Abstract
MicroRNAs (miRNAs) function as sequence-specific guides that control gene expression by post-transcriptional gene silencing. Many miRNAs influence plant development by regulating the accumulation of transcripts that encode transcription factors. Mutants defective in miRNA accumulation, such as dcl1, hen1, hyl1 and ago1, have pleiotropic developmental phenotypes. The serrate-1 (se-1) mutant of Arabidopsis also shows a highly pleiotropic phenotype, which overlaps with the phenotypes of mutants defective in miRNA accumulation. Although it has been proposed that SERRATE (SE) functions specifically in miRNA-mediated repression of the leaf polarity genes PHABULOSA and PHAVOLUTA, microarray analysis shows upregulation of many genes known to be the targets of miRNAs in se-1. We show that SE is a general regulator of miRNA levels affecting the processing of primary miRNA to miRNA.
Keywords: development, miRNA, plant, SERRATE
Introduction
MicroRNAs (miRNAs) and short interfering RNAs (siRNAs) function as sequence-specific guides to silence genes, transposons and viruses, and to modify the chromatin structure. MiRNAs are involved in the control of various plant developmental processes, including leaf morphogenesis (Palatnik et al, 2003), floral development (Chen, 2004), root development (Guo et al, 2005), vascular development (Kim et al, 2005) and the transition from vegetative to reproductive phases (Lauter et al, 2005). Many miRNAs regulate plant development by delimiting the regions of accumulation of transcripts encoding transcription factors that function in development (Kidner & Martienssen, 2005). This explains the pleiotropic developmental phenotypes of plant mutants defective in miRNA accumulation, such as dicer-like1 (dcl1), hua enhancer 1 (hen1), hyponastic leaves 1 (hyl1) and argonaute 1 (ago1) (Park et al, 2002; Boutet et al, 2003; Vaucheret et al, 2004; Vazquez et al, 2004). The maturation of both types of small RNA is catalysed by double-stranded RNA (dsRNA)-specific RNaseIII-like enzymes called DICER-LIKE (Park et al, 2002). DCL1 processes miRNA precursors (Park et al, 2002; Kurihara & Watanabe, 2004) with two other proteins HEN1 (Boutet et al, 2003; Yu et al, 2005) and HYL1 (Han et al, 2004). The short dsRNAs produced by DCL activity are assembled into effector complexes and they guide sequence-specific degradation of complementary target messenger RNAs, translational repression of target mRNAs or condensation of heterochromatin (Meister & Tuschl, 2004). ARGONAUTE (AGO) proteins are components of these silencing effector complexes.
The serrate-1 (se-1) mutant shows a highly pleiotropic phenotype that overlaps those of miRNA-defective mutants. se-1 has abnormal embryogenesis like short integument 1-2 (sin1-2; Ray et al, 1996); is hypersensitive to germination inhibition by abscisic acid (ABA) like hyl1 (Lu & Fedoroff, 2000; Bezerra et al, 2004); and has delayed leaf initiation, accelerated phase change and aberrant phyllotaxy in the inflorescence like hasty (hst) alleles (Clarke et al, 1999; Prigge & Wagner, 2001; Bollman et al, 2003). Mutations in SERRATE (SE) also lead to defects in leaf number, leaf shape and flower development. Despite its pleiotropic effects on development, se-1 is thought to cause only a partial loss of SE function. Two other se alleles (se-2 and se-3) show more severe defects in leaf development, with adaxial leaf curling, loss of asymmetric differentiation of abaxial and adaxial cell types, and development of trumpet-shaped or radial leaves (Grigg et al, 2005). These effects are correlated with increased expression of the adaxial determinant PHABULOSA (PHB), an HD-ZIP III gene, and with reduced levels of two miRNAs, miR165 and miR166, which mediate cleavage of HD-ZIP III transcripts.
We investigated those genes whose expression is altered in response to changes in SE activity. Many upregulated genes are targets of miRNA regulation. Increased levels of transcripts of these target genes are correlated with decreased levels of at least eight mature miRNAs and a consequent decrease of target gene miRNA cleavage products. The levels of most primary miRNAs (pri-miRNAs) are increased in se-1. A new allele of se is embryonic lethal, which serves to underline the importance of miRNA-based regulation in all stages of plant development.
