Abstract
Background
Many filarial nematodes harbour Wolbachia endobacteria. These endobacteria are transmitted vertically from one generation to the next. In several filarial species that have been studied to date they are obligatory symbionts of their hosts. Elimination of the endobacteria by antibiotics interrupts the embryogenesis and hence the production of microfilariae. The medical implication of this being that the use of doxycycline for the treatment of human onchocerciasis and bancroftian filariasis leads to elimination of the Wolbachia and hence sterilisation of the female worms. Wolbachia play a role in the immunopathology of patients and may contribute to side effects seen after antifilarial chemotherapy. In several studies Wolbachia were not observed in Loa loa. Since these results have been doubted, and because of the medical significance, several independent methods were applied to search for Wolbachia in L. loa.
Methods
Loa loa and Onchocerca volvulus were studied by electron microscopy, histology with silver staining, and immunohistology using antibodies against WSP, Wolbachia aspartate aminotransferase, and heat shock protein 60. The results achieved with L. loa and O. volvulus were compared. Searching for Wolbachia, genes were amplified by PCR coding for the bacterial 16S rDNA, the FTSZ cell division protein, and WSP.
Results
No Wolbachia endobacteria were discovered by immunohistology in 13 male and 14 female L. loa worms and in numerous L. loa microfilariae. In contrast, endobacteria were found in large numbers in O. volvulus and 14 other filaria species. No intracellular bacteria were seen in electron micrographs of oocytes and young morulae of L. loa in contrast to O. volvulus. In agreement with these results, Wolbachia DNA was not detected by PCR in three male and six female L. loa worms and in two microfilariae samples of L. loa.
Conclusions
Loa loa do not harbour obligatory symbiotic Wolbachia endobacteria in essential numbers to enable their efficient vertical transmission or to play a role in production of microfilariae. Exclusively, the filariae cause the immunopathology of loiasis is patients and the adverse side effects after antifilarial chemotherapy. Doxycycline cannot be used to cure loiais but it probably does not represent a risk for L. loa patients when administered to patients with co-infections of onchocerciasis.
Background
Rickettsia-like intracytoplasmatic bacteria were first observed in filarial nematodes using electron microscopy in the 1970's [1,2]. Later it was shown that these endobacteria are closely related to intracellular bacteria found in many arthropods and they were all grouped together in the genus Wolbachia [3,4]. Over the last few years the filarial Wolbachia have received increased attention since it was shown that in those filarial species that harbour them they are obligatory symbionts needed for all stages of embryogenesis (from oocytes in the ovary to microfilariae, infective larvae, male and female worms) [5]. The term 'obligatory symbiosis' was introduced for the association of Wolbachia with the wasp Asobara tabida, because following treatment with antibiotics, (which cleared the endobacteria in the insect), it became sterile due to a blockade of oocyte production [6]. Similarly, the removal of Wolbachia by administration of doxycycline and other antibiotics leads to an interruption of embryogenesis and probably permanent sterilisation of the female filariae [7]. Langworthy et al., reported a macrofilaricidal activity of prolonged oxytetracycline treatment against Onchocerca ochengi in cattle [8]. It has been shown for onchocerciasis and bancroftian filariasis that doxycycline therapy may be a new treatment strategy at least for individual patients or for small groups [9,10].
Wolbachia have been observed in most filarial species that have been examined. The absence of endobacteria has been reported for four species based on the results of several independent methods of investigation: Acanthocheilonema viteae [1,11,12], Onchocerca flexuosa [13,14], Loa loa, and very recently Setaria equina [15]. For the three animal parasites these findings have been accepted. The absence of endobacteria from the human parasite L. loa has been reported by several authors based on electron microscopy [1,16-18], on immunohistology [19] and PCR [20]. However, because of its medical importance and the small number of samples used in most studies, the statements regarding L. loa haven been repeatedly doubted.
For the interruption of transmission of Onchocerca volvulus in areas endemic for onchocerciasis and loiasis an alternative treatment with doxycycline may be useful when ivermectin and diethylcarbamazine cannot be used for onchocerciasis patients with high microfilariae loads of L. loa [21-23]. For such a strategy it is crucial to know whether L. loa have endobacteria that may cause serious adverse side effects when co-infections are to be treated.
By using electron microscopy, histology, immunohistology, and PCR, we present evidence that there are not sufficient numbers of Wolbachia endobacteria in L. loa, if any, to live in an obligatory symbiotic association.
Methods
Filariae and insects
Nematode samples from infected humans and animals were obtained with the approval of the ethics committees and regulatory authorities of all institutions and countries involved in this study.
Specimens with blood containing L. loa microfilariae, adult L. loa worms and skin biopsies extirpated by ophthalmologists and other physicians from human patients had been sent to the Bernhard Nocht Institute for diagnosis. Several specimens of a spleen extirpated from a 24-year-old German, who had been travelling in Nigeria and Cameroon, were kindly supplied by Prof. GD Burchard [24]. Further adult worms and a sample of spleen were collected in Cameroon from an experimentally infected two-year-old drill (Mandrillus leucophaeus) born in captivity.
Third stage larvae were collected from Chrysops silacea fed on microfilaremic human volunteers from Cameroon, who had expressed informed consent. Infective larvae from these C. silacea were used to infect the drill and seven months later adult L. loa worms and the spleen were recovered. Samples of the spleen were embedded in Paris and sent to Hamburg. Fragments of additional adult L. loa worms from previous experimental infections of monkeys were available in Paris. Six of these worms belonged to the human strain and three to a monkey strain.
Onchocercomas with microfilariae and adult O. volvulus and specimens of other filarial worms embedded in paraffin were available from several previous studies [13,25]. To test the reactivity of the antibodies against Wolbachia proteins from various hosts, we also examined females of the sand flea Tunga penetrans removed from patients in Ghana [26] and the mosquito Culex pipiens from a laboratory colony maintained at the Istituto di Parassitologia, Università die Roma La Sapienza.
Electron microscopy
Electron micrographs that had been taken during previous studies on L. loa [17,18,27] and on O. volvulus were re-examined ([28] and unpublished studies). The L. loa worms had been collected in Lambarene (Gabon) during herniotomies or they had been sent to the Bernhard Nocht Institute for diagnosis by physicians in northern Germany. The O. volvulus worms had been removed from patients in Liberia. The filariae had been fixed in 2% buffered glutaraldehyde and 1% osmium tetroxide, embedded in araldite, epon or Spurr's ERL medium and processed for electron microscopy as usual. Two years ago, L. loa microfilariae from the blood of a Cameroonian patient were processed for immunogold electron microscopy [19,26]. For light microscopic controls, semi-thin sections had been stained with azure II and methylene-blue. Larger pieces of onchocercomas were also embedded in methacrylate and stained by methylene-blue.
