Abstract
Objective
The spontaneously hypertensive rat (SHR), often used to study cardiovascular disease processes, may also be utilized to model certain central nervous system changes associated with memory disorders. Previous work in our laboratory indicated that central nicotinic acetylcholine receptors are markedly diminished and that memory-related task performance is impaired in this rodent phenotype. Due to the well-documented importance of the central cholinergic system to memory processes and its vulnerability to the effects of aging, it was of interest to measure other cholinergic markers and to further evaluate memory function in older SHRs.
Method
Radial arm maze performance was used to assess working memory, quantitative receptor autoradiography with [3H]-pirenzipine, [3H]-AFDX-384 and [3H]-epibatidine (combined with cytisine) was used to determine the densities of muscarinic-M1 and -M2 and nicotinic cholinergic α3 receptors, respectively. Immunoblotting experiments were also used to determine the expression of the presynaptic cholinergic markers, choline acetyltransferase and the vesicular acetylcholine transporter.
Results
Radial arm maze performance was impaired in hypertensive (compared with normotensive Wistar and Wistar-Kyoto) rats, regardless of age. M1 binding was increased in frontal and prefrontal cortical areas in SHR (p < 0.05), whereas M2 densities were higher in the hypertensive phenotype in the caudate putamen. A lower expression of !#!alpha;3-containing nicotinic receptors was observed in the superior colliculus in SHRs. Age-related differences in the expression of the vesicular acetylcholine transporter were noted in the hippocampus.
Conclusion
The SHR may be useful to model some aspects (particularly hypertension-related) of memory disorders, especially those in which cholinergic function is altered.
Medical subject headings: acetylcholine; aging; hypertension; memory disorders; models, animal; rats, inbred SHR; rats, inbred WKY; receptors, cholinergic
Abstract
Objectif
Le rat spontanément hypertendu (RSH), souvent utilisé pour étudier les phénomènes morbides cardiovasculaires, peut aussi servir à modéliser certains changements du système nerveux central associés à des troubles de la mémoire. Des travaux antérieurs réalisés dans notre laboratoire ont indiqué que l'activité des récepteurs centraux de l'acétylcholine nicotinique diminue sensiblement et que l'exécution des tâches reliées à la mémoire est réduite chez ce phénotype de rongeurs. En raison de l'importance bien documentée du système cholinergique central pour les processus de la mémoire et de sa vulnérabilité aux effets du vieillissement, il s'avérait intéressant de mesurer d'autres marqueurs cholinergiques et d'évaluer davantage la fonction mémoire chez des RSH plus âgés.
Méthodes
On a utilisé les résultats du test du labyrinthe en étoile pour évaluer la mémoire au travail, l'autoradiographie des récepteurs quantitatifs avec la [3H]-pirenzipine, [3H]-AFDX-384 et [3H]-épibatidine (combinée à la cytisine) pour déterminer les densités des récepteurs muscariniques M1 et M2 et cholinergiques nicotiniques α3 respectivement. On a aussi utilisé des expériences de transfert pour déterminer l'expression des marqueurs cholinergiques présynaptiques, de la choline-acétylase et du transporteur vésiculaire de l'acétylcholine.
Résultats
Les résultats du test du labyrinthe en étoile ont diminué chez les rats hypertendus (comparativement à des rats Wistar et Wistar-Kyoto normotendus), sans égard à l'âge. La fixation de M1 dans les régions du cortex frontal et préfontal était accrue chez les RSH (p < 0,05), tandis que les densités de M2 étaient plus élevées dans le putamen caudé chez le phénotype hypertendu. On a observé une expression réduite des récepteurs nicotiniques contenant de l'α3 dans le colliculus supérieur chez les RSH. Dans l'hippocampe, on a constaté des différences liées à l'âge dans l'expression du transporteur de l'acétylcholine vésiculaire.
Conclusion
Le RSH peut être utile pour modéliser certains aspects des troubles de la mémoire (reliés particulièrement à l'hypertension), spécialement dans les cas où la fonction cholinergique est modifiée.
Introduction
A number of epidemiologic studies support the premise that hypertension is a risk factor for late life cognitive impairment.1,2 Although the risk for memory dysfunction and dementia associated with strokes resulting from hypertension and vascular disease (i.e., vascular dementia3) is clearly apparent, evidence is emerging that these factors may also play a role in the pathogenesis of other forms of dementia such as Alzheimer's disease (AD),4,5 progressive supranuclear palsy6,7 and dementia with Lewy bodies.8 In vascular dementia and hypertension-related dementia, the contribution of factors other than overt infarcts is also gaining interest. These include alterations in cerebrovascular autoregulation, cytokine activation and increased inflammatory processes in the brain, atherosclerosis, elevated plasma homocysteine and white matter changes.4,9 It is conceivable that any of these processes could negatively alter neural transmission and thus play either a direct or indirect role in cognitive dysfunction. It is important, therefore, to further investigate the role of these factors, as well as other hypertension-related CNS changes, in appropriate animal models.
For several decades, the spontaneously hypertensive rat (SHR) has been used extensively as a model of human hypertension and cardiovascular disease. Certain unique phenotypic characteristics (e.g., hyperactivity, deficits in sustained attention) have also resulted in the use of the SHR as a model of attention-deficit hyperactivity disorder.10,11 In addition, observations of inferior performance on a variety of memory-related tasks, including conditioned avoidance12,13,14 and appetitively15,16,17 and non-appetitively motivated18,19,20 spatial learning tasks, have led to interest in using the SHR to study the effects of hypertension on cognitive function. Few studies have, however, investigated the effects of aging on memory function in the SHR.
As early as 6 months of age, SHRs demonstrate hypertensive brain damage (e.g., cytoskeletal breakdown, astrogliosis and atrophy in the hippocampus) reminiscent of vascular dementia.21,22 SHRs show other signs of significant morbidity and spontaneous deaths after 15 months of age, as well as a reduced mean lifespan23,24,25 compared with other rat strains. These findings combined with observations of significant deficits in central nicotinic cholinergic receptors (nAChRs) that worsen with age20 suggest that older SHRs (in particular) could be used to model certain aspects of several age-related human diseases in which cholinergic function is altered.
The cholinergic system appears to be particularly vulnerable to the effects of aging, as determined by immunostaining techniques, neurotransmitter turnover studies and receptor measurements26,27,28,29,30 in aged animals and humans, as well those who suffer from age-related neurodegenerative diseases such as AD.31,32 There is also evidence of a decline in the integrity of the cholinergic system in patients with vascular dementia (e.g., decreased choline acetyltransferase [ChAT] and a depletion of cerebrospinal fluid acetylcholine levels.33,34,35). Recent clinical trials indicate that acetylcholinesterase inhibitors (e.g., galantamine) may be effective in improving memory function in patients with vascular dementia.1,36,37 These observations highlight the value of identifying animal models that show age-related cardiovascular changes and deficits in cholinergic function and memory. Such models will be useful for investigate the interactions among these factors as well as for preclinical drug testing.
