Abstract
Objective
To assess the hypnotic effect of melatonin in patients with primary insomnia.
Method
Ten patients (mean age 50 yr, range 30–72 yr) who met the DSM-IV criteria for primary insomnia received, in random order, 0.3 mg of melatonin, 1.0 mg of melatonin or placebo 60 minutes before bedtime. A crossover design was used so that each patient received each of the 3 treatments for a 7-day period (with a 5-day washout period between). After each 7-day treatment, night time electroencephalographic (EEG) records were collected, and each morning, subjects completed sleep logs and analogue-visual scales to document the amount and subjective quality of sleep.
Results
There were no significant differences in sleep EEG, the amount or subjective quality of sleep or side effects between the placebo, 0.3-mg melatonin or 1.0-mg melatonin treatments.
Conclusion
Melatonin did not produce any sleep benefit in this sample of patients with primary insomnia.
Medical subject headings: melatonin; sleep disorders, intrinsic; sleep initiation and maintenance disorders; sleep stages
Abstract
Objectif
Évaluer l'effet hypnotique de la mélatonine chez les patients qui souffrent d'insomnie primaire.
Méthode
Dix patients (âge moyen de 50 ans, intervalle de 30 à 72 ans) qui satisfaisaient aux critères relatifs à l'insomnie primaire selon le DSM-IV ont reçu, au hasard, 0,3 mg de mélatonine, 1,0 mg de mélatonine ou un placebo 60 minutes avant le coucher. On a suivi un plan croisé afin que chaque patient reçoive chacun des trois traitements pendant une période de sept jours (traitements séparés par une période d'élimination de cinq jours). Les sujets se sont soumis à un électroencéphalogramme (EEG) chaque soir et ont rempli des registres sur le sommeil et des échelles analogiques-visuelles pour consigner la durée du sommeil et sa qualité subjective.
Résultats
Il n'y avait pas de différences importantes au niveau de l'EEG, de la durée du sommeil ou de sa qualité subjective, ou des effets secondaires entre le traitement au placebo, à 0,3 mg de mélatonine ou à 1,0 mg de mélatonine.
Conclusion
La mélatonine n'a produit aucun avantage pour le sommeil chez cet échantillon de patients qui souffraient d'insomnie primaire.
Introduction
Research in animals and humans suggests that melatonin plays a role in diverse functions such as controlling circadian rhythms, inhibiting reproductive processes, increasing immune response, reducing cellular damage and promoting sleep.1,2 Although many studies have been done on this pineal hormone, the psychopharmacologic actions are still unclear. Moreover, more empirical evidence is necessary to determine whether melatonin might benefit specific clinical populations such as patients with primary insomnia.
Soon after the discovery of melatonin by Lerner and colleagues in 1958, the hypnotic effects of melatonin were described in healthy volunteers.3 However, the neurobiological mechanisms responsible for these hypnotic effects are still being investigated. Sack et al4 proposed that the sleep-promoting effect of melatonin might be mediated by melatonin Mel 1a receptors in the suprachiasmatic nucleus (SCN) of the hypothalamus,2 through either a phase shift in the pacemaker at the SCN or a decrease in cortical and behavioural activation dependent on the SCN. Redman et al5 described melatonin as a “chronobiotic” hormone after demonstrating that daily injections of melatonin could entrain circadian rhythms in the rat. Hajak et al6 reported lower levels in blood melatonin in patients with insomnia than in healthy controls, and Haimov et al7 found lower levels of urinary 6-sulphatoxymelatonin in elderly patients with insomnia than in healthy elderly subjects.
The results of many reports and clinical trials on melatonin suggest that it has a hypnotic or sleep-promoting effect. However, most studies to date8,9,10,11,12,13,14,15,16 have been done with healthy adults, and only 29,15 included simultaneous objective (e.g., sleep electroencephalograms [EEG]) and subjective (e.g., sleep log) measures of sleep quality and amount. In addition, structured psychiatric interviews were not conducted to rule out other psychiatric disorders.