Light microscopy
The worms and the biopsies from organs had been fixed in 80% ethanol or 4% buffered formaldehyde. They were embedded in paraffin and stained conventionally with haematoxylin & eosin. Biopsies from organs also were stained with Giemsa or Pappenheim stain. Sections of all adult L. loa worms selected for this study (Table 1) and selected onchocercoma sections were stained with silver using the Warthin-Starry method conducted under strictly controlled staining conditions [29]. For immunohistology, the alkaline phosphatase anti-alkaline phosphatase (APAAP) method was applied according to the manufacturer's recommendations (Dako Diagnostika, Hamburg, Germany). Antisera against filarial, wolbachial or Yersinia enterocolitica proteins or against human leukocytes were used as primary antibodies. We applied as secondary antibodies anti-rabbit mouse immunoglobulins (clone MR12/53, Dako Diagnostika, Hamburg, Germany) for rabbit sera against filarial and bacterial proteins and anti-mouse antibodies for the monoclonal immunoglobulins against the proteins of human immune cells. Fast Red TR salt (Sigma, Deisenhofen, Germany) was used as chromogen and haematoxylin (Merck, Darmstadt, Germany) functioned as the counterstain.
Table 1.
L. loa used for histology and immunohistology
| Stage of worms | Number of worms | Quality of worms | Host | Country of origin |
| Microfilariae | dozens | in blood | human | Cameroon |
| hundreds | in spleen | human | Cameroon or Nigeria | |
| dozens | in spleen | drill | Cameroon | |
| Female worms with embryos | 2 | complete worms | human | unknown |
| 5 | complete worms | drill | Cameroon | |
| 3 | fragments | human | Cameroon and Gabon | |
| 6 | fragments | monkey | Cameroon and unknown | |
| Male worms | 2 | complete worms | human | unknown |
| 4 | complete worms | drill | Cameroon | |
| 2 | fragments | human | Nigeria and unknown | |
| 3 | fragments | monkey | Cameroon and unknown |
As primary antibodies for all selected L. loa worms (Table 1), O. volvulus and other filarial worms we used rabbit antisera against the following recombinant proteins: Wolbachia surface protein of Dirofilaria immitis Wolbachia (Wol-Di-WSP, dilution 1:1 000 – 1:4 000, [30,31]), aspartate aminotransferase of Wolbachia from O. volvulus (Wol-Ov-AAT, dilution 1:60 – 1:200, [32]), and Y. enterocolitica heat shock protein-60 (Y-HSP60, dilution 1:1 000, [33]), which had been supplied by Prof. IB Autenrieth, Tübingen. The specificity of the antisera against Wol-Di-WSP and Y-HSP60 to label Wolbachia had been shown by immuno electron microscopy [26]. Wol-Di-WSP labelled only WSP, Y-HSP60 also labelled filarial HSP60 in mitochondria and other tissues, and Wol-OV-AAT labelled both wolbachial and the filarial AAT especially in sperms. In addition, for selected sections we applied antibodies against human HSP60 (clone LK2, Sigma, dilution 1:5), HSP60 from O. volvulus Wolbachia (Wol-Ov-HSP, dilution 1:500, [19]) supplied by PD Dr. KD Erttmann, a catalase from O. volvulus Wolbachia (Wol-Ov-CAT, dilution 1:30 – 1:50, [14]) supplied by PD Dr. K. Henkle-Dührsen, Düsseldorf, and monoclonal antibodies against human neutrophil granulocyte elastase (Dako Diagnostika, dilution 1:150, [34]), and neutrophil defensins (Dianova, Hamburg, Germany, dilution 1:1 000 – 1:4 000, [34]). Furthermore, antibodies against proteins of eosinophil granulocytes, macrophages [34], mast cells and other filarial and wolbachial proteins were applied. As positive control a Wolbachia-positive onchocercoma section was included for each stained set of L. loa sections.
PCR
DNA was prepared from ethanol preserved or paraffin embedded adult L. loa worms using the Dneasy Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Microfilariae were isolated from two patients from Cameroon with confirmed loiasis by blood filtration using a 5 μm polycarbonate filter. Filters with approxiamtely 500 microfilariae each were washed using 500 μl TE buffer (0.01 M Tris, 0.1 mM EDTA), lysed in 250 μl DSP buffer (0.02 M Tris, 0.05 M KCL, 2.5 mM MgCl2, 0.5% tween 20, 150 μg/ml proteinase k) and 1 μl template was used in a 50 μl PCR assay. Three different sets of primers were used to amplify Wolbachia DNA: one Wolbachia-specific primer pair targeting the 16S rDNA, one primer pair targeting the gene encoding the FTSZ cell cycle protein and one primer pair targeting the gene encoding the WSP as described in detail previously [26]. These primers were known to amplify not only DNA of Wolbachia from filariae but also from T. penetrans. As positive control, DNA of untreated O. volvulus worms was used. In addition, to test the quality of the DNA template, the 5S rDNA of the filarial host was amplified by PCR as previously described [35].
Results
Characterisation of obligatory symbiotic Wolbachia
Onchocerca volvulus represents a typical filaria that contains obligatory symbiotic Wolbachia. We have therefore examined the characteristics of the obligatory symbiotic Wolbachia in more than 800 adult O. volvulus worms and in several other filaria species. It was found that each intact living worm harboured Wolbachia in large numbers (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5). This concerned worms from patients from Uganda, Cameroon, Benin, Togo, Ghana, Burkina Faso, Mali, Liberia, Mexico, Guatemala, Brazil, and Yemen. We observed numerous endobacteria in the hypodermis of female worms in all portions from the nerve ring to the posterior end.
Figure 1.
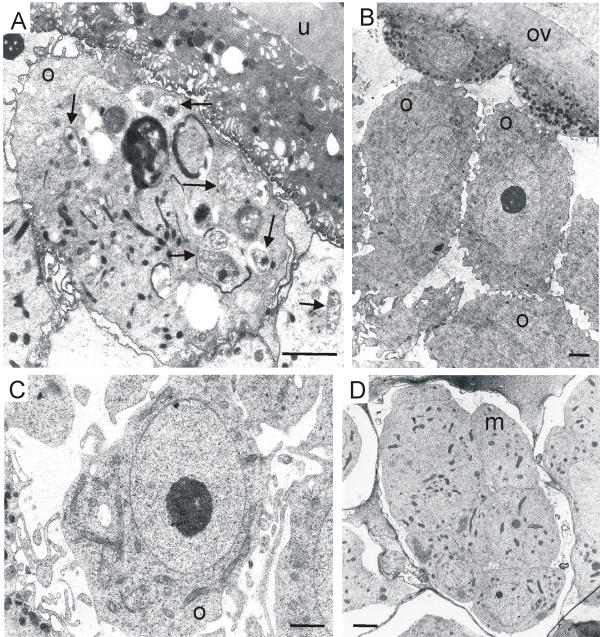
Electron micrographs of oocytes and a morula of O. volvulus and L. loa. (A) Oocyte with several intracellular Wolbachia bacteria (arrows) and uterus wall of O. volvulus. (B, C) Oocytes without any bacteria in the ovary and uterus of L. loa. (D) Young morula without endobacteria in the uterus of L. loa. m, morula, o, oocytes; ov, ovary; u, uterus; scale bar = 2 μm.
Figure 2.