Given the well-documented importance of the central cholinergic system to memory processes and the unique phenotypic features of SHRs, we were interested in extending our previous investigations on memory performance and central cholinergic function in older SHRs. We evaluated SHRs in a memory-related task known to depend on cholinergic function (the radial arm maze38) and measured the expression of muscarinic cholinergic receptors (mAChRs) and α3-containing nAChRs via quantitative receptor autoradiography. Immunoblotting experiments were conducted to evaluate potential phenotypic and age-related differences in the expression of 2 well described (presynaptic) cholinergic markers:39,40 ChAT and the vesicular acetylcholine transporter (VAChT).
Methods
Male SHRs and Wistar-Kyoto rats (WKYs), 3 months old and 9-month-old retired breeders, were purchased from Taconic Farms (Germantown, NY). Male Wistar rats (3 months old) were purchased from Harlan (Indianapolis, Ind.). Animals were housed individually in a temperature-controlled room (25οC) with a 12-hour light–dark cycle. Upon arrival, each animal was provided with water and food (Teklad rodent feed or Purina Rat Chow) ad libitum. Retired breeder WKY and SHRs were allowed to age an additional 5 months. All rats were handled for 2 weeks before behavioural testing or whole brain tissue extraction.
All procedures employed during this study were reviewed and approved by the Medical College of Georgia Committee on Animal Use for Research (CAURE) and the Veterans Affairs Medical Center Subcommittee on Animal Use and were consistent with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines.
Unless otherwise noted, all non-radioactive chemicals were obtained from Sigma–Aldrich (St. Louis) and all radiolabelled compounds were obtained from Perkin Elmer (formerly NEN Lifesciences, Boston).
Radial arm maze
Three days before radial arm maze (RAM) testing, rats were restricted to a daily feeding of 18 g (approximately 80% of their ad libitum consumption); they were immediately returned to free food access upon completion of RAM testing. RAM testing was conducted in an 8-arm maze constructed of polyvinyl chloride plastic and plexiglass. Each arm (12 cm х 70 cm) extended radially from a central arena (30-cm diameter) and had a food cup 2 cm from the distal end. The maze was positioned 90 cm above the floor in a testing room with a number of extra-maze cues. Rats were tested 7 days per week, 2 trials per day. Before testing, each arm was baited with pieces of sugar-coated cereal.
Results were manually recorded by the experimenter, who was blinded to the phenotype and age of the rats, was located within 30 cm of the maze and able to clearly observe the food cups and entry points of each maze arm and remained stationary between the same 2 arms for all trials. At the beginning of the study, each rat was placed in the central arena and given one 10-min session each day until 2 food pieces were consumed (i.e., during 1 session). These initial sessions were used to allow a period of acclimation (shaping) to the novel environment. Actual testing began on the day after the rat reached criterion.
Test sessions began by placing the rat in the central arena and recording arm choices as the rat entered each arm to consume food rewards. A correct entry was recorded when the rat located and consumed a reward; reinforcements were not replaced during the session. All subsequent entries into an arm (defined as all 4 paws past the threshold of the proximal end) were scored as incorrect entries (errors). A session continued until all 8 pellets were consumed or until 5 minutes elapsed. The dependent measures were the percent correct of the total arms entered and “efficiency,” defined as the number of correct (i.e., reinforced) entries of the first 8 arms entered.
Blood pressure (BP) measurements
Systolic BPs (10 readings/rat) were obtained via a tail-cuff method after behavioural testing. Rats were placed in a restraining chamber and warmed to an ambient temperature of approximately 37οC, typically taking about 30 minutes. Automatic data collection was performed using a MacLab (World Precision Instruments, Sarasota, Fla.) system synchronized to trigger an electrosphygmomanometer (Narco Biosystems, Austin, Tex.) and to inflate and deflate the tail-cuff to a calibrated pressure at 2-minute intervals. Tail pressure pulsations were detected with a pneumatic pulse transducer.
Brain tissue preparation
Rats were killed by decapitation at 4 and 15 months of age for whole brain tissue extraction. Brains were removed immediately and frozen in isopentane at –35οC. All brains were stored at –70οC for at least 24 hours before sectioning for quantitative autoradiography or dissection of cortex and hippocampus for immunoblotting.
Quantitative receptor autoradiography
Preparation of standards
To define the response of the radiosensitive films to increasing amounts of radioactivity, tissue paste standards containing increasing amounts of radioactivity were prepared and included in all film exposures. Whole rat brains were homogenized in ice-cold phosphate buffer (50 mmol/L sodium phosphate). Aliquots of [3H]-choline were individually added to prepare a range of 0.5–30.0 nCi/mg (1 Bq = 2.7 х 10–11 Ci) of brain homogenate. The specific activity of each standard was determined using a liquid scintillation counter. Standards were flash frozen and 16-μm slices were serially sectioned onto chrome-alum gelatin coated slides in an IEC-Minotome cryostat held at –18°C. Tissue paste standard slides were stored at –70°C until used.
Tissue preparation and sectioning
Frozen whole brains from 15-month-old WKY (n = 6) and SHR (n = 6) were sectioned at a thickness of 16 μm using a Leica-Jung 1800 cryostat/microtome set at –18οC. Each brain was coronally sectioned from prefrontal cortex through the medulla onto chrome-alum gelatin coated microslides. All slides were stored overnight in a dessicator at 4οC and then stored at –70οC until all brains had been sectioned. All slides containing brain sections were stored for at least 24 hours at –70οC before undergoing radioligand binding assays.
Radioligand binding assay
[3H]-Epibatidine and cytisine: Measurement of α3-containing nAChRs was accomplished via the method of Marks et al41 in which [3H]-epibatidine ([3H]-EPB) and cytisine are combined. Slides were preincubated with 50 mmol/L Tris–HCl buffer containing NaCl, KCl, CaCl2 and MgCl2 at pH 7.5 for 10 minutes at 25οC. After preincubation, slides were incubated in 450 pmol/L [3H]-EPB and 150 nmol/L cytisine for 60 minutes at 25οC. Incubation with the radioligand was followed by two 5-minute washes in Tris–HCl buffer at 0οC. Nonspecific binding was determined by adding 300 μmol/L nicotine bitartrate to the incubation buffer prior to the ligand.
[3H]-Pirenzipine and AFDX-384: Comparisons between the rodent phenotypes were made for M1 and M2 mAChR subtypes using [3H]-pirenzipine ([3H]-PRZ) and [3H]-AFDX 384 ([3H]-AFX), respectively. Slides were preincubated in 50 mmol/L Tris–HCl buffer (pH 7.4) for 15 minutes. After preincubation, slides were incubated with 5 nmol/L [3H]-PRZ or 10 nmol/L [3H]-AFX for 90 minutes at room temperature. Incubation with the radioligand was followed by a series of washes at 4οC: three 4-minute 50 mmol/L Tris–HCl washes, one 5-minute 5 mmol/L Tris–HCl and one 10-second in deionized water. Nonspecific binding was determined by adding 10 μmol/L atropine to the incubation buffer before the muscarinic radioligands.
