Abstract
Molecular and intra-cellular mechanisms involved in the regulation of apoptosis processes in endometrial cells are poorly understood and documented. We have investigated the possibility that Akt survival pathway might be involved in the regulation of apoptosis in the uterus during the estrous cycle. Rats with regular estrous cycle (4 days) were killed at different days of estrous cycle (diestrus, proestrus, estrus and metestrus). Uteri were collected and fixed for immunohistochemical staining (IHC) and apoptotic cell death detection by [TdT]-mediated deoxyuridinetriphosphate nick end-labelling (TUNEL) or endometrial protein extracts collected for Western analysis. TUNEL analysis revealed that apoptosis was mainly found at estrus compared to other day of estrous cycle. TUNEL positive cells were apparent in luminal epithelial cells only. No apoptotic cells were observed at proestrus. In contrast, proliferation was maximal at proestrus as confirmed with the expression of CDC47/MCM7 (a cell proliferation marker). Intact form of caspase-3 was maximal at proestrus and was reduced only at estrus. Likewise, presence of a specific cleaved caspase-3 fragment was observed only at estrus and IHC revealed that cleaved caspase-3 signal was found in luminal epithelial cells. PTEN protein, a phosphatase involved in the regulation of Akt phosphorylation, was present at all days of estrous cycle and showed no significant regulation in relation to cycle. Expression of phospho-Akt (the activated form of Akt) was present at metestrus, diestrus, and proestrus but decreased significantly at estrus. Akt protein expression was maximal at estrus. IHC revealed that Akt expression was high in both stromal and epithelial cells at estrus. Further studies using ovariectomized rats demonstrated that 17β-estradiol increased endometrial cell proliferation which was accompanied by an increase of both Akt expression and phosphorylation. These results suggest that increased Akt expression and activity in response to estradiol may be an important mechanism to protect endometrial cells from apoptotic triggering and to induce endometrial cell proliferation, whereas inhibition of Akt activity leads to caspase-3 activation and apoptosis in endometrial cells.
Introduction
Apoptosis is a mechanism by which uterine luminal epithelium and glands degenerate in the absence of embryonic factors and regenerate in a cycling fashion through the estrous cycle. Sex steroids (estrogen and progesterone) are directly responsible for the histological and morphogical changes in the uterus during estrous cycle. Studies have shown highest apoptosis expression in lining epithelium at estrus in mouse [1] and rat .[2,3]. Other studies have shown that estrogen induces uterine epithelial cell proliferation and estrogen withdrawal results in cell death [4-6]. However, little is known about the cellular and molecular mechanisms involved in the regulation of apoptosis in the uterus. Since menses are absent in rodents as compared to humans and primates, there must be important mechanisms controlling the balance between survival and death factors in order to maintain integrity of endometrium throughout the estrous cycle, particularly following estrogen-induced proliferation and absence of embryonic signal.
Akt, a serine/threonine protein kinase also known as PKB, is activated by phosphorylation at threonine 308 and serine 473 in response to growth factors or cytokines .[7-9] through phosphatidylinositol 3-kinase (PI 3-K). Once phosphorylated Akt has been shown to 1) phosphorylate and block the action of several pro-apoptotic proteins such as Bad [8], and 2) block cytochrome C release from the mitochondria through the regulation of Bcl-2 [10]. This is supported by the observation that an activated form of Akt is able to block apoptosis [11]. PTEN (phosphatase and tensin homolog deleted in chromosome 10) gene encodes a 403-amino acid polypeptide with lipid phosphatase activity. The PTEN protein dephosphorylates position D3 of phosphatidylinositol 3,4,5-triphosphate (PIP3) and generates inactive PIP2 [12]. PIP3 is a direct product of PI 3-K and regulates PDK1, a kinase that phosphorylates and activates Akt. Thus, PTEN is a key negative regulator of Akt activity [13]. Studies using MCF-7 cells showed that 17β-estradiol effects are mediated through PI 3-K pathway and induced Akt phosphorylation [14,15] indicating that this survival pathway is important for estrogen signaling.
Caspases are well known and documented proteases involved in the activation of apoptosis. Once activated from their proactive forms, caspases target important proteins involved in cell proliferation and survival (for a review see [16]). Caspase-3 is one of the key executioner of apoptosis. During the execution phase of apoptosis, caspase-3 is responsible or in part for the proteolysis of a large number of substrates, each of which contains a common Asp-Xaa-Xaa-Asp (DXXD) motif originally described by Lazebnik et al. [17]. We have recently shown that Akt is also a new target for caspase-3 cleavage, indicating that Akt survival pathway inhibition is an important mechanism for apoptosis activation [18].
Although the regulation and importance of Akt has been described in other systems, the presence and role of Akt has not been documented in the cycling uterus. Recent studies revealed that Akt phosphorylation on serine 473 as well as its nuclear translocation are stimulated by prolactin in decidual cells and act through PI-3K pathway to exert antiapoptotic effect in rat deciduas [19]. It is suggested that Akt may also be an important and regulated survival factor in endometrium during the rat estrous cycle. In the present study, regulation of apoptosis was measured in rat uterus during the four stages of estrous cycle (proestrus, estrus, metestrus and diestrus). Regulation of Akt survival pathway and caspase-3 activation were investigated using immunohistochemistry (IHC) and Western analysis using phospho-specific and specific-cleaved-caspase-3 antibodies to determine their involvement in the regulation of apoptotic processes.
Materials and Methods
Reagents
PhosphoPlus Akt (Ser473), PhosphoPlus Akt (Ser473) IHC specific, Akt, PTEN, procaspase-3 and cleaved specific caspase-3 antibodies were obtained from New England Biolabs (Mississauga, ON). CDC47/MCM7 antibody was obtained from Medicorp (Montréal, QC). β-actin antibody was purchased through Cedarlane (Milton, ON). Vectastain ABC Kit for rabbit IgG was purchased from Vector Laboratories Inc. (Burlingame, CA). In Situ Cell Death detection, Protease Inhibitor Cocktail Tablets, POD and DAB substrate were purchases from Roche (Laval, QC). 17β-Estradiol (E2) was purchased from Laboratoire Mat (Québec, QC).
