Abstract
Thyroid hormone receptors generally activate transcription of target genes in the presence of thyroid hormone (T3) and repress their transcription in its absence. Here, we investigated the role of unliganded thyroid hormone receptor (TR) during vertebrate development using an amphibian model. Previous studies led to the hypothesis that before production of endogenous T3, the presence of unliganded receptor is essential for premetamorphic tadpole growth. To test this hypothesis, we generated a Xenopus laevis TR β mutant construct ineffective for gene repression owing to impaired corepressor NCoR recruitment. Overexpression by germinal transgenesis of the mutant receptor leads to lethality during early development with numerous defects in cranio-facial and eye development. These effects correlate with TR expression profiles at these early stages. Molecular analysis of transgenic mutants reveals perturbed expression of genes involved in eye development. Finally, treatment with iopanoic acid or NH-3, modulators of thyroid hormone action, leads to abnormal eye development. In conclusion, the data reveal a role of unliganded TR in eye development.
Keywords: eye development, thyroid hormone receptor, Xenopus laevis
Introduction
Thyroid hormone (T3, or 3,5,3′-triiodothyronine) plays important roles in many biological processes from development to homeostasis in adults (Yen, 2001). T3 acts through thyroid hormone receptors (TRs), nuclear receptors that control the transcriptional activity of target genes. A basic model proposed by Wolffe (1997) states that both liganded and unliganded TRs can bind to target genes, through cis-acting sequences, thyroid hormone response elements (T3REs). On positively regulated T3REs, unliganded TRs repress transcription and liganded TRs relieve this repression, activating transcription. Most functional analyses of TR action during development have examined liganded TR-mediated transcriptional activation (Yen, 2001). Although unliganded TR has been hypothesized to exert roles during development, no studies have yet addressed this possibility.
Thus, despite much work on mechanisms underlying TR activation and repression of target genes, few studies have investigated the physiological roles of TR-mediated repression in vivo. One of the most striking developmental processes involving T3 and TRs is amphibian metamorphosis (Shi, 1999). Metamorphosis requires the induction of a genetic program that is entirely T3-dependent. However, current knowledge suggests that a molecular repressor mechanism could be in place before metamorphosis, functionally repressing metamorphic-inducing genes (Puzianowska-Kuznicka et al, 1997; Sachs and Shi, 2000; Sachs et al, 2002; Havis et al, 2003). But it is not known whether this process is vital to normal physiological development of the embryo or tadpole.
Here, we exploited the accessibility of amphibian development to analyse the physiological role of TR repressor function before metamorphosis. Considering that one of the functions of unliganded TR is their ability to recruit corepressors, an elegant approach to suppress this TR activity is to abrogate corepressor recruitment. The ligand-binding domain (LBD) of TR is known to be essential to NCoR recruitment (Marimuthu et al, 2002). We generated a Xenopus laevis TRβ mutated in the LBD (TRI188K) and show that it is impaired both for gene repression and activation. We analysed the effects of its overexpression by transgenesis in X. laevis, in comparison with those resulting from overexpression of wild-type TRβ and a TRβ mutant form impaired only for gene activation. Overexpression of TRI188K is lethal and affects eye development at embryonic stages. The use of molecules that interfere with normal TR function, NH-3 and iopanoic acid (IOP), also affect eye development. Together, the results show an important, and unexpected, role of unliganded TR during embryogenesis.
Results
TRs are expressed in spatio-temporal-specific manners during embryogenesis
We first analysed the expression profiles of both TRα and TRβ mRNA during early development. Reverse transcription–polymerase chain reaction (RT–PCR) analyses were carried out on total RNA extracts isolated from embryos at different stages from gastrulation (stage NF10) to larval stages (stage NF40). As shown in Figure 1A, TRα mRNA levels doubled at stage NF28 (P<0.01) followed by a second, two-fold, increase after stage NF34 (P<0.01). In contrast, we found that the increase in TRβ mRNA level, after stage NF24 (P<0.05) was not followed by another significant increase (Figure 1B).
Figure 1.
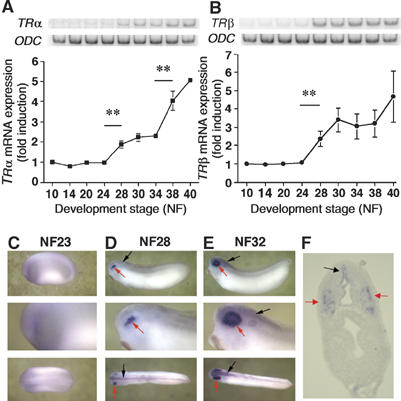
TRα and TRβ gene transcripts are present during embryonic development. Total RNA from 20 embryos at stage NF10–40 were isolated and used for RT–PCR analyses of TRα (A) and TRβ RNA levels (B). ODC RNA level was used as an internal control. Blots were quantified and TR levels normalised against the ODC expression level. The average values±s.e.m. of three independent experiments are expressed as fold induction, where 1 is equal to normalised TR expression at stage NF10. TRβ expression was localised in stage NF23 (C), NF28 (D, F) and NF32 (E) by whole-mount ISH. Red arrows and black arrows indicate, respectively, expression in the eyes and neural tube. (F) Transverse section at stage NF28 showing TRβ expression profile within the head.
Next, whole-mount in situ hybridisation (ISH) was carried out to localise expression of TRα and TRβ mRNA. TRα expression was not detected above background levels observed with the sense probe (data not shown). TRβ expression increased between stages NF24 and 28 (compare Figure 1C with D). At stage NF28, TRβ mRNAs were strongly expressed in the optic vesicle (Figure 1D, red arrow) and to a lesser degree in the neural tube (Figure 1D, black arrow). Transversal sections of stage NF28 embryos confirmed expression in the optic vesicle (Figure 1F, red arrow) and the neural tube with a clearly defined localisation in the dorsal ventricle (Figure 1F, black arrow). At stage NF32, expression of TRβ mRNA was maintained in the neural tube (Figure 1E, black arrow) and was stronger in the retina (Figure 1E, red arrow).
The TRI188K mutant shows impaired transcriptional repression and activation
Polymerase chain reaction (PCR) and ISH data show TRs mRNAs to be present well before the production of endogenous T3 (Yaoita and Brown, 1990). These findings triggered the hypothesis that before metamorphosis, TRs act to repress T3-response genes. Given the importance of the residue I280 in the LBD of human TR for corepressor recruitment (Marimuthu et al, 2002) and the conservation of this site in Xenopus TR, we created a X. laevis TRβ variant (TRI188K) that should lose its potential to recruit corepressor (Supplementary Figure S1). We also used another TRβ variant (TRΔAF2) deleted for the last nine C-terminal amino acids that correspond to the activation function 2 (AF2) domain of Xenopus TR (Puzianowska-Kuznicka et al, 1997; Wong et al, 1997). To visualise expression of each TRβ variant, their cDNAs were fused to the 3′ end of GFP (Supplementary Figure S1). Expression vectors for green fluorescent protein (GFP) with cytoplasmic or nuclear localisation were used as controls (Cts) (Supplementary Figure S1).