The purpose of this study was to compare the efficiency and safety of melatonin (0.3 mg and 1.0 mg) and placebo in patients with primary insomnia.
Methods
Ten patients who met the DSM-IV criteria for primary insomnia and were being treated in an outpatient setting at the Instituto Nacional de PsiquiatrÍa “Ramón de la Fuente Muñiz,” in Mexico City from 1997 to 1998 were enrolled in the study. All patients with circadian rhythm sleep disorders were excluded. Participants, at the time of the study, were not medically ill and had not suffered from any of the following medical conditions: vascular disease (e.g., arterial hypertension, coronary disease), diabetes mellitus, hyperlipidemic disease, obesity (body mass index over 30) or dermatologic, gynecologic or endocrine diseases. Patients who used beta-adrenergic antagonists (e.g., propanolol, atenolol, metoprolol) and those with a substance abuse problem or drug dependence (e.g., alcohol, nicotine) were also excluded.
Patients who were taking psychotropic medication (e.g., antidepressants, benzodiazepines, antipsychotics) or a hypnotic drug (e.g., zolpidem, zopiclone, chloral hydrate, antihistamines) were included in the study only if they stopped taking their medication at least 4 weeks before the study began. This was confirmed by medical records and in an interview with their psychiatrist.
All participants gave their written informed consent. The study was approved by the Research and Ethics Committee of the Instituto Nacional de PsiquiatrÍa “Ramón de la Fuente Muñiz.”
Clinical assessments
For a precise psychiatric diagnosis and to rule out affective, anxiety, psychotic or substance abuse disorders, participants completed the Structured Clinical Interview of the DSM-III-R (SCID-I).17 This was conducted by a psychiatrist trained and experienced in the use of the instrument. In addition, the Hamilton Depression Rating Scale, 17-item version (HDRS 17), Carroll Depression Scale (CDS), Beck Depression Inventory (BDI) and Hamilton Anxiety Rating Scale (HARS) were administered.
Patients were asked to complete a sleep log every morning, which included the following questions:
· At what time did you switch off the light?
· How long did it take you to fall asleep?
· How many times did you wake up during the night?At what time did you get up?
· How many hours did you sleep?
· Rate your sleep on a scale of 0 (poor) to 5 (excellent).
· Did you take a nap during the day? If yes, how long did it last?
Additionally, analogue-visual scales were used to assess the quality and amount of sleep. Blood and urine samples were obtained for red and white blood cell counts, and the following tests were performed: complete urine test, serum electrolyte assay, hepatic function test, thyroid function test, lipid profile, electrocardiogram, EEG, computed tomographic (CT) scan and a urinary screening test for illegal drugs.
Polisomnographic studies
Two all-night polisomnographic studies were performed at the sleep lab in the chronobiology department before random assignment. This included an EEG recording following the international 10-20 system of electrode placement (F8-T4, T4-T6, T3-T5, C4-A1, O1-O2), an electro-oculogram and an electromyogram, with electrode placement according to Rechtschaffen and Kales,18 a nasal thermistor, thoracic and abdominal bands, 2 electrodes placed in the anterior tibial region and an electrocardiogram.
Procedure
All patients were randomly assigned by means of a 3 х3 Latin square design to receive 0.3 mg of melatonin, 1.0 mg of melatonin or placebo in identical capsules 60 minutes before bedtime. Sustained-release synthetic melatonin was used (Cronocaps, Productos Medix, Ejidos de Santa Ursula, México). According to the manufacturer, the initial concentration in plasma is reached in 30 minutes, the maximal concentration is reached in 60 minutes, and the stable state is reached in 6 hours. The capsules produce a sustained release over 8 hours and can produce serum concentrations over 10 hours. This product has been approved for human consumption by the SecretarÍa de Salud (Mexican Department of Health, registration no. 591M97, LEAR-21836/6R97).