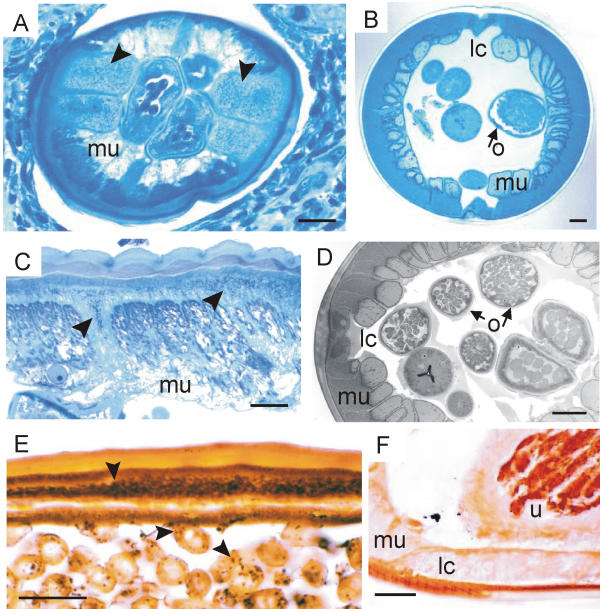
Silver and methylene-blue staining of Wolbachia endobacteria. (A, C) Cross sections of female O. volvulus show Wolbachia (arrow-heads) in the lateral cords of the hypodermis. (E) Longitudinal section of O. jakutensis with black endobacteria in hypodermis and oocytes (arrowheads). (B, D, F) No endobacteria are found in the lateral cords or in oocytes of female L. loa worms. lc, lateral cord; o, oocytes in the ovary; u, uterus. A – D, semi-thin sections stained with azure II and methylene-blue; E, F, sections stained with silver, Warthin-Starry method; scale bar = 25 μm.
Figure 3.
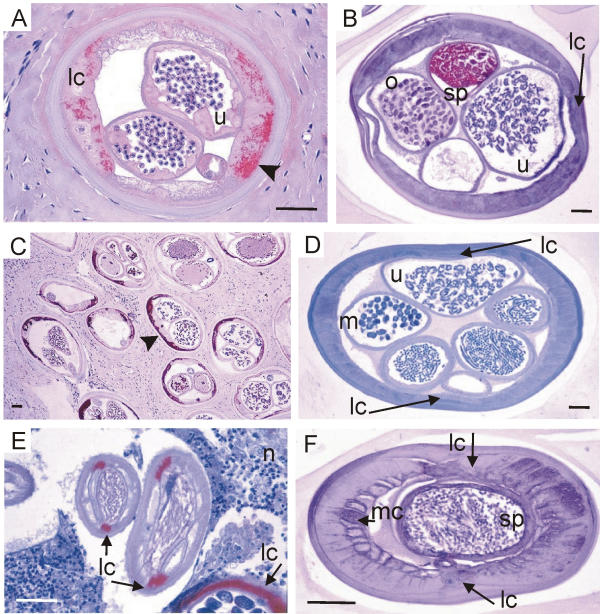
Cross sections of Onchocerca and L. loa stained with three antisera against Wolbachia. (A, B) Antiserum against Wol-Ov-AAT labels Wolbachia endobacteria (arrow-head in A) in a female O. volvulus but no bacteria are seen in a female L. loa, although sperms in the uterus are well labelled. (C, D) Antiserum against Y-HSP60 stains Wolbachia in the hypodermis of all sections of a female O. volvulus in an onchocercoma (arrow-head in C) but no bacteria can be detected in hypodermis or embryos of a female L. loa (D). (E, F) Antiserum against Wol-Di-WSP stains the bacteria in the lateral cords (arrows) of a male O. dukei (E). No bacteria are seen in the lateral or median cords of a male L. loa (F). lc, lateral cord; m, morulae: mc, median cord; n, neutrophil granulocytes; o, oocytes in the uterus; sp, sperms; u, uterus; scale bar = 50 μm.
Figure 4.
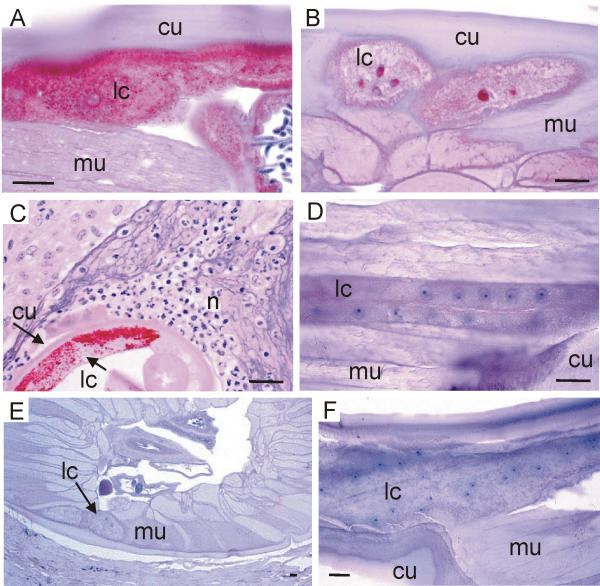
Lateral cords of Onchocerca and L. loa stained with three antisera against Wolbachia. (A, B) Antiserum against Wol-Ov-AAT stains numerous bacteria in a female O. fasciata (A). Whereas no bacteria are seen in the otherwise well stained lateral cords of a female L. loa from Gabon (B). (C, D) Antiserum against Y-HSP60 stains the bacteria in the hypodermis of a female O. ochengi to which many neutrophil granulocytes are attached (C). No bacteria are found in the lateral cords of a male L. loa. (D, F) No Wolbachia are seen in the lateral cords of a female L. loa from Gabon (E, cross section in skin) and in a male L. loa (longitudinal section) stained with Wol-Di-WSP. No neutrophil granulocytes are found attached to the cuticle of L. loa (E). cu, cuticle; lc, lateral cord; mu, muscles; n, neutrophil ganulocytes; scale bar = 25 μm.
Figure 5.
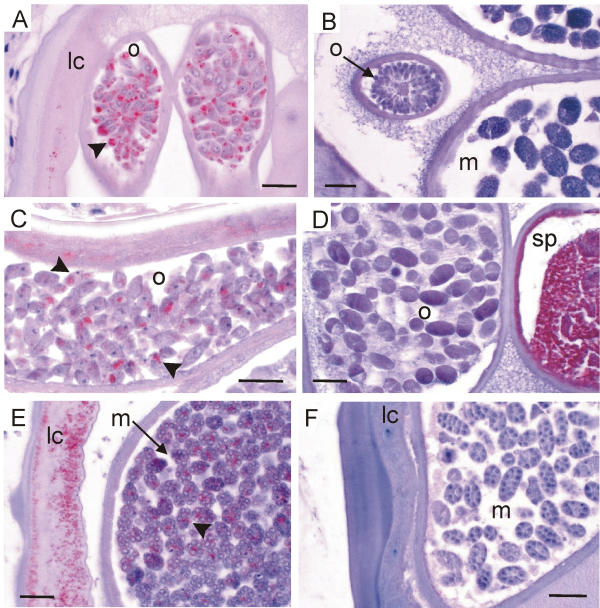
Oocytes and young morulae of Onchocerca and L. loa stained with three antisera against Wolbachia. (A, B) Antiserum against Wol-Di-WSP stains the Wolbachia (arrowhead in A) in all primary oocytes in the ovary of O. volvulus whereas no bacteria are seen in the oocytes in the ovary or in the morulae of a L. loa female (B). (C, D) Antiserum against Wol-Ov-AAT stains the Wolbachia (arrow-heads in C) in all mature oocytes in the uterus of O. ochengi but no bacteria are seen in the uterus of L. loa. The L. loa worm indicates its reactivity to the antiserum by well-labelled sperms. (E, F) Antiserum against Y-HSP60 stains the Wolbachia (arrow-head in E) in the lateral cord and in all young morulae of an O. gibsoni female but no bacteria can be detected in the morulae or in the lateral cord of a L. loa female (F). lc, lateral cord; m, morulae; o, oocytes; sp, sperms; scale bar = 25μm.