An in vivo somatic gene transfer approach was employed to investigate the functionality of the TRI188K mutant in modulating T3-dependent gene regulation. The plasmids pCG, pCGT, pCGTI188K and pCGTΔAF2 were microinjected into dorsal tail muscle or into brain of X. laevis tadpoles at stage NF55. The luciferase gene was used as a reporter system driven by a T3-dependent promoter (T3RE-tk-Luc). Overexpression of TR both in the muscle (Figure 2A) and brain (Figure 2B) led to a dramatic decrease of luciferase expression compared to Cts that only overexpress GFP (P<0.01). This decrease demonstrates the repressive effect of TR in the absence of ligand (Sachs et al, 2002). Injection of pCGTI188K induced a lesser decrease of luciferase expression in the muscle (Figure 2A, P<0.05) and gave no repression in the brain (Figure 2B). Coexpression of TRI188K with RXRα in the dorsal muscle gave similar results (data not shown). TRΔAF2 retained its ability to repress transcription, as the overexpression of pCGTΔAF2 led to a strong decrease of luciferase expression, as did TR (Figure 2A, P<0.01). These results indicate that TR and TRΔAF2 can repress transcription as expected. However, the TRI188K form has lost most of its capacity to repress transcription.
Figure 2.
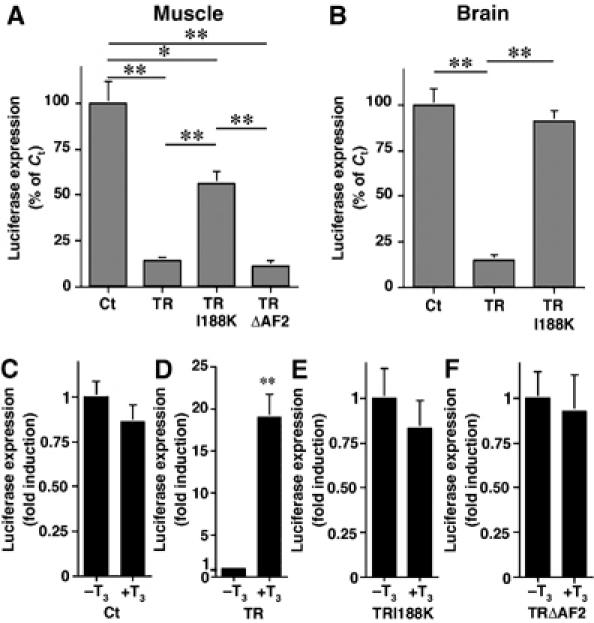
Functional analysis of TR variant-GFP fusion proteins shows the TRI188K mutant is impaired for both transcriptional repression and activation. (A) Overexpression, in tadpole tail muscle, of TR and TRΔAF2, but not TRI188K strongly decreases the expression of T3RE-tk-Luc. pCG, pCGT, pCGTI188K and pCGTΔAF2 vectors (all 0.5 μg) were coinjected with 0.5 μg of the T3RE-tk-Luc. (B) Overexpression, in tadpole brain, of TR, but not TRI188K decreases the expression of T3RE-tk-Luc. The experiments were performed as in (A) except that the plasmids were complexed with polyethylenimine. (C–F) T3 does not affect expression of T3RE-tk-Luc in presence of GFP (C), TRI188K (E) or TRΔAF2 (F), but increases expression of T3RE-tk-Luc in presence of TR (D). One microgram of pCG, pCGT, pCGTI188K or pCGTΔAF2 was coinjected with 0.2 μg of T3RE-tk-Luc in tadpole tail muscle. Following injection, tadpoles either received 10 nM T3 (+T3) for 48 h, or were used as Cts (−T3). For all the experiments, luciferase was assayed 48 h after transfection, and expressed as % of the Ct value (100%) or fold induction with −T3 value equal 1. Means±s.e.m. are given. Each point represents at least seven animals. Significant changes *P<0.05 and **P<0.01) are indicated as compared to Ct or −T3 value.
To determine whether TRI188K retained its ability to activate transcription, the injections in tadpole dorsal muscle were repeated with T3 treatment. Owing to the low levels of endogenous TR in cell muscles, luciferase expression was not affected by 48 h of 10 nM T3 treatment when only GFP was overexpressed (Figure 2C). However, overexpression of wild-type TR led, in the presence of T3, to a strong increase of luciferase expression compared to untreated Cts (Figure 2D, P<0.01). In contrast, overexpression of TRI188K or TRΔAF2 had no effect on luciferase expression compared to their respective Cts (respectively Figure 2E and F). Again, coexpression of TRI188K with RXRα did not improve its capacity to activate transcription (data not shown). Thus, TRI188K was impaired for both transcriptional repression and activation.
The TRI188K mutant binds DNA and is impaired for corepressor recruitment in vivo
We next tested the capacity of the TR variants to bind to T3 response gene regulatory regions in vivo by chromatin immunoprecipitation (ChIP) assay (Figure 3). The Cts included ChIP at stage NF40 tadpoles as a positive Ct (Figure 3) and PCR was carried out on isolated DNA before ChIP to verify that the amount of chromatin used for each group was not different (Figure 3, lane 5). First, an antibody recognising both TRs was used to immunoprecipitate chromatin from embryos overexpressing either GFP or the GFP fusion proteins with TR or TRI188K. Tadpoles transgenic for GFP alone with either cytoplasmic or nuclear localisation were used as Cts. These experimental conditions will reflect the binding of endogenous TR. The TR-bound DNA fragments were analysed by semiquantitative PCR (Figure 3, lane 1). Primers flanking the T3REs were used for the TRβ and TH/bZIP regulatory regions (Havis et al, 2003). The EF1α promoter does not bind TR and was thus used as a negative Ct (Havis et al, 2003). As shown in Figure 3, lane 1, in Ct embryos overexpressing GFP, TR was found on the TRβ and TH/bZIP, but not the EF1α regulatory regions. When GFP-fused-TR and TRI188K proteins were overexpressed, TR was present on TRβ and TH/bZIP regulatory regions and was still absent from the EF1α promoter (Figure 3, lane 1).
Figure 3.
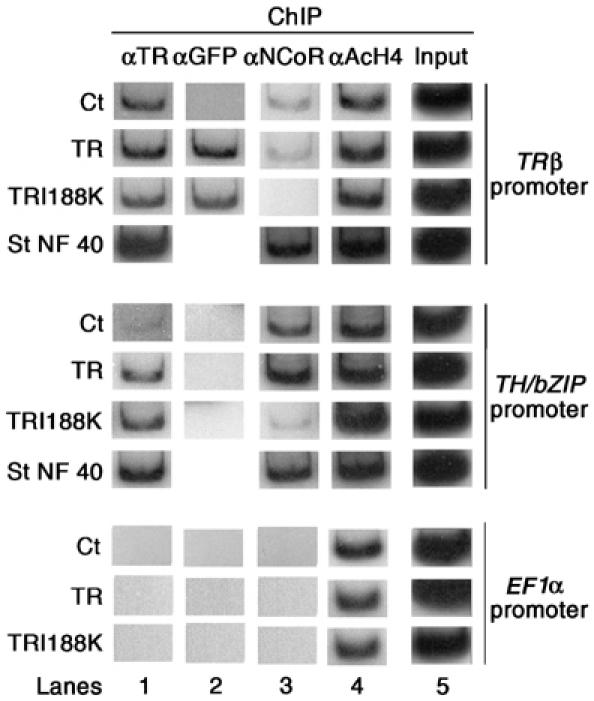
The TRI188K mutant binds DNA and is impaired for corepressor recruitment. TRI188K expression leads to binding of and release of xNCoR from T3 response gene promoters. Two-day-old transgenic embryos nuclei were isolated and subjected to ChIP assays with antibodies against TR (lane 1), GFP (lane 2), NCoR (lane 3) or AcH4 (lane 4). ChIP products were analysed by PCR for the presence of T3RE-containing fragments of TRβ and TH/bZIP promoters or for the presence of EF1α promoter, which is not T3 regulated. Aliquots of chromatin before immunoprecipitation were used for PCR as Cts of DNA quantity (Input, lane 5). Each transgenic group represented 100 embryos.