The crossover design ensured that each subject underwent each of the 3 treatments for a 7-day period. After each period, a night of polisomnographic study was performed. Between each 7-day period, a single-blind placebo was used for 5 days to prevent residual effects from the previous procedure. Weekly visits were scheduled for physical examinations and to assess side effects.
Each polisomnographic study was scored according to the Rechtschaffen and Kales criteria18 by an experienced physician who was blind to the treatment. Once scored, all data were entered into a computer software program (WinSleep), which generated all the statistical data (i.e., number of wakings, phase 1, phase 2, delta sleep and REM sleep stages; total time of wakings, phase 1, phase 2, delta sleep and REM sleep stages; average length of wakings, phase 1, phase 2, delta sleep and REM sleep stages; percent of wakings, phase 1, phase 2, delta sleep and REM sleep stages in the total sleep time and total recording time; latency for each sleep stage; total recording time and total sleep time; average length of the NREM–REM cycle and sleep efficiency).
Statistical analysis
All polisomnographic and subjective variables were compared by multivariate analysis of variance (MANOVA), and the chi-square test was used to assess side effects.
Results
The mean age of the 6 men and 4 women was 50 (standard deviation [SD] 12.7 yr, range 30–72 yr). All patients had been previously taking benzodiazepine medications, and only 2 reported at least 1 major depressive episode in the past. The mean duration of insomnia complaint was 12.8 years (SD 12.1 yr, range 0.5–32 yr). Seven patients had terminal insomnia, 6 had mild insomnia and 6 had initial insomnia. Seven rated their insomnia as a severe problem.
The mean score on the HDRS was 9.4 (SD 3.5), on the CDS was 15 (SD 5.8), on the BDI was 9.9 (SD 5.6) and on the HARS was 12.5 (SD 5.2).
Polisomnographic data
As shown in Fig. 1, there were no significant differences in sleep efficiency between the placebo, 0.3-mg melatonin and 1.0-mg melatonin treatments (p = 0.92). As shown in Fig. 2, there were no significant differences in total sleep time between the 3 treatments (p = 0.77). Also, there were no statistical differences in latency to any sleep stage between placebo, 0.3 mg and 1.0 mg of melatonin (stage 1, p = 0.72; stage 2, p = 0.14; delta stage, p = 0.42) (Fig. 3).
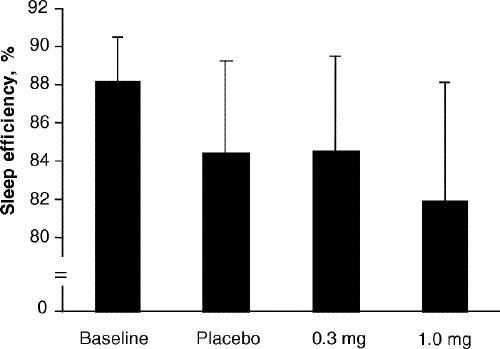
Fig. 1: Mean (and standard deviation) sleep efficiency at baseline and with placebo, 0.3-mg melatonin and 1.0-mg melatonin treatments.
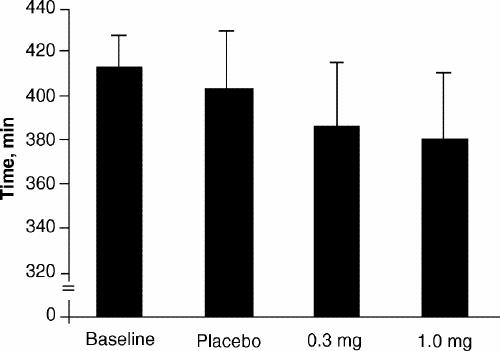
Fig. 2: Mean (and standard deviation) total sleep time at baseline and with placebo, 0.3-mg melatonin and 1.0-mg melatonin treatments.
