Abstract
The activities of carbapenems against Pseudomonas aeruginosa decreased in the presence of siliconized latex urinary catheters (SLUCs). This effect was associated with the loss of OprD. The zinc that eluted from SLUCs is responsible for this phenomenon. We have found that zinc exerts a negative effect on the expression of OprD, the porin responsible for carbapenem entry into P. aeruginosa.
Pseudomonas aeruginosa is an important cause of urinary tract infections in patients with urinary catheters (17). This organism is able to colonize the surface of the catheter, forming a biofilm that interferes with the activities of antimicrobial agents and host defense mechanisms (7, 13). In vitro P. aeruginosa adheres more efficaciously to siliconized latex urinary catheters (SLUCs) than to other plastic materials (7, 10, 11). SLUCs elute substances that can be used as nutrients by this organism (10). Moreover, the eluate from SLUCs decreases the activities of carbapenems against P. aeruginosa (16). The last effect is not due to drug inactivation or increased beta-lactamase activity but is associated with changes in the outer membrane protein (OMP) profile, that is, the loss of an OprD-like protein and the expression of a new OMP of about 50 kDa. This phenomenon is reversible, which indicates that, by means of some unknown mechanism, the eluate regulates the physiology of P. aeruginosa rather than selects for mutants (9).
OprD is a protein whose primary role is the passive uptake of basic amino acids across the outer membrane of P. aeruginosa, but it forms pores that are also permeable to carbapenems (4, 21, 22). OprD loss reduces the activities of carbapenems against these bacteria (18, 21). OprD expression is very much influenced by environmental conditions. Different amino acids such as arginine, histidine, glutamate, and alanine strongly induce the expression of this porin when they are the only sources of carbon or nitrogen in the environment (14). Weak aromatic acids like acetyl salicylate or benzoate reduce the expression of OprD (15, 20). None of several previously identified organic compounds in SLUC eluates, however, affect OprD expression or the susceptibilities of P. aeruginosa to carbapenems (9).
This study was undertaken in order to investigate which of the SLUC components are responsible for the decreased activities of carbapenems against P. aeruginosa grown in the presence of SLUCs.
To investigate the presence of different elements in urinary catheters, 0.5-cm segments of SLUCs (two-way pediatric silicone-coated latex Foley catheter 8FR/CH; Kendall Co., Kangar, Malaysia) were analyzed by a particle-induced X-ray emission (PIXE) technique (8). After removal of the organic matrix, elemental analysis was carried out with 2.5-MeV protons generated by a Tandem Van de Graff accelerator (9SDH-2; NEC, Middleton, Wis.). The induced X rays were simultaneously detected with a 350-μm-thick Mylar absorber (Dupont, Wilmington, Del.) with an LEGe and Si(Li) detector. The spectra obtained by the PIXE technique were analyzed with an AXIL personal computer (Canberra Packard, Zelik, Belgium). Zinc was the most abundant element found in all the samples, followed by silicon, phosphorus, sulfur, and chlorine. Finally, traces of copper, titanium, chromium, and manganese were also detected.
The eluates from the SLUCs were also investigated for the presence of zinc. For this purpose, eluates were prepared by incubating segments of SLUCs in sterile cation-adjusted Mueller-Hinton broth (MH) at 37°C for 24 h (four 1-cm segments per milliliter of medium). The zinc contents in MH and in the eluates from the SLUCs were determined by inductively coupled plasma atomic emission spectrometry with a sequential multielement instrument (ARL 3410; FISONS Instruments, Valencia, Calif.) (3). The analyses were performed in duplicate and were repeated in three different batches of media. The zinc contents in MH and the eluate were 0.29 ± 0.1 and 36.8 ± 3.2 μg/ml, respectively.
The MICs of imipenem (IPM; Merck Sharp & Dohme, Madrid, Spain) for P. aeruginosa PAO1 and its OprD-deficient mutant were determined by a microdilution assay according to the guidelines of NCCLS (12). The media used were MH, the eluate from the SLUCs, and MH supplemented with zinc acetate dihydrate (Sigma, Madrid, Spain) to achieve final concentrations of 73.6, 36.8, 18.4, 11.2, 5.7, 3.0, 1.65, and 0.97 μg of zinc/ml of medium. Zinc concentrations in MH lower than 5.7 μg/ml did not affect the activity of IPM against P. aeruginosa PAO1, while zinc concentrations between 5.7 and 18.9 μg/ml caused a fourfold increase in the MIC. Finally, when the zinc concentration in MH was equal to that found in the eluate (36.8 μg/ml), the activities of IMP were the same in both MH and the eluate (8 μg/ml). On the other hand, the MICs of IPM for the OprD-deficient mutant were the same (8 μg/ml) in all the media tested.