To discriminate DNA binding of overexpressed GFP-fused proteins versus endogenous TR, an antibody recognising GFP was used (Figure 3, lane 2). As expected, GFP was present on the TRβ regulatory region when TR and TRI188K fusion proteins were expressed but not when GFP alone was expressed. Further, we never observed TR-GFP binding on the constitutively expressed EF1α promoter (Figure 3, lane 2). However, we were also unable to see GFP on the TH/bZIP regulatory region (Figure 3, lane 2). It was unclear why the GFP antibody did not precipitate the TH/bZIP regulatory region in TR and TRI188K transgenics. A possibility is that the conformation of the GFP-TR fusion protein on this response element did not permit recognition of the GFP epitope by the antibody. Another possibility is that the anti-GFP antibody has a lower affinity for the fusion protein than has the anti-TR antibody. Also, Buchholz et al (2005) have also reported lower binding of TR on the TH/bZIP regulatory region versus the TRβ regulatory region. However, this lack of GFP-antibody precipitation of GFP-TRs did not modify our conclusion regarding TR and TRI188K binding to the TH/bZIP regulatory region, as this capacity was validated by ChIP with the TR antibody. So taken together, our data indicate that GFP-fused TR and TRI188K bind to T3RE in chromatin in vivo.
Of particular interest in this experimental context was the possibility to address corepressor recruitment by TRI188K. We carried out a ChIP assay with a polyclonal antibody to X. laevis NCoR corepressor. In embryos overexpressing GFP or wild-type TR and in stage NF40 tadpoles, NCoR was present on the TRβ and TH/bZIP regulatory regions, but absent from the EF1α promoter (Figure 3, lane 3). In contrast, in TRI188K transgenic embryos, NCoR was hardly detectable on the TH/bZIP regulatory region and absent from the TRβ and EF1α regulatory regions (Figure 3, lane 3). These results indicated that TRI188K was impaired for NCoR recruitment in vivo. Regarding coactivator recruitment, we investigated using ChIP assays the histone acetylation status of the promoters because many coactivators possess intrinsic histone acetyl transferase activity and because coactivator recruitment is T3-reponse gene promoter and tissue specific (Havis et al, 2003; Buchholz et al, 2005). As shown in Figure 3, lane 4, using a ChIP assay with an antibody specific to acetylated histone H4 (AcH4), acetylation was unchanged on all regulatory regions examined (TRβ, TH/bZIP and EF1α) whether GFP or GFP fusion protein with TR or TRI188K were overexpressed (Figure 3, lane 4). Thus, we observed in vivo that overexpression of TR or TRI188K had no effect on the histone acetylation level of T3 response genes.
TRI188K overexpression is deleterious to embryogenesis
To analyse the requirement of TR-mediated repression in embryonic development, germinal transgenesis was employed and the effect of GFP with nuclear localisation signal (nls), TR, TRI188K and TRΔAF2 overexpression on development of embryos and tadpoles was compared. TRΔAF2, which is only impaired for transcriptional activation, was used to distinguish whether TRI188K effects were the result of repression or activation defects. The rate of transgenic embryos determined by GFP observation varied around 85% from one experiment to another (data not shown). RT–PCR and Western blot analysis were used, respectively, to control that the level of expression of the different TR variants mRNA (Supplementary Figure S2A), and protein (Supplementary Figure S2B) was similar. The survival rate was analysed after gastrulation (24 h post-transgenesis, stage NF12) because of high variability before this time (from 40 to 90%), which was probably the result of inconsistent egg quality from frog to frog. At 48 h post-transgenesis, when embryos reached tail-bud stage (NF28), there was no significant difference in survival rate between the four conditions tested (Figure 4A). However, at 72 h (stage NF40), the survival rate was unchanged for Ct, TR and TRΔAF2 transgenic embryos, whereas it dropped significantly for TRI188K embryos (Figure 4A, P<0.01). At later stages of development from NF40 to NF45, the survival rate was maintained for each condition tested (Figure 4A). These results indicate that overexpression of TRI188K is lethal during embryonic development.
Figure 4.
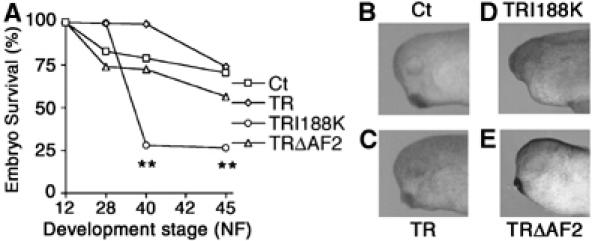
TRI188K transgenic embryos die during early development and display various phenotypes. (A) The survival rate in transgenic embryos expressing either GFP or GFP fused to TR, TRI188K or TRΔAF2 were analysed up to 120 h following transgenesis. The number of live embryos were measured and transformed as % of total number of embryos in each group. Means±s.e.m. are given. Each point represents results from three independent experiments with at least 300 animals per group. **Indicates significant changes (P<0.01) compared to Ct values. (B–E) Overexpression of TRI188K results in eye abnormalities. One transgenic embryo representative of the major phenotype observed was photographed 48 h after transgenesis (stage NF28).
Next, the proportion of normal embryos for each condition was assessed. The rate of normal embryos produced during germinal transgenesis was usually around 70% and this was consistently observed for Ct, TR and TRΔAF2 transgenic embryos (Supplementary Figure S3A), the 30% abnormal embryos being inherent to the transgenic method (Kroll and Amaya, 1996). A markedly different result was obtained when overexpressing TRI188K. The proportion of normal embryos decreased to around 30% for each time point analysed (Supplementary Figure S3A, P<0.01). Thus, TRI188K overexpression affects the morphological development of the embryos.
To refine the investigation of the defects in the abnormal TRI188K embryos compared with Ct, TR and TRΔAF2 transgenic embryos at stage NF28, we focused on the head region where TR is expressed. More than 60% of TRI188K embryos showed head abnormalities (Supplementary Figure S3D), whereas less than 30% of Ct, TR and TRΔAF2 (respectively Supplementary Figure S3B, S3C and S3E) embryos showed the same phenotype. At higher magnification, most Ct, TR and TRΔAF2 transgenic embryos were seen to display normal head morphology with correctly formed eyes and cement gland (respectively Figure 4B, C and E). However, TRI188K embryos had abnormal head morphologies, either lacking or with underdeveloped eyes (Figure 4D).
TRI188K overexpression affects the expression of genes involved in eye development
To confirm the lack of eye structure in TRI188K transgenic embryos, whole-mount ISH analysis was carried out with probes against either Pax6, Rx, Otx2 or xhh mRNA, four genes involved in eye development (Harris and Perron, 1998). Pax6 and Rx code for homeobox-containing proteins, expressed early in the future eye for specification of the eye field. Otx2 codes for a homeodomain protein that later specifies the eye field. Xhh codes for a morphogene that is expressed in different parts of the head, but not in the eye before stage NF34. Earlier, xhh is implicated in eye field separation and proximo-distal axis formation. Whole-mount ISH at stage NF28 showed Pax6, Rx and Otx2 to be expressed in the developing eye of Ct, TR and TRΔAF2 transgenic embryos, but there were only weak signals in the heads of TRI188K embryos (Figure 5). Furthermore, xhh was expressed in the head of Ct, TR and TRΔAF2 transgenic embryos, but only a weak signal was seen in TRI188K embryos (Figure 5). The smaller size of the eyes was confirmed using Pax6 expression limits. Indeed, the diameter was significantly smaller in TRI188K transgenic embryos compared to Ct, TR and TRΔAF2 transgenic embryos (Supplementary Figure S4, P<0.01). This latter result emphasises the abnormal eye organogenesis in TRI188K embryos.