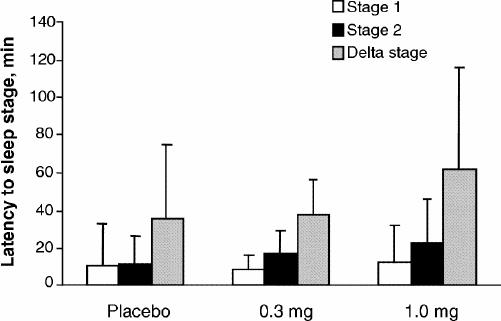
Fig. 3: Mean (and standard deviation) latency to stage 1, stage 2 and delta stage with placebo, 0.3-mg melatonin and 1.0-mg melatonin treatments.
Table 1 shows percentage of waking stage in total recording time, number of awakenings and total time of waking stage for each treatment. Again, there were no significant differences.
Table 1
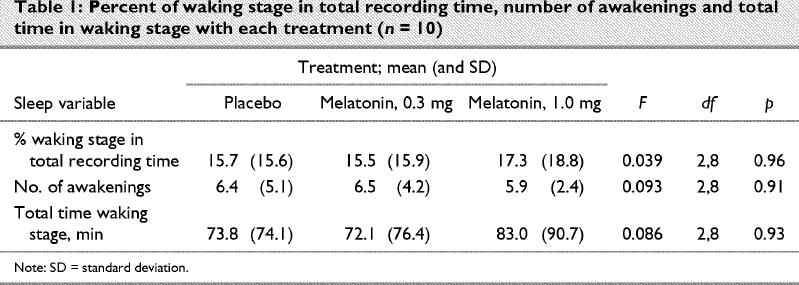
There were also no statistical differences in total recording time; average length of the NREM–REM cycle; percent of stage 1, 2, delta sleep and REM sleep in total sleep time; number of each sleep stage and total time of each sleep stage.
Subjective measures of sleep amount and quality
With the 1.0-mg melatonin dose, the mean lights off time was 2316 (SD 0.6) hours, with 0.3 mg melatonin 2319 (SD 1.1) hours and with placebo 2352 (SD 0.4) hours (p = 0.24). The hour at which patients got up was advanced by 18 minutes with 1.0 mg of melatonin in contrast to placebo and 17 minutes in contrast with 0.3 mg of melatonin (p = 0.83).
Sleep latency was 57.4 (SD 47.2) minutes with 1.0 mg of melatonin, 84.4 (SD 89.4) minutes with 0.3 mg of melatonin and 69.2 (SD 29.1) minutes with placebo (p = 0.11). The mean number of awakenings subjects recorded in their logs was 1.3 (SD 0.8) with placebo, 1.3 (SD 0.8) with 0.3 mg and 1.5 (SD 0.9) with 1.0 mg of melatonin (p = 0.53). Total sleep time was 5.1 (SD 2.3) hours with 1.0 mg of melatonin, 4.0 (SD 1.3) hours with 0.3 mg of melatonin and 4.5 (SD 1.4) hours with placebo (p = 0.70).
On a scale of 0 (poor) to 5 (excellent), sleep quality was rated a mean score of 2.2 (SD 1.6) with 1.0 mg of melatonin, 2.4 (SD 1.1) with 0.3 mg of melatonin and 2.0 (SD 1.3) with placebo (p = 0.33).
There were no significant differences in sleep quality or amount between the 3 treatments on visual-analogue scales, and there were no differences in reported side effects.
Discussion
We found no difference between the effects of placebo and 2 doses of melatonin on polisomnographic and subjective measures of sleep quality in patients with primary insomnia. This indicates that the physiological action of melatonin is likely the entrainment of internal rhythms with the light–dark cycle and that melatonin is not directly responsible for sleep onset.19
We observed an advance (non-significant) in bedtime induced by 1.0 mg of melatonin (36 min) and 0.3 mg of melatonin (33 min), which supports the well known capacity of this hormone to change biological rhythms.4,20,21,22 Unfortunately, this was not helpful to our patients because they still experienced poor sleep quality.
