Abstract
Although glucocorticoid (GC)-induced nongenomic effects have been reported, the underlying mechanisms remain unexplained. We previously described that lymphocyte-specific protein tyrosine kinase (LCK) and FYN oncogene related to SRC, FGR, YES (FYN) mediate GC-induced inhibition of T-cell-receptor (TCR) signalling. Here we characterize the underlying molecular mechanism. The present study shows that the GC receptor is part of a TCR-linked multiprotein complex containing heat-shock protein (HSP)90, LCK and FYN, which is essential for TCR-dependent LCK/FYN activation. Experiments with cells transfected with GC-receptor short interfering RNA (siRNA) showed that the GC receptor is an essential component of the TCR signalling complex. Short-term GC treatment induces dissociation of this protein complex, resulting in impaired TCR signalling as a consequence of abrogated LCK/FYN activation. HSP90siRNA-transfected cells are not able to assemble this TCR-associated multiprotein complex, and accordingly HSP90siRNA treatment mimics GC effects on LCK/FYN activities. These observations support a model for nongenomic GC-induced immunosuppression on the basis of dissolution of membrane-bound GC-receptor multiprotein complexes after GC-receptor ligation.
Keywords: glucocorticoids, glucocorticoid receptor, signal transduction, T cell, nongenomic
Introduction
Glucocorticoids (GCs) mediate their immunosuppressive effects through cytosolic ligand-inducible receptors (Buttgereit et al, 2004). Inactive GC receptors (GRs) are associated with (co)chaperones, such as heat-shock protein 90 (HSP90), which dissociate after GR ligation, followed by nuclear translocation of GR and regulation of gene transcription (Franchimont, 2004; Pratt et al, 2004). GCs also evoke nongenomic effects on cellular function, which occur in minutes (Baus et al, 1996; Croxtall et al, 2000; Rhen & Cidlowski, 2005). Cardiovascular protective effects of GCs that could not be mediated by genomic mechanisms have been reported, because they occurred too fast and could not be blocked by a transcription inhibitor (Hafezi-Moghadam et al, 2002). Recently, we generated a comprehensive profile of such GC-induced rapid effects on signal transduction using activated human CD4+ T lymphocytes and a peptide array for kinome analysis (Lowenberg et al, 2005). The results showed marked early effects of GC treatment, in particular suppressed phosphorylation of LCK/FYN kinase consensus substrates. Further studies showed impaired recruitment of LCK and FYN to the T-cell-receptor (TCR) complex, resulting in reduced LCK/FYN enzymatic activities and impaired TCR signalling after short-term GC treatment. Although these studies identified LCK and FYN as cellular targets for nongenomic GC activities, the underlying mechanism remains unexplained.
LCK and FYN are members of the SRC family of non-receptor tyrosine kinases and have a key role in TCR signalling. TCR activation results in membrane translocation of LCK and FYN in an HSP90-dependent manner (Bijlmakers & Marsh, 2000; Zamoyska et al, 2003; Palacios & Weiss, 2004). LCK predominantly associates with CD4 or CD8 cell-surface receptors and FYN binds to CD3 co-receptors, resulting in LCK and FYN kinase activation. Once activated, LCK and FYN phosphorylate immunoreceptor tyrosine-based activation motifs (ITAMs) on the TCR, allowing downstream signal transduction and eventually T-cell cytokine production, proliferation and differentiation. GRs and SRC-like kinases share a requirement for HSP90 for proper functioning, opening the possibility that GR action on LCK and FYN activities is mediated by HSP90. The present study shows that GCs cause disruption of TCR-associated multiprotein complexes containing GR, HSP90, LCK and FYN, leading to reduced LCK/FYN enzymatic activities and impaired TCR signalling.