In many oocytes, embryos and microfilariae the endobacteria were not detected by immunohistology. However, when the endobacteria had expressed the respective proteins, for which the antisera applied were specific, all live (intact) oocytes or morulae presented endobacteria (Figure 5A, 5C, 5E). Using electron microscopy we counted up to 14 bacteria in one oocyte section. We assume that all live embryos developing later to microfilariae harbour at least ten bacteria (and probably more since an ultra-thin section of 0.1 μm covers only a thin layer of the oocyte). As far as it can be concluded from the limited numbers of worms examined, we assume that this occurrence of numerous endobacteria found in O. volvulus applies also to O. ochengi (Figure 4C; Figure 5C), O. dukei (Figure 3E), O. gibsoni (Figure 5E), O. fasciata (Figure 4A), O. jakutensis (Figure 2E[36]), Litomosoides sigmodontis [12], Wuchereria bancrofti, Dirofilaria immitis, and Dirofilaria repens.
Based on these findings, we define a filaria species harbouring obligatory symbiotic Wolbachia as one with numerous endobacteria in each adult worm and several bacteria in each oocyte and embryo that will develop to a mature microfilaria. The studies described in the following paragraphs aimed to search for Wolbachia in the L. loa worms in numbers, as they were observed in the above-mentioned filaria species containing obligatory symbiotic Wolbachia.
Electron microscopy
Screening several dozen electron micrographs from the previous studies on L. loa and O. volvulus [17,18,27,28] we often found endobacteria in the oocytes of O. volvulus (Figure 1A). In contrast, no endobacteria were observed in the oocytes of the ovary (Figure 1B), the uterus (Figure 1C) or in the early morulae (Figure 1D) of L. loa. Immunogold electron microscopy using the anti-Y-HSP60 serum showed well-labelled mitochondria but no endobacteria in the cells of L. loa microfilariae.
Histology
In semi-thin sections stained with azure II and methylene-blue we detected granular structures in the hypodermis (Figure 2A,2C), oocytes and embryos of dozens of O. volvulus worms but never any in L. loa worms (Figure 2B,2D). Using silver staining of paraffin sections, we found endobacteria-like granules in consecutive sections of O. volvulus and O. jakutensis (Figure 2E) precisely where Wolbachia were seen after labelling with specific antisera against Wolbachia antigens. In contrast, none of the L. loa worms selected for this study (Table 1) displayed such silver-stained granules (Figure 2F). Furthermore, no endobacteria-like granules stained by haematoxylin or Giemsa stain were seen in the hypodermis of L. loa.
Immunohistology
The reactivity of Wolbachia with our antisera was examined in various hosts. The antiserum against Wol-Di-WSP reacted strongly with Wolbachia belonging to wolbachial clade C: D. immitis, D. repens, O. volvulus, O. gutturosa, O. dukei, O. gibsoni, O. fasciata, O. armillata, O. ochengi, O. jakutensis, O. tarsicola; to clade D: Brugia malayi, Brugia pahangi, L. sigmodontis, W. bancrofti, and three other filarial species and with the Wolbachia from the insects Cx. pipiens (clade B) and T. penetrans. The antiserum against Y-HSP60 reacted well with all the Wolbachia of the above-mentioned filariae and T. penetrans (except that it was not tested with those of B. pahangi and Cx. pipiens). We conclude that these two antisera react with all Wolbachia. The other antisera were examined with some or most but not all of the above mentioned Wolbachia.
Having shown the suitability of these anti-wolbachial sera, L. loa worms were compared with O. volvulus and other Wolbachia-positive filariae. Using these antisera against Wolbachia we easily detected endobacteria in many filariae in the hypodermis of male (Figure 3E) and female worms (Figure 3A,3C; Figure 4A,4C; Figure 5E). We found the Wolbachia well labelled by the antisera in the oocytes of the ovary (Figure 5A) and in portions of the uterus (Figure 5C), in zygotes, in morulae (Figure 5E) or in other embryos and microfilariae (Figure 6A), when the respective wolbachial proteins had been expressed. However, to interpret these photos and other previously published photos [12,14,32,36-39] it has to be taken into account that it would have been easy to present as many photos from other sections of the same worms, in which no endobacteria occurred or where the endobacteria had not expressed the respective proteins. Such non-reactive endobacteria were often well stained by haematoxylin, Giemsa or toluidine-blue.
Figure 6.
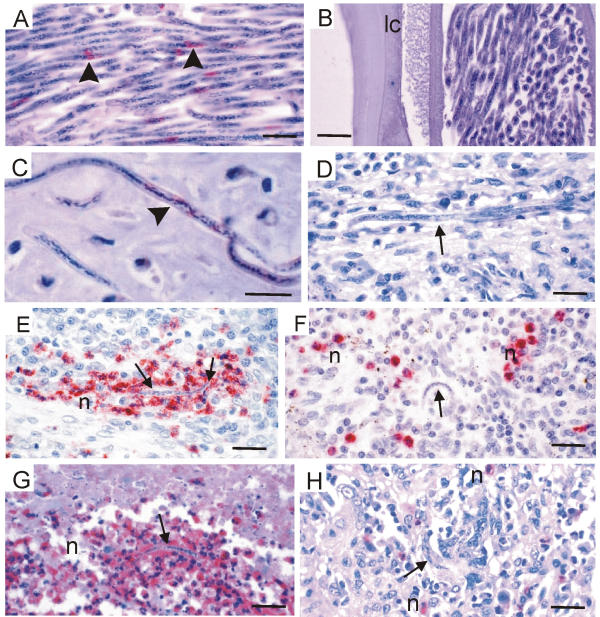
Wolbachia in microfilariae of Onchocerca but not of L. loa and the reaction of neutrophils. (A – D) Antiserum against Wol-Di-WSP stains the Wolbachia (arrowheads) of microfilariae in the uterus of O. volvulus (A) and in an onchocercoma (C) whereas no bacteria are seen in intact microfilariae of L. loa in the uterus (B) or in human spleen tissue (D). (E – H) Neutrophil granulocytes are attracted to degenerating microfilariae (arrows) of O. volvulus in lymph node tissue 24 hours after a dose of ivermectin (E, anti-neutrophils elastase) or in an onchocercoma of an untreated patient (G, anti-neutrophils defensins). Neutrophils are not attracted by degenerated L. loa microfilariae (arrows) in spleen tissue from a human patient not treated with microfilaricidal drugs (F, anti-neutrophils elastase) or from an experimentally infected monkey (H, anti-neutrophils defensins). n, neutrophil granulocytes; scale bar = 25 μm.