Film exposure and development
After rinsing, slides were air-dried at room temperature and stored overnight in a vacuum dessicator. Autoradiograms were made by exposing the slides to [3H]-sensitive Amersham Hyperfilm in Fisher Biotech aluminum autoradiographic cassettes for 20 days ([3H]-PRZ), 4 weeks ([3H]-AFX) and 10 weeks ([3H]-EPB and cytisine). All films were manually developed in Kodak D-19 Developer (5 min), Indicator Stop Bath (30 s) and Rapid Fixer (5 min) according to package instructions.
Quantification of receptor binding (densitometry)
Autoradiographic analyses were made using NIH Image Software and an imaging station (Macintosh Power PC 8100/100I computer, Data Translation QuickCapture imaging board, Sony SC-77 CCD camera and a Northern Lights Precision Desktop Illuminator). Receptor binding was quantified as optical density in all brain areas and nuclei that had a signal greater than background. Each area was measured bilaterally in at least 4 sections for each rat, with an average number of 20 measurements per area per rat. A calibration curve (optical density versus the known molar quantities of radioligand) was generated from the tissue paste standards. From the curve, molar quantities of bound ligand were obtained. To better visualize and discriminate between structures and boundaries, brain sections were stained with cresyl violet. All brain images were referenced to Paxino and Watson's Rat Brain in Stereotaxic Coordinates, 4th ed.42
Immunoblotting
The hippocampus and cortex were dissected from 4- and 15-month-old WKY and SHR (n = 3 per group) and then homogenized in ice-cold lysis buffer (50 mmol/L Tris [pH 7.4], 150 mmol/L NaCl, 10% glycerol, 1 mmol/L ethylene glycol-bis(2-aminoethylether)-N,N,N1,N1-tetraacetic acid [EGTA], 1 mmol/L sodium orthovanadate, 5 μmol/L ZnCl2, 100 mmol/L NaF, 1% Triton X-100, 10 μg/mL aprotonin, 1 μg/mL leupeptin and 1 mmol/L phenylmethanesulfonyl fluoride [PMSF]). Homogenates were centrifuged at 16 000 x g for 15 minutes at 4οC. The protein content of the supernatants was determined using a colorimetric method with Bradford reagent (Bio-Rad Laboratories, Hercules, Calif.). Total hippocampal (50 μg) or total cortical (100 μg) protein was boiled for 90 seconds in loading/sample buffer (0.5 mol/L Tris–HCl, 20% glycerol, 10% sodium dodecyl sulfate [SDS], 1% bromophenol blue and 5% 2-mercaptoethanol). Samples were size-fractionated on 10% SDS polyacrylamide gels, then transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, Mass.) at 30 V overnight at 4οC. Membranes were blocked overnight in 5% nonfat milk (Bio-Rad) in Tris-buffered saline (TBS; 20 mmol/L Tris, 500 mmol/L NaCl, pH 7.6) and then probed overnight at 4οC incubation with 1:500 goat anti-ChAT (Chemicon International, Temecula, Calif.) or 1:1000 goat anti-VAChT (Santa Cruz Biotechnology, Santa Cruz, Calif.) in milk–TBS. Membranes were then washed with TBS, and the primary antibody was detected using a horseradish peroxidase (HRP)-conjugated secondary antibody in milk–TBS (1:10 000, 60 min, 25οC). HRP activity was revealed with the enhanced chemiluminescence procedure, according to manufacturer's instructions (Supersignal West Pico Substrate, Pierce Endogen, Rockford, Ill.). Chemiluminescent signal was detected using Amersham Hyperfilm-ECL, and films were developed in an automatic Kodak X-OMAT processor. Band densitometries were measured using the same imaging station used for quantitative autoradiography experiments.
Statistical analyses
Comparisons of rat phenotypes and age for RAM (overall) efficiency scores, systolic blood pressures and immunoblotting experiments were made using 2-way analysis of variance (ANOVA). In the case of daily RAM performance comparisons, group differences, the effects of the day of testing and group by day interactions were compared using 2-way repeated measures ANOVA. Densitometry measurements from autoradiographic experiments were compared using 1-way ANOVA, since only older WKY and SHRs were compared. Statistical significance was assessed at an alpha level of 0.05, except in the case of the autoradiographic results where an alpha level of 0.01 was utilized, to account for multiple measurements. The Student-Newman-Keuls method for post hoc analysis was used for all multiple comparisons.
Results
Radial arm maze
The ability of younger and older WKY and SHRs to navigate an 8-arm RAM for food reinforcement across 7 days of training is depicted in Fig. 1A. A group of young Wistar rats were included in the experiments so that comparisons between WKY and SHRs and a normal (outbred) rat strain (known to efficiently perform the RAM test) could be made. Each group (with the exception of older SHRs) learned to enter the baited arms to locate food with progressively fewer errors of re-entry (i.e., into unbaited arms) on successive days of training. For the percent correct of the total arms entered (by day) comparisons, statistical analyses revealed the following: group effect F4,48 = 5.20, p < 0.001; day effect F6,24 = 29.49, p < 0.001; group by day interaction F284,366 = 3.25, p < 0.001. Post hoc comparisons indicated that both WKY and SHRs (regardless of age) exhibited a significantly (p < 0.05) lower level of performance on several days than the younger Wistar rats. All other group differences the were not statistically significant (p > 0.05). For the overall efficiency comparisons (collapsed across the days of testing), however, (Fig. 1B) both younger and older SHRs demonstrated inferior performance (p < 0.01) compared with younger Wistar and WKYs of both age groups. There were no age-related differences among SHRs.
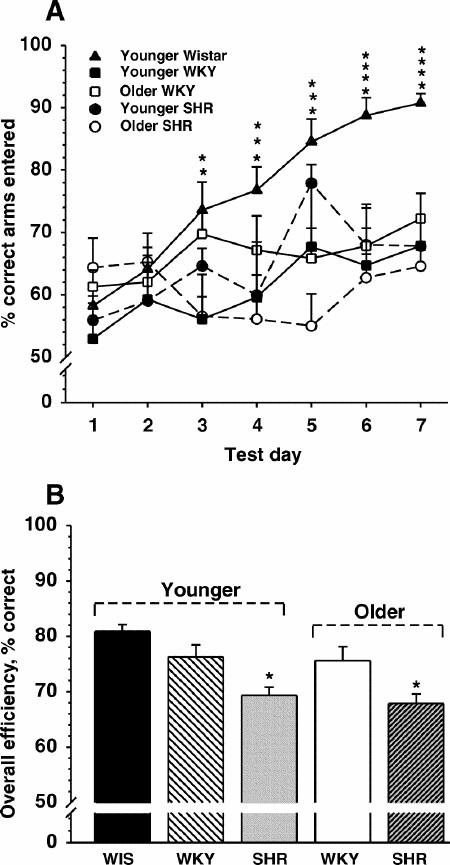
Fig. 1A: Performance of a win-shift task in an 8-arm radial arm maze across 7 consecutive days of testing (2 trials per day per animal) by the various rat groups (n = 9–12). Each point represents the mean percent correct (and SEM) of the total arms entered during the 5-min trial. * = Wistar performance significantly different from 1 or more of the other groups.