Figure 5.
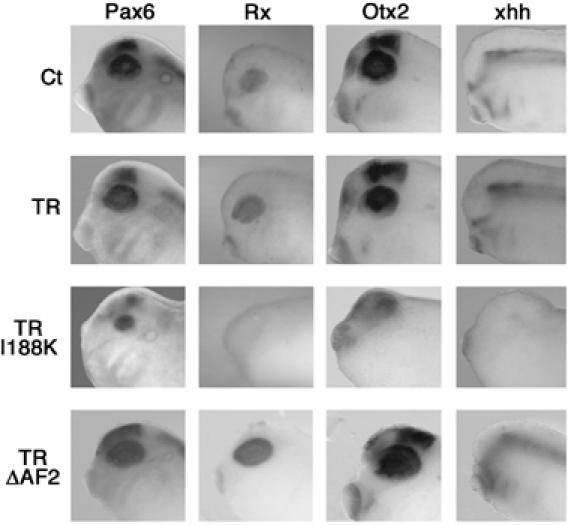
Effects of TRI188K overexpression on Pax6, Rx, Otx2 and xhh gene expression in transgenic embryos. Gene expression localised by whole-mount ISH in the head for one stage NF28 embryo representative of the major phenotype observed for each type of transgene used. Panels show a higher magnification of the head for one embryo representative of the ISH signal observed. A colour version of this figure is available at the EMBO Journal Online.
IOP treatment recapitulates the deleterious effects of TRI188K overexpression
The physiological function of unliganded TR could be questioned because physiological T3 treatment of embryos neither produces morphological effects nor modification of T3 response gene expression during early embryogenesis (Yaoita and Brown, 1990; Puzianowska-Kuznicka et al, 1997). One explanation could be the presence of the inactivating deiodinase D3 in certain tissues during early development (Marsh-Armstrong et al, 1999; Morvan-Dubois et al, 2006) degrading any T3 present or introduced. Indeed, in the retina, a few cells expressing D3 mRNA are seen as early as stage NF22 when TRβ starts to be expressed (Marsh-Armstrong et al, 1999). A means of revealing deiodinase activity is to use the inhibitor iopanoic acid (IOP). We used IOP at 5 μM, a concentration shown to block deiodinase activities in amphibians (Marsh-Armstrong et al, 1999). Starting treatment with IOP at the morula stage led to embryonic mortality at gastrula (data not shown). When the IOP treatment was started at the end of gastrulation (stage NF11-12), 100% of the embryos examined 24 h later (stage NF28) showed many developmental defects including developmental delay, oedema, lower motility and absence of observable eyes or smaller eyes (Figure 6A). As for the TRI188K transgenic embryos (Figure 4A), 55% of these IOP-treated embryos died within 72 h of treatment. Molecular analysis by whole-mount ISH at stage NF28 showed Pax6, Rx, Otx2 and xhh to be expressed in the eye of Ct embryos, but there were weaker signals and the shapes were different in IOP-treated embryos (Figure 6B). The smaller size of the eyes was confirmed using Pax6 localisation. Indeed, eye diameter was significantly smaller (Supplementary Figure 5SB, P<0.01 compare Ct versus IOP). Thus, IOP treatment induces eye malformation.
Figure 6.
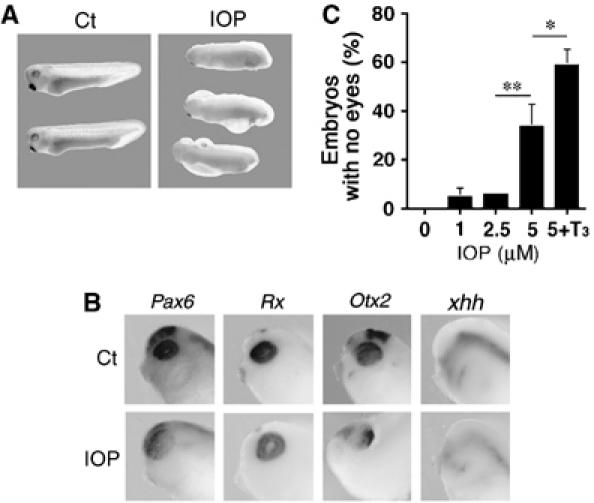
Effects of IOP on embryonic development. (A) At stage NF31, several phenotypes were identified in embryos treated with IOP. Embryos at stage NF11–12 either received 5 μM IOP for 24 h or were used as Cts in ethanol 0.1% (Ct). (B) Pax6, Rx, Otx2, and xhh expression localised at stage NF28 by whole-mount ISH in the head for one embryo representative of the major phenotype following IOP treatment. (C) T3 treatment increases the effect of IOP treatment. Embryos at stage NF11–12 were treated for 24 h with 0, 1, 2.5 or 5 μM IOP (in 0.1% ethanol) and one group received 5 μM IOP and 10 nM T3 (in 0.1% ethanol). Means±s.e.m. are given. Each point represents at least 25 animals. Significant changes *P<0.05 and **P<0.01 are indicated. A colour version of this figure is available at the EMBO Journal Online.
IOP treatment inhibits both activating and inactivating deiodinases, affecting both T3 production and T3 degradation. To distinguish between these two processes, embryos were treated with T3 and/or IOP. A phenotype owing to IOP will be rescued by T3 treatment if T3 production is involved. In contrast, T3 treatment will aggravate a phenotype where IOP affects mainly T3 degradation. IOP treatment induces a significant (P<0.01) increase of embryos with no visible eyes (Figure 6C). T3 treatment alone does not have any effect on eye number compared to Ct embryos (data not shown). However, the combination of 10 nM T3 with IOP, during the same period of time, increases further the number of embryos lacking visible eyes (Figure 6C, P<0.05) suggesting that the IOP effect is played out by blocking T3 degradation. To further analyse IOP and T3 effects on eye development, whole-mount ISH for Pax-6 was carried out. In IOP- or IOP plus T3-treated embryos, the retina (red arrows) was reduced and did not completely surround the lens (blue arrows) (Supplementary Figure S5A; black arrows). Furthermore, eye diameter was significantly smaller (Supplementary Figure S5B, P<0.01). Treatment with both IOP and T3 led to a stronger effect on eye shape (Supplementary Figure S5A; compare Ct and IOP versus IOP plus T3) and another significant (P<0.05) decrease of eye height (Supplementary Figure S5B). Interestingly, whole-mount ISH with Pax-6 probe revealed an effect of T3 alone, not on eye shape (Supplementary Figure S5A; compare Ct versus T3), but on eye height with a small, but significant (P<0.05), decrease compared to Ct embryos (Supplementary Figure S5B). Taken together, these results suggest that unliganded TR has a physiological role in embryogenesis. They also explain earlier observations on lack of T3 effects at these stages.
