Abstract
The intermediate filament protein, nestin, has been implicated as an organizer of survival-determining signaling molecules. When nestin expression was related to the sensitivity of neural progenitor cells to oxidant-induced apoptosis, nestin displayed a distinct cytoprotective effect. Oxidative stress in neuronal precursor cells led to downregulation of nestin with subsequent activation of cyclin-dependent kinase 5 (Cdk5), a crucial kinase in the nervous system. Nestin downregulation was a prerequisite for the Cdk5-dependent apoptosis, as overexpression of nestin efficiently inhibited induction of apoptosis, whereas depletion of nestin by RNA interference had a sensitizing effect. When the underlying link between nestin and Cdk5 was analyzed, we observed that nestin serves as a scaffold for Cdk5, with binding restricted to a specific region following the alpha-helical domain of nestin, and that the presence and organization of nestin regulated the sequestration and activity of Cdk5, as well as the ubiquitylation and turnover of its regulator, p35. Our data imply that nestin is a survival determinant whose action is based upon a novel mode of Cdk5 regulation, affecting the targeting, activity, and turnover of the Cdk5/p35 signaling complex.
Keywords: apoptosis, Cdk5 kinase, nestin, oxidative stress, signaling scaffold
Introduction
The intermediate filament (IF) protein nestin is specifically expressed in progenitor cells of the central nervous system (CNS) and myogenic tissue (Lendahl et al, 1990; Sejersen and Lendahl, 1993). Upon differentiation, nestin expression is downregulated (Lendahl et al, 1990; Sejersen and Lendahl, 1993), with the exception of specific sites, such as the neuromuscular (NMJ) and myotendinous junctions (MTJ) in adult muscle (Vaittinen et al, 1999, 2001). Nestin expression is upregulated in the adult under pathological conditions, such as the formation of the glial scar after injury to the CNS (Frisen et al, 1995), during regeneration of injured muscle tissue (Vaittinen et al, 1999, 2001), and in neuroepithelial tumors (Dahlstrand et al, 1992; Tohyama et al, 1992). Although very little is known about the functions of nestin, recent evidence indicate that nestin may be important in the distribution and organization of critical cellular factors regulating cell proliferation, survival, and differentiation (Shen et al, 2002; Bieberich et al, 2003, 2004; Chou et al, 2003; Sahlgren et al, 2003).
An emerging function of IF-networks is their role as scaffolds upon which intracellular kinases are organized (Toivola et al, 2005). These interactions not only regulate the phosphorylation and assembly state of the IFs but also regulate the activity of the kinases. One of the proteins associated with the IF-protein nestin is cyclin-dependent kinase 5 (Cdk5), which is a key regulator of neuronal development (Ohshima et al, 1996) and normal neuronal function (Cheung and Ip, 2004; Cruz and Tsai, 2004). The activity of Cdk5 is regulated through association with specific protein activators, including p35, p39, and p67 (Lew et al, 1994; Tang et al, 1995; Veeranna et al, 1997; Humbert et al, 2000). In addition to its regulatory roles in neurons, Cdk5 is important in regulating the differentiation, organization, and signal transduction in muscle tissue (Lazaro et al, 1997; Philpott et al, 1997; Fu et al, 2001). Disturbances in the regulation of Cdk5 have detrimental effects, as demonstrated by studies linking deregulation of Cdk5 to several neurodegenerative disorders, including Alzheimer's disease (Pei et al, 1998; Ahlijanian et al, 2000), Parkinson's disease (Brion and Couck, 1995), and amyotrophic lateral sclerosis (Bajaj et al, 1999; Nguyen et al, 2001). Deregulated Cdk5 activity has also been associated with neuronal cell death under normal as well as pathological conditions (Ahuja et al, 1997; Zhang et al, 1997; Patrick et al, 1999; Zhang and Johnson, 2000; Gao et al, 2001). Various neurotoxic insults, including ischemia and oxidative stress, induce hyperactivation of Cdk5 with fatal consequences for the cell (Lee et al, 2000; Strocchi et al, 2003). On the other hand, lack of Cdk5 activity also results in neuronal cell death (Ko et al, 2001) emphasizing that appropriate control of Cdk5 activity is crucial for normal cellular homeostasis.
The activity of Cdk5 is regulated by protein–protein interactions with both regulatory and target molecules (Grant et al, 2001). We have previously shown that Cdk5 is associated with nestin in differentiating myoblasts, in progenitor cells of the CNS, and at NMJs (Sahlgren et al, 2003). Inhibition of Cdk5 activity induced an increased association of p35 with nestin, indicating that there is a continuous turnover of Cdk5/p35 activity on a scaffold formed by nestin (Sahlgren et al, 2003).
Prompted by the previously observed association between nestin and Cdk5, and we wanted to determine how this interaction affects the organization and operation of Cdk5. The physiological significance of the interplay between Cdk5 and nestin is here demonstrated by experiments in which degradation of nestin is shown to be a prerequisite for activation of Cdk5 and induction of apoptosis during oxidative stress. Nestin serves as a scaffold for Cdk5, and the organization and presence of this scaffold affects the organization, stability, and activity of the Cdk5/p35 signaling complex.
Results
Oxidative stress in neuronal precursor cells leads to proteasome-mediated degradation of nestin and induction of apoptosis
When neuronal progenitor ST15A cells were treated with hydrogen peroxide (H2O2) (100 μM) to induce oxidative stress, the cells rapidly downregulated nestin with almost complete nestin depletion at 5–6 h of treatment (Figure 1A). Following nestin depletion, apoptotic execution was triggered in the cells, as reflected by the cleavage of vimentin and poly[ADP-ribose] polymerase (PARP), both of which are good markers for caspase-3 activity (Figure 1A). Apoptosis, as the mode of cell death induced by H2O2, was further verified by chromatin condensation as visualized by DAPI staining (Figure 1A) and flow cytometry analysis of propidium iodide-labeled cells (Supplementary Figure 1). Nestin downregulation preceded the activation of effector caspases, as at the time of nestin downregulation, only very weak cleavage of PARP or vimentin could be observed, and only a few apoptotic cells (<10%) were present (Figure 1A). Downregulation of the coexpressed IF-proteins, nestin, and vimentin, proceeded with different kinetics. While no intact nestin could be detected in the completely detached (apoptotic) cells, both intact and caspase-cleaved vimentin products were detected (Figure 1B). Also the mechanism of downregulation was different. Conversely to vimentin cleavage, which occurred as a caspase-dependent process as previously described (Byun et al, 2001), the observed downregulation of nestin was not due to caspase activity. Nestin downregulation could not be inhibited by the general caspase inhibitor Z-VAD (Figure 1C) and analysis of the nestin sequence revealed that nestin, in contrast to vimentin, lacks consensus sites for caspase cleavage. Instead, nestin depletion was efficiently inhibited by the proteasome inhibitor MG-132 (Figure 1C), indicating that the degradation of nestin is a proteasome-dependent process.
Figure 1.
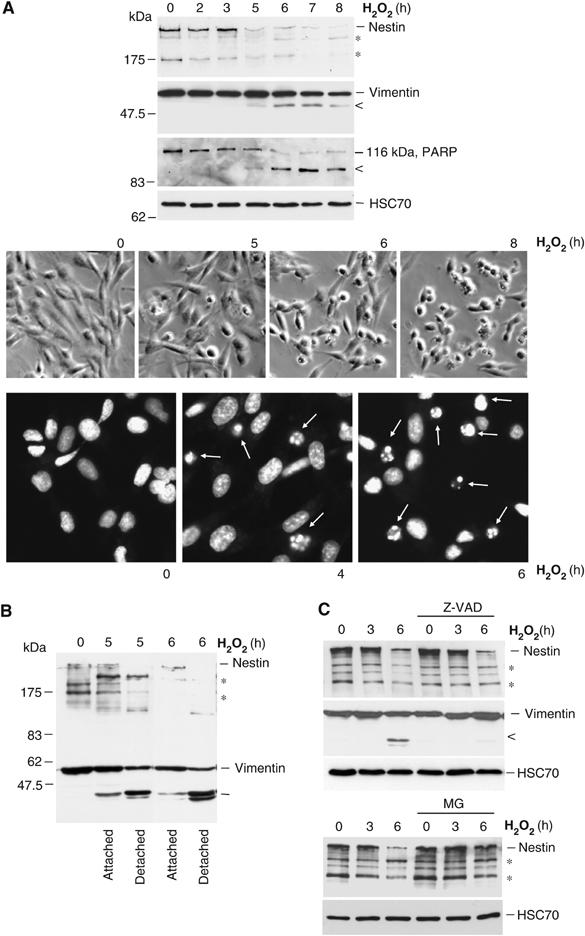
Downregulation of nestin during oxidative stress-induced cell death. ST15A cells were treated with H2O2 for the indicated time periods. (A) WCEs were resolved by SDS–PAGE and analyzed by Western blotting with antibodies for nestin, vimentin, PARP antibodies, and HSC70 (loading control). Lower molecular weight bands on the nestin immunoblot (*) correspond to normal degradation products of nestin. The lower molecular weight bands of vimentin (<) and PARP (<) correspond to caspase cleavage products of the respective protein. Cells treated with H2O2 for the indicated time periods were viewed under phase contrast microscopy to visualize cells with apoptotic morphology (upper panel). Chromatin condensation of apoptotic cells were visualized by DAPI-labeling and analyzed with immunofluorescence microscopy (lower panel). (B) Cell lysates of apoptotic (detached) and surviving (attached) cells were resolved by SDS–PAGE and analyzed by Western blotting with nestin and vimentin antibodies. (C) The effect of caspase inhibition by Z-VAD and proteasome inhibition by MG-132 on nestin and vimentin during oxidative stress was analyzed by Western blotting.