B: Overall efficiency (collapsed across all days) defined as the number of correct (i.e., reinforced) entries of the first 8 arms entered.
Blood pressure (BP)
Systolic BPs were measured after behavioural testing (Fig 2). There were both phenotypic (F95.55, p < 0.0001) and age-related (F7.12, p < 0.02) differences in systolic BP. Post-hoc comparisons indicated: systolic BPs were significantly (p < 0.05) higher in SHRs than WKYs regardless of age, although the margin of difference was wider among old animals, and systolic BPs were higher in older than younger SHRs (p < 0.05).
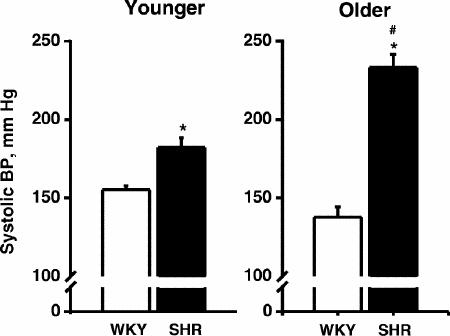
Fig. 2: Mean (and SEM) systolic blood pressure of young and old 15-month-old WKY and SHR rats obtained via a tail cuff method after behavioural training (n = 10–12 rats per group).
* = significant difference between the 2 rat phenotypes (p < 0.05); # = significant age-related difference (p < 0.05).
Receptor autoradiography
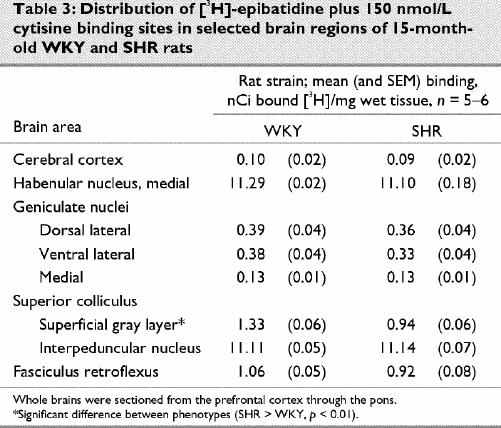
Representative autoradiograms and receptor densitometry comparisons between older (15 month old) WKY and SHR for are presented in Fig. 3 and in Table 1 ([3H]-PRZ), Table 2 ([3H]-AFX) and Table 3 ([3H]-EPB + cytisine).
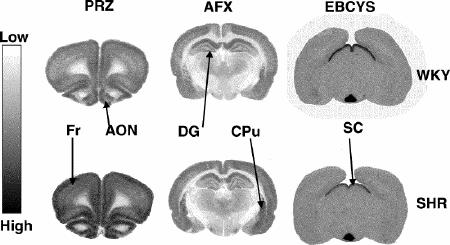
Fig. 3: Representative autoradiograms illustrating receptors labeled by [3H]-pirenzipine (PRZ, M1 muscarinic cholinergic receptors), [3H]-AFDX-384 (AFX, M2 muscarinic cholinergic receptors) and [3H]-epibatidine + 150 nM cytisine (EBCYS, α3-containing nicotinic cholinergic receptors) in coronal sections of brains from 15-month-old SHR and age-matched controls (WKY). Fr = frontal cortex, AON = anterior olfactory nucleus, DG = dentate gyrus, CPu = caudate putamen, SC = superior colliculus. Sections in which significantly different binding densities between SHR and WKY were found are presented.
* = significant difference between the 2 rat phenotypes (p < 0.05).
Table 1
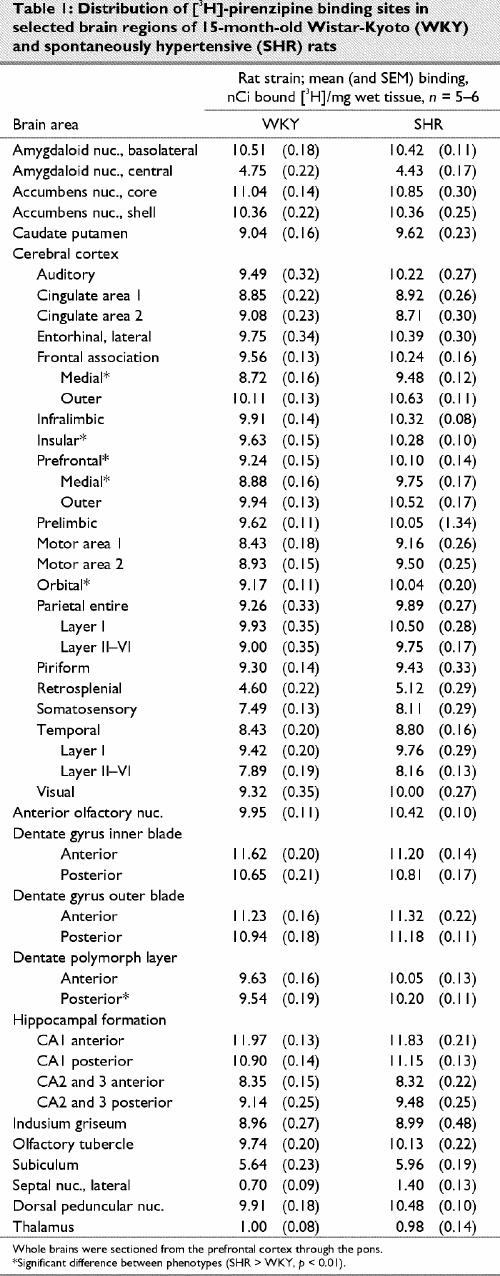
Table 2
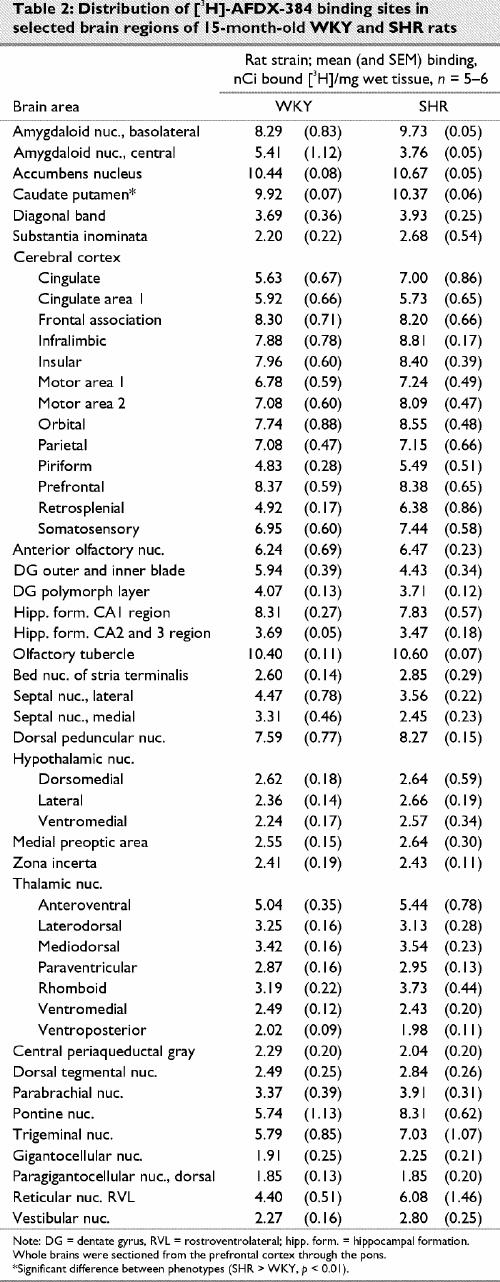
Table 3
[3H]-Pirenzipine
The autoradiographic distribution of [3H]-PRZ binding to central M1 mAChRs in older WKY and SHRs was similar. Binding was widely distributed in the neocortex and hippocampal formation and minimally represented in the thalamus, hypothalamus and midbrain. The highest [3H]-PRZ binding densities were observed in the CA1 region of the hippocampus, dentate gyrus, nucleus accumbens and the basolateral amygdala. Moderate binding was observed in the cortex, caudate putamen, olfactory tubercle, anterior olfactory nuclei and dorsal peduncular nuclei. The lowest [3H]-PRZ binding densities were found in the subiculum, lateral septal nuclei, centromedial amygdaloid nuclei and thalamus. This distribution profile is similar to what has been reported in other studies where SHRs and other rat strains were examined.43 Overall, SHRs demonstrated higher binding densities in 38 of the 46 areas measured; however, significant differences (p < 0.05) were limited to cortical areas.
[3H]-AFDX 384