Results
DEX inhibits GR–HSP90–LCK/FYN interactions
We examined the action of short-term treatment with the synthetic fluorinated GC dexamethasone (DEX) on the physical interactions of HSP90–LCK and HSP90–FYN. Cell lysates were subjected to LCK and FYN immunoprecipitation and analysed on western blot for the presence of HSP90. These experiments showed HSP90–LCK and HSP90–FYN interactions (Fig 1A), confirming previous studies (Hartson et al, 1996; Bijlmakers & Marsh, 2000; Yun & Matts, 2005). DEX treatment (30 min) resulted in the disappearance of these HSP90–LCK and HSP90–FYN complexes, suggesting that DEX rapidly inhibits HSP90–LCK and HSP90–FYN associations. Next, we investigated whether these DEX-sensitive HSP90–LCK and HSP90–FYN complexes contained GR. Cells were pretreated for 10 min with DEX or dimethyl sulphoxide (DMSO), followed by 15 min activation using CD3 and CD28 antibodies. LCK and FYN immunoprecipitates were immunoblotted for the presence of GR, showing GR–LCK and GR–FYN complexes in activated cells, and these associations disappeared after short-term DEX treatment (Fig 1B). These observations indicate that LCK, FYN, HSP90 and GR are part of a multiprotein complex the integrity of which is sensitive to DEX treatment.
Figure 1.
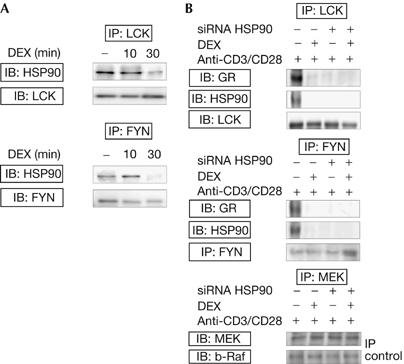
Dexamethasone rapidly inhibits GR–HSP90–LCK and GR–HSP90–FYN associations. (A) Cells were incubated for 10 or 30 min with DEX (1 μM) or solvent-supplemented media (−). LCK and FYN immunoprecipitates were analysed on western blot for the presence of HSP90, and total LCK/FYN levels were analysed to test for equal protein loading. (B) HSP90siRNA-transfected or nontransfected cells were pretreated for 10 min without (−) or with (+) DEX and activated for 15 min with CD3 and CD28 antibodies. Lysates were subjected to LCK and FYN immunoprecipitation followed by western blotting for the presence of GR or HSP90. Equal loading was verified using LCK and FYN antibodies. Supernatants were subjected to immunoprecipitation using MEK antibody, followed by immunoblotting for MEK and b-Raf (negative immunoprecipitation control). Three independent experiments were performed and reproducible results were obtained. DEX, dexamethasone; FYN, FYN oncogene related to SRC, FGR, YES; GR, glucocorticoid receptor; HSP90, heat-shock protein 90; IB, immunoblotting; IP, immunoprecipitation; LCK, lymphocyte-specific protein tyrosine kinase; MEK, mitogen-activated protein kinase kinase.
GR and HSP90-dependent LCK–CD4 or FYN–CD3 binding
HSP90-dependent cellular distribution of LCK and FYN is essential for efficient TCR signalling. To determine the role of HSP90 in LCK–CD4 and FYN–CD3 associations, cells were transfected with HSP90 short interfering RNA (HSP90siRNA), followed by 10 min DEX pretreatment and 15 min activation. LCK–CD4 and FYN–CD3 interactions were detected in CD4 and CD3 immunoprecipitates prepared from activated cells (Fig 2A). These interactions were disrupted on treatment with HSP90siRNA, irrespective of DEX stimulation. Furthermore, GR–CD4 and GR–CD3 associations were seen in activated cells, and treatment with DEX or HSP90siRNA resulted in reduced GR–CD4 and GR–CD3 binding (Fig 2A). Cell lysates were subjected to GR immunoprecipitation, confirming DEX- and HSP90-sensitive GR–LCK and GR–CD4 interactions (supplementary Fig 1 online). In addition, cells were pretreated with the HSP90 inhibitor geldanamycin for 30 min, followed by 10 min DEX treatment and 15 min stimulation. LCK and FYN immunoprecipitates were immunoblotted for the presence of GR, confirming reduced LCK–GR and FYN–GR bindings owing to HSP90 inhibition (Fig 2B). These observations indicate that TCR-associated multiprotein complexes are formed in activated cells (containing GR, HSP90, LCK and FYN), which depend on the presence of HSP90, and are disrupted on short-term DEX incubation. Complex dissolution does not lead to LCK or FYN degradation, as total LCK/FYN protein levels were not affected by HSP90siRNA or DEX (data not shown). Given that HSP90 associates readily with unfolded proteins (such as ZAP70) and can therefore be nonspecifically immunoprecipitated, we studied whether DEX interferes with ZAP70–HSP90 bindings. These data indicated that the observed effects of DEX are specific for SRC-family kinases, as DEX did not inhibit HSP90–ZAP70 interactions in activated T cells (supplementary Fig 2 online). These results were supported by the lack of effect of either DEX or HSP90siRNA on the integrity and functional activity of b-Raf/mitogen-activated protein kinase kinase protein complexes (supplementary Fig 3 online). Hence, DEX treatment does not interfere with protein complex formation per se, but specifically targets LCK–HSP90 and FYN–HSP90 formations. The transfection procedure was evaluated by immunoblotting cellular extracts prepared from cells transfected with or without HSP90siRNA (supplementary Fig 4 online). To investigate further whether the presence of GR is required for efficient TCR signalling, GRsiRNA-transfected cells were pretreated for 10 min with DEX followed by 15 min stimulation. Western blotting showed impaired TCR signalling in GRsiRNA-transfected cells, as indicated by suppressed phosphorylation of several downstream TCR signalling intermediates (Fig 2C). These findings provide direct evidence that the GR is important in mediating efficient TCR signalling. As GRsiRNA might induce nonspecific effects, cells were transfected with control siRNA, demonstrating that the observed effects on TCR signalling are not a result of nonspecific GRsiRNA action (supplementary Fig 5 online).