Downregulation of nestin sensitizes neuronal progenitor cells to oxidant-induced cell death
As our data indicated that nestin downregulation could be a prerequisite for execution of oxidant-induced cell death, we employed RNA interference to analyze the effect of nestin depletion prior to subjecting the cells to oxidative stress. Downregulation of nestin was confirmed by Western blotting, (Figure 2A). A scrambled vector was used as a control throughout the experiments. Downregulation of nestin clearly sensitized the cells to oxidant-induced cell death. Already at 4 h of exposure to H2O2, there was a clear increase in the number of apoptotic cells among nestin-depleted cells (35–45% apoptotic cells), as compared to untransfected cells and cells transfected with the scrambled vector (<10% apoptotic cells; Figure 2B). The effect was even more obvious at 6 h of H2O2 treatment (>50% apoptosis among nestin-depleted cells; Figure 2B). These results corresponded well to the results obtained with a cell viability assay, using MTT (3-(4,5-dimetyltiazol-2-yl)-2,5-difenyltetrazoliumbromid). The latter assay showed that at 4 h of H2O2 exposure, cell survival in nestin-depleted cells was 60%, as compared to more than 90% in cells transfected with the scrambled vector, and at 6 h of H2O2 treatment, cell survival of nestin-depleted cells was 40% as compared to 65% in control cells (Figure 2C). The nestin depletion-mediated sensitization to apoptosis was further verified by measuring cleavage of PARP (Figure 2D).
Figure 2.
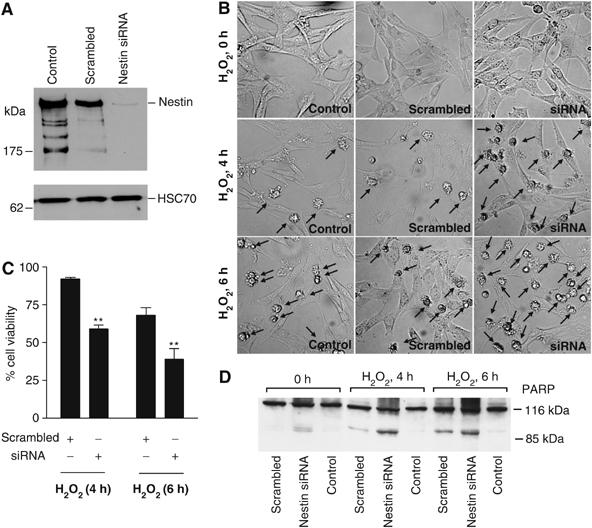
Downregulation of nestin sensitizes ST15A cells to oxidant-induced cell death. (A) Downregulation of nestin using transfection with an siRNA plasmid for nestin. A scrambled vector was used as a control. ST15A cells were transiently transfected and WCEs were prepared 48 h post-transfection to analyze nestin levels by Western blotting. HSC70 was used as loading control. (B) Transiently transfected cells were treated with H2O2 for the indicated time periods and viewed by phase contrast microscopy to analyze for the presence of apoptotic cells. Arrows denote cells with apoptotic morphology. (C) The sensitivity of nestin-depleted cells to oxidant induced cytotoxicity was analyzed by MTT assay (**P<0.01). The survival of cells is presented as a percentage of survival related to untreated cells. (D) Apoptosis was analyzed by Western blot analysis of PARP, with caspase-induced cleavage product of PARP (85 kDa) as an apoptosis indicator.
The presence of nestin protects neuronal progenitor cells from cell death mediated by oxidative stress
In order to further investigate a possible protective role for nestin, we transfected ST15A cells with GFP-tagged wild-type nestin (nest-1893) and subjected the cells to H2O2-treatment. Cell death was specifically determined in cells positive for nest-1893. Cell death among cells not expressing GFP-tagged nestin in the same cell population was used as reference. Cell death among a mock and GFP transfected cell population was used as an additional control (Supplementary Figure 2). Apoptosis was determined by chromatin condensation visualized by DAPI staining. Expression of nestin had a protective effect with <2% cell death in cells expressing nest-1893 as compared to 47% in cells not expressing nest-1893 (Figure 3). Apoptosis in mock-transfected cells was 49% corresponding to the amount of cell death in cells not expressing GFP-tagged nestin among the transfected population (Supplementary Figure 2).
Figure 3.
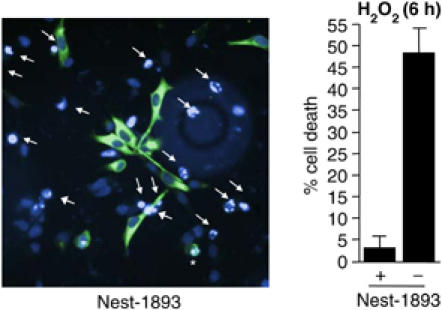
Expression of nestin protects ST15A cells from oxidant-induced cell death. ST15A cells were transfected with GFP-tagged full-length nestin, nest-1893. Apoptosis of nest-1893 expressing cells as compared to cells not expressing nest-1893 was determined by chromatin condensation visualized by DAPI-labeling and analyzed with immunofluorescence microscopy. Arrows denote cells with apoptotic nuclear morphology not expressing the nestin construct and asterisks denote apoptotic cells expressing nest-1893. The graph represents the mean value of four different fields in three different experiments.
Nestin downregulation is associated with increased Cdk5 activity
IFs provide a framework for a vast number of signaling molecules, and have been shown to regulate essential cellular processes through the interactions with critical signaling components. As recent reports indicate that Cdk5 is activated upon oxidative stress and has an apoptosis-promoting role in neurotoxicity (Gong et al, 2003; Strocchi et al, 2003; Shea et al, 2004; Zambrano et al, 2004) and we have previously shown that nestin can serve as a scaffolding molecule for Cdk5 (Sahlgren et al, 2003), we wanted to determine whether the nestin-mediated modulation of oxidant-induced cell death could be a consequence of nestin–Cdk5 interactions. Cdk5 activity increased during oxidative stress as determined by increasing levels of p35, the protein activator of Cdk5, and by kinase assay. Cdk5 activity showed an inverse relationship to the nestin levels, as both the expression of p35 and the activity of Cdk5 started to increase at 5 h of H2O2 exposure, with a marked elevation at 6 h (Figure 4A), when nestin was completely depleted (Figure 1A). As nestin has previously been shown to be a substrate for Cdk5 (Sahlgren et al, 2003), we tested whether nestin degradation could be regulated by Cdk5. Inhibition of Cdk5 activity by roscovitine or transfection with dnCdk5 reduced nestin degradation, indicating that Cdk5 is involved in regulation of nestin turnover (Supplementary Figure 3).
Figure 4.