The autoradiographic distribution of [3H]-AFX binding to central M2 mAChRs in 15-month-old WKY and SHRs was also quite similar. Like [3H]-PRZ binding, [3H]-AFX binding was widely distributed in the cortex and hippocampal formation for both phenotypes. Unlike [3H]-PRZ binding, [3H]-AFX binding was also distributed across the thalamus, hypothalamus and hindbrain. The highest [3H]-AFX binding densities were observed in the caudate putamen, nucleus accumbens and olfactory tubercle. Moderate binding was found in the cortex, basolateral amygdala and hippocampal formation. Lower [3H]-AFX binding densities were found in the hypothalamus, thalamus and hindbrain. This pattern of [3H]-AFX binding is similar to that reported in previous studies.43 In the present study, a significant difference in binding density was detected in only the caudate putamen (SHR > WKY, p = 0.003).
[3H]-Epibatidine and cytisine
The autoradiographic distributions of [3H]-EPB and cytisine binding to central α3-containing nAChRs in 15-month-old WKY and SHRs were again similar. The highest [3H]-EPB and cytisine binding densities were observed in the medial habenular nuclei and interpeduncular nuclei, and moderate binding densities were found in the fasciculus retroflexus. Lower [3H]-EPB and cytisine binding densities were found in the geniculate nuclei and mammillary nuclei. This pattern of binding is similar to that reported in previous studies.2 Of the 10 areas measured, a statistically significant difference was found only in the superior colliculus, with the WKY rats having greater mean density (p < 0.001). Binding was slightly higher (p > 0.05) than SHR in 7 areas of the 10 areas measured, however.
Immunoblotting
Choline acetyltransferase
The antibody to ChAT recognized a 70-kD band in both the cortex and hippocampus of younger and older WKY and SHRs (Fig. 4B and 4C). There were no significant age- or phenotype-related differences detected in ChAT immunoreactivity in the cortex or hippocampus. There was a notable trend (p < 0.1) toward higher ChAT immunoreactivity in the cortex in younger WKYs compared with younger SHRs, however.
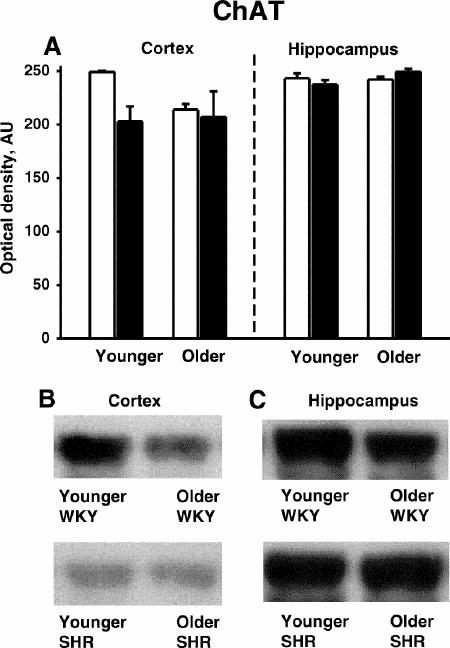
Fig. 4: Choline acetyltransferase (ChAT) immunoreactivity in the cortex and hippocampus of younger (4-month-old) and older (15-month-old) SHR (black bars) and WKY (white bars) rats (n = 3 per group).
A: Quantitative densitometry results from ChAT immunoblotting experiments.
B: Representative immunoblots of ChAT in the cortex.
C: Representative immunoblots of ChAT in the hippocampus.
AU = arbitrary densitometric units.
Vesicular acetylcholine transporter
The antibody to VAChT recognized a 70-kD band in both the cortex and hippocampus of younger and older WKY and SHRs (Fig. 5B and 5C). Within the hippocampus, VAChT immunoreactivity significantly (p < 0.05) decreased with age for both phenotypes (Fig. 5A). In addition, VAChT immunoreactivity was somewhat higher in older SHRs than older WKYs in the hippocampus. Significant differences in VAChT immunoreactivity between age and phenotype were not observed in the cortex.