Before we selected the L. loa worms for our study, we examined them with several antisera against filarial proteins. Antisera against the ankyrin-related protein [40] and glutathione S-transferase of O. volvulus did not react with any of the L. loa worms. Y-HSP60 cross-reacting with filarial proteins [36,39], reacted weakly with L. loa proteins. However, using immuno electron microscopy, mitochondria of microfilariae were distinctly labelled by this antibody. Good cross-reactivity was achieved by the antiserum against Wol-Ov-AAT, which labelled the Wolbachia-free sperms of L. loa and with two other antisera. All L. loa worms that did not react well with Wol-Ov-AAT were excluded as unsuitable for immunohistology. Those that were well labelled (Figure 3B; Figure 4B; Figure 5D) were selected for the study. As shown in Table 1, seven complete female L. loa worms producing embryos and microfilariae, six complete male worms, and some fragments of 14 other adult worms were examined. Each complete worm had been cut into small pieces before embedding in one block that was cut until nothing was left. All sections were analysed by either anti-Wol-Di-WSP or anti-Y-HSP60, except for a few sections stained with anti-Wol-Ov-AAT or by the silver method. This procedure yielded about 20 slides from each L. loa worm, each slide containing many worm sections. No Wolbachia was detected in any of these sections (Figure 3B, Figure 3D,3F; Figure 4B,4D,4E,4F; Figure 5B,5D,5F; Figure 6B,6D). Furthermore, no Wolbachia were observed in L. loa when antisera against human HSP60, Wol-Ov-HSP60, Wol-Ov-CAT and another antiserum were applied.
Wolbachia could easily be observed with all antisera in microfilariae in onchocercomas (Figure 6C), skin, and lymph nodes. In contrast, no Wolbachia were found in several hundred L. loa microfilariae from the spleen of a patient and of an experimentally infected monkey. Sections of L. loa microfilariae from a sample of human blood were also non-reactive with the antisera.
Neutrophil granulocytes as an indicator of Wolbachia
Previously it has been shown that the accumulation of neutrophil granulocytes around adult O. volvulus worms is dependent on Wolbachia [36]. Such accumulation could also be seen around the other endobacteria-positive filariae: O. jakutensis [36], O. dukei (Figure 3E), O. ochengi (Figure 4C), O. gibsoni, and D. repens in subcutaneous nodules of human patients. In contrast, no neutrophil granulocytes were found around the two female L. loa worms in skin biopsies from patients (Figure 4E). Since live adult L. loa are mobile these observations were not sufficient for a conclusion. Therefore, degenerated microfilariae in the spleen were examined. We had previously shown the activity of neutrophils against O. volvulus microfilariae in onchocercomas [41] and in the skin after treatment with diethylcarbamazine [34]. Such activity of neutrophils against microfilariae could clearly be shown after staining with antisera specific for proteins of neutrophils (Figure 6E,6G). However, neutrophils present in human and monkey spleen tissue, where degenerating L. loa microfilariae could be observed, were not attached to the parasites (Figure 6F,6H). The degenerated or disintegrating microfilariae were attacked only by eosinophil granulocytes, macrophages and small giant cells, as shown previously by Duke [42]. We conclude from these findings that the microfilariae of L. loa do not contain sufficient numbers of Wolbachia, if any, to attract neutrophil granulocytes.
PCR
The 5S rDNA spacer of L. loa was amplified from all samples included in the study, indicating the absence of significant PCR inhibitor in the samples. Using the Wolbachia 16S rDNA primers, the ftsZ primers and the wsp primers, PCR products of about 530 bp, 510 bp and 490 bp, respectively, were obtained in all O. volvulus samples, which functioned as controls. In contrast, no bands were visible on the ethidium bromide stained agarose gel when the DNA from the three males and six female L. loa worms and two batches of L. loa microfilariae samples were tested.
Discussion
Positive findings, when Wolbachia endobacteria are detected by any of the methods used in this study, need only a few worms to demonstrate the reproducibility of the results. Negative findings, such as the absence of Wolbachia, can only be presented with a certain probability and even this evidence requires larger numbers of worms and repeated experiments. Negative electron microscopic findings of Wolbachia in Loa microfilariae have been based on observations of "several hundred" [1] or "many thousand" sections [16]. These numbers are needed since large portions of bacteria-positive microfilariae are free of Wolbachia. The screening of adult L. loa worms has to focus on microfilariae producing female worms since they would harbour the largest numbers of endobacteria in oocytes and embryos. Using electron microscopy we observed up to 14 bacteria in oocytes of O. volvulus, eight or more bacteria were found in an oocyte of W. bancrofti [43] and electron micrographs of D. immitis showed 15 – 25 bacteria in embryos [1]. In Mansonella ozzardi microfilariae, ten or more bacteria were reported [44]. Since all these figures are based on single ultra-thin sections, and regarding our light microscopic observations of several bacteria clusters in oocytes and microfilariae, we estimate that oocytes, embryos and microfilariae of filaria species with essential numbers of Wolbachia for vertical transmission harbour 30–50 or more endobacteria. Assuming only one or ten Wolbachia per embryo and a minimal number of 50,000 oocytes and embryos in an 8 cm long L. loa female worm (we calculated more than 200,000 based on the number of more than 100 embryos per cross section) a microfilariae producing L. loa female would harbour 50,000 or 500,000 endobacteria plus those in the hypodermis, if essential numbers would be present. These figures indicate that electron microscopic searches for endobacteria should focus on the oocytes, zygotes and young morulae as far as possible and not on the hypodermis.
The main problem for immunohistology is the reactivity of the wolbachial proteins with the antisera. The proteins may not have been expressed or they may have been destroyed by processing the specimens for microscopy. To overcome these problems and to achieve a rather high degree of probability for the absence of Wolbachia in L. loa, we used 27 adult worms from different sources, and applied several anti-wolbachial antisera that had resulted in reliable findings for 21 filarial or insect hosts of Wolbachia. The analysis of seven microfilariae producing female worms that were completely examined should especially provide a high degree of probability that we did not miss Wolbachia, even if they would have been present in only small numbers.
In insects, the density of Wolbachia can vary between different host populations. For example, in the mosquito Aedes albopictus a higher density of Wolbachia was found in populations from Houston (USA) compared to those from Mauritius or Koh Samui (Thailand) [45]. Furthermore, a changing Wolbachia density is also observed after experimental transinfection to a novel host, and it was suggested that too high densities in the ovaries may result in reduction of reproductive fitness [46]. However, in arthropods a minimal level of bacterial infection is assumed to be required to cause effects like cytoplasmatic incompatibility or for vertical transmission in an obligatory symbiosis [6,47].
The completely different molecular biological analysis of nine adult L. loa worms and two batches of microfilariae by PCR comprises by itself a high degree of probability that the negative result is correct. As calculated above, in an adult worm at least 50,000 – 500,000 copies of the Wolbachia target sequence should be present, which can be easily amplified by a single PCR using 35 cycles. It cannot be excluded however, that the primer sets used did not hybridise to the target sequences, though our previous results have shown that these primers amplified Wolbachia DNA of all species that have been examined so far including the sand flea T. penetrans [26]. Furthermore, our results are in line with those reported from PCR analysis of microfilariae from two patients infected with L. loa [20].