Figure 2.
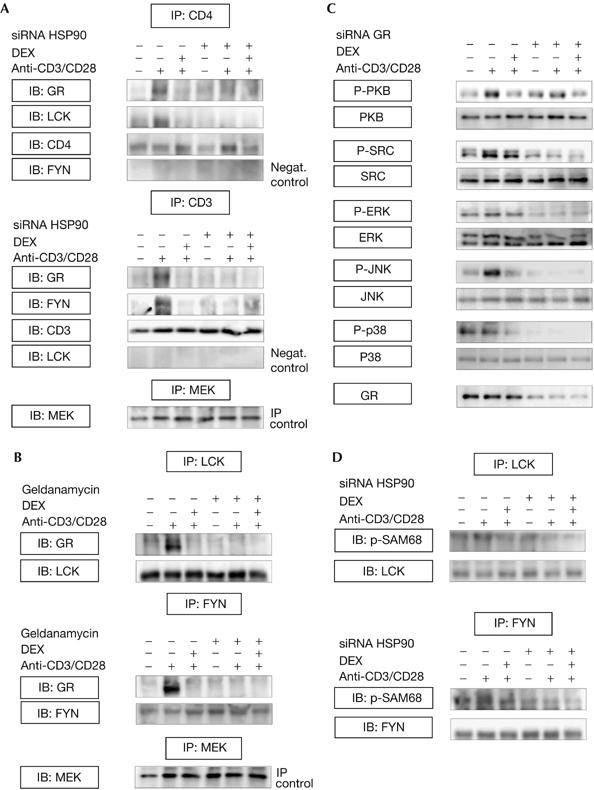
GR and HSP90-dependent LCK–CD4 and FYN–CD3 formations. (A) HSP90siRNA-transfected (+) and nontransfected (−) cells were pretreated for 10 min in the presence (+) or absence (−) of DEX (1 μM) and incubated with (+) or without (−) CD3/CD28 antibodies for 15 min. CD3 and CD4 immunoprecipitates were immunoblotted using the indicated antibodies. To ensure specificity in these experiments, the presence of FYN and LCK was analysed in CD4 and CD3 immunoprecipitates, respectively, demonstrating that specific complexes had been immunoprecipitated containing either FYN and CD3 or LCK and CD4. (B) Cells were pretreated for 30 min with geldanamycin (5 μM), followed by 10 min DEX treatment and 15 min activation (CD3/CD28 antibodies). LCK/FYN immunoprecipitates were immunoblotted for the presence of GR, and LCK and FYN antibodies were used to evaluate for equal loading. An irrelevant protein (that is, MEK) was immunoprecipitated from these lysates followed by western blotting using MEK antibody (immunoprecipitation controls). (C) GRsiRNA-transfected or nontransfected cells were pretreated with DEX (10 min), followed by 15 min activation. Lysates were immunoblotted using phosphospecific antibodies against TCR signalling intermediates, and appropriate antibodies were used to test for equal loading. (D) Cell lysates, treated as described above, were subjected to LCK/FYN immunoprecipitation followed by in vitro kinase assays using SAM68 as a substrate. Phosphorylated SAM68 was immunoblotted with PY20, and equal loading was verified using LCK and FYN antibodies. Experiments were performed three times and representative results are shown. DEX, dexamethasone; ERK, extracellular-signal-regulated kinase; FYN, FYN oncogene related to SRC, FGR, YES; GR, glucocorticoid receptor; IB, immunoblotting; IP, immunoprecipitation; JNK, Jun amino-terminal kinase; LCK, lymphocyte-specific protein tyrosine kinase; MEK, mitogen-activated protein kinase kinase; P, phosphorylated; SAM68, SRC-associated in mitosis.