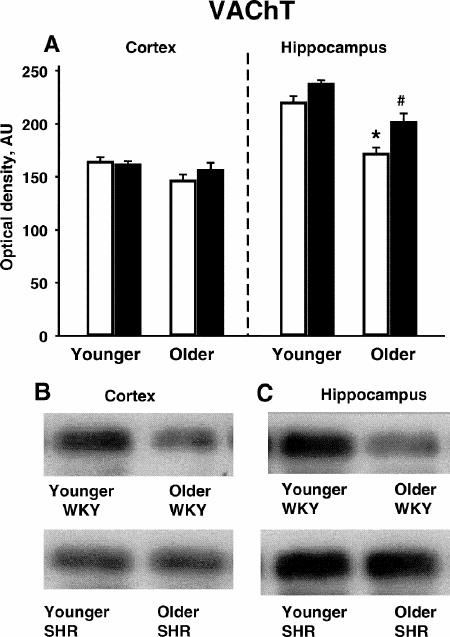
Fig. 5: Vesicular acetylcholine transporter (VAChT) immunoreactivity in the cortex and hippocampus of younger (4-month-old) and older (15-month-old) SHR (black bars) and WKY (white bars) rats (n = 3 per group).
* = younger SHR > older SHR (p < 0.05); # = younger WKY > older WKY (p < 0.05).
A: Quantitative densitometry results from VAChT immunoblotting experiments.
B: Representative immunoblots of VAChT in the cortex.
C: Representative immunoblots of VAChT in the hippocampus.
Discussion
In previous experiments, we demonstrated that older SHRs exhibit both cognitive deficits in a water maze task (compared with normotensive controls) and a decrease in α4-containing nAChRs with increasing age.20 An objective of this study was to extend these findings and evaluate both age-related and phenotypic differences in memory performance in an appetitively motivated spatial learning task (i.e., the RAM). The results (of overall efficiency in the RAM) indicated that memory was impaired in SHRs compared with normotensive animals, regardless of age.
One potential confounding factor in this analysis was the observation that younger and older WKYs were unable to reach an asymptotic level of performance (as did Wistar rats) over the 7 days of training. Furthermore, both younger and older WKYs were difficult to shape to the novel test environment in the RAM. In contrast to younger and older SHRs (and younger Wistar rats), which generally habituated to the RAM in only 1 or 2 days, WKYs of both ages took several days on average. A reduced level of investigatory behaviour has been observed in this strain of rat previously.44 Furthermore, daily variation in RAM performance was also quite high in the WKYs. These observations highlight possible limitations of using the WKY rats as a so-called “normal control” for SHRs (particularly in some behavioural tasks).
Other factors that should be considered when interpreting memory-related behavioural differences between SHR and WKY strains include level of anxiety, attentional processes and level of motivation. SHRs have been reported to have lower anxiety levels than WKYs, arguing against a major role of anxiety in impaired RAM performance.45,46 However, SHRs do demonstrate poorly sustained attention and impaired reward/reinforcement mechanisms,47,48,49 which could, in fact, contribute to decreased efficiency in the RAM. Many researchers contend that acetylcholine plays a pivotal role in attentional processes;50 therefore, observations of deficits in attention and reduced expression of cholinergic markers in SHRs may be of particular importance to the interpretation of memory-related task performance.
The use of the WKY as a normotensive control for SHRs has been challenged on the basis of the variety of genetic differences observed both between the strains and within colonies of each strain (many of which may not be hypertension related). From a different perspective, it has also been hypothesized that the normotensive Wistar strain may be more appropriate as controls for hypertension-related changes, because the SHR strain was derived from WKY and thus likely carries some of the same genes responsible for hypertension.51 In the present study, normotensive Wistar rats were clearly superior in performance in the RAM compared with both SHRs and WKYs, although the effects of aging were not assessed.
A second objective of the study was to further analyze the cholinergic system in SHRs to compare the relative densities of (G-protein coupled) mAChRs and α3-containing nAChRs.52 M1 and M2 receptors are the most prominent of the 5 known mAChR subtypes (M1–M5). Post-synaptic M1 mAChRs have a high affinity for the M1 agonist pirenzipine and stimulate phosphatidyl inositol turnover. M2 muscarinic receptors are thought to primarily exist as presynaptic autoreceptors that bind AFDX-384 with high affinity and inhibit the release of adenylate cyclase.34,53 Both of these receptors are known to play an important role in a number of mnemonic processes.34,52,54,55 Although the functional role of α3-containing nAChRs in the CNS is poorly understood at present outside of developmental and autonomic processes,56,57,58 the need to accurately differentiate the expression of these receptors (in hypertensive animals) will become increasingly important as more functional data are obtained.
The density of M1 binding sites was slightly higher in SHRs than in WKYs across most of the brain regions analyzed (p < 0.05 in prefrontal and frontal cortical areas). However, given the modest nature of these differences (i.e., 7%–8%), the functional significance of this finding is unclear. The slightly increased level of M1 receptor expression in SHRs might represent a post-synaptic upregulation after depletion of cholinergic projections in the cortex with age or, alternatively, a compensatory mechanism that follows the nAChR deficiency in older SHRs. Interestingly, an increase in M1 AChR expression is observed in humans with dementia with Lewy bodies,59 a disease noted for its decrease in nAChRs and other cholinergic markers. As opposed to the observed differences in M1 binding sites, the phenotypic difference in the density of M2 binding sites was minimal; only the caudate putamen was statistically different (i.e., SHR > WKY).
Only 1 area was significantly different in α3-containing nAChRs (i.e., binding in the superior colliculus was markedly higher in WKYs). Interestingly, the density of α4- and α7-containing receptors was also reported to be higher in older WKYs20 in this brain region. Although the functional significance of these differences is unknown, nAChRs in the superior colliculus have been documented to play a significant role in visual responses.60 However, studies in our laboratory demonstrated no significant differences in performance of a water maze visible platform task between SHRs and WKYs at either 4 or 15 months of age.18,19,20
Immunoblotting experiments showed no statistically significant phenotypic or age-related differences in cortical and hippocampal ChAT, although there was a strong trend toward a lower expression of ChAT in the cortex of young SHRs (see Fig. 4A). Both strains demonstrated a loss of VAChT in the hippocampus with age, however. In cholinergic presynaptic terminals, ChAT is responsible for synthesizing acetylcholine, and VAChT is responsible for the transport of acetylcholine into synaptic vesicles for regulated exocytotic release.61 Decreases in VAChT in an area important for memory, such as the hippocampus, could contribute to the inferior performance of SHRs compared with WKYs often observed in memory-related tasks.
In summary, the autoradiographic results of this study (measuring α3-containing nAChRs and mAChRs) and previous studies (in which α4- and α7-containing nAChRs were measured20) indicate no marked differences between SHRs and WKYs (at a younger or older age) in the expression of α3- and α7-containing nAChRs or mAChRs in important memory areas. However, age-related alterations in α4-containing nAChRs and the cholinergic marker VAChT in important memory areas, combined with the behavioural results of this study, indicate that the SHR may be useful to model some aspects (particularly hypertension-related) of memory disorders, especially those in which cholinergic function is altered.
Acknowledgments
We thank Dr. Jennifer Waller for biostatistical consults.