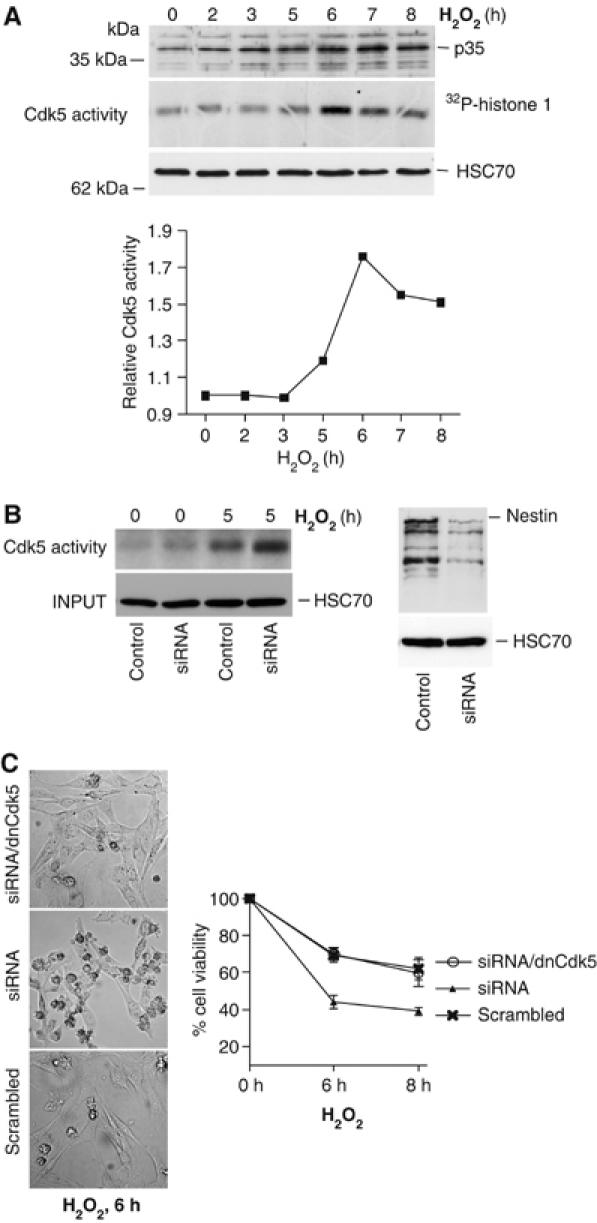
Oxidative stress leads to activation of Cdk5 subsequent to nestin downregulation. (A) Cdk5 activity during H2O2 treatment was analyzed by the presence of p35, the protein activator of Cdk5, and by an in vitro Cdk5 kinase assay using histone 1 as a substrate (upper panel). Cdk5 activity during the H2O2 treatment was quantified by phosphoimager analysis (lower panel). (B) Cdk5 kinase assay of control and nestin depleted cells at 0 and 5 h of H2O2 treatment (left panel). Western blotting of WCEs shows nestin levels in scrambled (control) and siRNA transfected cells (right panel). (C) Nestin depleted cells and nestin depleted cells expressing dnCdk5, treated with H2O2 for 6 h, were viewed under phase contrast microscopy to check for cells with apoptotic morphology. The sensitivity of nestin-depleted cells as compared to nestin depleted cells overexpressing dnCdk5 to oxidant induced cytotoxicity was analyzed by MTT assay. The graph represents the mean values of five experiments.
Enhanced sensitivity of nestin-depleted cells is associated with increased activity of Cdk5
While nestin-depleted cells displayed a marked sensitization to cell death mediated by oxidative stress, we aimed at determining whether the increased sensitivity could be related to changes in Cdk5 activity. Depletion of nestin did not affect Cdk5 activity in untreated cells, but the nestin-depleted cells showed a clearly elevated activity at 5 h of H2O2 treatment (Figure 4B), as compared to cells with normal nestin expression. For quantification of the effect of nestin depletion on Cdk5 activity, see Supplementary Figure 4. Furthermore, the increased sensitivity of nestin-depleted cells to oxidative stress was reversed by expression of dnCdk5 (Figure 4C), placing Cdk5 downstream of nestin in the sequence of signaling events leading to oxidant-induced cell death.
Analysis of Cdk5-binding domains on nestin
In order to identify the Cdk5-binding domains on nestin, we generated a series of deletion mutants of nestin. The series of mutants comprised the α-helical IF rod domain (nest-314), two constructs with increasing length of the C-terminal tail (nest-640 and nest-1177, respectively), and a construct corresponding to the last 332 amino acids (nest-T332) of the C-terminal domain in addition to the previously generated full-length nestin (nest-1893) (see Figure 5A). These constructs were transiently transfected into ST15A cells and their subcellular organization, assembly properties, and protein stability were analyzed. Most of the endogenous nestin occurs as filaments with a small pool of soluble protein (Figure 5B, left panel). Full-length nestin, nest-1893, and nest-314, were both incorporated into filaments (detergent-insoluble pellet, P) with little protein in the soluble fraction (detergent-soluble supernatant, S) (Figure 5B, right panel). Nest-T332 was not able to form filaments, and remained soluble (Figure 5B, right panel), which is not surprising, as it does not contain the IF-specific alpha-helical domain, which is required for filament formation. The truncated mutants showed an altered polymer equilibrium, as nest-640 and 1177 were distributed between both soluble and insoluble pool, resulting in a clear increase of soluble nestin present in the cell (Figure 5B, right panel). These results were further corroborated by immunofluorescence microscopy (data not shown).
Figure 5.
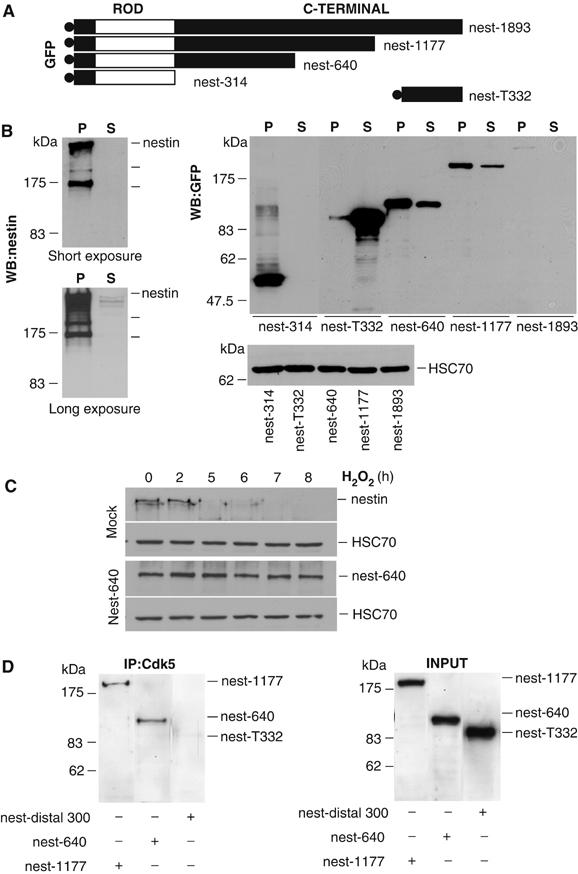
Characterization of nestin mutants. (A) The following GFP-tagged nestin constructs were used; full-length nestin (nest-1893), nestin with C-terminal tail deletions (nest-1177, nest-640), nestin lacking the C-terminal tail (nest-314), and the distal 332 amino acids of the C-terminal tail domain (nest-T332). The construct name denotes the amino acid sequences of the mutants. (B) Solubility properties of nestin mutants when expressed in ST15A cells were determined by Western blotting of Triton-X insoluble (P) and soluble (S) fractions of ST15A cells with a nestin-specific antibody detecting endogenous nestin (left panels show short (upper) and long (lower) exposures; typical nestin fragments are indicated by lines below the main protein band) and a GFP-specific antibody detecting the GFP-tagged deletion mutants, nest-1893, nest-1177, nest-640, nest-314, and nest-T332 (right panel). Lower panel to the right shows HSC70 levels to control for protein concentration prior to fractionation. (C) WCEs of mock and nest-640 transfected cells treated with H2O2 were resolved by SDS–PAGE and analyzed by Western blotting with antibodies for nestin, GFP to detect nest-640 and HSC70 (loading control). Nest-640 shows a clear stabilization as compared to endogenous nestin during oxidative stress. (D) Cdk5 was immunoprecipitated from ST15A cells expressing the GFP-tagged nestin mutants. The affinity of the different nestin mutants to Cdk5 was analyzed by Western blotting of the immunoprecipitates using a GFP antibody. The presence of the nestin mutants in the cell lysate prior to immunoprecipitation is shown in the panel at the right (INPUT).
The truncations in the nest-640 and nest-1177 had an apparent stabilizing effect on these proteins, with approximate half-lives of >4 h, as compared to the endogenous nestin, which has an exceptionally short half-life <1 h, as measured by cycloheximide chase assays (Tao He, Cecilia Sahlgren, Adolfo Rivero-Muller, Mikko Nieminen, Hanna-Mari Pallari and John E Eriksson, unpublished results). The stabilization was reflected as very high expression levels of the truncated proteins, as compared to full-length nestin (Figure 5B, right panel). ST15A cells transfected with nest-640 were subjected to H2O2 treatment. The mutant showed a marked stability during oxidative stress as compared to endogenous nestin, and the protein levels remain unchanged up to 8 h of H2O2 treatment (Figure 5C). Similar results were obtained with nest-1177 (data not shown).
