, F. Wang, and L. Corey. 2004. Targeting virological core groups: a new paradigm for controlling herpes simplex virus type 2 epidemics. J. Infect. Dis. 190:1610-1617. [DOI] [PubMed] [Google Scholar]
- 10.Catalone, B. J., T. M. Kish-Catalone, L. R. Budgeon, E. B. Neely, M. Ferguson, F. C. Krebs, M. K. Howett, M. Labib, R. Rando, and B. Wigdahl. 2004. Mouse model of cervicovaginal toxicity and inflammation for preclinical evaluation of topical vaginal microbicides. Antimicrob. Agents Chemother. 48:1837-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2002. Division of HIV/STD prevention annual report. Centers for Disease Control and Prevention, Atlanta, Ga.
- 12.Cubitt, C. L., R. N. Lausch, and J. E. Oakes. 1995. Differences in interleukin-6 gene expression between cultured human corneal epithelial cells and keratocytes. Investig. Ophthalmol. Vis. Sci. 36:330-336. [PubMed] [Google Scholar]
- 13.Duerst, R. J., and L. A. Morrison. 2003. Innate immunity to herpes simplex virus type 2. Viral Immunol. 16:475-490. [DOI] [PubMed] [Google Scholar]
- 14.Elias, C. J., and C. Coggins. 1996. Female-controlled methods to prevent sexual transmission of HIV. AIDS 3:S43-S51. [PubMed] [Google Scholar]
- 15.Finberg, R. W., D. M. Knipe, and E. A. Kurt-Jones. 2005. Herpes simplex virus and toll-like receptors. Viral Immunol. 18:457-465. [DOI] [PubMed] [Google Scholar]
- 16.Garnett, G. P., G. Dubin, M. Slaoui, and T. Darcis. 2004. The potential epidemiological impact of a genital herpes vaccine for women. Sex. Transm. Infect. 80:24-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giantonio, B. J., H. Hochster, R. Blum, P. H. Wiernik, G. R. Hudes, J. Kirkwood, D. Trump, and M. M. Oken. 2001. Toxicity and response evaluation of the interferon inducer poly ICLC administered at low dose in advanced renal carcinoma and relapsed or refractory lymphoma: a report of two clinical trials of the Eastern Cooperative Oncology Group. Investig. New Drugs 19:89-92. [DOI] [PubMed] [Google Scholar]
- 18.Halperin, S. A., G. Van Nest, B. Smith, S. Abtahi, H. Whiley, and J. J. Eiden. 2003. A phase I study of the safety and immunogenicity of recombinant hepatitis B surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide adjuvant. Vaccine 21:2461-2467. [DOI] [PubMed] [Google Scholar]
- 19.Harandi, A. M., K. Eriksson, and J. Holmgren. 2003. A protective role of locally administered immunostimulatory CpG oligodeoxynucleotide in a mouse model of genital herpes infection. J. Virol. 77:953-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heikenwalder, M., M. Polymenidou, T. Junt, C. Sigurdson, H. Wagner, S. Akira, R. Zinkernagel, and A. Aguzzi. 2004. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat. Med. 10:187-192. [DOI] [PubMed] [Google Scholar]
- 21.Herbst, M. M., and R. B. Pyles. 2003. Immunostimulatory CpG treatment for genital HSV-2 infections. J. Antimicrob. Chemother. 6:887-889. [DOI] [PubMed] [Google Scholar]
- 22.Herold, B. C. 1997. Therapeutic blockade of sexually transmitted pathogens. Pediatr. Infect. Dis. J. 16:925-931. [DOI] [PubMed] [Google Scholar]
- 23.Katze, M. G., Y. He, and M. Gale, Jr. 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2:675-687. [DOI] [PubMed] [Google Scholar]
- 24.Kunder, S. C., K. M. Kelly, and P. S. Morahan. 1993. Biological response modifier-mediated resistance to herpesvirus infections requires induction of alpha/beta interferon. Antivir. Res. 21:129-139. [DOI] [PubMed] [Google Scholar]
- 25.Latz, E., A. Schoenemeyer, A. Visintin, K. A. Fitzgerald, B. G. Monks, C. F. Knetter, E. Lien, N. J. Nilsen, T. Espevik, and D. T. Golenbock. 2004. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5:190-198. [DOI] [PubMed] [Google Scholar]
- 26.Maguire, R. A., N. Bergman, and D. M. Phillips. 2001. Comparison of microbicides for efficacy in protecting mice against vaginal challenge with herpes simplex virus type 2, cytotoxicity, antibacterial properties, and sperm immobilization. Sex. Transm. Dis. 28:259-265. [DOI] [PubMed] [Google Scholar]
- 27.Malmgaard, L., J. Melchjorsen, A. G. Bowie, S. C. Mogensen, and S. R. Paludan. 2004. Viral activation of macrophages through TLR-dependent and -independent pathways. J. Immunol. 173:6890-6898. [DOI] [PubMed] [Google Scholar]
- 28.Marcelo, P., and F. Lefevre. 2002. Induction of the IFN-gamma gene and protein is linked to the establishment of cell polarity in a porcine epithelial cell line. Exp. Cell Res. 280:33-44. [DOI] [PubMed] [Google Scholar]