Animals
Mature Sprague-Dawley female rats (200–225 g) were obtained from Charles River Laboratories Canada. Animals were maintained on standard chow and water, which were available ad libitum , in animal facilities illuminated on a normal 12 hour cycle. All procedures were performed in accordance with guidelines of the Canadian Council on Animal Care for the handling and training of laboratory animals and the Good Health and Animal Care Committee of the Université du Québec à Trois-Rivières. Stages of the estrous cycle were confirmed by vaginal smears. Rats with three regular cycles of 4 days were used in these experiments and killed at various stages of the estrous cycle (diestrus, proestrus, estrus and metestrus). Uteri were collected and fixed for immunohistochemical staining (IHC) and apoptotic cell death detection by [TdT]-mediated deoxyuridinetriphosphate nick end-labelling (TUNEL) or endometrial protein extracts collected by scraping the endometrium for Western analysis. To determine the effect of estrogen, rats were ovariectomized for at least 10 days and then injected with E2. Animals were treated for a total of 3 days and killed after hormone treatment according to previous preliminary time-course studies done in our laboratory (unpublished information). E2 was dissolved with sesame oil, and administered by subcutaneous injection. Sesame oil was injected into control animals. The dose administered was 40 μg/kg/day (E2).
Immunohistochemistry
The uterus was fixed in 4% paraformaldehyde solution and embedded in paraffin. Tissue sections 7 μm thick were mounted on polylysine-coated slides, deparaffinized, rehydrated, and then heated with 10 mM citrate buffer (pH 6). After two wash with PBS, slides were then incubated with 0.3 % hydrogen peroxide in methanol for 30 min to quench endogenous peroxidase activity. After washing with PBS, tissues were incubated with blocking serum (Vectastain ABC Kit) at room temperature for 1 h. Then, a primary antibody (Akt 1:100; CDC47/MCM7 1:200; Phospho-Akt IHC 1:50; PTEN 1:100 and cleaved caspase-3 1:50) was added to the slides and incubated at 4° C overnight. After washing 5 min in PBS, tissue sections were incubated for 30 min with 3 μg/ml biotinylated antibody (anti-rabbit or anti-mouse). Subsequently, slides were washed with PBS and incubated with avidin-biotin complex reagent containing horseradish peroxidase for 30 min. Again washed with PBS for 5 min and color development was achieved using DAB substrate. The tissue sections were counterstained with haematoxylin. Negative controls were performed using the same protocol without primary antibody.
Terminal deoxynucleotidyl transferase-mediated nick end-labeling (TUNEL)
Tissue sections were deparaffinized, rehydrated and rinsed with PBS. They were incubated with proteinase K (20 μg/ml) for 30 min at room temperature. Slides were washed two times with PBS, the endogenous peroxidase was inactivated with 0.3 % hydrogen peroxide in methanol for 30 min. Slides were rinsed and incubated with 10 mM citrate solution, two min on ice. Then, tissue sections were rinsed with PBS and incubated with TdT labelling reaction (In Situ Cell Death Detection, POD) for 30 min at 37°C in humidified environment. Slides were washed three times in PBS and tissue sections were blocked with 3% BSA for 20 min at room temperature. Converter-POD solution was added to the slides and incubated 30 min at 37°C in humidified environment. Slides were washed 5 min in PBS, colour development was achieved using DAB substrate and counterstained with haematoxylin. Negative control was performed using the same protocol without TdT enzyme.
Protein extraction and Western analysis
Endometrium from each uterus was scraped using a glass microscope slide and homogenized using a pipette in the lysis buffer (PBS 1X pH 7.4; 1% Nonidet P-40; 0.5% Sodium deoxycholate; 0.1% SDS; Protease Inhibitor Cocktail Tablets (Roche)). Homogenates were centrifuged (12,000 × g for 20 min at 4°C) to remove insoluble material. The supernatant was recovered and stored at -20°C pending analysis. Protein content was determined with the Bio-Rad DC Protein Assay. Concentrations of reagents found in the lysis buffer were chosen to avoid any interference with the protein assay. Protein extracts (50 μg) were heated at 94°C for 3 min, resolved by 10% SDS-PAGE and electrotransferred to nitrocellulose membranes using a semidry transfer (Bio-Rad, Mississauga, ON). The membranes were then blocked 2 h at room temperature with PBS containing 5 % milk powder, then incubated with Akt 1:1000 ; Procaspase-3 1:1000; CDC47/MCM7 1:1000; Phospho-Akt 1:250; PTEN 1:500 and cleaved caspases 1:1000 and subsequently with horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody (1:3000; room temperature for 45 min). All membranes were reprobed with a antibody specific to β-actin which was used as an internal standard. Densitometrical analyses were performed on both films (protein of interest and β-actin) using the GelDoc 2000 and the Quantity One software (Bio-Rad, Mississauga, ON). Results are expressed as a ratio protein of interest/β-actin to correct for loading for each endometrial sample.
Statistical analysis
Western analyses of cycling animals were repeated six to eight times (6 to 8 different rats/endometrial extract per day of estrous cycle). Endometrial extracts from each rats was assessed individually. Western analyses of ovariectomized rats treated with E2 were repeated 5 times (5 different rats/ endometrial extracts per group including control). Endometrial extracts from each rats was assessed individually for both studies. Results subjected to statistical analyses were expressed as means ± SEM. Data were subjected to one-way ANOVA (PRISM software version 4.0; GraphPad, San Diego, CA). Differences between experimental groups were determined by the Tukey's test.