Our results agree with the previous reports on the absence of Wolbachia in L. loa worms [1,16-20]. Other filaria species for which the absence of endobacteria has been shown by at least two independent methods are O. flexuosa [13,14], A. viteae [1,11,12], and S. equina [15]. Less certain is the absence in Acanthocheilonema setariosa (reported as Dipetolonema setariosum, [1]). Possibly no obligatory symbiotic Wolbachia occur in Mansonella perstans since Fischer and co-workers did not detect any Wolbachia DNA examining three batches of microfilariae by PCR using the same primers as for L. loa (unpublished data). In contrast, among the filariae infecting man are O. volvulus, W. bancrofti, B. malayi, Brugia timori, M. ozzardi, D. repens, and D. immitis bacteria-positive species [48,49].
The medical significance of the Wolbachia endobacteria concerns their role in the immunopathology [50-52] including adverse side effects after antifilarial chemotherapy [53,54] and the feasibility of antifilarial treatment using already registered antibiotics [7,9]. These aspects have been discussed in detail in recent meetings and they have been summarised in several reviews [4,5,55,56].
Conclusions
Using electron microscopy, histology, immunohistology and PCR, no direct evidence was found that L. loa filariae harbour Wolbachia endobacteria in numbers required for vertical transmission of the bacteria or embryogenesis of the filariae. These findings exclude only the occurrence of obligatory symbiotic filarial Wolbachia. They do not exclude that endobacteria in small numbers may occasionally be detected in L. loa, e.g. acquired from any infected vectors. Even if they would be detected in a local population of L. loa, they would not be obligatory for microfilariae production. Hence, only the filariae and not any Wolbachia cause the immunopathology of loiasis patients or the adverse side effects after antifilarial chemotherapy seen in patients with high microfilarial loads [21-23]. This is confirmed by the lack of any reaction of neutrophil granulocytes to L. loa microfilariae.
Unlike onchocerciasis and lymphatic filariasis, loiasis cannot be treated by antibiotics. This statement is in accordance with the findings of Brouqui et al., [20].
The African Programme for the Control of Onchocerciasis (APOC) uses ivermectin to eliminate onchocerciasis as a public health problem [57]. In a few African countries, co-endemicity of onchocerciasis and loiasis exists [58,59]. Co-infected patients with high L. loa microfilarial loads cannot be treated with ivermectin without a risk of serious side effects. Since the number of such patients is very small, the onchocerciasis of these individuals can probably be treated with doxycycline without any additional risk due to their loiasis, because doxycycline acts mainly in an indirect manner, eliminating the Wolbachia from the O. volvulus.
Competing interests
None declared.
Author's contributions
DWB conceived the study, participated in drafting the manuscript, and carried out the morphological analysis of L. loa and Onchocerca.
SW performed the monkey experiments to collect L. loa worms and spleen.
CB produced the rabbit antiserum against Wol-Di-WSP and examined the reactivity of it for Wolbachia of filariae and of Culex.
OB collected and identified L. loa worms.
PF participated in drafting the manuscript, produced the rabbit antiserum against Wol-Ov-AAT, examined the reactivity of it for filariae and sand fleas, and performed the PCR analysis.
Acknowledgments
Acknowledgements
We thank I. Bonow, I. Albrecht, K. Fischer and C. Schmetz for excellent technical assistance. We are grateful for the supply of worm samples to Prof. G.D. Burchard, PD Dr. A. Hörauf, Prof. M. Omar, Dr. A. Plenge-Bönig, Prof. P. Racz, PD Dr. A. Renz, Prof. H. Schulz-Key, and to Prof. M. Coluzzi for the mosquitoes. We are obliged to Prof. I.B. Autenrieth, PD. Dr. K.D. Erttmann, PD Dr. Henkle-Dührsen, and PD Dr. E. Liebau for sharing specific antibodies with us. P. F. received a scholarship of the "Vereinigung der Freunde des Tropeninstitutes, Hamburg".
Contributor Information
Dietrich W Büttner, Email: Buettner@bni-hamburg.de.
Samuel Wanji, Email: swanji@yahoo.fr.
Chiara Bazzocchi, Email: chiara.bazzocchi@unimi.it.
Odile Bain, Email: bain@cimrs1.mnhn.fr.
Peter Fischer, Email: Pfischer@bni-hamburg.de.
References
- McLaren DJ, Worms MJ, Laurence BR, Simpson MG. Micro-organisms in filarial larvae (Nematoda) Trans R Soc Trop Med Hyg. 1975;69:509–514. doi: 10.1016/0035-9203(75)90110-8. [DOI] [PubMed] [Google Scholar]
- Kozek WJ, Figueroa Marroquin H. Intracytoplasmic bacteria in Onchocerca volvulus. Am J Trop Med Hyg. 1977;26:663–678. doi: 10.4269/ajtmh.1977.26.663. [DOI] [PubMed] [Google Scholar]
- Sironi M, Bandi C, Sacchi L, Di Sacco B, Damiani G, Genchi C. Molecular evidence for a close relative of the arthropod endosymbiont Wolbachia in a filarial worm. Mol Biochem Parasitol. 1995;74:223–227. doi: 10.1016/0166-6851(95)02494-8. [DOI] [PubMed] [Google Scholar]
- Bandi C, Trees AJ, Brattig NW. Wolbachia in filarial nematodes: evolutionary aspects and implications for the pathogenesis and treatment of filarial diseases. Vet Parasitol. 2001;98:215–238. doi: 10.1016/S0304-4017(01)00432-0. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Hoerauf A. A new approach to the treatment of filariasis. Curr Opin Infect Dis. 2001;14:727–731. doi: 10.1097/00001432-200112000-00011. [DOI] [PubMed] [Google Scholar]
- Dedeine F, Vavre F, Fleury F, Loppin B, Hochberg ME, Bouletreau M. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc Natl Acad Sci U S A. 2001;98:6247–6252. doi: 10.1073/pnas.101304298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerauf A, Adjei O, Buttner DW. Antibiotics for the treatment of onchocerciasis and other filarial infections. Curr Opin Investig Drugs. 2002;3:533–537. [PubMed] [Google Scholar]