The Cdk5-binding properties of the truncated proteins were analyzed by co-immunoprecipitation assays. Nest-640 and nest-1177 co-immunoprecipitated with Cdk5 (Figure 5D). In contrast, the nestin peptide nest-T332 (Figure 5D) did not interact with Cdk5. Cdk5 did not interact with the nestin fragment nest-314, as shown by the inability of the insoluble nest-314 fragment to co-IP with Cdk5 or to sequester Cdk5 to the filament pellet fraction (for results and experimental details see Supplementary Figure 5). While nest-640 bound to Cdk5 equally well as nest-1177, these results restricted the Cdk5 binding to a region (314–640aa) on the C-terminal, following the alpha-helical domain of nestin.
Expression of nestin protects neuronal progenitor cells from oxidative stress-induced apoptosis through negative regulation of Cdk5 death promoting activity
As nest-640 and nest-1177 interacted with Cdk5 and showed increased stability in oxidative stress, expression of these mutants seemed to be a promising basis for further elucidating the role of the Cdk5-nestin interplay in modulating the sensitivity to oxidative stress induced cell death. To specifically address the protective effect of the truncated mutants, ST15A cells were transfected with nest-640 or nest-1177 and apoptosis was determined (Figure 6). The truncated mutants showed similar inhibition of apoptosis as the full-length protein (Figure 3), as approximately 7% of the cells expressing nest-640 as compared to 42% of cells not expressing nest-640 showed condensed chromatin at 6 h of treatment (Figure 6). The result was similar when nest-1177 expressing cells were compared to non-expressing cells, 12 versus 37% (Figure 6).
Figure 6.
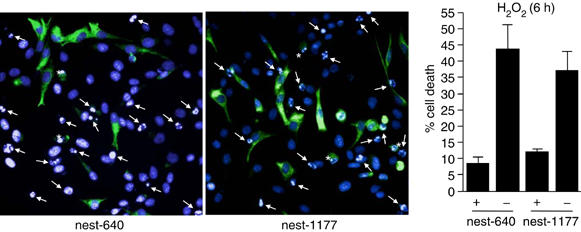
Expression of nestin deletion mutants protects ST15A cells from oxidant-induced cell death. Apoptosis of ST15A cells expressing the GFP-tagged nest-1177, nest-640, as compared to control cells, was determined by chromatin condensation visualized by DAPI-labeling and analyzed with immunofluorescence microscopy. Arrows denote cells with apoptotic nuclear morphology not expressing the nestin constructs and asterisks denote apoptotic cells expressing the nestin peptides. The graph represents the mean value of four different fields in three different experiments.
To further address the regulatory roles of the Cdk5-nestin complex in oxidant-mediated apoptosis, we analyzed the overall effect of combined or separate transfections of nest-640, nest-1177, Cdk5, and dnCdk5. The transfected cells were subjected to H2O2 treatment; overall cell viability was analyzed by the MTT assay and activation of the caspase machinery by cleavage of PARP. In line with the cell viability assay in Figure 4C, dnCdk5 protected the cells from oxidant-induced cytotoxicity. Survival in dnCdk5 expressing cells was approximately 75%, as compared to 65% in mock-transfected cells at 6 h (Figure 7A). Expression of the Cdk5-interacting nestin peptides 640 and 1177 also had a protective effect and increased survival to 79 and 78%, respectively, at 6 h as compared to 65% in mock transfected cells (Figure 7A). The effect of nestin overexpression could be reversed by co-transfection with Cdk5, showing that the effect is due to inhibition of the proapoptotic Cdk5 activity in cells expressing the nestin peptides (Figure 7A). These results were further supported by PARP cleavage assay, in which the degree of PARP cleavage corresponded to the observed reductions in cell viability (Figure 7B).
Figure 7.
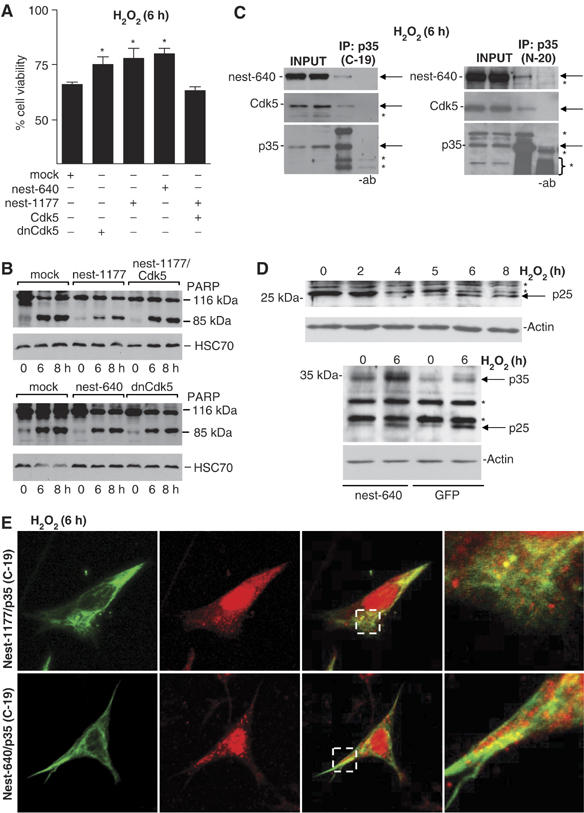
The relationship between nestin, Cdk5, p35, and cell viability. (A) Cell viability of ST15A cells transfected with mock, dnCdk5, nest-1177, nest-640, or co-transfected with nest-1177 and Cdk5, was analyzed by MTT assay and the results are presented as relative cell viability as related to the control (untreated population at 0 h) of each transfection. The graph represents the mean values of six experiments (*P<0.05). (B) Apoptosis in ST15A cells transfected with mock, dnCdk5, nest-1177, nest-640 or co-transfected with nest-1177, and Cdk5 was analyzed by Western blot analysis of PARP, with caspase-induced cleavage product of PARP (85 kDa) as an apoptosis indicator. (C) Co-immunoprecipitation of Cdk5 and nest-640 with p35 using the p35 antibodies C-19 and N-20 at 6 h of H2O2 treatment (note that the specific p35 band overlaps to some extent with unspecific primary antibody bands in the N-20 immunoblot). Arrows denote exact position of nest-640, Cdk5, and p35, respectively, and asterisks denote unspecific bands. (D) Generation of p25 during oxidative stress. Western blotting of untransfected cells (upper panel) and of control and H2O2-treated GFP and nestin expressing cells (lower panel) using a p35/p25-specific (C-19) antibody. Arrows denote p35 or p25, and asterisks denote unspecific bands (note that the unspecific second band un the upper panel disappears while p25 is generated). (E) Analysis of the colocalization of p35/p25 with nest-640 and nest-1177 at 6 h of H2O2-treatment by confocal microscopy (for further analysis of the nuclear localization of p25, see Supplementary Figure 6B).
The observed protective effect of nestin could be based upon an interaction between p35, Cdk5, and nestin during oxidative stress. This assumption was supported by co-immunoprecipitation analysis (Figure 7C), which showed that Cdk5, p35, and nestin occur in the same complex in stressed cells. We then analyzed whether this interaction could affect the cleavage of p35 to p25, which has been shown to be a central component of neuronal death induced by various neurotoxic insults (O'Hare et al, 2005). Generation of p25 leads to deregulated Cdk5 activity by the enhanced stability of the Cdk5/p25 complex and a switch in the normal localization of Cdk5 activity. We detected the presence of p25 in oxidant-treated cells, with increasing p25 levels after 4–6 h of H2O2-treatment (Figure 7D, upper panel). The generation of p25 at 4–6 h of H2O2-treatment (Figure 7D) corresponded well to the kinetics of nestin depletion (Figure 1A) and Cdk5 activation (Figure 4A). In contrast, the generation of p25 was clearly reduced in cells overexpressing nestin, indicating that nestin increases the stability of p35 and blocks cleavage to p25 (Figure 7D, lower panel).
The neurotoxic action of Cdk5 has been linked to a change in the subcellular distribution of Cdk5 activity, mediated by p25. It has been proposed (O'Hare et al, 2005) that nuclear localization of Cdk5–p35/p25 would be toxic whereas cytoplasmic Cdk5/p35 would have a protective effect. Oxidative stress induced an accumulation of overall p35 immunoreactivity in the nucleus associated with a decrease of cytoplasmic labeling (Supplementary Figure 6A). The effect of nestin on the subcellular organization of p35/p25 during oxidative stress was examined by transfecting ST15A cells with p35 together with nest-640 or nest-1177 and treated with H2O2 for 6 h. Analysis of p35 colocalization with nest-640 and nest-1177 (Figure 7E) indicates that the overexpressed nestin has the ability to sequester p35, as p35 remained colocalized in the cytoplasm together with nestin, while the cytoplasmic labeling was lost in GFP transfected cells (Supplementary Figure 6A). To further examine the effects of nestin on p35 dynamics, a nuclear enrichment protocol was used to determine whether p25 was accumulating in the nuclear fractions of control and H2O2-treated GFP and nestin expressing cells. In cells transfected with GFP, p25 was detected in the nuclear fraction at 6 h of oxidant-treatment. In contrast, in nestin-overexpressing cells, the level of p25 in this fraction was clearly reduced (Supplementary Figure 6B).