Footnotes
This work was supported in part by the American Heart Association (Southeastern Affiliate), the American Psychological Association's Minority Fellowship in Neuroscience (MH#18882) Program, the American Foundation for Pharmaceutical Education's Pre-doctoral Fellowship Program and the Medical College of Georgia Foundation.
Competing interests: None declared.
Correspondence to: Dr. Alvin V. Terry Jr., University of Georgia College of Pharmacy and Medical College of Georgia Alzheimer's Research Center, CJ-1020, Medical College of Georgia, Augusta, GA 30912-2450; fax 706 721-3994; aterry@mail.mcg.edu
Submitted Sept. 11, 2002 Revised Dec. 30, 2002 Accepted Jan. 9, 2003
References
- 1.Elias MF, Wolf PA, D'Agostino RB, Cobb J, White LR. Untreated blood pressure level is inversely related to cognitive functioning: the Framingham Study. Am J Epidemiol 1993;138:353-64. [DOI] [PubMed]
- 2.Launer LJ, Masaki K, Petrovich H, Foley D, Havlik RJ. The association between midlife blood pressure level and late-life cognitive function: the Honolulu-Asia Aging Study. JAMA 1995;274:1846-51. [PubMed]
- 3.Hebert R, Lindsay J, Verreault R, Rockwood K, Hill G, Dubois MF. Vascular dementia: incidence and risk factors in the Canadian study of health and aging. Stroke 2000;31:1487-93. [DOI] [PubMed]
- 4.Breteler MMB. Vascular risk factors for Alzheimer's disease: an epidemiologic perspective. Neurobiol Aging 2000;21:153-60. [DOI] [PubMed]
- 5.Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, et al. 15-year longitudinal study of blood pressure and dementia. Lancet 1996;347:1141-5. [DOI] [PubMed]
- 6.Akashi T, Arima K, Maruyama N, Ando S, Inose T. Severe cerebral atrophy in progressive supranuclear palsy: a case report. Clin Neuropathol 1989;9:195-9. [PubMed]
- 7.Ghika J, Bogousslavsky J. Presymptomatic hypertension is a major feature in the diagnosis of progressive supranuclear palsy. Arch Neurol 1997;54:1104-8. [DOI] [PubMed]
- 8.Barber R, Gholkar A, Scheltens P, Ballard C, McKeith IG, O'Brien JT. MRI volumetric correlates of white matter lesions in dementia with Lewy bodies and Alzheimer's disease. Int J Geriatr Psychiatry 2000;15(10):911-6. [DOI] [PubMed]
- 9.Petrovich H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery W, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Neurobiol Aging 2000;21:57-62. [DOI] [PubMed]
- 10.Meneses A, Hong E. Spontaneously hypertensive rats: a potential model to identify drugs for treatment of learning disorders. Hypertension 1998;31:968-72. [DOI] [PubMed]
- 11.Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit hyperactivity disorder (AD/HD). Neurosci Biobehav Rev 2000;24:31-9. [DOI] [PubMed]
- 12.Knardahl S, Karlsen K. Passive-avoidance behavior of spontaneously hypertensive rats. Behav Neural Biol 1984;42:9-22. [DOI] [PubMed]
- 13.Sutterer JR, Devito WJ, Rykaszewski I. Developmental aspect of 2-way shuttle box avoidance in the spontaneously hypertensive rat and normotensive rat. Dev Psychobiol 1981;14:405-14. [DOI] [PubMed]
- 14.Sutterer JR, Perry J, Devito W. Two-way shuttlebox avoidance in the spontaneously hypertensive and normotensive rat. J Comp Physiol Psychol 1980;94:155-63. [DOI] [PubMed]
- 15.Mori S, Kato M, Fujishima M. Impaired maze learning and cerebral glucose utilization in aged hypertensive rats. Hypertension 1995;25:545-53. [DOI] [PubMed]
- 16.Nakamura-Palacios EM, Caldas CK, Fiorini A, Chagas KD, Chagas KD, Vasquez ED. Deficits of spatial learning and working memory in spontaneously hypertensive rats. Behav Brain Res 1996;74:217-21. [DOI] [PubMed]
- 17.Wyss JM, Fisk G, van Groen T. Impaired learning and memory in mature spontaneously hypertensive rats. Brain Res 1992;592:135-40. [DOI] [PubMed]
- 18.Gattu M, Pauly JR, Boss KL, Summers JB, Buccafusco JJ. Cognitive impairment in spontaneously hypertensive rats: role of central nicotinic receptors I. Brain Res 1997;771:89-103. [DOI] [PubMed]
- 19.Gattu M, Terry AV, Pauly JR, Buccafusco JJ. Cognitive impairment in spontaneously hypertensive rats: role of central nicotinic receptors II. Brain Res 1997;771:104-14. [DOI] [PubMed]
- 20.Terry AV, Hernandez CM, Buccafusco JJ, Gattu M. Deficits in spatial learning and nicotinic-acetylcholine receptors in older, spontaneously hypertensive rats. Neurosci 2000;101:357-68. [DOI] [PubMed]
- 21.Sabbatini M, Catalani A, Consoli C, Marletta N, Tomassoni D, Avola R. The hippocampus in spontaneously hypertensive rats: an animal model of vascular dementia? Mech Ageing Dev 2002;123:547-59. [DOI] [PubMed]
- 22.Sabbatini M, Strocchi P, Vitaioli L, Amenta F. The hippocampus in spontaneously hypertensive rats: a quantitative microanatomical study. Neurosci 2000;100:251-8. [DOI] [PubMed]
- 23.Linz W, Jessen T, Becker BA, Schoelkens BA, Wiemer G. Long-term ACE inhibition doubles lifespan of hypertensive rats. Circulation 1997;96:3164-72. [DOI] [PubMed]
- 24.Linz W, Wohlfart P, Schoelkens BA, Becker RHA, Malinski T, Wiemer G. Late treatment with ramipril increases survival in old spontaneously hypertensive rats. Hypertension 1999;34:291-5. [DOI] [PubMed]
- 25.Okamoto K, editor. Spontaneous hypertension: its pathogenesis and complications. New York: Springer-Verlag; 1972.