The mixed T3 agonist/antagonist NH-3 strongly affects eye formation
To target more specifically endogenous TR signalling, NH-3, a synthetic T3 agonist/antagonist was used to treat embryos. NH-3 was initially described for its property to bind TR and block TR coactivator recruitment. However, in addition to its antagonist effect, NH-3 has an agonist effect because NH-3 also induces release of corepressor (Nguyen et al, 2002). These agonist/antagonist effects were also observed in tadpoles (Lim et al, 2002). The authors showed that μM treatment of NH-3 alone increases slightly, but significantly, the expression (10% of the T3 induced mRNA level) of collagenase-3 mRNA, an endogenous T3 target gene, yet blocks T3 upregulation of this target gene. Thus, TR with NH-3 will mimic effects of TR mutant I188K. Like TRI188K, the presence of NH-3 produces a TR that is unable to repress or activate transcription, but NH-3 treatment has the added advantage of only targeting cells expressing endogenous TR. This specific effect will not be achieved by overexpression of TRI188K under the control of a ubiquitous promoter.
Treatment at the morula stage with 6 μM NH-3 (data not shown) or 10 μM NH-3 (Figure 7A) strongly altered embryonic development compared to Ct embryos (100% of the embryos were affected). Effects were seen as soon as they reached stage NF28 (48 h) with delayed development, as well as slight oedema, lower motility and absent or small eyes. At stage NF40 (72 h, Figure 7A), eye and tail were clearly affected, as was lower motility. At stage NF45 (5 days after fertilisation), treated embryos displayed significant ventral oedema, abnormal heads, smaller and abnormally formed and positioned eyes, malformed tails and intestines (Figure 7A). Unlike TRI188K expression and IOP treatment, NH-3 had no effect on embryo viability. Molecular analysis by whole-mount ISH at stage NF28 showed Pax6, Rx, Otx2 and xhh to be expressed in eye of Ct embryos, but there were weaker signals and the shapes were different in NH-3-treated embryos (Figure 7B). The smaller eye size was confirmed using Pax6 localisation (Supplementary Figure S6A, P<0.01 compare Ct versus IOP). Focusing on later eye development, eye position was affected in NH-3-treated embryos (Figure 7A, dorsal view). First, the inter-eye distance was shortened and second, eyes were located rostrally not laterally (Figure 7A, dorsal view, black arrows). Interestingly, the position and orientation of the eyes in NH-3-treated embryos was similar to the position of the eyes observed in juvenile or adult frogs. To confirm the observed difference, parameters such as the height, the width and the inter-eye distance were measured, and were seen to be affected significantly in a dose-dependent manner by NH-3 at all developmental stages analysed (Supplementary Figure S6B at stage NF45 and data not shown). Furthermore, the shape of the eyes was also affected (Figure 7C). The eyes of NH-3-treated embryos had an apparently normal lens (blue arrows), but the lens was not completely surrounded by the retina (the black pigmented area, red arrows) as it was in Ct embryos (Figure 7C). In many embryos treated with NH-3, the eyes had a second pigmented area close to the lens (Figure 7C). Histological analysis confirmed the presence of a duplicated pigmented area (Figure 7D, the two red arrows in eye section of NH-3-treated embryos). Moreover, the retina was thinner and most of the organisation into multiple layers was absent, indicating that retinal differentiation was defective (Figure 7D, compare Ct versus NH-3, retina indicated by red arrows). Finally, some NH-3-treated embryos showed potentially duplicated retinal areas (two red arrows in Figure 7D NH-3).
Figure 7.
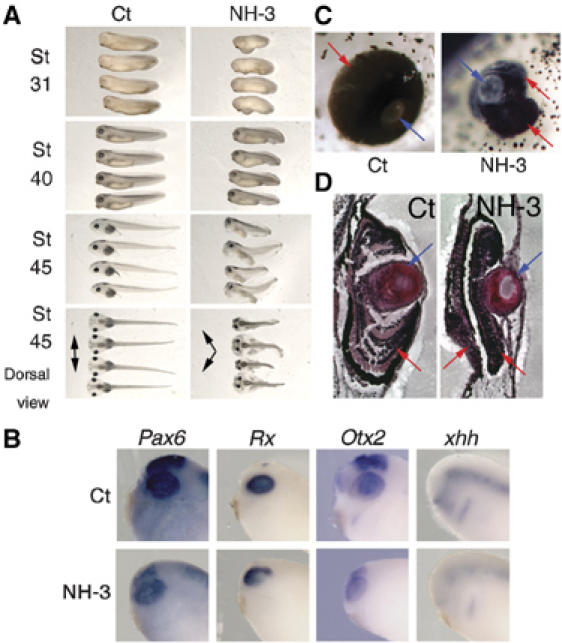
NH-3, a TR antagonist inhibits eye development. Embryos at the morula stage were treated (48 h) with either 0.1% ethanol, 10 μM NH-3 or were treated with 0.1% ethanol (Ct). (A) NH-3-treated embryos had abnormal embryonic development. Development was observed at stage NF28, NF40 or NF45. Black arrows indicated the orientation of the eyes. (B) Pax6, Rx, Otx2 and xhh expression localised at stage NF28 by whole-mount ISH in the head for one embryo representative of the major phenotype following NH-3 treatment. (C) High magnifications of tadpole eyes (stage NF45) following NH-3 treatment as in panel A are presented for one tadpole representative of the major phenotype observed compared to control tadpoles (Ct). Blue arrows indicate the lens and red arrows indicate the retina. (D) The organisation of the eye structure is affected by NH-3. Some of the embryos presented in panels A and C were sectioned to analyse the organisation of the eye. The blue and red arrows are used as in panel C.
Discussion
The potential roles of active transcriptional repression by unliganded TR in developmental and pathological processes have received increasing attention in recent years (Privalsky, 2004). Here, studying amphibian development, we provide in vivo evidence that TRβ is expressed in a tissue-specific manner during embryogenesis, at stages when the thyroid gland is inactive. The results suggest a new physiological role of unliganded TR, before its participation in the repression of metamorphic T3 response genes during larval development. Further, the results indicate that unliganded TRβ is implicated in eye formation.
TRs are expressed in tissue-specific manners during embryogenesis
The expression of TRα and TRβ during Xenopus embryogenesis has been observed previously (Banker et al, 1991), but has always been considered minor, mainly because TH treatment of embryos has no morphological effect (Yaoita and Brown, 1990; Puzianowska-Kuznicka et al, 1997). The more detailed TRs expression profiles described herein show expression of TRα and TRβ mRNA during embryogenesis. TRα mRNA levels increase after stage NF24 and are maintained at levels two-fold of those seen at stage NF24 until larval stages (stage NF35), when their levels show another increase. Unfortunately, TRα expression could not be localised by whole-mount ISH owing to ubiquitous expression and probably because this genomic area is transcribed in both orientations giving both sense and antisense ISH signals. TRβ mRNA levels are low but detectable until stage NF24, then increase three-fold at stage NF30, after which they are maintained at a constant level. ISH shows TRβ expression to be localised in the neural tube and the developing eyes.
TR functions in eye formation
TRI188K transgenic embryos displayed markedly abnormal head development with, in most cases, abnormal eye development. The absence of TRβ expression in the developing cranio-facial area (other than the retina) suggests that the observed cranio-facial phenotype is the result of the interference of TRI188K or the effects of NH-3 and IOP treatment with TRα, as its localisation is as yet undefined or the result of gastrulation defects that are dependent on TRβ.