Our results agree with those of James et al,8 Ellis et al,14 Hughes et al23 and Dawson et al,16 who also studied patients with insomnia, but do not agree with results obtained with healthy subjects. This suggests that patients with insomnia have a pathophysiologic disturbance that is not reversed by melatonin. Reports of increased heart rate, core body temperature, muscle tone and 24-hour metabolic rate in patients with insomnia (compared with controls)24 suggest that hyperactivity of waking mechanisms is an inherent pathophysiologic component of insomnia that results in a failure of sleep mechanisms to express normally. Thus, we believe that insomnia is not primarily a disorder of sleep mechanisms, but rather a disturbance of waking mechanisms.
One limitation of this study is that the negative findings observed are difficult to interpret because of the variability of the sample (e.g., age). We are aware of the ontogenesis of sleep and the changes in normal sleep patterns over the lifespan. In this study, however, we used as a dependent variable the DSM-IV definition of primary insomnia, which does not include age as inclusion or exclusion criteria for a diagnosis. In addition, because of the crossover design of this study, each subject was compared with himself or herself and served as his or her own control.
Finally, 5 patients showed a first REM sleep latency in under 65 minutes on at least 3 nights. This was not related to a particular treatment but raised 2 questions: Is this shorter REM sleep latency because a REM sleep rebound is produced when patients sleep in a different environment (e.g., sleep lab), such as is seen in psychophysiological insomnia? Or does it represent a non-specific “biological marker” of primary insomnia, as is seen in major depressive disorder,25 borderline personality disorder26 and schizophrenia?27 If the latter is true, we propose that patients with primary insomnia may represent a subset of mood disorder whose symptom severity and amount do not reach a DSM-IV threshold for the formal diagnosis of major depressive disorder or an anxiety disorder.
Acknowledgments
We thank Dr. Augusto Fernández Guardiola, I. Rodrigo Fernández Mass, Dr. Gloria Benitez King, Lourdes Montes, Isidoro Camacho GarcÍa, Fernando Jiménez Peña, Jennifer Bonelli and Gouran Roger, D.M.A.
Footnotes
Research and Ethics Committee: Dr. Ramón de la Fuente Muñiz, Dr. MarÍa Elena Medina Mora, Dr. Augusto Fernández Guardiola, Dr. Carlos Campillo Serrano, Dr. Humberto Nicolini Sánchez.
Competing interests: None declared.
Correspondence to: Dr. Luis G. Almeida Montes, Ignacio Mariano de las Casas No. 34, Colonia Cimatario, Querétaro, Querétaro México CP 76030; fax 555 655 04 11; almeidal@prodigy.net.mx
Submitted Jan. 29, 2002 Revised Aug. 7, 2002; Dec. 11, 2002 Accepted Dec. 17, 2002
References
- 1.Nagtegaal JE, Kerkhof GA, Smits MG. A placebo-controlled crossover study on the effects of melatonin administered five hours before the individual dim light melatonin onset. J Sleep Res.1998;7:135-43. [DOI] [PubMed]
- 2.Brzezinski A. Melatonin in humans. N Engl J Med 1997;336:186-95. [DOI] [PubMed]
- 3.Lerner AB, Case JD. Melatonin. Fed Proc 1960;19:590-2.