HSP90siRNA inhibits LCK and FYN activities
To investigate the functional consequences of dissolution of these multiprotein complexes on TCR-induced activation of LCK and FYN, we examined whether HSP90siRNA affects LCK/FYN activities, using LCK and FYN immunoprecipitates and in vitro phosphorylation of SAM68 (SRC-associated in mitosis). TCR stimulation resulted in enhanced SAM68 phosphorylation compared with control cells, confirming TCR-dependent activation of LCK and FYN in these experiments (Fig 2D). Similar to DEX treatment, LCK and FYN immunoprecipitated from HSP90siRNA-transfected cells did not have the capacity to phosphorylate SAM68, indicating that HSP90siRNA and DEX impair the enzymatic activities of LCK and FYN. These observations suggest that LCK/FYN-mediated TCR signalling depends on the presence of both HSP90 and GR.
DEX or HSP90siRNA reduce GR–LCK/FYN localization
Cellular localizations of GR, LCK and FYN were studied using confocal fluorescence microscopy. Double stainings for GR and LCK or GR and FYN demonstrated cytoplasmic staining of LCK, FYN and GR in quiescent cells (Fig 3). LCK, FYN and GR staining were mainly detected in the cell periphery on TCR stimulation (Fig 3, dotted lines). GR–LCK and GR–FYN cytoplasmic colocalization (indicated by yellow staining) was seen in quiescent cells, which was increased on TCR stimulation (Fig 3, solid lines). Reduced GR–LCK and GR–FYN colocalization was observed in activated cells treated with DEX or HSP90siRNA (indicated by diminished yellow staining), which supports the immunoprecipitation data that DEX and HSP90siRNA disrupt GR–LCK and GR–FYN interactions. Owing to the small cytoplasm volume compared with the nuclear volume, no conclusions can be drawn about the precise cytoplasmic or membrane localization of LCK or FYN in the different conditions. Specificity controls for the immunofluorescent stainings are shown as supplementary information online (supplementary Fig 6 online).
Figure 3.
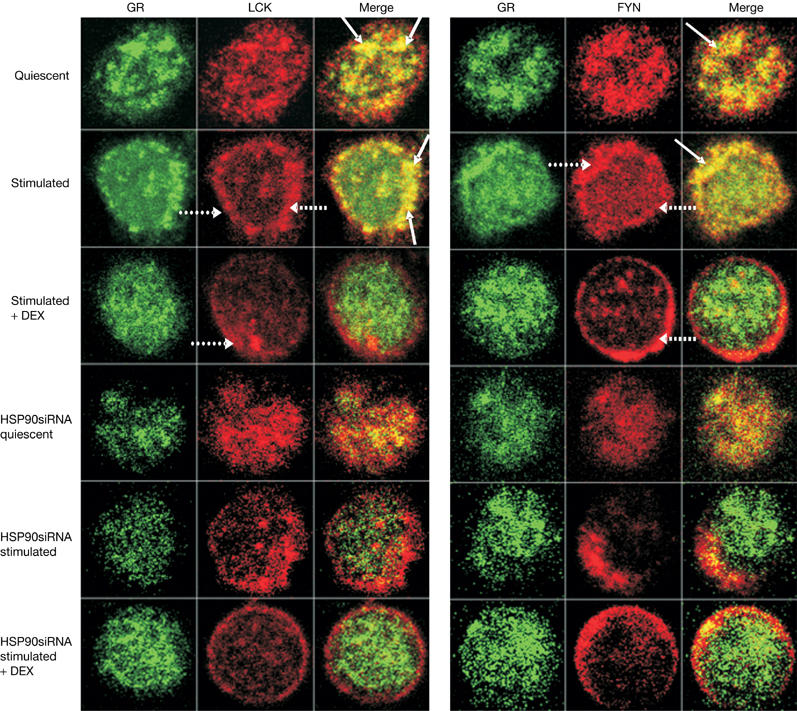
Dexamethasone and HSP90siRNA interfere with GR–LCK and GR–FYN colocalization. HSP90siRNA-transfected or nontransfected cells were pretreated with or without DEX (1 μM; 10 min) and activated with CD3 and CD28 antibodies (15 min). Cells were studied by confocal fluorescence microscopy and immunofluorescent double stainings of GR (fluorescein isothiocyanate-labelled, green) together with LCK or FYN (tetramethylrhodamine isothiocyanate-labelled, red) are shown. Cytoplasmic colocalization between GR and LCK or GR and FYN is indicated by yellow staining (solid lines). The nucleus was visualized with 4,6-diamidino-2-phenylindole (not shown). Panels of optical cross-sections through T cells are depicted; scanned area for all images: 9 μm × 9 μm. Three independent experiments were performed and reproducible results were obtained. At least 20 cells under each condition were scanned, and representative cross-sections are indicated. DEX, dexamethasone; FYN, FYN oncogene related to SRC, FGR, YES; GR, glucocorticoid receptor; HSP90, heat-shock protein 90; LCK, lymphocyte-specific protein tyrosine kinase; siRNA, short interfering RNA.