Results
Apoptosis and proliferation analyses through the estrous cycle
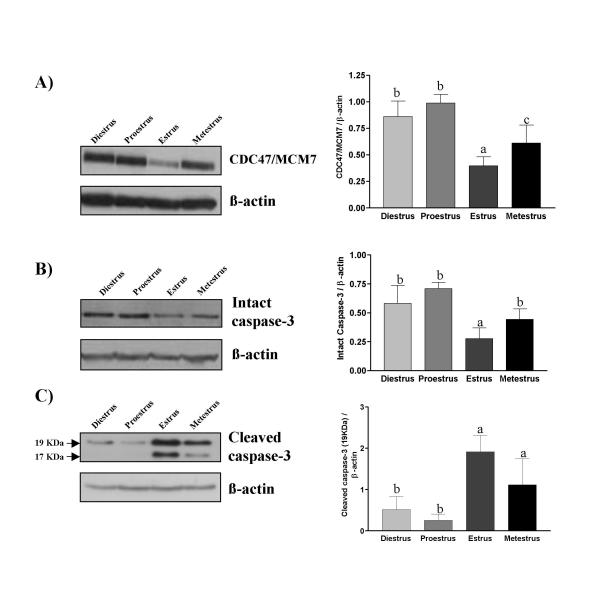
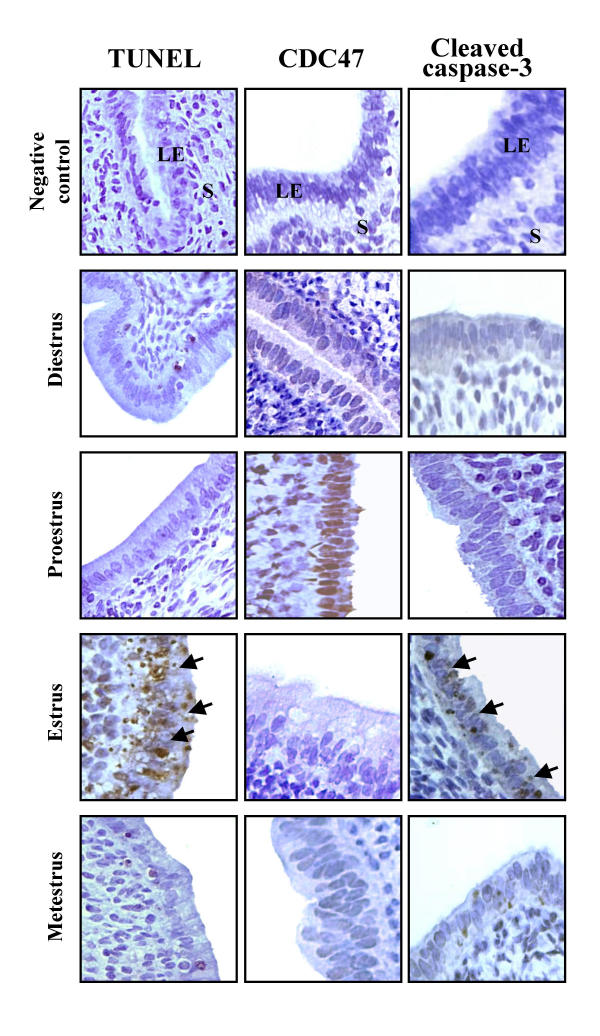
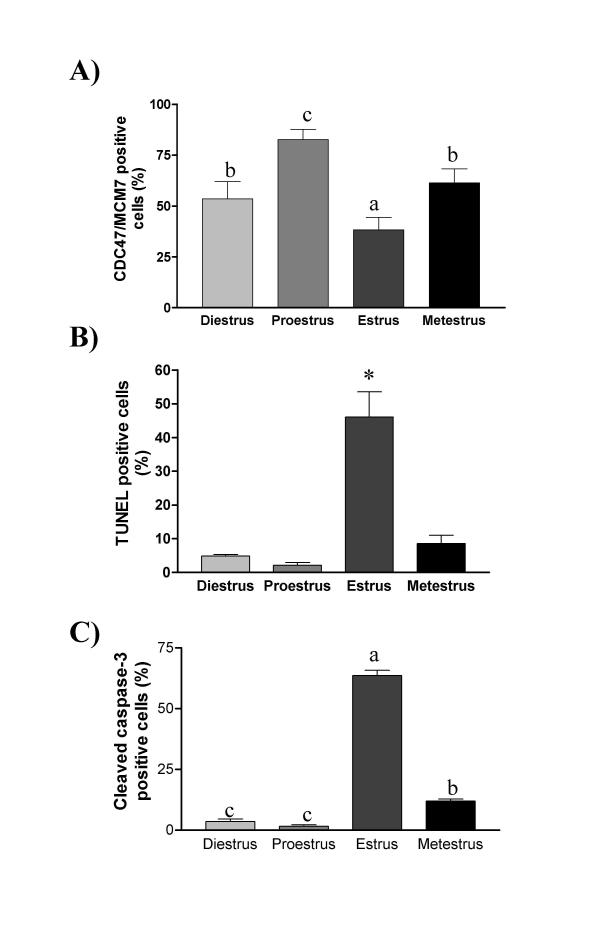
To confirm proliferation and cell death status of endometrial cells in our current model, uteri of cycling rats were recovered to perform TUNEL, IHC and Western analyses. Estrous cycle determination was carried out using vaginal smear and animals were classified according to the type of cell present in the smear. TUNEL analysis showed that apoptosis was maximal at estrus, present at metestrus and weakly detectable at diestrus and proestrus (Fig. 1, 2 and 3). Apoptosis was mainly located in the luminal epithelial cells at estrus. These results were in accordance with results obtained previously [3]. On the other hand, cell proliferation was maximal at proestrus as determined by the expression of CDC47/MCM7 (cell proliferation marker) and was significantly reduced at estrus. Intact form of caspase-3 (Procaspase-3) was maximal at proestrus, metestrus and diestrus but was reduced only at estrus (Fig. 1 and 3). Caspase-3 was active mainly at estrus, as demonstrated by the presence of a specific cleaved fragment using Western and IHC analyses. As shown by IHC, Western analysis revealed that CDC47/MCM7 was reduced at estrus compared to other days of the estrous cycle.