The observed effects on eye development correlate with the strong expression of TRβ in the retina. At later stages of eye development, during metamorphosis, T3 has been shown to influence asymmetric growth and development of the retina by inducing cells to proliferate and differentiate (Marsh-Armstrong et al, 1999). In mice, T3 and TR have also been shown to play important roles in eye development (Harpavat and Cepko, 2003) such as morphological development or photoreceptor differentiation (Ng et al, 2001). The molecular analyses reported here reveal that treated embryos have reduced levels of Pax6, Rx, Otx2 and xhh mRNA compared to Ct embryos, which correlates with eye abnormality. A link between TR and sonic hedgehog signalling exists as xhh mRNA is induced by T3 during amphibian metamorphosis (Stolow and Shi, 1995) and both sonic hedgehog and T3 induce oligodendrocyte differentiation in mammals (Rogister et al, 1999). Thus, numerous lines of evidence confer that T3 and TR appear to play critical, yet not fully understood, roles in the early development of the retina.
In such a complex developmental process, other molecular pathways besides those identified here could also be involved in determining the phenotype of TRI188K transgenics or NH-3- and IOP-treated embryos. Recently, DNA array analysis revealed that the initial response of T3 induction, during Xenopus brain metamorphosis, is cell proliferation (Das et al, 2006). Some genes shown to be differentially regulated are involved in embryonic retinal development, such as Notch and Otx2 (Harris and Perron, 1998). Other data from Xenopus and other species point to several other candidates that could be implicated in relaying TR signalling during eye morphogenesis. For example, cyclin D1 is known to be involved in cell proliferation and mice lacking cyclin D1 show defects in eye development (Fantl et al, 1995). During amphibian development, cyclin D1 expression is stage and tissue specific, being found in the neural plate and eye vesicles, as in mammals (Tanaka et al, 2003). Moreover, cyclin D1 is differentially regulated during amphibian metamorphosis (Das et al, 2006). Thus, it is tempting to speculate that the deregulation of cell cycle and differentiation signalling affects neurogenesis in specific brain areas, thereby contributing to the observed phenotype.
The physiological role of unliganded TR
Based on the expression studies of TRs and their mechanism of action in X. laevis, a dual function model for the role of TR during amphibian development has been proposed (Puzianowska-Kuznicka et al, 1997). Unliganded TRs function as transcriptional repressors of T3 response genes before metamorphosis and liganded TR as transcriptional activators of T3 response genes in presence of T3 during metamorphosis. However, no pertinent data have revealed a fundamental, physiological role for unliganded TR.
Analysis of the TRI188K-expressing embryos raises the following key question: is unliganded TR-mediated gene repression exerting a physiological role during early development? Our data and previous reports (Schreiber et al, 2001; Buchholz et al, 2003) on overexpression of wild-type TR or dominant-negative TR impaired for gene activation showed no effect on early development, suggesting that the effects of TRI188K observed here indeed result from abrogation of repression and not from failure to activate target genes. Moreover, much data suggest that the TRs expressed at these early stages are unliganded. First, low levels of thyroid hormones are stored in the yolk of amphibian eggs and T4 is converted to T3 in early embryos (Morvan-Dubois et al, 2006). However, there are no data on the local availability of T3 and T4 during early embryonic stages. Thus, it is currently impossible to rule out low, highly localised presence of T3 from maternal origin with very specific functions, such as that described in embryonic mammals (Zoeller and Rovet, 2004). We can also presume that in some tissues, any T3 present would be rapidly degraded because the inactivating type III deiodinase D3 is found in the retina as early as stage NF22 when TRβ starts to be expressed (Marsh-Armstrong et al, 1999). This presence of D3 could explain why T3 treatment of embryos produces neither morphological effects nor modification of T3 response gene expression during early embryogenesis (Sachs and Shi, 2000). Second, the decrease of some T3 target genes expression (xhh or Otx2) could be explained by the loss of tissues where they are normally expressed, such as eye in TRI188K transgenics and NH-3- or IOP-treated embryos. Finally, another explanation could involve cell-specific regulation of TR target genes. TR is implicated in processes as varied as cell proliferation and cell differentiation according to cell competence. A given T3 response gene will be differently regulated as a function of cellular context and constraints. Cyclin D1 is again a good example, being upregulated by T3 in dividing cells (Barrera-Hernandez et al, 1999) and downregulated by T3 during cell differentiation (Garcia-Silva et al, 2002). Thus, taken together, these observations strongly argue for a role of unliganded TR in embryonic development.
Analysis of mice lacking TRs has not so far revealed a fundamental physiological role for unliganded TR during development (Chassande, 2003). However, a role of unliganded TR during rodent heart development has been shown (Mai et al, 2004). The essential role of unliganded TR might thus be specific to amphibians or species that have a larval developmental period between embryonic stages and juvenile stages. In such cases, TR repression would ensure correct larval development before metamorphosis. Another possibility is that redundant systems, such as retinoic acid receptors, can substitute for TR repression in certain situations or species.
In conclusion, our results suggest that TR with functional corepressor recruitment is essential for X. laevis embryogenesis. The head area, in particular, the retina and neural tube are specific targets, although the physiological role remains to be defined. Finally, because retinal development is well characterised and has much in common with other developing neural structures, it offers a number of experimental advantages to further our understanding of this phenomenon and will probably provide key insights into the mechanisms of T3 action in the developing nervous system.
Materials and methods
Animals and treatments
South African clawed frogs (X. laevis) were obtained from the CNRS UPR1086 facility (Montpellier, France). Wild-type embryos were obtained by in vitro fertilisation as previously described (Puzianowska-Kuznicka et al, 1997). Tadpoles were raised and maintained in 0.1% perchlorate to keep them hypothyroid (de Luze et al, 1993). Developmental stages were determined according to Nieuwkopp and Faber (NF) (1956). For IOP, NH-3 and/or T3 treatment, embryos were maintained 24 or 48 h, as indicated, in 5 ml of MMR 0.1 × (Marc's modified Ringers) with 1, 2.5 or 5 μM IOP (TCI Europe, Zwijndrecht, Belgium) and/or 6 or 10 μM NH-3 (Department of Pharmaceutical Chemistry, UCSF, San-Francisco) and/or finally 10 nM T3 (Sigma, St Quentin Fallavier, France). Following somatic in vivo gene transfer, stage NF55 tadpoles were maintained for 48 h in 5 l of dechlorinated tap water with or without 10 nM T3. Tadpoles were killed by decapitation after anaesthesia (0.01% MS222, Sigma) before tissue isolation. Animal care was in accordance with institutional and national guidelines.
DNA constructs
pCG and pCGnls express GFP under the control of the cytomegalovirus promoter (CMV). pCG is the commercially available plasmid pS65T from BD Biosciences Clontech (Ozyme, Saint Quentin en Yvelines, France) and provides cytoplasmic expression of GFP. pCGnls leads to nuclear localisation of GFP and was obtained by substituting the GFP cDNA sequence of pCG by an nls fused to the GFP sequence from pCS2nlsGFP construct (Kroll and Amaya, 1996). pCGT expresses from the CMV promoter the TRβ protein fused to the C-terminal part of GFP. pCGTI188K expresses from the CMV promoter the TRβ mutant I188K protein fused to GFP. The point mutation was introduced by directed mutagenesis in pCGT using the Quickchange™ XL site-directed mutagenesis kit according to the manufacturer's recommendations (Stratagene, Amsterdam, Netherlands). The primers used are forward: 5′-GCCAGTTTACAAAAA TAATCACCCCAGCAAAGACAAGAGTTGTTG-3′ and reverse: 5′-CAA CAACTCTTGTCTTTGCTGGGGTGATTATTTTTGTA AACTGGC-3′. pCGTΔAF2 expresses from the CMV promoter the TRβΔAF2 protein fused to GFP. A schematic representation of these DNA constructs is given (Supplementary Figure S1).