Discussion
GCs mediate well-defined genomic effects through GR-dependent transcriptional changes. Clinical and experimental evidence for rapid nongenomic GC action have accumulated, but the underlying molecular mechanisms remain unexplained. Oestrogens can induce rapid signalling through intracellular transmembrane oestrogen receptors, providing evidence for nongenomic effects of steroids on cellular physiology (Revankar et al, 2005). Similarly, membrane-bound GRs have been identified in lymphocytes (Gametchu et al, 1999; Gametchu & Watson, 2002) and in human peripheral blood mononuclear cells (Bartholome et al, 2004), but their functional relevance remains unclear. It is known that, at high concentrations, GCs increase GR saturation in a dose-dependent manner, which intensifies the therapeutically relevant genomic GC activities (Buttgereit et al, 2002). Although saturation of cytosolic GRs is almost complete with 100 mg prednisone equivalent a day, the use of higher dosages (100–1,000 mg) is common and successful in daily clinical practice (Buttgereit et al, 2004), possibly as a consequence of nongenomic effects (Buttgereit et al, 1998). Nongenomic GC action is thought to be mediated through cytosolic or membrane-bound GRs and/or through nonspecific physicochemical interactions with membranes.
Nongenomic GC-induced inhibition of LCK/FYN-mediated TCR signalling has been reported (Lowenberg et al, 2005), but the underlying mechanism remains unexplained. A relationship between GC stimulation and LCK/FYN kinases is supported by previous work, showing that prolonged DEX stimulation (enabling GC-induced genomic effects) disturbed the submembrane localization of LCK and FYN in murine T cells (Van Laethem et al, 2001). It is possible that HSP90-dependent regulation of LCK and FYN contributes to these effects, but further studies are needed to clarify the role of GR and HSP90 in regulating TCR signalling.
On the basis of the present work, we propose a model arguing that, in the absence of ligand, the GR sustains a TCR-associated complex containing HSP90, LCK and FYN (Fig 4). On GR–ligand binding, this membrane-bound multiprotein complex is dissociated, leading to a cellular redistribution of LCK and FYN and an inability of LCK/FYN to participate in TCR signalling. Our data show an essential role of HSP90 and GR in the formation of TCR-associated protein complexes and in the regulation of efficient TCR signalling. Overall, these results provide new insight into the nongenomic mechanism of GC-induced immunosuppression in T cells. Selective inhibition of proximal TCR signalling through LCK/FYN presents opportunities for the development of novel immunosuppressive therapies.
Figure 4.