- 26.Muir JL. Acetylcholine, aging, and Alzheimer's disease. Pharmacol Biochem Behav 1997;56:687-96. [DOI] [PubMed]
- 27.Taylor L, Griffith WH. Age-related decline in cholinergic synaptic transmission in hippocampus. Neurobiol Aging 1993;14(5):509-15. [DOI] [PubMed]
- 28.Bowen DM, Benton JS, Spillane JA, Smith CC, Allen SJ. Choline acetyltransferase activity and histopathology of frontal neocortex from biopsies of demented patients. J Neurol Sci 1982;57(2-3):191-202. [DOI] [PubMed]
- 29.Efange SMN, Garland EM, Staley JK, Share AB, Mash DC. Vesicular acetylcholine transporter density and Alzheimer's disease. Neurobiol Aging 1997;18:407-13. [DOI] [PubMed]
- 30.Kish SJ, Distefano LM, Docik S, Robitaille Y, Rajput A, Deck JHN, et al. [3H] Vesamicol binding in human brain cholinergic deficiency disorders. Neurosci Lett 1990;177:347-52. [DOI] [PubMed]
- 31.Bartus RT. On neurodegenerative diseases, models and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol 2000;63:495-529. [DOI] [PubMed]
- 32.Sihver W, Gillberg PG, Svensson AL, Nordberg A. Autoradiographic comparison of [3H](-)nicotine, [3H]cytisine and [3H]epibatidine binding in relation to vesicular acetylcholine transport sites in the temporal cortex in Alzheimer's disease. Neurosci 1999;94:685-96. [DOI] [PubMed]
- 33.Gottfries CG, Blennow K, Karlsson I, Wallin A. The neurochemistry of vascular dementia. Dementia 1994;5:163-7. [DOI] [PubMed]
- 34.Tohgi H, Abe T, Kimura M, Saheki M, Takahashi S. Cerebrospinal fluid acetylcholine and choline in vascular dementia of Binswanger and multiple small infarct types as compared with Alzheimer's type dementia. J Neural Transm 1996;103: 1211-20. [DOI] [PubMed]
- 35.Wallin A, Blennow K, Gottfries CG. Neurochemical abnormalities in vascular dementia. Dementia 1989;1:120-30.
- 36.Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomized trial. Lancet 2002;359:1283-90. [DOI] [PubMed]
- 37.Maelicke A. The pharmacological rationale for treating vascular dementia with galantamine (ReminylTM). Int J Clin Prac 2001;120:24-8. [PubMed]
- 38.McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res Rev 1993;18:33-49. [DOI] [PubMed]
- 39.Oda Y. Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system. Pathol Int 1999;49:921-37. [DOI] [PubMed]
- 40.Roghani A, Shirzadi A, Butcher LL, Edwards RH. Distribution of the vesicular transporter for acetylcholine in the rat central nervous system. Neurosci 1998;82:195-212. [DOI] [PubMed]
- 41.Marks MJ, Smith KW, Collins AC. Differential agonist inhibition identifies multiple epibatidine binding sites in mouse brain. J Pharmacol Exp Ther 1998;285:377-86. [PubMed]
- 42.Paxinos G, Watson CR. Rat brain in stereotaxic coordinates. 4th ed. San Diego (CA): Academic Press; 1998.
- 43.Gattu M, Pauly JR, Urbanawiz S, Buccafusco JJ. Autoradiographic comparison of muscarinic M1 and M2 binding sites in the CNS of spontaneously hypertensive and normotensive rats. Brain Res 1997;771:173-83. [DOI] [PubMed]
- 44.Pare WP. Investigatory behavior of a novel conspecific by Wistar-Kyoto, Wistar and Sprague-Dawley rats. Brain Res Bull 2000;53:759-65. [DOI] [PubMed]
- 45.Durand M, Berton O, Aguerre S, Edno L, Combourieu I, Mormede P, et al. Effects of repeated fluoxetine on anxiety-related behaviours, central serotonergic, and the corticotropic axis in SHR and WKY rats. Neuropharmacology 1999;38(6):893-907. [DOI] [PubMed]
- 46.Kulikov A, Aguerre S, Berton O, Ramos A, Mormede P, Chaouloff F. Central sertonergic systems in the spontaneously hypertensive and Lewis rat strains that differ in the elevated plus-maze test of anxiety. J Pharmacol Exp Ther 1997;281:775-84. [PubMed]
- 47.Russell VA, Wiggins TM. Increased glutamate-stimulated norepinephrine release from prefrontal cortex slices of spontaneously hypertensive rats. Metab Brain Dis 2000;15:294-304. [DOI] [PubMed]
- 48.Russell VA. The nucleus accumbens motor-limbic interface of the spontaneously hypertensive rat as studied in vitro by the superfusion slice technique. Neurosci Biobehav Rev 2000;24:133-6. [DOI] [PubMed]
- 49.Meneses A, Castillo C, Ibarra M, Hong, E. Effects of aging and hypertension on learning, memory and activity in rats. Physiol Behav 1996;60:341-5. [PubMed]
- 50.Blokland A. Acetylcholine: a neurotransmitter for learning and memory? Brain Res Rev 1996;21:285-300. [DOI] [PubMed]
- 51.Doggrell SA, Brown L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc Res 1998;39:89-105. [DOI] [PubMed]
- 52.van der Zee EA, Luiten PGM. Muscarinic acetylcholine receptors in the hippocampus, neocortex and amygdala: a review of immunocytochemical localization in relation to learning and memory. Prog Neurobiol 1999;58:409-71. [DOI] [PubMed]
- 53.Caufield MP. Muscarinic receptors — characterization, coupling and function. Pharmacol Ther 1993;58:319-79. [DOI] [PubMed]
- 54.Flynn DD, Ferrari-DiLeo G, Levey AI, Mash DC. Differential alterations in muscarinic receptor subtypes in Alzheimer's disease: implications for cholinergic-based therapies. Life Sci 1995;56:869-76. [DOI] [PubMed]
- 55.Flynn DD, Ferrari-DiLeo G, Mash DC, Levey AI. Differential regulation of molecular subtypes of muscarinic receptors in Alzheimer's disease. J Neurochem 1995;64:1888-91. [DOI] [PubMed]
- 56.Picciotto MR, Caldarone BJ, Brunzell DH, Zachariou V, Stevens TR, King SR. Neuronal nicotinic receptor subunit knockout mice: physiological and behavioral phenotypes and possible clinical implications. Pharmacol Ther 2001;92:89-108. [DOI] [PubMed]
- 57.Zoli M, Lena C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors using beta2 mutant mice. J Neurosci 1998;18(12):4461-72. [DOI] [PMC free article] [PubMed]
- 58.White P, Hiley DR, Goodhardt MJ, Carrasco LH, Keet JP, Williams IE, et al. Neocortical cholinergic neurons in elderly people. Lancet 1977;1(8013):668-71. [DOI] [PubMed]
- 59.Shiozaki K, Iseki E, Uchiyama H, Watanebe Y, Haga T, Kameyama K, et al. Alternations of muscarinic acetylcholine receptor subtypes in diffuse Lewy body disease: relation to Alzheimer's disease. J Neurol Neurosurg Psychiatry 2000;68:253-4. [DOI] [PMC free article] [PubMed]
- 60.Binns KE, Salt TE. The functional influence of nicotinic cholinergic receptors on the visual responses of neurons in the superficial superior colliculus. Vis Neurosci 2000;17(2):283-9. [DOI] [PubMed]
- 61.Parsons SM, Prior C, Marshall IG. Acetylcholine transport, storage, and release. Int Rev Neurobiol 1993;35:279-390. [DOI] [PubMed]