Nestin affects the sequestration and turnover of the active Cdk5/p35 complex
Our data indicate that the presence of nestin has an inhibitory effect on the proapoptotic action of Cdk5 during oxidative stress and points to sequestration of the Cdk5/p35 complex as a possible mechanism for the protective effect of nestin. To further elucidate if nestin has the ability to sequester the Cdk5/p35 complex, we utilized the apparent stability and the changed polymer equilibrium of the nestin mutants. The active Cdk5/p35 complex is normally translocated to the membrane due to a myristylation signal on p35 (Patrick et al, 1999). To determine if expression of the partially soluble nestin peptides affects the subcellular distribution of the active Cdk5/p35 complex, p35 was cotransfected with the different nestin constructs into ST15A cells followed by fractionation of detergent extractions. The subcellular localization of Cdk5 activity was determined by the presence of p35 (Figure 8A) in the different fractions (whole-cell extract (WCE) prior to centrifugation, soluble fraction after centrifugation, S, and insoluble pellet after centrifugation, P) and by a kinase activity assay (Figure 8B) of the soluble fraction (S) in comparison to the overall kinase activity following preclearing of the WCE (as outlined in Materials and methods). p35 was normally associated with the detergent insoluble fraction (Figure 8A). In contrast, the distribution of p35 was significantly altered if p35 was coexpressed with the soluble nestin fragment nest-640 (Figure 8A), with the protein shifted from the pellet to the soluble fraction in the presence of nest-640. Cdk5 kinase assays further corroborated that the stabilized (and to a significant extent soluble) nestin mutants had the capacity to alter the distribution of the Cdk5/p35 complex. Control cells showed no or very low Cdk5 activity, whereas p35-transfected cells displayed a marked increase in the overall Cdk5 activity in ST15A cells (Figure 8B). No change in the overall Cdk5 activity in the whole-cell lysate was detected when p35 was co-transfected with either nest-T332 or nest-640 (Figure 8B, right side of the panel). However, when the activity in the soluble fraction was analyzed, in contrast to extracts from mock-transfected cells, in which Cdk5 activity was associated with the insoluble cytoskeletal fraction (as evident by the absence of activity in the soluble fraction), the Cdk5 activity in cells overexpressing nest-640 was primarily shifted to the soluble fraction (Figure 8B, left side of the panel). The nest-1177 nestin fragment had a similar effect on the distribution of p35 and Cdk5 activity (data not shown), whereas overexpression of the soluble nestin fragment nest-T332, which does not bind to Cdk5 (Figure 4C), did not affect the distribution of Cdk5 activity (Figure 8B). Coimmunoprecipitation experiments of cells show that p35 interacts with nest-640 (Figure 8C) and nest-1177 (data not shown); further supporting the indication that nestin has the ability to sequester the active Cdk5/p35 complex.
Figure 8.
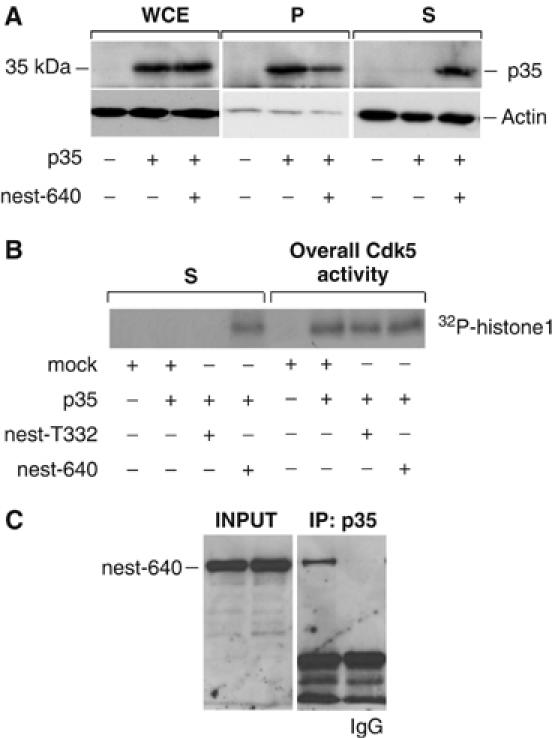
Nestin affects the distribution of the active Cdk5/p35 complex. (A) The effect of nest-640 overexpression on the subcellular distribution of the Cdk5/p35 complex was analyzed by Western blotting with a p35 antibody of WCE and detergent soluble (S) and insoluble (P) fractions of ST15A cells. Western blotting of the fractions with an actin antibody was performed to control for equal loading. (B) Total kinase activity (measured in WCE after preclearing with sepharose beads) and kinase activity assays of the soluble fractions (S) of ST15A cells expressing p35 alone and cells coexpressing p35 and nest-640 or nest-T332. (C) p35 was immunoprecipitated from ST15A cells expressing the GFP-tagged nest-640. The interaction of p35 with nest-640 was analyzed by Western blotting of the immunoprecipitates using a GFP antibody. The presence of the nest-640 in the cell lysate prior to immunoprecipitation is shown in the left panel.
Cdk5/p35 activity is subjected to a negative feedback regulation through the Cdk5-mediated ubiquitylation and subsequent degradation of p35 (Patrick et al, 1998). Based on our observations of the sequestering properties of nestin, we examined the role of nestin fragments on the turnover of p35. ST15A cells were co-transfected with nest-640 and HA-ubiquitin and treated with or without the proteasome inhibitor MG-132. The analysis demonstrated that p35 was considerably less ubiquitylated in cells overexpressing nest-640, as demonstrated both for the whole-cell lysate and co-immunoprecipitations of HA-ubiquitin and p35 (Figure 9A). In agreement with its effect on ubiquitylation, nest-640 had a considerable stabilizing effect on p35. In cells cotransfected with nest-640, the p35 expression levels were consistently higher than in cells transfected with p35 alone (Figure 9B, 0 h). Furthermore, in cycloheximide chase assays, nest-640 coexpression resulted in significantly increased half-life of p35 (Figure 9B).
Figure 9.
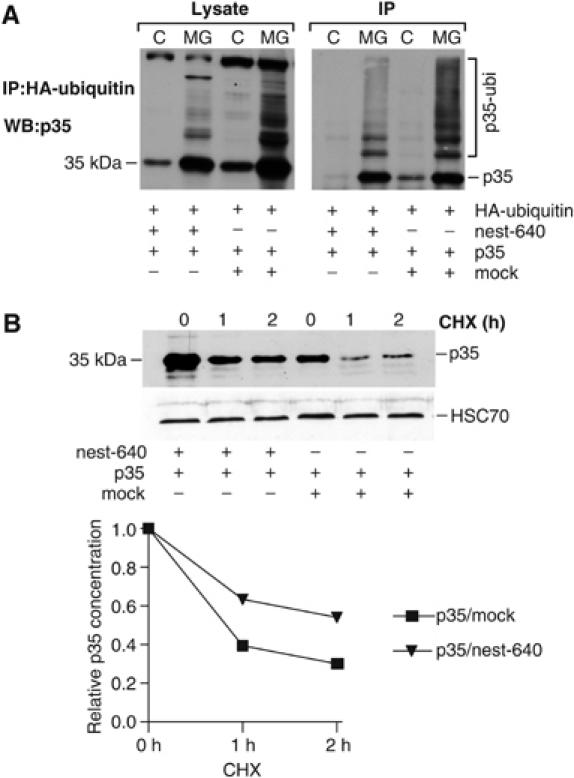
Overexpression of a soluble nestin mutant affects the turnover and ubiquitylation of p35. (A) HA-tagged ubiquitin, p35, and nest-640 were transfected into ST15A cells as indicated and cells were treated with and without MG-132. Cell lysates (Lysate) were run directly on SDS–PAGE or immunoprecipitated with anti-HA antibody (IP) and immunoblotted with a p35 antibody. (B) ST15A cells were transfected with p35 with or without nest-640 and the half-life of p35 was analyzed by a CHX chase assay. Cell lysates were separated on SDS–PAGE and blotted with anti-p35 (upper panel). The graph shows quantification of the relative half-life of p35 from samples transfected with p35 alone or together with nest-640 (lower panel).