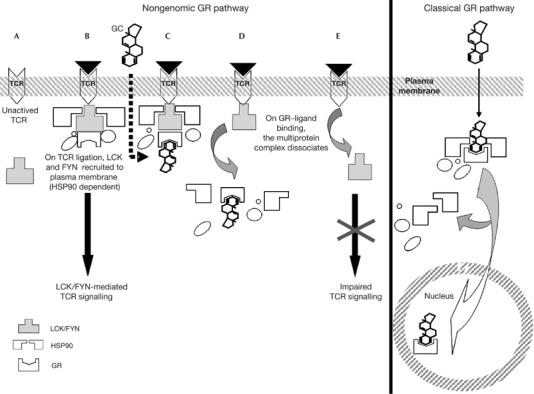
Model for nongenomic GC-induced immunosuppression in T cells. (A,B) On TCR ligation, LCK and FYN are recruited to the TCR complex, resulting in LCK/FYN kinase activation and initiation of downstream TCR signalling. The present study shows TCR-linked GR multiprotein complexes containing HSP90, LCK and FYN in activated T cells. (C,D) On GR–ligand binding, this multiprotein complex dissociates. (E) LCK and FYN are released from the TCR complex, leading to impaired TCR signalling as a consequence of a cellular redistribution and abrogated activation of LCK and FYN. FYN, FYN oncogene related to SRC, FGR, YES; GC, glucocorticoid; GR, glucocorticoid receptor; HSP90, heat-shock protein 90; LCK, lymphocyte-specific protein tyrosine kinase; TCR, T-cell receptor.
Methods
Cell culture. CD4+ T cells were purified from human peripheral blood mononuclear cells and maintained as described previously (Lowenberg et al, 2005).
Reagents and antibodies. A complete list of the antibodies can be found in the supplementary information online. DEX and geldanamycin were obtained from Sigma-Aldrich (Zwijndrecht, The Netherlands). HSP90α/βsiRNA, GRsiRNA, control siRNA and SAM68 were purchased from Santa Cruz (Heidelberg, Germany). Kinase buffer and lysis buffer (supplemented with 1 μg/ml NaF, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 10 mM Na3VO4, 1 mM pefabloc) were from Cell Signaling Technology (Beverley, CA, USA).
Cell transfection. Cells were transfected by electroporation using routine procedures (Amaxa, Cologne, Germany). Each nucleofection sample contained 5 × 106 cells, 2 μg of highly purified siRNA and 100 μl of human T-cell Nucleofector. To determine transfection efficiency, green fluorescent protein (GFP)-tagged DNA (Amaxa) was used for electroporations, showing cells that were 40–50% GFP-positive 16 h after transfection, as assessed by fluorescence-activated cell sorting analysis (data not shown).
Immunoprecipitation, in vitro kinase assay and immunoblotting. Cells were incubated at 37°C in six-well plates (5–10 × 106 cells/well) for 2 h followed by a 10 min pretreatment with 1 μM DEX dissolved in dimethyl sulphoxide (DMSO) or DMSO-supplemented media (control). Cells were subsequently activated for 15 min with CD3 antibodies (immobilized on plastic) and soluble CD28 antibodies (3 μg/ml). Cells were centrifuged (1,750 r.p.m., 10 min), lysed in non-denaturing lysis buffer and subjected to immunoprecipitation followed by in vitro kinase assay and western blotting. The protocol for these procedures can be found in the supplementary information online.
Fluorescent double stainings. After in vitro stimulations, cells were centrifuged for 5 min at 1,750 r.p.m. and pellets were resuspended in PBS. SuperFrost® plus microscope slides were pretreated with 0.01% poly-L-lysine for 10 min, cell suspensions were subsequently incubated on the slides for 30 min (final concentration 2 × 105 cells/slide) and 3.7% paraformaldehyde was added for 30–60 min. Sections were washed in PBS/Triton X-100 0.1% (PBS-T), followed by a 1 h blocking step with 10% FCS in PBS-T. After overnight incubation with a GR antibody at 4°C, antibodies against LCK or FYN were added for 2–3 h. Slides were washed and incubated for 60 min with fluorescein isothiocyanate- and tetramethylrhodamine isothiocyanate-conjugated secondary antibodies (Heverlee, Belgium) diluted in 3% BSA/PBS-T. Alternatively, sections were incubated overnight at 4°C with an HSP90 antibody. Sections were mounted in Vectashield (Vector Laboratories Inc., Burlingame, CA, USA) supplemented with 4,6-diamidino-2-phenylindole and sealed with coverslips. The protocol of the imaging technique can be found in the supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We thank M. Scheffer for technical support and G. van den Brink for a critical reading of the manuscript. D.W.H. is a clinical fellow of The Netherlands Organization for Health Research and Development. M.P.P. is supported by the Dutch Digestive Disease Foundation. The work of F.B. is supported by the Deutsche Forschungsgemeinschaft (Bu 1015/4-1) and by the Bundesministerium für Bildung und Forschung (01GS0110;160;413). We have no conflicting financial interests.