Figure 1.
Proliferation and apoptosis in the rat endometrium during the estrous cycle as demonstrated by Western analyses. Rats were killed at different days of estrous cycle (diestrus, proestrus, estrus and metestrus) and total endometrial proteins were collected. A) Proliferation as determined by Western analysis of CDC47/MCM7. B) Apoptosis as determined by Western analysis of intact and cleaved caspase-3. Data represent the mean ± SEM of six independent experiments for CDC47/MCM7 and five for intact and cleaved caspase-3. β-actin blots shown were used as controls to correct for loading in each lane. Blots shown are from one representative experiment. Graphics represent Western blots densitometrical analysis. Columns with different superscript are significantly different (different letters are different from each other) (p < 0.05).
Figure 2.
Proliferation and apoptosis in the rat endometrium during the estrous cycle as demonstrated by TUNEL and IHC. Rats were killed at different days of estrous cycle (diestrus, proestrus, estrus and metestrus). Uteri were collected, fixed and sectioned for detection of apoptotic cells by TUNEL and IHC of CDC47/MCM7 and cleaved caspase-3. IHC and TUNEL shown are from one representative experiment and were repeated 6 times using 6 different uterine sections from 6 different rats per day of estrous cycle. Arrows indicate example of apoptotic cells. L = lumen, LE = luminal epithelium, S = stroma.
Figure 3.
Apoptotic and proliferative endometrial cell count during the estrous cycle. TUNEL and IHC of CDC47/MCM7 were used to count positive apoptotic and proliferative luminal epithelial cells as shown in figure 2. A minimum of 200 luminal epithelial cells per day of estrous cycle were counted in each experiment and results are presented as the percentage of proliferative positive-cells (A), % of TUNEL apoptotic-positive cells (B), and % of cleaved caspase-3 positive cells (C). Data represent the mean ± SEM of six different rat endometrial sections per day of estrous cycle. Columns with different superscript are significantly different (different letters are different from each other) (p < 0.05). *Significantly different (p < 0.05).
Regulation of Akt and PTEN expression and activity during the estrous cycle
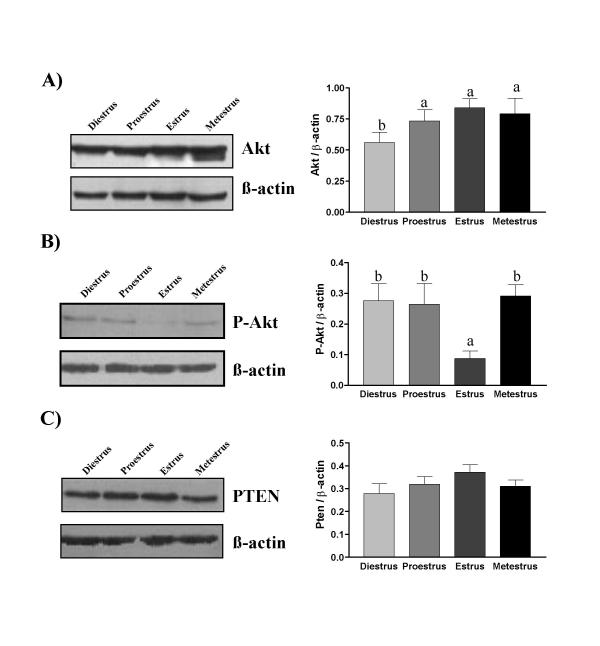
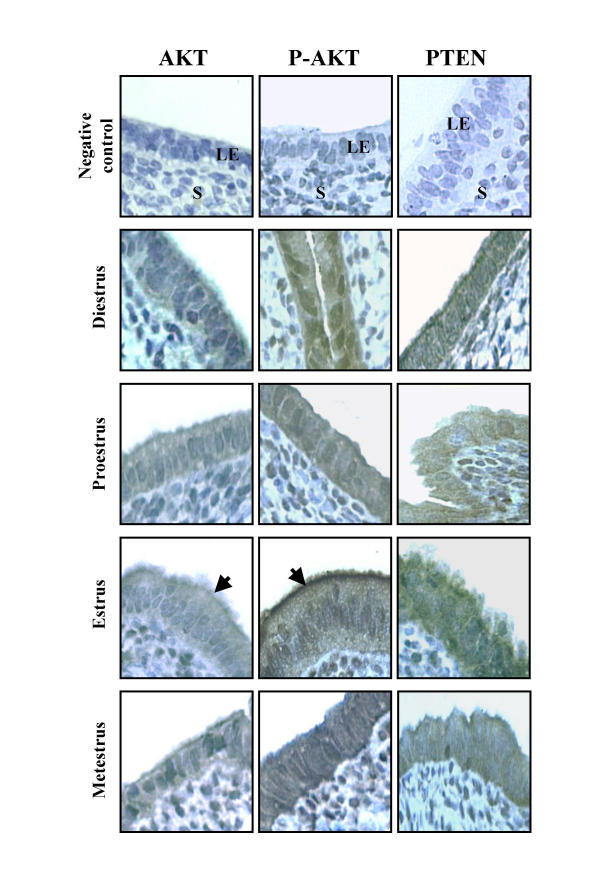
In order to determine the possibility that Akt and PTEN might be regulated by sex steroids during estrous cycle, Akt and PTEN protein abundance were measured by Western analyses and localized by IHC. PTEN protein was present at all days of estrous cycle and the estrous cycle did not influence expression of this protein significantly (Fig. 4 and 5). However, expression of total Akt protein was increased from proestrus to estrus. Akt and PTEN proteins were mainly localized in the luminal and glandular epithelium as demonstrated by IHC (Fig. 5). Phospho-Akt (the activated form of Akt) was present at metestrus, diestrus and proestrus but was significantly reduced 3.1 fold at estrus (Fig. 4). IHC revealed that phospho-Akt was primarily located in the luminal and glandular epithelial cells (Fig. 5). Interestingly, as shown by Western analysis, IHC confirmed that phospho-Akt expression was lower at estrus and that phospho-Akt was predominantly localized at the membrane level facing the uterine lumen. In other days of estrous cycle (metestrus, diestrus and proestrus), phospho-Akt signal was found mainly in the cytoplasm and nucleus. These results clearly demonstrate a redistribution of Akt protein from the cytoplasm to cell membrane only at estrus.