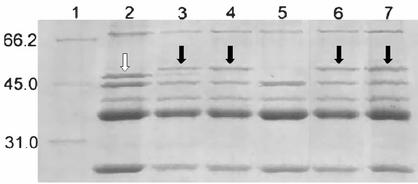
The effect of zinc on the OMP profile of P. aeruginosa grown in MH, eluate, or zinc-supplemented MH (final zinc concentration, 36.8 μg/ml) was evaluated. OMPs were prepared as described previously (5); separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with the buffers described by Laemmli (6), with 10% (wt/vol) acrylamide and 0.1% (wt/vol) bisacrylamide in the running gel; and stained with Coomassie blue. Both P. aeruginosa PAO1 and its OprD-deficient mutant showed the same OMP profiles when they were grown in either the eluate or zinc-supplemented MH (Fig. 1). The OprD-like protein and the 50-kDa protein expressed in the eluate and in zinc-supplemented MH were further analyzed by matrix-assisted laser desorption-ionization time-of-flight (MALDI-TOF) mass spectrometry after in-gel trypsin digestion. Digestion of the proteins (in the gel) was performed as described previously (19), with minor modifications. The digested proteins were analyzed on a Reflex III MALDI-TOF mass spectrometer (Bruker-Franzen Analytic GmbH, Bremen, Germany) equipped with the SCOUT source in the positive ion reflector mode. The ion acceleration voltage was 20 kV. The equipment was first externally calibrated with protonated mass signals from a peptide mixture covering the 1,000- to 4,000-m/z range, and then every spectrum was internally calibrated by using signals arising from trypsin autoproteolysis. Analysis of tryptic fragments from the OMP lost in the eluate and in zinc-supplemented MH showed that they matched OprD (formerly OprD2), and the new OMP expressed in those media was identified as OprD3, as inferred from the PAO genome (PA2505; http://pseudomonas.com).
FIG. 1.
SDS-PAGE showing OMP profiles of P. aeruginosa grown in MH (lanes 2 and 5), eluates from SLUCs (lanes 3 and 6), and zinc-supplemented MH (final zinc concentration, 36.8 μg/ml) (lanes 4 and 7). Lanes: 1, molecular weight markers; 2 to 4, OMP profile of PAO1; 5 to 7, OMP profile of OprD-negative PAO1. White arrow, OprD2; black arrow, OprD3. Numbers on the left are in kilodaltons.
The expression of OprD3 seemed to be unrelated to the decreased susceptibilities of the two P. aeruginosa strains to carbapenems when they were grown in the eluate, as it was expressed by both strains when they were grown in the eluate. Similar results were found for P. aeruginosa strains with different levels of expression of the AmpC beta-lactamase, OprD, and MexA-MexB-OprM and were even found for those strains without changes in carbapenem susceptibility (M. C. Conejo, L. Martínez-Martínez, I. García, and A. Pascual, Abstr. 12th Eur. Congr. Clin. Microbiol., abstr. P1402, 2002). The expression of OprD3 might be a compensatory change that allows the entry of amino acids into P. aeruginosa when OprD2 is repressed.
Different investigators have reported that the concentration of zinc in MH significantly affects the susceptibility of P. aeruginosa to IPM (1, 2). The reason for this observation was unknown. We have found that zinc exerts a negative effect on the expression of OprD2, the porin responsible for carbapenem entry into P. aeruginosa. New studies are in progress in order to evaluate the role of zinc in the regulation of OMP expression in P. aeruginosa. The in vivo relevance of the loss of OprD from P. aeruginosa strains forming biofilms on SLUCs needs to be evaluated.
Acknowledgments
We thank María Dolores Ynsa (Centro Nacional de Aceleradores, Parque Tecnológico Cartuja 93, Seville, Spain) for determinations by the PIXE technique and Juan Antonio Ocaña (Department of Analytical Chemistry, University of Seville) for measurement of the zinc concentrations in the media. The Proteomics Facility of the Centro Nacional de Biotecnología de Madrid (CNB) is acknowledged for its help with the MALDI-TOF mass spectrometry analysis. We are grateful to José Luis Martínez (CNB) for helpful advice throughout this work.
This work was supported by the Dirección General de Investigación del Ministerio de Ciencia y Tecnología (project SAF2000-035).