Discussion
A nestin scaffold links Cdk5 signaling to oxidative stress-induced cell death
Recent evidence point to a role for nestin in the distribution and organization of critical cellular factors regulating cell proliferation, survival, and differentiation (Shen et al, 2002; Bieberich et al, 2003, 2004; Chou et al, 2003). Our previous observations on the association between nestin and Cdk5 and recent data on the involvement of Cdk5 in oxidative stress-induced cell death prompted us to explore whether this interaction could constitute a mechanism for regulating Cdk5, and thereby influence the outcome of oxidant-induced stress. In our study, downregulation of nestin was a prerequisite for activation of Cdk5 and induction of apoptosis during oxidative stress. Aberrant expression of nestin altered normal sequestration of Cdk5 activity, thereby preventing Cdk5 from inducing apoptosis. Depletion of nestin sensitized the neuronal precursor cells to oxidative stress by unleashing the control of Cdk5 activity from nestin-mediated regulation. Cell viability is not affected in nestin-depleted cells in the absence of oxidative stress, indicating that depletion of nestin is not sufficient to induce death. Thus, the low level of Cdk5 activity normally present in the cell does not seem to be enough to induce the apoptotic cascade, although the signaling complex would be released by the absence of nestin, but requires an additional stimulus provided by oxidative stress. Increased expression of p35, generation of p25, and relocalization of Cdk5 activity are events likely to be critical for the death-promoting activity of Cdk5. This is supported by our data showing cleavage of p35 to generate p25 and nuclear accumulation of p25 during H2O2-tretament. The presence of nestin retains the Cdk5/p35 complex in cytoplasm, increases the stability of p35, and reduces generation of p25. Hence, nuclear localization of Cdk5, mediated by the generation of p25 could be the basis of cytotoxic action of Cdk5 and the presence of a nestin scaffold prevents this action by increasing the stability of p35 and sequestering Cdk5 activity in the cytoplasm. Recently, O'Hare and colleagues (2005) implicated that nuclear localization of Cdk5 activity would be toxic whereas cytoplasmic Cdk5 activity would have a protective effect. We show that scaffolding of the p35/Cdk5 complex by nestin prevents the generation of p25 and shifts the balance between a protective p35/Cdk5 complex and a toxic p25/Cdk5 complex. Hence, our results demonstrate nestin downregulation as being one important factor in the cascade of events leading to aberrant Cdk5 activation and cell death.
Nestin as a signaling scaffold for Cdk5
IFs provide a scaffold for different signaling molecules (Toivola et al, 2005) and alterations in the normal subunit stochiometry and composition of IFs affect the signaling pathways that these molecules are involved in. The role of nestin as a signaling scaffold for Cdk5 and the need for proper regulation of the interaction between nestin and Cdk5 are clearly manifested by the effects of stabilized nestin fragments on p35 distribution and turnover. p35 is a short-lived protein with a rapid turnover, and phosphorylation of p35 by the activated Cdk5 plays an autoregulatory role in the ubiquitin-mediated proteolysis of p35 (Patrick et al, 1998). Although the overall Cdk5 activity was not affected in cells overexpressing the partially soluble truncated nestin mutants, the intracellular localization of the Cdk5/p35 complex was changed. Moreover, the stabilized nestin had also a marked effect on the p35 ubiquitylation and turnover, demonstrating that nestin expression and distribution together with Cdk5 activity will determine the sequestration and turnover of p35. Recent data have shown that the phosphorylation state and thereby the proteolytic processing and turnover of p35 are altered during postnatal brain development, although Cdk5 displays the same activity (Saito et al, 2003). These observations indicate that, apart from Cdk5, there are other factors with the capacity to regulate p35 turnover.
Our study shows that, in contrast to vimentin, nestin downregulation is not caspase-dependent but a consequence of proteasome-mediated degradation. We have preliminary data to indicate that the degradation of nestin is an actively regulated process, involving both ubiquitylation and phosphorylation (Tao He, Cecilia Sahlgren, Adolfo Rivero-Muller, Mikko Nieminen, Hanna-Mari Pallari and John E Eriksson, unpublished results), implying that both the regulator (p35) and the scaffold (nestin) of the signaling complex would be controlled by orchestrated phosphorylation and ubiquitylation. Inhibition of Cdk5 activity during oxidative stress protected nestin from degradation, pointing to a possible involvement of Cdk5 in regulation of nestin turnover. Whether this occurs directly by Cdk5-dependent regulation of nestin turnover, for example, by targeting nestin for ubiquitylation and proteasomal degradation, or whether the regulation is indirect is not known. The possible direct involvement of Cdk5 in regulating nestin degradation is consistent with the observation that the nestin mutants lacking the Cdk5 specific phosphorylation site thr-1495 are significantly more stable than the full-length nestin. Future studies aim at elucidating the degradation mechanisms, and the involvement of Cdk5 in regulating nestin turnover both in the context of oxidative stress and also during myogenic differentiation where a similar interplay between nestin and Cdk5 occurs (Sahlgren et al, 2003).
Based on our previous results (Sahlgren et al, 2003) and the results in the present study, the interaction between nestin and Cdk5 involves three different regulatory modes: (i) spatial sequestration of Cdk5 to nestin-confined areas, (ii) dynamic turnover of the nestin-Cdk5/p35 complex, involving phosphorylation of nestin by the activated Cdk5/p35 complex with subsequent release of the complex from nestin and degradation of p35, and (iii) degradation or downregulation of nestin (possibly involving Cdk5), which comprises a negative regulatory loop to abrogate the inhibitory/targeting effects of the nestin scaffold.
Context-dependent roles for the nestin–Cdk5 interaction
The present study shows that the formation of a nestin/Cdk5 complex is important in targeting and regulation of the Cdk5/p35 signaling complex. While the Cdk5/nestin equilibrium was a survival-determinant in the cell model used in the present study, the Cdk5–nestin interplay could be employed also for other purposes. An inverse relationship between nestin levels and Cdk5 activity, analogous to our study, has also been observed during neuronal (Lendahl et al, 1990; Tsai et al, 1993) and muscle (Sejersen and Lendahl, 1993; Lazaro et al, 1997; Fu et al, 2002) differentiation, when nestin is downregulated and Cdk5 is activated upon terminal differentiation. A close spatiotemporal association between nestin and Cdk5 is also observed in the adult at the NMJs and the MTJs (Vaittinen et al, 1999). Cdk5 was recently shown to be involved in neuromuscular synapse formation providing the negative signal dispersing Acetylcholine receptor (AchR) clusters that are not stablized by agrin produced by the inervating nerve terminal (Lin et al, 2005). As we have previously shown that nestin is associated with Cdk5 in NMJ (Sahlgren et al, 2003) and that nestin is redistributed together with AchRs after denervation of muscle tissue (Vaittinen et al, 1999), it seems likely that nestin could be involved in the Cdk5-mediated equilibrium that will determine stabilization versus dispersion of AchR clusters. It is tempting to speculate that nestin may function as a scaffolding protein for AchRs, regulating the stability of AchR clusters, through its ability to target Cdk5 activity. On the other hand, Cdk5 could regulate the assembly of the scaffold through specific phosphorylation of nestin (Sahlgren et al, 2003). Furthermore, coexpression of Cdk5 and nestin occurs at the growth cones of migrating neurons (Yan et al, 2001). The association between nestin and Cdk5 at these distinct localizations is a likely mechanism for targeting Cdk5 and to constrain both regulation and activity to limited areas.
Taken together, our data imply that nestin is a survival determinant during oxidative stress whose action is based upon a novel mode of Cdk5/p35 regulation, affecting the targeting, activity, and stability of this signaling complex. The concept of nestin as a potent survival determinant has important ramifications into understanding the regulation of cell survival during neuronal and muscle development and regeneration.
Materials and methods
Cell cultures, treatments, and transfection
Immortalized CNS ST15A precursor cells were cultured as previously described (Sahlgren et al, 2003). For transfections the following constructs were used: HA-tagged ubiquitin (Treier et al, 1994; kindly provided by Dirk Bohmann, EMBL, Heidelberg, Germany), GFP-tagged nestin constructs including the first 314 (nest-314), 640 (nest-640), 1177 (nest-1177), and 1893 (nest-1893, corresponding to the full-length nestin) amino acids of the rat glioma nestin sequence. In addition, the last 332 amino-acid residues of the C-terminal (nest-T332) of the tail domain of hamster nestin sequence (all cDNAs were cloned into pEGFP-C1 vector); wild-type Cdk5, p35, and dominant-negative Cdk5 (all cDNAs cloned into pcDNA3.1His vector expressing a His6 tag and aXpress epitope; kindly provided by Dr. Harish Pant, National Institutes of Health). Cells were transfected by electroporation as previously described (Sahlgren et al, 2003). Cycloheximide CHX (30 μg/ml) (Sigma) was used to inhibit protein synthesis and 20 μM MG-132 (Peptide institute, Inc.) to inhibit proteosome function and 20 μM Z-VAD to inhibit caspases. Oxidative stress was induced by 100 μM of H2O2. ‘Mitotic shake-off' (which is typically used to collect mitotic rounded-up cells) was used to collect apoptotic and preapoptotic cells. The cell culture flask was tapped 20 times against a pile of tissue paper, the medium containing the detached cells was collected, cell culture flasks were rinsed with twice with PBS to collect the remaining detached cells, and the detached cells were pelleted by centrifugation.