- Langworthy NG, Renz A, Mackenstedt U, Henkle-Duhrsen K, de Bronsvoort MB, Tanya VN, Donnelly MJ, Trees AJ. Macrofilaricidal activity of tetracycline against the filarial nematode Onchocerca ochengi: elimination of Wolbachia precedes worm death and suggests a dependent relationship. Proc R Soc Lond B Biol Sci. 2000;267:1063–1069. doi: 10.1098/rspb.2000.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerauf A, Buttner DW, Adjei O, Pearlman E. Onchocerciasis. BMJ. 2003;326:207–210. doi: 10.1136/bmj.326.7382.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerauf A, Mand S, Fischer K, Kruppa T, Marfo-Debrekyei Y, Debrah AY, Pfarr KM, Adjei O, Buttner DW. Doxycycline as a novel strategy against bancroftian filariasis – depletion of Wolbachia endosymbionts from Wuchereria bancrofti and stop of microfilaria production. Med Microbiol Immunol (Berl) 2003. [DOI] [PubMed]
- Bandi C, Anderson TJ, Genchi C, Blaxter ML. Phylogeny of Wolbachia in filarial nematodes. Proc R Soc Lond B Biol Sci. 1998;265:2407–2413. doi: 10.1098/rspb.1998.0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerauf A, Nissen-Pahle K, Schmetz C, Henkle-Duhrsen K, Blaxter ML, Buttner DW, Gallin MY, Al-Qaoud KM, Lucius R, Fleischer B. Tetracycline therapy targets intracellular bacteria in the filarial nematode Litomosoides sigmodontis and results in filarial infertility. J Clin Invest. 1999;103:11–18. doi: 10.1172/JCI4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenge-Bonig A, Kromer M, Buttner DW. Light and electron microscopy studies on Onchocerca jakutensis and O. flexuosa of red deer show different host-parasite interactions. Parasitol Res. 1995;81:66–73. doi: 10.1007/BF00932419. [DOI] [PubMed] [Google Scholar]
- Henkle-Duhrsen K, Eckelt VH, Wildenburg G, Blaxter M, Walter RD. Gene structure, activity and localization of a catalase from intracellular bacteria in Onchocerca volvulus. Mol Biochem Parasitol. 1998;96:69–81. doi: 10.1016/S0166-6851(98)00109-1. [DOI] [PubMed] [Google Scholar]
- Chirgwin SR, Porthouse KH, Nowling JM, Klei TR. The filarial endosymbiont Wolbachia sp. is absent from Setaria equina. J Parasitol. 2002;88:1248–1250. doi: 10.1645/0022-3395(2002)088[1248:TFEWSI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kozek WJ, Orihel TC. Ultrastructure of Loa loa microfilaria. Int J Parasitol. 1983;13:19–43. doi: 10.1016/s0020-7519(83)80063-0. [DOI] [PubMed] [Google Scholar]
- Franz M, Melles J, Buttner DW. Electron microscope study of the body wall and the gut of adult Loa loa. Z Parasitenkd. 1984;70:525–536. doi: 10.1007/BF00926694. [DOI] [PubMed] [Google Scholar]
- Weber P. The fine structure of the female reproductive tract of adult Loa loa. Int J Parasitol. 1987;17:927–934. doi: 10.1016/0020-7519(87)90010-5. [DOI] [PubMed] [Google Scholar]
- Koszarski A. Klonierung und Charakterisierung eines potentiellen Endobakterien-Hitzeschockproteins aus Onchocerca volvulus LEUCKART. PhD dissertation, University of Hamburg, Germany. 1999.
- Brouqui P, Fournier PE, Raoult D. Doxycycline and eradication of microfilaremia in patients with loiasis. Emerg Infect Dis. 2001;7:604–605. doi: 10.3201/eid0707.010747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardon J, Gardon-Wendel N, Demanga-Ngangue , Kamgno J, Chippaux JP, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. 1997;350:18–22. doi: 10.1016/S0140-6736(96)11094-1. [DOI] [PubMed] [Google Scholar]
- Boussinesq M, Gardon J, Gardon-Wendel N, Kamgno J, Ngoumou P, Chippaux JP. Three probable cases of Loa loa encephalopathy following ivermectin treatment for onchocerciasis. Am J Trop Med Hyg. 1998;58:461–589. doi: 10.4269/ajtmh.1998.58.461. [DOI] [PubMed] [Google Scholar]
- Blum J, Wiestner A, Fuhr P, Hatz C. Encephalopathy following Loa loa treatment with albendazole. Acta Trop. 2001;78:63–65. doi: 10.1016/S0001-706X(00)00159-5. [DOI] [PubMed] [Google Scholar]
- Burchard GD, Reimold-Jehle U, Burkle V, Kretschmer H, Vierbuchen M, Racz P, Lo Y. Splenectomy for suspected malignant lymphoma in two patients with loiasis. Clin Infect Dis. 1996;23:979–982. doi: 10.1093/clinids/23.5.979. [DOI] [PubMed] [Google Scholar]
- Buttner DW, Albiez EJ, von Essen J, Erichsen J. Histological examination of adult Onchocerca volvulus and comparison with the collagenase technique. Trop Med Parasitol. 1988;39:390–417. [PubMed] [Google Scholar]
- Fischer P, Schmetz C, Bandi C, Bonow I, Mand S, Fischer K, Buttner DW. Tunga penetrans: molecular identification of Wolbachia endobacteria and their recognition by antibodies against proteins of endobacteria from filarial parasites. Exp Parasitol. 2003. [DOI] [PubMed]
- Melles J. Licht- und elektronenmikroskopische Untersuchungen von Loa loa (Nematoda: Filarioidea) MD dissertation, University of Hamburg, Germany. 1983.
- Buttner DW, Mac Donald A. The fine structure of adult Onchocerca volvulus recovered by collagenase digestion. Trop Med Parasitol. 1985;36:171–174. [PubMed] [Google Scholar]
- Luna LG, ed Manual of histologic staining methods of the Armed Forces Institute of Pathology. McGraw-Hill Book Company, New York, Toronto, London, Sydney. 1968. p. 238.
- Bazzocchi C, Jamnongluk W, O'Neill SL, Anderson TJ, Genchi C, Bandi C. Wsp gene sequences from the Wolbachia of filarial nematodes. Curr Microbiol. 2000;41:96–100. doi: 10.1007/s002840010100. [DOI] [PubMed] [Google Scholar]
- Casiraghi M, McCall JW, Simoncini L, Kramer LH, Sacchi L, Genchi C, Werren JH, Bandi C. Tetracycline treatment and sex-ratio distortion: a role for Wolbachia in the moulting of filarial nematodes? Int J Parasitol. 2002;32:1457–1468. doi: 10.1016/S0020-7519(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Fischer P, Bonow I, Buttner DW, Kamal IH, Liebau E. An aspartate aminotransferase of Wolbachia endobacteria from Onchocerca volvulus is recognized by IgG1 antibodies from residents of endemic areas. Parasitol Res. 2003;90:38–47. doi: 10.1007/s00436-002-0813-2. [DOI] [PubMed] [Google Scholar]
- Noll A, Roggenkamp A, Heesemann J, Autenrieth IB. Protective role for heat shock protein-reactive alpha beta T cells in murine yersiniosis. Infect Immun. 1994;62:2784–2791. doi: 10.1128/iai.62.7.2784-2791.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Pena EJ, Knab J, Buttner DW. Neutrophil granule proteins: evidence for the participation in the host reaction to skin microfilariae of Onchocerca volvulus after diethylcarbamazine administration. Parasitology. 1996;113:403–414. doi: 10.1017/s0031182000066543. [DOI] [PubMed] [Google Scholar]
- Fischer P, Buttner DW, Bamuhiiga J, Williams SA. Detection of the filarial parasite Mansonella streptocerca in skin biopsies by a nested polymerase chain reaction-based assay. Am J Trop Med Hyg. 1998;58:816–820. doi: 10.4269/ajtmh.1998.58.816. [DOI] [PubMed] [Google Scholar]