Figure 4.
Expression of Akt, Phospho-Akt and PTEN during the estrous cycle. Endometrial proteins were extracted from uteri at different days of estrous cycle for Western analysis: A) Akt, B) Phospho-Akt and C) PTEN. β-actin blots shown were used as controls to correct for loading in each lane. Graphics represent Western blots densitometrical analysis: Akt (mean ± SEM of seven independent experiments), Phospho-Akt (mean ± SEM of six independent experiments) and PTEN (mean ± SEM of eight independent experiments). Blots shown are from one representative experiment. Columns with different superscript are significantly different (different letters are different from each other) (p < 0.05).
Figure 5.
IHC of Akt, Phospho-Akt and PTEN in rat endometrium during the estrous cycle. IHC shown are from one representative experiment and were repeated 6 times using 6 different uterine sections from 6 different rats per day of estrous cycle. Arrows indicates strong redistribution of Akt and Phospho-Akt at the membrane level. LE = luminal epithelium, S = stroma.
Influence of 17β-estradiol in the regulation of Akt expression and phosphorylation
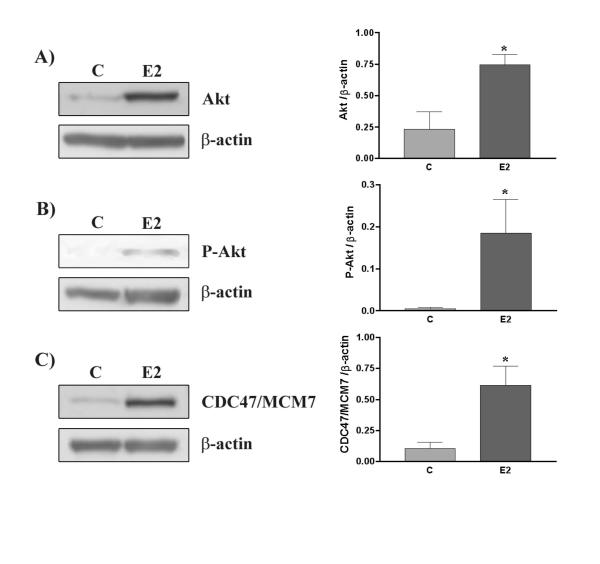
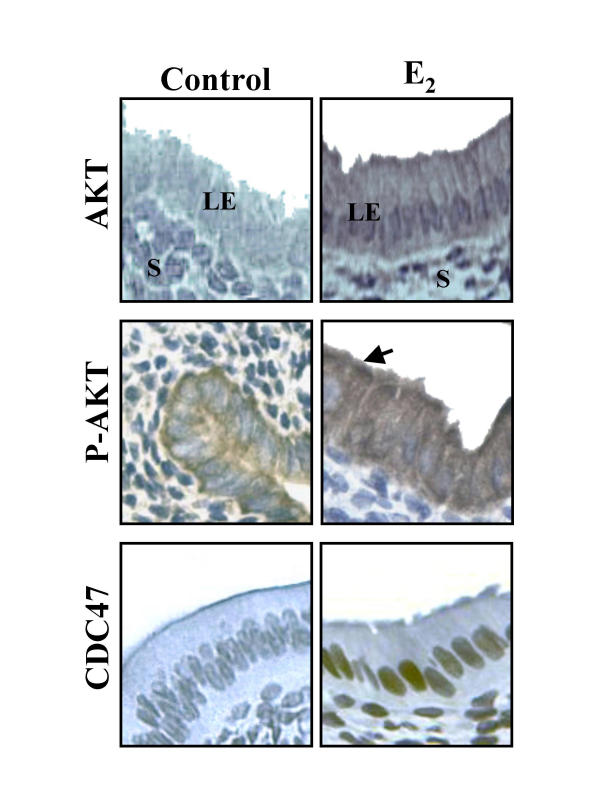
To further determine if 17β-estradiol might be involved in the regulation Akt phosphorylation/activity and expression, ovariectomized rats were treated with 17β-estradiol for 3 days. The results indicated that 17β-estradiol increased endometrial cell proliferation significantly (as determined by the expression of CDC47/MCM7) (Fig. 6). Furthermore, 17β-estradiol significantly induced both total Akt protein expression and phosphorylation. IHC revealed that 17β-estradiol induced both Akt expression and phosphorylation and was found at the cellular membrane as observed at estrus. IHC studies confirmed in ovariectomized rats treated by 17β-estradiol that both Akt expression and phosphorylation was increased in endometrial cells (Fig. 7). Increased endometrial cell proliferation (epithelial and stromal) in response to 17β-estradiol was confirmed using CDC47/ MCM7 proliferation marker (Fig. 7).
Figure 6.