Plasmid constructs for RNA interference transfections
The pSUPER vector (Oligoengine) was utilized for nestin knockdown experiments using the specific nestin sequence, CTCTCCCTGACTCTACTCC (Brummelkamp et al, 2002). The nestin siRNA vector was generated according to the manufacturer's protocol. The scrambled sequence that was used as a control does not correspond to any known gene in the databases, and has the following sequence: GCGCGCTTTGTAGGATTCG.
Separation of IF fractions following extraction of cells with Triton X-100
Transfected ST15A cells were lysed in Triton X-100 lysis buffer containing 25 mM HEPES, pH 7.6, 100 mM NaCl, 5 mM MgCl2, 5 mM EGTA, 0.5% Triton X-100, and complete protease inhibitor cocktail (Roche) for 30 min on ice. Cell lysates were separated into insoluble and soluble pools by centrifugation at 15 000 g at 4°C for 40 min. The supernatant and pellet fractions were resuspended in Laemmli sample buffer and analyzed by SDS–PAGE.
Immunofluorescence and Western blotting
Immunofluorescence with ST15A cells was performed as previously described (Sahlgren et al, 2001, 2003). Cells were labeled with antibodies against p35/p25 (C-19) and p35 (N-20, Santa Cruz Biotechnologies). Images were collected using a Zeiss LSM 510 META laser scanning confocal microscope (Zeiss, Jena, Germany) configured on an inverted Axiovert 200 M stand (Zeiss) equipped with a Plan-Apochromat × 63/1.4 oil DIC objective. Images are maxprojections generated from 8 to 12 (0.8–1 μm thick) z-sections using Zeiss LSM 3.0 software. For phase contrast cells were analyzed in a Zeiss Axiovert 200 widefield microscope. Western blotting were performed according to standard protocols described previously (Sahlgren et al, 2001, 2003).
Kinase assay
Cells were lysed in IP buffer (PBS pH 7.4, 1% NP40, 0.5% sodium deoxycholate, 1 mM EDTA, 1 mM EGTA, 20 mM NaF, 1 mM PMSF, complete protease inhibitor cocktail, and 0.5 mM DTT) for 30 min on ice. To obtain a crude WCE, samples were homogenized eight times with 26 G needle, and centrifuged at 14 000 r.p.m. for 15 min at 4°C. The protein concentration was determined using the Bradford assay, and the samples were normalized accordingly. For kinase assays of fractionated lysates, cells were lysed in fractionation buffer with 0.5% Triton X-100 for 30 min on ice, centrifuged 15000 g at 4°C for 40 min, and the supernatant was collected as the soluble fraction. Immunoprecipitation of Cdk5 and kinase reaction was performed as previously described (Sahlgren et al, 2003).
Co-immunoprecipitation
For immunoprecipitation of HA-ubiquitin, cells were treated with or without 20 μM MG-132 and lysed in lysis buffer (25 mM HEPES (pH 7.4), 150 mM NaCl, 5 mM EDTA, 0.5% Triton X-100, 20 mM β-glycerophosphate, 20 mM para-nitro-phenyl phosphate, 1 mM PMSF, 1 mM DTT, protease inhibitor cocktail, 5 μM MG-132, and 20 mM N-ethylmaleimide). The immunocomplex was retained by protein G Sepharose beads (Amersham Biosciences) precoupled with anti-HA antibody (Santa Cruz Biotechnology). For immunoprecipitation of Cdk5, ST15A cells transfected with GFP-tagged nestin peptides were lysed in RIPA buffer (20 mM HEPES, pH 7.4, 140 mM NaCl, 10 mM pyrophosphate, 5 mM EDTA, 0.4% NP-40, 100 mM PMSF, and protease inhibitor cocktail) for 30 min on ice. The lysates were lightly sonicated and centrifuged 10 000 r.p.m. for 10 min. The supernatant was precleared with protein A sepharose beads, followed by Cdk5 immunoprecipitation with a polyclonal Cdk5 antibody (Santa Cruz). and captured by protein A sepharose. For immunoprecipitation of p35, ST15A cells were transfected with nest-640, p35 and Cdk5 before H2O2 treatment. At indicated time points, medium containing dead cells was discarded and living cells still attached to the plate were lysed in lysis buffer containing 50 mM Tris pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.05% SDS, 5 mM EDTA, 5 mM EGTA, and protease inhibitor cocktail. Lysates were centrifugated for 15 000 g 10 min at 4°C. The supernatant was precleared with protein A sepharose beads following immunoprecipitation with p35 C-19 and N-20 antibodies (Santa Cruz).
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Acknowledgments
We thank Helena Saarento and Adolfo Rivero-Müller for excellent technical assistance. This work was supported by the Academy of Finland, the Research Institute of Åbo Akademi University, the Foundation of the Åbo Akademi University, and the Foundation for Swedish Culture in Finland.
References
- Ahlijanian MK, Barrezueta NX, Williams RD, Jakowski A, Kowsz KP, McCarthy S, Coskran T, Carlo A, Seymour PA, Burkhardt JE, Nelson RB, McNeish JD (2000) Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci USA 97: 2910–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja HS, Zhu Y, Zakeri Z (1997) Association of cyclin-dependent kinase 5 and its activator p35 with apoptotic cell death. Dev Genet 21: 258–267 [DOI] [PubMed] [Google Scholar]
- Bajaj NP, al-Sarraj ST, Leigh PN, Anderson V, Miller CC (1999) Cyclin dependent kinase-5 (CDK-5) phosphorylates neurofilament heavy (NF-H) chain to generate epitopes for antibodies that label neurofilament accumulations in amyotrophic lateral sclerosis (ALS) and is present in affected motor neurones in ALS. Prog Neuropsychopharmacol Biol Psychiatry 23: 833–850 [DOI] [PubMed] [Google Scholar]
- Bieberich E, MacKinnon S, Silva J, Noggle S, Condie BG (2003) Regulation of cell death in mitotic neural progenitor cells by asymmetric distribution of prostate apoptosis response 4 (PAR-4) and simultaneous elevation of endogenous ceramide. J Cell Biol 162: 469–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberich E, Silva J, Wang G, Krishnamurthy K, Condie BG (2004) Selective apoptosis of pluripotent mouse and human stem cells by novel ceramide analogues prevents teratoma formation and enriches for neural precursors in ES cell-derived neural transplants. J Cell Biol 167: 723–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion JP, Couck AM (1995) Cortical and brainstem-type Lewy bodies are immunoreactive for the cyclin-dependent kinase 5. Am J Pathol 147: 1465–1476 [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553 [DOI] [PubMed] [Google Scholar]
- Byun Y, Chen F, Chang R, Trivedi M, Green KJ, Cryns VL (2001) Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death Differ 8: 443–450 [DOI] [PubMed] [Google Scholar]
- Cheung ZH, Ip NY (2004) Cdk5: mediator of neuronal death and survival. Neurosci Lett 361: 47–51 [DOI] [PubMed] [Google Scholar]
- Chou YH, Khuon S, Herrmann H, Goldman RD (2003) Nestin promotes the phosphorylation-dependent disassembly of vimentin intermediate filaments during mitosis. Mol Biol Cell 14: 1468–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz JC, Tsai LH (2004) A Jekyll and Hyde kinase: roles for Cdk5 in brain development and disease. Curr Opin Neurobiol 14: 390–394 [DOI] [PubMed] [Google Scholar]
- Dahlstrand J, Collins VP, Lendahl U (1992) Expression of the class VI intermediate filament nestin in human central nervous system tumors. Cancer Res 52: 5334–5341 [PubMed] [Google Scholar]
- Frisen J, Johansson CB, Torok C, Risling M, Lendahl U (1995) Rapid, widespread, and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J Cell Biol 131: 453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu AK, Fu WY, Cheung J, Tsim KW, Ip FC, Wang JH, Ip NY (2001) Cdk5 is involved in neuregulin-induced AChR expression at the neuromuscular junction. Nat Neurosci 4: 374–381 [DOI] [PubMed] [Google Scholar]
- Fu WY, Fu AK, Lok KC, Ip FC, Ip NY (2002) Induction of Cdk5 activity in rat skeletal muscle after nerve injury. Neuroreport 13: 243–247 [DOI] [PubMed] [Google Scholar]