- Brattig NW, Buttner DW, Hoerauf A. Neutrophil accumulation around Onchocerca worms and chemotaxis of neutrophils are dependent on Wolbachia endobacteria. Microb Infect. 2001;3:439–446. doi: 10.1016/S1286-4579(01)01399-5. [DOI] [PubMed] [Google Scholar]
- Hoerauf A, Volkmann L, Hamelmann C, Adjei O, Autenrieth IB, Fleischer B, Buttner DW. Endosymbiotic bacteria in worms as targets for a novel chemotherapy in filariasis. Lancet. 2000;355:1242–1243. doi: 10.1016/S0140-6736(00)02095-X. [DOI] [PubMed] [Google Scholar]
- Hoerauf A, Mand S, Adjei O, Fleischer B, Buttner DW. Depletion of Wolbachia endobacteria in Onchocerca volvulus by doxycycline and microfilaridermia after ivermectin treatment. Lancet. 2001;357:1415–1416. doi: 10.1016/S0140-6736(00)04581-5. [DOI] [PubMed] [Google Scholar]
- Hoerauf A, Mand S, Volkmann L, Buttner M, Marfo-Debrekyei Y, Taylor M, Adjei O, Buttner DW. Doxycycline in the treatment of human onchocerciasis: kinetics of Wolbachia endobacteria reduction and of inhibition of embryogenesis in female Onchocerca worms. Microb Infect. 2003;5:261–273. doi: 10.1016/S1286-4579(03)00026-1. [DOI] [PubMed] [Google Scholar]
- Erttmann KD, Gallin MY, Eggert P, Buttner DW. Immunohistological studies on an Onchocerca volvulus ankyrin (EI) Trop Med Int Health. 1996;1:558–574. doi: 10.1111/j.1365-3156.1996.tb00081.x. [DOI] [PubMed] [Google Scholar]
- Wildenburg G, Korten S, Buttner DW. Mast cell distribution in nodules of Onchocerca volvulus from untreated patients with generalized onchocerciasis. Parasitology. 1998;116:257–268. doi: 10.1017/S0031182097002278. [DOI] [PubMed] [Google Scholar]
- Duke BOL. Studies on loiasis in monkeys. III. – The pathology of the spleen in drills (Mandrillus leucophaeus) infected with Loa loa. Ann Trop Med Parasitol. 1960;54:141–146. [PubMed] [Google Scholar]
- Peixoto CA, Silva LF, Teixeira KM, Rocha A. Ultrastructural characterization of intracellular bacteria of Wuchereria bancrofti. Trans R Soc Trop Med Hyg. 2001;95:566–568. doi: 10.1016/s0035-9203(01)90042-2. [DOI] [PubMed] [Google Scholar]
- Kozek WJ, Raccurt C. Ultrastructure of Mansonella ozza rdi microfilaria, with a comparison of the South American (simuliid-transmitted) and the Caribbean (culicoid-transmitted) forms. Tropenmed Parasitol. 1983;34:38–53. [PubMed] [Google Scholar]
- Sinkins SP, Braig HR, O'Neill SL. Wolbachia pipientis: bacterial density and unidirectional cytoplasmatic incompartibility between infected populations of Aedes albopictus. Exp Parasitol. 1995;81:284–291. doi: 10.1006/expr.1995.1119. [DOI] [PubMed] [Google Scholar]
- McGraw EA, Merritt DJ, Droller JN, O'Neill Wolbachia density and virulence attenuation after transfer to a novel host. Pro Nat Acad Sci. 2002;99:2918–2923. doi: 10.1073/pnas.052466499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breewer JAJ, Werren JH. Cytoplasmatic incompartibility and bacterial infection in Nasonia vitripennis. Genetics. 1993;135:565–574. doi: 10.1093/genetics/135.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Hoerauf A. Wolbachia bacteria of filarial nematodes. Parasitol Today. 1999;15:437–442. doi: 10.1016/S0169-4758(99)01533-1. [DOI] [PubMed] [Google Scholar]
- Fischer P, Wibowo H, Pischke S, Ruckert P, Liebau E, Ismid IS, Supali T. PCR-based detection and identification of the filarial parasite Brugia timori from Alor Island, Indonesia. Ann Trop Med Parasitol. 2002;96:809–821. doi: 10.1179/000349802125002239. [DOI] [PubMed] [Google Scholar]
- Brattig NW, Rathjens U, Ernst M, Geisinger F, Renz A, Tischendorf FW. Lipopolysaccharide-like molecules derived from Wolbachia endobacteria of the filaria Onchocerca volvulus are candidate mediators in the sequence of inflammatory and antiinflammatory responses of human monocytes. Microb Infect. 2000;2:1147–1157. doi: 10.1016/S1286-4579(00)01269-7. [DOI] [PubMed] [Google Scholar]
- Saint André A, Blackwell NM, Hall LR, Hoerauf A, Brattig NW, Volkmann L, Taylor MJ, Ford L, Hise AG, Lass JH, Diaconu E, Pearlman E. The role of endosymbiontic Wolbachia bacteria in the pathogenesis of river blindness. Science. 2002;295:1892–1895. doi: 10.1126/science.1068732. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Cross HF, Bilo K. Inflammatory responses induced by the filarial nematode Brugia malayi are mediated by lipopolysaccharide-like activity from endosymbiotic Wolbachia bacteria. J Exp Med. 2000;191:1429–1436. doi: 10.1084/jem.191.8.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser PB, Reynolds SM, Awadzi K, Ottesen EA, Taylor MJ, Nutman TB. Bacterial endosymbionts of Onchocerca volvulus in the pathogenesis of posttreatment reactions. J Infect Dis. 2002;185:805–811. doi: 10.1086/339344. [DOI] [PubMed] [Google Scholar]
- Cross HF, Haarbrink M, Egerton G, Yazdanbakhsh M, Taylor MJ. Severe reactions to filarial chemotherapy and release of Wolbachia endosymbionts into blood. Lancet. 2001;358:1873–1875. doi: 10.1016/S0140-6736(01)06899-4. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Bandi C, Hoerauf AM, Lazdins J. Wolbachia bacteria of filarial nematodes: a target for control? Parasitol Today. 2000;16:179–180. doi: 10.1016/S0169-4758(00)01661-6. [DOI] [PubMed] [Google Scholar]
- Hoerauf A, Walter RD, Remme H, Lazdins J, Fleischer B. Call to consolidate achievements for onchocerciasis and lymphatic filariasis control. Trends Parasitol. 2001;17:566–567. doi: 10.1016/S1471-4922(01)02192-4. [DOI] [PubMed] [Google Scholar]
- Richards FO, Jr, Boatin B, Sauerbrey M, Seketeli A. Control of onchocerciasis today: status and challenges. Trends Parasitol. 2001;17:558–563. doi: 10.1016/S1471-4922(01)02112-2. [DOI] [PubMed] [Google Scholar]
- Esum M, Wanji S, Tendongfor N, Enyong P. Co-endemicity of loiasis and onchocerciasis in the South West Province of Cameroon: implications for mass treatment with ivermectin. Trans R Soc Trop Med Hyg. 2001;95:673–676. doi: 10.1016/s0035-9203(01)90112-9. [DOI] [PubMed] [Google Scholar]
- Wanji S, Tendongfor N, Esum M, Ndindeng S, Enyong P. Epidemiology of concomitant infections due to Loa loa, Mansonella perstans, and Onchocerca volvulus in rain forest villages of Cameroon. Med Microbiol Immunol (Berl) 2003;192:15–21. doi: 10.1007/s00430-002-0154-x. [DOI] [PubMed] [Google Scholar]