References
- Bartholome B et al. (2004) Membrane glucocorticoid receptors (mGCR) are expressed in normal human peripheral blood mononuclear cells and up-regulated after in vitro stimulation and in patients with rheumatoid arthritis. FASEB J 18: 70–80 [DOI] [PubMed] [Google Scholar]
- Baus E, Andris F, Dubois PM, Urbain J, Leo O (1996) Dexamethasone inhibits the early steps of antigen receptor signaling in activated T lymphocytes. J Immunol 156: 4555–4561 [PubMed] [Google Scholar]
- Bijlmakers MJ, Marsh M (2000) Hsp90 is essential for the synthesis and subsequent membrane association, but not the maintenance, of the Src-kinase p56(lck). Mol Biol Cell 11: 1585–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttgereit F et al. (2002) Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: current questions and tentative answers in rheumatology. Ann Rheum Dis 61: 718–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttgereit F, Straub RH, Wehling M, Burmester GR (2004) Glucocorticoids in the treatment of rheumatic diseases: an update on the mechanisms of action. Arthritis Rheum 50: 3408–3417 [DOI] [PubMed] [Google Scholar]
- Buttgereit F, Wehling M, Burmester GR (1998) A new hypothesis of modular glucocorticoid actions: steroid treatment of rheumatic diseases revisited. Arthritis Rheum 41: 761–767 [DOI] [PubMed] [Google Scholar]
- Croxtall JD, Choudhury Q, Flower RJ (2000) Glucocorticoids act within minutes to inhibit recruitment of signalling factors to activated EGF receptors through a receptor-dependent, transcription-independent mechanism. Br J Pharmacol 130: 289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchimont D (2004) Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann NY Acad Sci 1024: 124–137 [DOI] [PubMed] [Google Scholar]
- Gametchu B, Watson CS (2002) Correlation of membrane glucocorticoid receptor levels with glucocorticoid-induced apoptotic competence using mutant leukemic and lymphoma cells lines. J Cell Biochem 87: 133–146 [DOI] [PubMed] [Google Scholar]
- Gametchu B, Chen F, Sackey F, Powell C, Watson CS (1999) Plasma membrane-resident glucocorticoid receptors in rodent lymphoma and human leukemia models. Steroids 64: 107–119 [DOI] [PubMed] [Google Scholar]
- Hafezi-Moghadam A et al. (2002) Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med 8: 473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartson SD, Barrett DJ, Burn P, Matts RL (1996) Hsp90-mediated folding of the lymphoid cell kinase p56lck. Biochemistry 35: 13451–13459 [DOI] [PubMed] [Google Scholar]
- Lowenberg M, Tuynman J, Bilderbeek J, Gaber T, Buttgereit F, van Deventer S, Peppelenbosch M, Hommes D (2005) Rapid immunosuppressive effects of glucocorticoids mediated through Lck and Fyn. Blood 106: 1703–1710 [DOI] [PubMed] [Google Scholar]
- Palacios EH, Weiss A (2004) Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene 23: 7990–8000 [DOI] [PubMed] [Google Scholar]
- Pratt WB, Galigniana MD, Morishima Y, Murphy PJ (2004) Role of molecular chaperones in steroid receptor action. Essays Biochem 40: 41–58 [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER (2005) A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307: 1625–1630 [DOI] [PubMed] [Google Scholar]
- Rhen T, Cidlowski JA (2005) Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med 353: 1711–1723 [DOI] [PubMed] [Google Scholar]
- Van Laethem F, Baus E, Smyth LA, Andris F, Bex F, Urbain J, Kioussis D, Leo O (2001) Glucocorticoids attenuate T cell receptor signaling. J Exp Med 193: 803–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun BG, Matts RL (2005) Differential effects of Hsp90 inhibition on protein kinases regulating signal transduction pathways required for myoblast differentiation. Exp Cell Res 307: 212–223 [DOI] [PubMed] [Google Scholar]
- Zamoyska R, Basson A, Filby A, Legname G, Lovatt M, Seddon B (2003) The influence of the Src-family kinases, Lck and Fyn, on T cell differentiation, survival and activation. Immunol Rev 191: 107–118 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