- Gao C, Negash S, Wang HS, Ledee D, Guo H, Russell P, Zelenka P (2001) Cdk5 mediates changes in morphology and promotes apoptosis of astrocytoma cells in response to heat shock. J Cell Sci 114: 1145–1153 [DOI] [PubMed] [Google Scholar]
- Gong X, Tang X, Wiedmann M, Wang X, Peng J, Zheng D, Blair LA, Marshall J, Mao Z (2003) Cdk5-mediated inhibition of the protective effects of transcription factor MEF2 in neurotoxicity-induced apoptosis. Neuron 38: 33–46 [DOI] [PubMed] [Google Scholar]
- Grant P, Sharma P, Pant HC (2001) Cyclin-dependent protein kinase 5 (Cdk5) and the regulation of neurofilament metabolism. Eur J Biochem 268: 1534–1546 [PubMed] [Google Scholar]
- Humbert S, Dhavan R, Tsai L (2000) p39 activates cdk5 in neurons, and is associated with the actin cytoskeleton. J Cell Sci 113 (Part 6): 975–983 [DOI] [PubMed] [Google Scholar]
- Ko J, Humbert S, Bronson RT, Takahashi S, Kulkarni AB, Li E, Tsai LH (2001) p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J Neurosci 21: 6758–6771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro JB, Kitzmann M, Poul MA, Vandromme M, Lamb NJ, Fernandez A (1997) Cyclin dependent kinase 5, cdk5, is a positive regulator of myogenesis in mouse C2 cells. J Cell Sci 110 (Part 10): 1251–1260 [DOI] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH (2000) Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405: 360–364 [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD (1990) CNS stem cells express a new class of intermediate filament protein. Cell 60: 585–595 [DOI] [PubMed] [Google Scholar]
- Lew J, Huang QQ, Qi Z, Winkfein RJ, Aebersold R, Hunt T, Wang JH (1994) A brain-specific activator of cyclin-dependent kinase 5. Nature 371: 423–426 [DOI] [PubMed] [Google Scholar]
- Lin W, Dominguez B, Yang J, Aryal P, Brandon EP, Gage FH, Lee KF (2005) Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron 46: 569–579 [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Lariviere RC, Julien JP (2001) Deregulation of Cdk5 in a mouse model of ALS: toxicity alleviated by perikaryal neurofilament inclusions. Neuron 30: 135–147 [DOI] [PubMed] [Google Scholar]
- O'Hare MJ, Kushwaha N, Zhang Y, Aleyasin H, Callaghan SM, Slack RS, Albert PR, Vincent I, Park DS (2005) Differential roles of nuclear and cytoplasmic cyclin-dependent kinase 5 in apoptotic and excitotoxic neuronal death. J Neurosci 25: 8954–8966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna, Pant HC, Brady RO, Martin LJ, Kulkarni AB (1996) Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA 93: 11173–11178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick GN, Zhou P, Kwon YT, Howley PM, Tsai LH (1998) p35, the neuronal-specific activator of cyclin-dependent kinase 5 (Cdk5) is degraded by the ubiquitin-proteasome pathway. J Biol Chem 273: 24057–24064 [DOI] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH (1999) Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402: 615–622 [DOI] [PubMed] [Google Scholar]
- Pei JJ, Grundke-Iqbal I, Iqbal K, Bogdanovic N, Winblad B, Cowburn RF (1998) Accumulation of cyclin-dependent kinase 5 (cdk5) in neurons with early stages of Alzheimer's disease neurofibrillary degeneration. Brain Res 797: 267–277 [DOI] [PubMed] [Google Scholar]
- Philpott A, Porro EB, Kirschner MW, Tsai LH (1997) The role of cyclin-dependent kinase 5 and a novel regulatory subunit in regulating muscle differentiation and patterning. Genes Dev 11: 1409–14219192869 [Google Scholar]
- Sahlgren CM, Mikhailov A, Hellman J, Chou YH, Lendahl U, Goldman RD, Eriksson JE (2001) Mitotic reorganization of the intermediate filament protein nestin involves phosphorylation by cdc2 kinase. J Biol Chem 276: 16456–16463 [DOI] [PubMed] [Google Scholar]
- Sahlgren CM, Mikhailov A, Vaittinen S, Pallari HM, Kalimo H, Pant HC, Eriksson JE (2003) Cdk5 regulates the organization of Nestin and its association with p35. Mol Cell Biol 23: 5090–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Onuki R, Fujita Y, Kusakawa G, Ishiguro K, Bibb JA, Kishimoto T, Hisanaga S (2003) Developmental regulation of the proteolysis of the p35 cyclin-dependent kinase 5 activator by phosphorylation. J Neurosci 23: 1189–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejersen T, Lendahl U (1993) Transient expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci 106 (Part 4): 1291–1300 [DOI] [PubMed] [Google Scholar]
- Shea TB, Zheng YL, Ortiz D, Pant HC (2004) Cyclin-dependent kinase 5 increases perikaryal neurofilament phosphorylation and inhibits neurofilament axonal transport in response to oxidative stress. J Neurosci Res 76: 795–800 [DOI] [PubMed] [Google Scholar]
- Shen Q, Zhong W, Jan YN, Temple S (2002) Asymmetric Numb distribution is critical for asymmetric cell division of mouse cerebral cortical stem cells and neuroblasts. Development 129: 4843–4853 [DOI] [PubMed] [Google Scholar]
- Strocchi P, Pession A, Dozza B (2003) Up-regulation of cDK5/p35 by oxidative stress in human neuroblastoma IMR-32 cells. J Cell Biochem 88: 758–765 [DOI] [PubMed] [Google Scholar]
- Tang D, Yeung J, Lee KY, Matsushita M, Matsui H, Tomizawa K, Hatase O, Wang JH (1995) An isoform of the neuronal cyclin-dependent kinase 5 (Cdk5) activator. J Biol Chem 270: 26897–26903 [DOI] [PubMed] [Google Scholar]
- Tohyama T, Lee VM, Rorke LB, Marvin M, McKay RD, Trojanowski JQ (1992) Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest 66: 303–313 [PubMed] [Google Scholar]
- Toivola DM, Tao GZ, Habtezion A, Liao J, Omary MB (2005) Cellular integrity plus: organelle-related and protein-targeting functions of intermediate filaments. Trends Cell Biol 15: 608–617 [DOI] [PubMed] [Google Scholar]
- Treier M, Staszewski LM, Bohmann D (1994) Ubiquitin-dependent c-Jun degradation in vivo is mediated by the delta domain. Cell 78: 787–798 [DOI] [PubMed] [Google Scholar]
- Tsai LH, Takahashi T, Caviness VS Jr, Harlow E (1993) Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development 119: 1029–1040 [DOI] [PubMed] [Google Scholar]
- Vaittinen S, Lukka R, Sahlgren C, Hurme T, Rantanen J, Lendahl U, Eriksson JE, Kalimo H (2001) The expression of intermediate filament protein nestin as related to vimentin and desmin in regenerating skeletal muscle. J Neuropathol Exp Neurol 60: 588–597 [DOI] [PubMed] [Google Scholar]
- Vaittinen S, Lukka R, Sahlgren C, Rantanen J, Hurme T, Lendahl U, Eriksson JE, Kalimo H (1999) Specific and innervation-regulated expression of the intermediate filament protein nestin at neuromuscular and myotendinous junctions in skeletal muscle. Am J Pathol 154: 591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranna, Grant P, Pant HC (1997) Expression of p67 (Munc-18), Cdk5, P-NFH and syntaxin during development of the rat cerebellum. Dev Neurosci 19: 172–183 [DOI] [PubMed] [Google Scholar]
- Yan Y, Yang J, Bian W, Jing N (2001) Mouse nestin protein localizes in growth cones of P19 neurons and cerebellar granule cells. Neurosci Lett 302: 89–92 [DOI] [PubMed] [Google Scholar]
- Zambrano CA, Egana JT, Nunez MT, Maccioni RB, Gonzalez-Billault C (2004) Oxidative stress promotes tau dephosphorylation in neuronal cells: the roles of cdk5 and PP1. Free Radical Biol Med 36: 1393–1402 [DOI] [PubMed] [Google Scholar]
- Zhang J, Johnson GV (2000) Tau protein is hyperphosphorylated in a site-specific manner in apoptotic neuronal PC12 cells. J Neurochem 75: 2346–2357 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Ahuja HS, Zakeri ZF, Wolgemuth DJ (1997) Cyclin-dependent kinase 5 is associated with apoptotic cell death during development and tissue remodeling. Dev Biol 183: 222–233 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6