- 29.Milligan, G. N., D. I. Bernstein, and N. Bourne. 1998. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J. Immunol. 160:6093-6100. [PubMed] [Google Scholar]
- 30.Milligan, G. N., K. L. Dudley, N. Bourne, A. Reece, and L. R. Stanberry. 2002. Entry of inflammatory cells into the mouse vagina following application of candidate microbicides: comparison of detergent-based and sulfated polymer-based agents. Sex. Transm. Dis. 29:597-605. [DOI] [PubMed] [Google Scholar]
- 31.Pyles, R. B., D. Higgins, C. Chalk, A. Zalar, J. Eiden, C. Brown, G. Van Nest, and L. R. Stanberry. 2002. Use of immunostimulatory sequence-containing oligonucleotides as topical therapy for genital herpes simplex virus type 2 infection. J. Virol. 76:11387-11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quayle, A. J. 2002. The innate and early immune response to pathogen challenge in the female genital tract and the pivotal role of epithelial cells. J. Reprod. Immunol. 57:61-79. [DOI] [PubMed] [Google Scholar]
- 33.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 34.Sacks, S. L., P. D. Griffiths, L. Corey, C. Cohen, A. Cunningham, G. M. Dusheiko, S. Self, S. Spruance, L. R. Stanberry, A. Wald, and R. J. Whitley. 2004. HSV-2 transmission. Antivir. Res. 63:S27-S35. [DOI] [PubMed] [Google Scholar]
- 35.Salem, M. L., A. N. Kadima, D. J. Cole, and W. E. Gillanders. 2005. Defining the antigen-specific T-cell response to vaccination and poly(I:C)/TLR3 signaling: evidence of enhanced primary and memory CD8 T-cell responses and antitumor immunity. J. Immunother. 28:220-228. [DOI] [PubMed] [Google Scholar]
- 36.Sato, A., and A. Iwasaki. 2004. Induction of antiviral immunity requires Toll-like receptor signaling in both stromal and dendritic cell compartments. Proc. Natl. Acad. Sci. USA 101:16274-16279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato, S., O. Takeuchi, T. Fujita, H. Tomizawa, K. Takeda, and S. Akira. 2002. A variety of microbial components induce tolerance to lipopolysaccharide by differentially affecting MyD88-dependent and -independent pathways. Int. Immunol. 14:783-791. [DOI] [PubMed] [Google Scholar]
- 38.Sin, J., J. J. Kim, C. Pachuk, C. Satishchandran, and D. B. Weiner. 2000. DNA vaccines encoding interleukin-8 and RANTES enhance antigen-specific Th1-type CD4(+) T-cell-mediated protective immunity against herpes simplex virus type 2 in vivo. J. Virol. 74:11173-11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sin, J. I., J. J. Kim, J. D. Boyer, R. B. Ciccarelli, T. J. Higgins, and D. B. Weiner. 1999. In vivo modulation of vaccine-induced immune responses toward a Th1 phenotype increases potency and vaccine effectiveness in a herpes simplex virus type 2 mouse model. J. Virol. 73:501-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slade, H. B., M. L. Owens, M. A. Tomai, and R. L. Miller. 1998. Imiquimod 5% cream (Aldara). Expert Opin. Investig. Drugs 7:437-449. [DOI] [PubMed] [Google Scholar]
- 41.Soderberg, K. A., G. W. Payne, A. Sato, R. Medzhitov, S. S. Segal, and A. Iwasaki. 2005. Innate control of adaptive immunity via remodeling of lymph node feed arteriole. Proc. Natl. Acad. Sci. USA 102:16315-16320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stanberry, L., A. Cunningham, G. Mertz, A. Mindel, B. Peters, M. Reitano, S. Sacks, A. Wald, S. Wassilew, and P. Woolley. 1999. New developments in the epidemiology, natural history and management of genital herpes. Antivir. Res. 42:1-14. [DOI] [PubMed] [Google Scholar]
- 43.Tabeta, K., P. Georgel, E. Janssen, X. Du, K. Hoebe, K. Crozat, S. Mudd, L. Shamel, S. Sovath, J. Goode, L. Alexopoulou, R. A. Flavell, and B. Beutler. 2004. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 101:3516-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson, K. A., D. R. Strayer, P. D. Salvato, C. E. Thompson, N. Klimas, A. Molavi, A. K. Hamill, Z. Zheng, D. Ventura, and W. A. Carter. 1996. Results of a double-blind placebo-controlled study of the double-stranded RNA drug polyI:polyC12U in the treatment of HIV infection. Eur. J. Clin. Microbiol. Infect. Dis. 15:580-587. [DOI] [PubMed] [Google Scholar]
- 45.Xu, F., J. A. Schillinger, M. R. Sternberg, R. E. Johnson, F. K. Lee, A. J. Nahmias, and L. E. Markowitz. 2002. Seroprevalence and coinfection with herpes simplex virus type 1 and type 2 in the United States, 1988-1994. J. Infect. Dis. 185:1019-1024. [DOI] [PubMed] [Google Scholar]