Expression of CDC47/MCM7 (A), Akt (B) and Phospho-Akt (C) in response to 17β-estradiol (E2) in rat endometrium. Animals were ovariectomized for at least 10 days and then injected daily with E2 (or vehicle for control group) for 3 days. Rats were killed and endometrial proteins were collected for Western analysis. β-actin blots shown were used as controls to correct for loading in each lane. Blots shown are from one representative experiment. Graphics represent western blots densitometrical analysis and are the mean ± SEM of five independent experiments (5 different rats/endometrial extract per treatment group). *Significantly different from control (p < 0.001).
Figure 7.
IHC of CDC47/MCM7, Akt and Phospho-Akt in response to 17β-estradiol (E2) in rat endometrium. Animals were ovariectomized for at least 10 days and then injected daily with E2 (or vehicle for control group) for 3 days. Rats were killed and their uteri collected, fixed and sectioned for IHC. IHC shown are from one representative experiment and were repeated five times using five different uterine sections from five different rats per day of estrous cycle. Arrow indicates strong redistribution of Phospho-Akt at the membrane level. LE = luminal epithelium, S = stroma.
Discussion
This study is the first to show the presence, activity and regulation of Akt in the non-pregnant rat uterus. This survival factor is well known in other systems for its inhibitory effect on apoptosis triggering. Apoptosis has been shown to be present in the rat .[2,3] and mouse [1] endometrium during the estrous cycle. Apoptotic index was found to be high at estrus which was accompanied with a low level of estrogen receptor-α and high level of progesterone receptor. Since in the endometrium, growth of epithelial cells is dependent on estrogens and progesterone, and removal of ovarian hormones has been reported to cause cell death in rabbit and hamster [20,21], sex steroids take part actively in the balance of proliferation versus cell death in this tissue. Indeed, the appearance of apoptosis in epithelial cells of the endometrium following ovariectomy or treatment with the antiprogestin RU486 has been described in pseudopregnant rabbits [22] and in primary cell culture [23,24]. However, molecular and cellular mechanisms involved in the regulation of apoptosis in the uterus are poorly documented in the literature. We have recently demonstrated in human ovarian cancer cells [18] and rat granulosa cells [25] the importance of Akt phosphorylation status in relation to cell survival and chemoresistance. Many other studies have demonstrated the involvement of Akt on apoptosis inhibition through the activation of several survival factors [26]. Since apoptosis has been found in the endometrium and that a recent study showed activation of Akt in response to estradiol [27], we hypothesized that Akt might be an important regulator of uterine function throughout the rat estrous cycle. Given that menses are absent in the rodents uterus, there must be precise intra-cellular systems involved in the regulation of cell death and cellular cleaning (proliferation versus apoptosis) in the endometrium after estrogen withdrawal in the absence of embryonic factors.
As demonstrated previously by others [3], apoptosis was evident in luminal epithelial cells at estrus and proliferation was maximal at proestrus. As hypothesized, Akt activity/phosphorylation was high at proestrus and Akt was strongly expressed and localized in the lining epithelium and glands. Indeed, Akt activity/phosphorylation decreased at estrus whereas cell proliferation was low as demonstrated by the decrease of CDC47/MCM7 proliferation marker. Although, Akt phosphorylation was reduced at estrus, IHC revealed a specific pattern of phospho-Akt expression in luminal epithelial cells and was distributed mainly at the membrane level facing the uterine lumen. Translocation at the plasma membrane of phospho-Akt from the nucleus and/or cytoplasm suggests that its presence at the membrane level may not allow phospho-Akt to be active in term of survival signaling. A recent study demonstrated that transient membrane association is required for the physiological activation of Akt indicating that correct subcellular localization is crucial for the activation of the kinase, and it may also allow its appropriate inactivation by phosphatases [28]. One example of such phosphatase is phosphatase and tensin homologue tumor suppressor PTEN [13], a phosphatase found and active at the plasma membrane .[29]. PTEN directly dephosphorylates the phospholipid PIP3 which is essential for Akt phosphorylation [12]. The present results clearly show that PTEN protein was present in the endometrium and was not influenced by hormonal changes observed during the estrous cycle. Indeed, recruitment of Akt at the membrane level may be a mechanism by which PTEN or unknown phosphatases act on Akt to inactivate its function.
Since the levels of estrogen are maximal at proestrus and further decrease at estrus, we have used ovariectomized rats to determine the possible involvement of 17β-estradiol in the regulation of Akt phosphorylation. Akt and estrogen have been shown to be involved in the regulation and downstream signaling in different systems such as EGF, IGF-1 and GH [30,31], endothelial nitric oxide synthase (eNOS) .[32], and FSH [33] regulation pathways. Recently, using both wild-type (WT) and ER alpha knockout (alpha ERKO) mice, it has been shown that ERα is necessary for IGF-1 induction of uterine nuclear proliferative responses and that IGF-1 signaling is dependent of Akt .[34]. Moreover in ERKO mice, lower levels of vascular nitric oxide has been found suggesting a crucial role of ERα in the regulation of these processes [35]. A recent study showed that there was an apparent increase in Akt activity upon brief stimulation with 17β-E2 in CHO cells transfected with ERα whereas 17β-E2 had no effect on Akt activity in cells transfected with control vector or ERβ [36]. The present results clearly showed an increase of Akt phosphorylation in response to 17β-estradiol. As shown in another system [36], it is possible that the activation of estrogen receptor may activate Akt phosphorylation through a signaling mechanism not yet identified in the endometrium. PTEN protein expression was constant through the stages of estrous cycle studied indicating that PTEN is a constitutively expressed protein. In the human endometrium, PTEN has been shown to be regulated by progesterone [37]. However, the rat uterus does not undergo decidualization during the estrous cycle as compared to the menstrual cycle in women. Thus, PTEN might be an important protein involved in embryo implantation processes when decidualization initiate rather than a role during the regulation of estrous cycle in the rat endometrium.