- 4.Sack RL, Hughes RJ, Edgar DM, Lewy AJ. Sleep-promoting effects of melatonin: at what dose, in whom, under what conditions, and by what mechanisms? Sleep 1997;20:908-15. [DOI] [PubMed]
- 5.Redman J, Armstrong S, Ng KT. Free-running activity rhythms in the rat: entrainment by melatonin. Science 1983;219:1089-91. [DOI] [PubMed]
- 6.Hajak G, Rodenbeck A, Staedt J, Bandelow B, Hueter G, Rüther E. Nocturnal plasma melatonin levels in patients suffering from chronic primary insomnia. J Pineal Res 1995;19:116-22. [DOI] [PubMed]
- 7.Haimov I, Laudon M, Zisapel N, Souroujon M, Nof D, Shlitner A, et al. Sleep disorders and melatonin rhythms in elderly people. BMJ 1994;309:167. [DOI] [PMC free article] [PubMed]
- 8.James SP, Sack RL, Rosenthal NE. Melatonin administration in insomnia. Neuropsychopharmacology 1990;3:19-23. [PubMed]
- 9.Dahlitz M, Alvarez B, Vignau J, English J. Delayed sleep phase syndrome response to melatonin. Lancet 1991;337:1121-4. [DOI] [PubMed]
- 10.MacFarlane JG, Cleghorn JM, Brown GM. The effects of exogenous melatonin on the total sleep time on daytime alertness of chronic insomniacs: a preliminary study. Biol Psychiatry 1991;30:371-6. [DOI] [PubMed]
- 11.Jan JE, Espezel H, Appleton RE. The treatment of sleep disorders with melatonin. Dev Ed Child Neurol 1994;36:97-107. [DOI] [PubMed]
- 12.Garfinkel D, Laudon M, Nof D, Zisapel N. Improvement of sleep quality in elderly people by controlled-release melatonin. Lancet 1995;346:541-4. [DOI] [PubMed]
- 13.Wurtman RJ, Zhdanova IV. Improvement of sleep quality by melatonin. Lancet 1995;346(8988):1491. [DOI] [PubMed]
- 14.Ellis CM, Lemmens G, Parkes D. Melatonin and insomnia. J Sleep Res 1996;5:61-5. [DOI] [PubMed]
- 15.Hughes RJ, Badia P. Sleep-promoting and hypothermic effects of daytime melatonin administration in humans. Sleep 1997;20: 124-31. [PubMed]
- 16.Dawson D, Rogers NL, Cameron J. Effect of a sustained nocturnal transbucal melatonin administration on sleep and temperature in elderly insomniacs. J Biol Rhythms 1998;13:532-8. [DOI] [PubMed]
- 17.Spitzer RL, Williams JB, Gibbon M. Structured Clinical Interview for DSM III-R Axis I disorders (SCID-I). Washington: American Psychiatric Press; 1990.
- 18.Rechtschaffen A, Kales A, editors. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subjects. Los Angeles: Brain Information service/Brain research Institute; 1968.
- 19.Axelrod J. The pineal gland: a neurochemical transducer. Science 1974;184:1341-8. [DOI] [PubMed]
- 20.Sack RL, Blood ML, Lewy AJ. Melatonin administration to night shift workers: an update [letter]. J Sleep Res 1995;24:539.
- 21.Sack RL, Lewy AJ, Blood ML. Melatonin administration to blind people: phase advances and entrainment. J Biol Rhythms 1991;6:249-61. [DOI] [PubMed]
- 22.Lewy AJ, Ahmed S, Jackson JML, Sack RL. Melatonin shifts circadian rhythms according to phase-response curve. Chronobiol Int 1992;9:380-92. [DOI] [PubMed]
- 23.Hughes RJ, Sack RL, Lewy AJ. The role of melatonin and circadian phase in age-related sleep-maintenance insomnia: assessment in a clinical trial of melatonin replacement. Sleep 1998;21:52-68. [PubMed]
- 24.Sateia MJ, Doghramji K, Hauri P, Morin C. Evaluation of chronic insomnia. Sleep 2000;23:243-305. [PubMed]
- 25.Cartwright RD, Kravitz HM, Eastman CI, Wood E. REM latency and the recovery from depression: getting over divorce. Am J Psychiatry 1991;148:1530-5. [DOI] [PubMed]
- 26.McNamara E, Reynolds CF, Soloff PH, Mathias R, Rossi A, Spiker D, et al. EEG sleep evaluation of depression in borderline patients. Am J Psychiatry 1984;141:182-6. [DOI] [PubMed]
- 27.Maixner S, Tandon R, Eiser A, Taylor S, DeQuardo JR, Shipley J. Effects of antipsychotic treatment on polysomnographic measures in schizophrenia: a replication and extension. Am J Psychiatry 1998;155:1600-2. [DOI] [PubMed]