REFERENCES
- 1.Cooper, G. L., A. Louie, A. L. Baltch, R. C. Chu, R. P. Smith, W. J. Ritz, and P. Michelsen. 1993. Influence of zinc on Pseudomonas aeruginosa susceptibilities to imipenem. J. Clin. Microbiol. 31:2366-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daly, J. S., R. A. Dodge, R. H. Glew, D. T. Soja, B. A. DeLuca, and S. Hebert. 1997. Effect of zinc concentration in Mueller-Hinton agar on susceptibility of Pseudomonas aeruginosa to imipenem. J. Clin. Microbiol. 35:1027-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Floyd, M. A., A. A. Halouma, R. W. Morrow, and R. B. Farrar. 1985. Rapid multielement analysis of water samples by sequential ICP-AES. Am. Lab. 17:84. [Google Scholar]
- 4.Huang, H., and R. E. Hancock. 1993. Genetic definition of the substrate selectivity of outer membrane porin protein OprD of Pseudomonas aeruginosa. J. Bacteriol. 175:7793-7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Köhler, T., M. Michéa-Hamzehpour, U. Henze, N. Gotoh, K. Curty, and J. C. Pechère. 1997. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol. Microbiol. 23:343-354. [DOI] [PubMed] [Google Scholar]
- 6.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680-685. [DOI] [PubMed] [Google Scholar]
- 7.López- López, G., A. Pascual, L. Martínez-Martínez, and E. J. Perea. 1991. Effect of a siliconized latex urinary catheter on bacterial adherence and human neutrophil activity. Diagn. Microbiol. Infect. Dis. 14:1-6. [DOI] [PubMed] [Google Scholar]
- 8.Maenhaut, W., and K. G. Malmqvist. 1993. Particle-induced X-ray emission analysis, p. 719-809. In R. E. Van Grieken and A. A. Markowicz (ed.), Handbook of X-ray spectrometry, practical spectroscopy series, vol. 14. Marcel Dekker, Inc., New York, N.Y.
- 9.Martínez-Martínez, L., A. Pascual, M. C. Conejo, L. Picabea, and E. J. Perea. 1999. Resistance of Pseudomonas aeruginosa to imipenem induced by eluates from siliconized latex urinary catheters is related to outer membrane protein alterations. Antimicrob. Agents Chemother. 43:397-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martínez-Martínez, L., A. Pascual, and E. J. Perea. 1990. Effect of three plastic catheters on survival and growth of Pseudomonas aeruginosa. J. Hosp. Infect. 16:311-318. [DOI] [PubMed] [Google Scholar]
- 11.Martínez-Martínez, L., A. Pascual, and E. J. Perea. 1991. Kinetics of adherence of mucoid and non-mucoid Pseudomonas aeruginosa to plastic catheters. J. Med. Microbiol. 34:7-12. [DOI] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.Nickel, J., I. Ruseska, J. B. Wright, and J. W. Costerton. 1985. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob. Agents Chemother. 27:619-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochs, M. M., C. D. Lu, R. E. Hancock, and A. T. Abdelal. 1999. Amino acid-mediated induction of the basic amino acid-specific outer membrane porin OprD from Pseudomonas aeruginosa. J. Bacteriol. 181:5426-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochs, M. M., M. P. McCusker, M. Bains, and R. E. W. Hancock. 1999. Negative regulation of the Pseudomonas aeruginosa outer membrane porin OprD selective for imipenem and basic amino acids. Antimicrob. Agents Chemother. 43:1085-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pascual, A., L. Martínez-Martínez, E. Ramírez de Arellano, and E. J. Perea. 1993. Susceptibility to antimicrobial agents of Pseudomonas aeruginosa attached to siliconized latex urinary catheters. Eur. J. Clin. Microbiol. Infect. Dis. 12:761-766. [DOI] [PubMed] [Google Scholar]
- 17.Pollack, M. 1990. Pseudomonas aeruginosa, p. 1980-2002. In G. L. Mandell, J. E. Bennet, and R. Dolin (ed.), Principles and practice of infectious diseases, 4th ed. Churchill Livingstone, New York, N.Y.
- 18.Quinn, J. P., E. J. Dudek, C. A. DiVicenzo, D. A. Lucks, and S. A. Lerner. 1986. Emergence of resistance to imipenem during therapy for Pseudomonas aeruginosa infections. J. Infect. Dis. 154:289-294. [DOI] [PubMed] [Google Scholar]
- 19.Shevchenko, A., M. Wilm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68:850-858. [DOI] [PubMed] [Google Scholar]
- 20.Sumita, Y., and M. Fukasawa. 1993. Transient carbapenem resistance induced by salicylate in Pseudomonas aeruginosa associated with suppression of outer membrane protein D2 synthesis. Antimicrob. Agents Chemother. 37:2743-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trias, J., and H. Nikaido. 1990. Outer membrane protein D2 catalyzes facilitated diffusion of carbapenems and penems through the outer membrane of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 34:52-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trias, J., and H. Nikaido. 1990. Protein D2 channel of Pseudomonas aeruginosa outer membrane has a binding site for basic amino acids and peptides. J. Biol. Chem. 265:15680-15684. [PubMed] [Google Scholar]