T3RE-tk-Luc, expresses the firefly (Photinus pyralis) luciferase reporter gene under the control of a promoter containing a T3RE made of two direct repeats of AGGTCA separated by four base pairs, and the minimal thymidine kinase (tk) promoter (Sachs et al, 2002).
The plasmids pBl-TRβ, pBl-TRα (kindly provided by D Brown, Carnegie, Baltimore), xRX-sp73, pXPax6 (kindly provided by M Perron, CNRS, Orsay, France), pCRII-xhh and pCRII-Otx2 were used to produce sense and antisense probes of, respectively, X. laevis TRβ, TRα, Rx, Pax6, xhh and Otx2. The PCR fragments corresponding to xhh and Otx2 were cloned in the pCRII vector using TA-cloning kit (Invitrogen, Cergy Pontoise, France) and verified by sequencing. All plasmids were amplified and purified using Qiagen Kits (Courtaboeuf, France).
Transgenesis and in vivo somatic gene transfer
Somatic gene transfer in the muscle and brain were performed as previously described (de Luze et al, 1993; Ouatas et al, 1998). Two days after injection, dorsal muscles or brains were collected and flash frozen. Homogenates were obtained by sonication in 500 or 200 μl of luciferase lysis buffer (Promega, Charbonnières les Bains, France) for the muscle and brain, respectively. The luciferase assay was performed on 20 μl of the homogenate, according to the manufacturer's recommendations (Promega).
Transgenic animals were generated by restriction enzyme-mediated integration nuclear transplantation (Kroll and Amaya, 1996) with slight modifications (Damjanovski et al, 2002). SfiI restriction enzyme was used to linearise all plasmid constructs. When the transgene was mixed with sperm nuclei and eggs extract, SfiI was also used at 0.25 U. After injection of sperm nuclei, embryos were maintained at 16°C until the second day, when they were transferred to room temperature. To select transgenic animals, fluorescent proteins were observed on anaesthetised (0.01% MS222) intact embryos, under a dissecting scope equipped with fluorescent light.
RNA extraction and RT–PCR analysis
Groups of 20 wild-type embryos at different embryonic stages were collected, flash frozen and stored at −80°C. RNA extraction, RT reactions and PCR analysis were performed as previously described (Sachs and Shi, 2000). PCRs were performed for 30 cycles. The primers used for TRβ cDNA are: forward 5′-CAGAAACCTGAACC CACACAA-3′ and reverse 5′-CACTTTTCCACCCTCGGGCGCATT-3′, and for ornithine decarboxylase (ODC): forward 5′-GTCAATGATG GAGTGTATGGATC-3′ and reverse 5′-TCCATTCCGCTCTCCTTGAG CAC-3′. The primers used for TRα are as previously described (Sachs et al, 2001). The expression of ODC, which is constant during embryogenesis (Duval et al, 1990), was used as an internal control. PCR products were resolved on a 6% acrylamide-TBE gel and visualised by autoradiography. PhosphoImager scanning (Molecular Dynamics) was used to quantify the signals.
Whole-mount ISH, vibratome sections and eyes histology
Whole-mount ISH was performed on stage NF28 embryos according to a standard protocol (Harland, 1991). Following ISH, some embryos were transferred in glycerol and observed under a dissecting scope. Other embryos were fixed for 3 h at 4°C in 4% paraformaldehyde in phosphate-buffered saline (PBS) pH 7.4 and were embedded in 4% agarose in PBS pH 7.4. Vibratome sections (100 μM) were cut, mounted in glycerol and observed under a microscope. Specificity of in situ signals was confirmed by the absence of staining by sense probes (data not shown). For eye histological analysis, embryos were fixed with 1% paraformaldehyde in PBS pH 7.4. After fixation, whole embryos were processed in an automatic tissue processor (Leica, TP1020) and embedded with paraplast. Wax blocks were cast under binocular in order to orientate the embryos. Paraffin sections (10 μm) were cut on a Leica microtome (RM 2235) and mounted on microscopic slides. The sections were dewaxed in two baths of safesolv for 20 min and then rehydrated in descending grades of ethanol. The sections were counterstained with Harris haematoxylin (Sigma) and eosin (Sigma). They were dehydrated in ascending grades of ethanol before being mounted in DPX (Fluka). All images were taken with a digital camera.
ChIP assay
ChIP was carried out as described (Sachs and Shi, 2000). Five microliters of anti-acetylated histone H4 antiserum (Upstate biotechnology), 5 μl of anti-GFP (Torrey Pines Biolabs, Houston, TX) or 8 μl of anti-X. laevis TR or NCoR anti-serum were used per 10 μg of sonicated chromatin. Immunoprecipitated DNA was analysed by semiquantitative PCR as described (Sachs and Shi, 2000).
Results quantification and statistical analysis
All the quantitative results correspond to means±s.e.m. presented as percentages, percentages of the Ct value or fold induction as indicated. ANOVA test, parametric Student's t or nonparametric Mann and Whitney tests were used where appropriate to assess statistical differences between means. All the data represent one of at least two independent experiments, providing similar conclusions.
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Figure S6
Acknowledgments
We thank Drs M Perron, N Becker, N Pollet and G Levi for helpful discussions. We are indebted to Drs M Perron and D Brown for providing plasmids. We are grateful to WatchFrog for technical assistance. We also thank G Benisti and J-P Chaumeil for animal care. The work was supported by the ‘Centre National de la Recherche Scientifique', the ‘Muséum National d'Histoire Naturelle', the European Coordinated Action ‘XOMICS', the European Network of Excellence ‘CASCADE' and the European Integrated Project ‘CRESCENDO'. EH was supported by the ‘Ministère de l'éducation nationale de la recherche et de la technologie' and the ‘Association pour la Recherche sur le Cancer'.