Recent studies demonstrated that the transcriptional nuclear factor kappa-B (NF-κB) is a direct downstream target of phosphorylated Akt [38]. NF-κB is sequestered (p65 and p50 subunits) in the cytoplasm by the IκBs inhibitors which are phosphorylation targets of Akt. Upon phosphorylation IκBs are released and degradated through ubiquitination and NF-κB enter nucleus for gene expression [39]. In the literature, we found that X-linked inhibitor of apoptosis protein (XIAP) promoter is a target for NF-κB [40,41]. Since we have shown previously in rat granulosa cells and human ovarian surface epithelial cancer cells that XIAP might be involved in the regulation of Akt activity .[18,25], it is possible that Akt may act on this family of inhibitor of apoptosis protein in order to block apoptosis processes. Whether XIAP or other inhibitor of apoptosis proteins are involved in this process remains to be investigated.
In conclusion, these results document for the first time the presence and regulation of Akt in the rat endometrium during the estrous cycle and further demonstrate that this survival factor is regulated by 17β-estradiol. Further analysis will be necessary to determine more specifically, the intra-cellular and molecular signal transducers involved in the process of apoptosis in the rat reproductive tract. Whether TNF-α or other cytokines/growth factors such as TGF-β, IGF-1 or EGF may be involved in the regulation of programmed cell death through Akt and inhibitors of apoptosis proteins in the rat uterus remain to be elucidated. Currently, investigations are carried out to further characterize the role and mechanism of Akt on cell survival/apoptosis using endometrial (epithelial and stromal cells) cultured in vitro .
Acknowledgments
Acknowledgments
This work has been supported by a grant from NSERC (238501-01). Eric Asselin is a chercheur-boursier from the Fond de la Recherche en Santé du Québec (FRSQ). We are grateful to Mrs Rollande Caron for her support and her precious advices in regards to animal care and maintenance.
Contributor Information
Marie-Claude Dery, Email: Marie-Claude_Dery@uqtr.ca.
Valerie Leblanc, Email: Valerie_Leblanc@uqtr.ca.
Carl Shooner, Email: Carl_Shooner@uqtr.ca.
Eric Asselin, Email: Eric_Asselin@uqtr.ca.
References
- Dharma SJ, Kholkute SD, Nandedkar TD. Apoptosis in endometrium of mouse during estrous cycle. Indian J Exp Biol. 2001;39:218–222. [PubMed] [Google Scholar]
- Lai MD, Lee LR, Cheng KS, Wing LY. Expression of proliferating cell nuclear antigen in luminal epithelium during the growth and regression of rat uterus. J Endocrinol. 2000;166:87–93. doi: 10.1677/joe.0.1660087. [DOI] [PubMed] [Google Scholar]
- Sato T, Fukazawa Y, Kojima H, Enari M, Iguchi T, Ohta Y. Apoptotic cell death during the estrous cycle in the rat uterus and vagina. Anat Rec. 1997;248:76–83. doi: 10.1002/(SICI)1097-0185(199705)248:1<76::AID-AR9>3.3.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Quarmby VE, Korach KS. The influence of 17 beta-estradiol on patterns of cell division in the uterus. Endocrinology. 1984;114:694–702. doi: 10.1210/endo-114-3-694. [DOI] [PubMed] [Google Scholar]
- Finn CA, Publicover M. Hormonal control of cell death in the luminal epithelium of the mouse uterus. J Endocrinol. 1981;91:335–340. doi: 10.1677/joe.0.0910335. [DOI] [PubMed] [Google Scholar]
- Martin L, Pollard JW, Fagg B. Oestriol, oestradiol-17beta and the proliferation and death of uterine cells. J Endocrinol. 1976;69:103–115. doi: 10.1677/joe.0.0690103. [DOI] [PubMed] [Google Scholar]
- Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, Gaffney PR, Reese CB, McCormick F, Tempst P, Coadwell J, Hawkins PT. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- Hayakawa J, Ohmichi M, Kurachi H, Kanda Y, Hisamoto K, Nishio Y, Adachi K, Tasaka K, Kanzaki T, Murata Y. Inhibition of BAD phosphorylation either at serine 112 via extracellular signal-regulated protein kinase cascade or at serine 136 via Akt cascade sensitizes human ovarian cancer cells to cisplatin. Cancer Res. 2000;60:5988–5994. [PubMed] [Google Scholar]
- Suzuki Y, Nakabayashi Y, Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Natl Acad Sci U S A. 2001;98:8662–8667. doi: 10.1073/pnas.161506698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MA, Koul D, Dhesi H, Berman R, McDonnell TJ, McConkey D, Yung WK, Steck PA. Regulation of Akt/PKB activity, cellular growth, and apoptosis in prostate carcinoma cells by MMAC/PTEN. Cancer Res. 1999;59:2551–2556. [PubMed] [Google Scholar]
- Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- Duan R, Xie W, Li X, McDougal A, Safe S. Estrogen regulation of c-fos gene expression through phosphatidylinositol-3-kinase-dependent activation of serum response factor in MCF-7 breast cancer cells. Biochem Biophys Res Commun. 2002;294:384–394. doi: 10.1016/S0006-291X(02)00499-0. [DOI] [PubMed] [Google Scholar]
- Castoria G, Migliaccio A, Bilancio A, Di Domenico M, de Falco A, Lombardi M, Fiorentino R, Varricchio L, Barone MV, Auricchio F. PI3-kinase in concert with Src promotes the S-phase entry of oestradiol-stimulated MCF-7 cells. EMBO J. 2001;20:6050–6059. doi: 10.1093/emboj/20.21.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- Asselin E, Mills GB, Tsang BK. XIAP regulates Akt activity and caspase-3-dependent cleavage during cisplatin-induced apoptosis in human ovarian epithelial cancer cells. Cancer Res. 2001;61:1862–1868. [PubMed] [Google Scholar]