References
- Banker DE, Bigler J, Eisenman RN (1991) The thyroid hormone receptor gene (c-erbAα) is expressed in advance of thyroid gland maturation during the early embryonic development of Xenopus laevis. Mol Cell Biol 11: 5079–5089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera-Hernandez G, Park KS, Dace A, Zhan Q, Cheng SY (1999) Thyroid hormone-induced cell proliferation in GC cells is mediated by changes in G1 cyclin/cyclin-dependent kinase levels and activity. Endocrinology 140: 5267–5274 [DOI] [PubMed] [Google Scholar]
- Buchholz DR, Hsia SC, Fu L, Shi Y-B (2003) A dominant-negative thyroid hormone receptor blocks amphibian metamorphosis by retaining corepressors at target genes. Mol Cell Biol 23: 6750–6758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DR, Paul BD, Shi Y-B (2005) Gene-specific changes in promoter occupancy by thyroid hormone receptor during frog metamorphosis. J Biol Chem 280: 41222–41228 [DOI] [PubMed] [Google Scholar]
- Chassande O (2003) Do unliganded thyroid hormone receptors have physiological functions? J Mol Endocrinol 31: 9–20 [DOI] [PubMed] [Google Scholar]
- Damjanovski S, Sachs LM, Shi Y-B (2002) Function of thyroid hormone receptors during amphibian development. Methods Mol Biol 202: 153–176 [DOI] [PubMed] [Google Scholar]
- Das B, Cai L, Carter MG, Piao Y-L, Sharov AA, Ko MSH, Brown DD (2006) Gene expression changes at metamorphosis induced by thyroid hormone in Xenopus laevis. Dev Biol 291: 342–355 [DOI] [PubMed] [Google Scholar]
- de Luze A, Sachs L, Demeneix B (1993) Thyroid hormone-dependent transcriptional regulation of exogenous genes transferred into Xenopus tadpole muscle in vivo. Proc Natl Acad Sci USA 90: 7322–7326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval C, Bouvet P, Omilli F, Roghi C, Dorel C, LeGuellec R, Paris J, Osborne HB (1990) Stability of maternal mRNA in Xenopus embryos: role of transcription and translation. Mol Cell Biol 10: 4123–4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C (1995) Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev 9: 2364–2372 [DOI] [PubMed] [Google Scholar]
- Garcia-Silva S, Perez-Juste G, Aranda A (2002) Cell cycle control by the thyroid hormone in neuroblastoma cells. Toxicology 181–182: 179–182 [DOI] [PubMed] [Google Scholar]
- Harland RM (1991) In situ hybridization: an improved whole mount method for Xenopus embryos. In Methods in Cell Biology, Kay BK, Peng HB (eds), Vol. 36, pp 685–695. San Diego: Academic Press [DOI] [PubMed] [Google Scholar]
- Harpavat S, Cepko CL (2003) Thyroid hormone and retinal development: an emerging field. Thyroid 13: 1013–1019 [DOI] [PubMed] [Google Scholar]
- Harris WA, Perron M (1998) Molecular recapitulation: the growth of the vertebrate retina. Int J Dev Biol 42: 299–304 [PubMed] [Google Scholar]
- Havis E, Sachs LM, Demeneix BA (2003) Metamorphic T3-response genes have specific co-regulator requirements. EMBO Rep 4: 883–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll KL, Amaya E (1996) Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development 122: 3173–3183 [DOI] [PubMed] [Google Scholar]
- Lim W, Nguyen N-H, Yang HY, Scanlan TS, Furlow JD (2002) A thyroid hormone antagonist that inhibits thyroid hormone action in vivo. J Biol Chem 277: 35664–35670 [DOI] [PubMed] [Google Scholar]
- Mai W, Janier MF, Allioli N, Quignodon L, Chuzel T, Flamant F, Samarut J (2004) Thyroid hormone receptor a is a molecular switch of cardiac function between fetal and postnatal life. Proc Natl Acad Sci USA 101: 10332–10337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marimuthu A, Feng W, Tagami T, Nguyen H, Jameson JL, Fletterick RJ, Baxter JD, West BL (2002) TR surfaces and conformations required to bind nuclear receptor corepressor. Mol Endocrinol 16: 271–286 [DOI] [PubMed] [Google Scholar]
- Marsh-Armstrong N, Huang H, Remo BF, Liu TT, Brown DD (1999) Asymmetric growth and development of the Xenopus laevis retina during metamorphosis is controlled by type III deiodinase. Neuron 24: 871–878 [DOI] [PubMed] [Google Scholar]
- Morvan-Dubois G, Sebillot A, Kuiper GGJM, Verhoelst CHJ, Darras VM, Visser TJ, Demeneix BA (2006) Deiodinase activity is present in Xenopus laevis during early embryogenesis. Endocrinology (in press) [DOI] [PubMed] [Google Scholar]
- Ng L, Hurley JB, Dierks B, Srinivas M, Salto C, Vennstrom B, Reh TA, Forrest D (2001) A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat Genet 27: 94–98 [DOI] [PubMed] [Google Scholar]
- Nguyen NH, Apriletti JW, Cunha-Lima S, Webb P, Baxter JD, Scanlan TS (2002) Rational design and synthesis of a novel thyroid hormone antagonist that blocks coactivator recruitment. J Med Chem 45: 3310–3320 [DOI] [PubMed] [Google Scholar]
- Nieuwkopp P, Faber J (1956) Normal Table of Xenopus laevis. Amsterdam, North Holland: North Holland Publishing [Google Scholar]
- Ouatas T, Le Mevel S, Demeneix BA, de Luze A (1998) T3-dependent physiological regulation of transcription in the Xenopus tadpole brain studied by polyethylenimine based in vivo gene transfer. Int J Dev Biol 42: 1159–1164 [PubMed] [Google Scholar]
- Privalsky ML (2004) The role of corepressors in transcriptional regulation by nuclear hormone receptors. Annu Rev Physiol 66: 315–360 [DOI] [PubMed] [Google Scholar]
- Puzianowska-Kuznicka M, Damjanovski S, Shi YB (1997) Both TR and RXR are required to efficiently mediate the effects of thyroid hormone on embryonic development and specific gene regulation in Xenopus laevis. Mol Cell Biol 17: 4738–4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogister B, Ben-Hur T, Dubois-Dalcq M (1999) From neural stem cells to myelinating oligodendrocytes. Mol Cell Neurosci 14: 287–300 [DOI] [PubMed] [Google Scholar]
- Sachs LM, Shi Y-B (2000) Targeted chromatin binding and histone acetylation in vivo by thyroid hormone receptor during amphibian development. Proc Natl Acad Sci USA 97: 13138–13143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs LM, Amano T, Rouse N, Shi Y-B (2001) Requirement of histone deacetylase at two distinct steps in thyroid hormone receptor mediated gene regulation during amphibian development. Dev Dyn 222: 280–291 [DOI] [PubMed] [Google Scholar]
- Sachs LM, Jones PL, Havis E, Rouse N, Demeneix BA, Shi Y-B (2002) Nuclear receptor corepressor recruitment by unliganded thyroid hormone receptor in gene repression during Xenopus laevis development. Mol Cell Biol 22: 8527–8538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AM, Das B, Huang H, Marsh-Armstrong N, Brown DD (2001) Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. Proc Natl Acad Sci USA 98: 10739–10744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y-B (1999) Amphibian Metamorphosis. From Morphology to Molecular Biology. New York, USA: John Wiley &Sons [Google Scholar]
- Stolow MA, Shi YB (1995) Xenopus sonic hedgehog as a potential morphogen during embryogenesis and thyroid hormone-dependent metamorphosis. Nucleic Acids Res 23: 2555–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Kubota M, Shinohara K, Yasuda K, Kato JY (2003) In vivo analysis of the cyclin D1 promoter during early embryogenesis in Xenopus. Cell Struct Funct 28: 165–177 [DOI] [PubMed] [Google Scholar]
- Wolffe AP (1997) Sinful repression. Nature 387: 16–17 [DOI] [PubMed] [Google Scholar]
- Wong J, Shi Y-B, Wolffe AP (1997) Determinants of chromatin disruption and transcriptional regulation instigated by thyroid hormone receptor: hormone-regulated chromatin disruption is not sufficient for transcriptional activation. EMBO J 16: 3158–3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen PM (2001) Physiological and molecular basis of thyroid hormone action. Physiol Rev 81: 1097–1142 [DOI] [PubMed] [Google Scholar]
- Yaoita Y, Brown DD (1990) A correlation of thyroid hormone receptor gene expression with amphibian metamorphosis. Genes Dev 4: 1917–1924 [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Rovet J (2004) Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol 16: 809–818 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Figure S6