- Tessier C, Prigent-Tessier A, Ferguson-Gottschall S, Gu Y, Gibori G. PRL antiapoptotic effect in the rat decidua involves the PI3K/protein kinase B-mediated inhibition of caspase-3 activity. Endocrinology. 2001;142:4086–4094. doi: 10.1210/endo.142.9.8381. [DOI] [PubMed] [Google Scholar]
- Nawaz S, Lynch MP, Galand P, Gerschenson LE. Hormonal regulation of cell death in rabbit uterine epithelium. Am J Pathol. 1987;127:51–59. [PMC free article] [PubMed] [Google Scholar]
- Sandow BA, West NB, Norman RL, Brenner RM. Hormonal control of apoptosis in hamster uterine luminal epithelium. Am J Anat. 1979;156:15–35. doi: 10.1002/aja.1001560103. [DOI] [PubMed] [Google Scholar]
- Nawaz S, Lynch MP, Galand P, Gerschenson LE. Hormonal regulation of cell death in rabbit uterine epithelium. Am J Pathol. 1987;127:51–59. [PMC free article] [PubMed] [Google Scholar]
- Gerschenson LE, Depaoli JR, Murai JT. Inhibition of estrogen-induced proliferation of cultured rabbit uterine epithelial cells by a cell density-dependent factor produced by the same cells. J Steroid Biochem. 1981;14:959–969. doi: 10.1016/0022-4731(81)90203-X. [DOI] [PubMed] [Google Scholar]
- Lynch MP, Nawaz S, Gerschenson LE. Evidence for soluble factors regulating cell death and cell proliferation in primary cultures of rabbit endometrial cells grown on collagen. Proc Natl Acad Sci U S A. 1986;83:4784–4788. doi: 10.1073/pnas.83.13.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin E, Wang Y, Tsang BK. X-linked inhibitor of apoptosis protein activates the phosphatidylinositol 3-kinase/Akt pathway in rat granulosa cells during follicular development. Endocrinology. 2001;142:2451–2457. doi: 10.1210/endo.142.6.8080. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Stoica GE, Franke TF, Wellstein A, Czubayko F, List HJ, Reiter R, Morgan E, Martin MB, Stoica A. Estradiol Rapidly Activates Akt via the ErbB2 Signaling Pathway. Mol Endocrinol. 2003. [DOI] [PubMed]
- Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 2002;109:599–610. doi: 10.1016/s0092-8674(02)00745-6. [DOI] [PubMed] [Google Scholar]
- Martin MB, Franke TF, Stoica GE, Chambon P, Katzenellenbogen BS, Stoica BA, McLemore MS, Olivo SE, Stoica A. A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology. 2000;141:4503–4511. doi: 10.1210/endo.141.12.7836. [DOI] [PubMed] [Google Scholar]
- Shoba LN, Newman M, Liu W, Lowe W.L.,Jr. LY 294002, an inhibitor of phosphatidylinositol 3-kinase, inhibits GH-mediated expression of the IGF-I gene in rat hepatocytes. Endocrinology. 2001;142:3980–3986. doi: 10.1210/endo.142.9.8394. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Varone G, Fornari L, Mannella P, Luisi M, Labrie F, Genazzani AR. Genomic and nongenomic mechanisms of nitric oxide synthesis induction in human endothelial cells by a fourth-generation selective estrogen receptor modulator. Endocrinology. 2002;143:2052–2061. doi: 10.1210/endo.143.6.8749. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Robayna IJ, Falender AE, Ochsner S, Firestone GL, Richards JS. Follicle-Stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-lnduced kinase (Sgk): evidence for A kinase-independent signaling by FSH in granulosa cells. Mol Endocrinol. 2000;14:1283–1300. doi: 10.1210/mend.14.8.0500. [DOI] [PubMed] [Google Scholar]
- Klotz DM, Hewitt SC, Ciana P, Raviscioni M, Lindzey JK, Foley J, Maggi A, DiAugustine RP, Korach KS. Requirement of estrogen receptor-alpha in insulin-like growth factor-1 (IGF-1)-induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross-talk. J Biol Chem. 2002;277:8531–8537. doi: 10.1074/jbc.M109592200. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Freay AD, Kauser K, Sukovich D, Burton G, Lubahn DB, Couse JF, Curtis SW, Korach KS. Vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J Clin Invest. 1997;99:2429–2437. doi: 10.1172/JCI119426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamoto K, Ohmichi M, Kurachi H, Hayakawa J, Kanda Y, Nishio Y, Adachi K, Tasaka K, Miyoshi E, Fujiwara N, Taniguchi N, Murata Y. Estrogen induces the Akt-dependent activation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem. 2001;276:3459–3467. doi: 10.1074/jbc.M005036200. [DOI] [PubMed] [Google Scholar]
- Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Eng C. Changes in endometrial PTEN expression throughout the human menstrual cycle. J Clin Endocrinol Metab. 2000;85:2334–2338. doi: 10.1210/jcem.85.6.6652. [DOI] [PubMed] [Google Scholar]
- Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/S0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- Foo SY, Nolan GP. NF-kappaB to the rescue: RELs, apoptosis and cellular transformation. Trends Genet. 1999;15:229–235. doi: 10.1016/S0168-9525(99)01719-9. [DOI] [PubMed] [Google Scholar]
- Stehlik C, de Martin R, Kumabashiri I, Schmid JA, Binder BR, Lipp J. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J Exp Med. 1998;188:211–216. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao CW, Ash K, Tsang BK. Nuclear factor-kappaB-mediated X-linked inhibitor of apoptosis protein expression prevents rat granulosa cells from tumor necrosis factor alpha-induced apoptosis. Endocrinology. 2001;142:557–563. doi: 10.1210/endo.142.2.7957. [DOI] [PubMed] [Google Scholar]