Results
MicroRNA target genes are misexpressed in serrate-1
We used ATH1 arrays (Affymetrix, Santa Clara, CA, USA) to compare the transcript profiles of se-1 with those of wild type. A total of 303 genes showed significantly changed expression between wild type and se-1 (at least a twofold change and a P-value cutoff of 0.01), with 135 being upregulated and 168 downregulated (supplementary Fig 1 and supplementary Table 1 online).
Analysis of upregulated genes showed a total of 20 genes (16% of the upregulated genes; Table 1) to be known targets of miRNAs and/or trans-acting siRNAs (ta-siRNAs), as well as two AGO genes (AGO2 and AGO3). None of the downregulated genes was a known target of miRNAs. The differentially expressed miRNA target genes included those encoding transcription factors involved in leaf growth and leaf morphology. As has been reported for se-2 and se-3 (Grigg et al, 2005), we observed an upregulation of PHB and PHAVOLUTA (PHV) in se-1. Another HD-Zip III gene, ATHB15, was also upregulated in se-1. Other miRNA and ta-siRNA targets upregulated in se-1 included the transcriptional activators GRF1 (AtGRF1) and GRF3 (AtGRF3) (Kim et al, 2003), which are targets of miR396, AUXIN RESPONSE FACTOR (ARF)3/ETTIN and ARF4 (Pekker et al, 2005; Fahlgren et al, 2006).
Table 1.
Changes in the expression levels of genes targeted by microRNA and trans-acting short interfering RNA assayed on ATH1 microarrays
| Accession number | Fold increase | Gene description | Targeted by |
|---|---|---|---|
|
miRNA and ta-siRNA target genes | |||
| At1g51670 |
6.35 |
Expressed protein |
ta-siRNAs 255 and 752 |
| At3g05690 |
4.66 |
AtHAP2B |
miRNA 169 |
| At1g62930 |
4.65 |
PPR repeat protein |
miRNA 161 |
| At2g02850 |
3.85 |
Plastocyanin-like protein |
miRNA 408 |
| At1g63080 |
3.18 |
PPR repeat protein |
miRNA 161 |
| At5g18040 |
3.00 |
Expressed protein |
ta-siRNAs 752, 255, 289 and 850 |
| At2g22840 |
2.70 |
GRF1 |
miRNA 396 |
| At2g33860 |
2.70 |
ETTIN/ARF3 |
ta-siRNAs 2141 and 2142 |
| At1g52150 |
2.68 |
Athb-15 |
miRNA 165/166 |
| At2g34710 |
2.62 |
Athb-14, PHB |
miRNA 165/166 |
| At5g60450 |
2.60 |
ARF4 |
ta-siRNAs 2141 and 2142 |
| At5g37020 |
2.43 |
ARF8 |
miRNA 167 |
| At1g63130 |
2.36 |
PPR repeat protein |
miRNA 161; ta-siRNA 2140 |
| At2g28190 |
2.33 |
Superoxide dismutase |
miRNA 398 |
| At1g30330 |
2.31 |
ARF6 |
miRNA 167 |
| At1g27340 |
2.18 |
F-box protein |
miRNA 394 |
| At5g50570 |
2.16 |
SPL13 |
miRNA 156/157 |
| At1g30490 |
2.12 |
Athb-9, PHV |
miRNA 165/166 |
| At2g36400 |
2.07 |
GRF3 |
miRNA 396 |
|
ARGONAUTE-like proteins | |||
| At1g31290 |
7.39 |
AGO3 |
Not known |
| At1g31280 | 4.15 | AGO2 | miRNA 403 |
AGO, ARGONAUTE; ARF, AUXIN RESPONSE FACTOR; Athb, Arabidopsis thaliana homeobox; GRF, GROWTH-REGULATING FACTOR; HAP, HEME ACTIVATOR PROTEIN; miRNA, microRNA; PHB, PHABULOSA; PHV, PHAVOLUTA; PPR, PENTATRICOPEPTIDE REPEAT; SPL, SQUAMOSA PROMOTER BINDING PROTEIN-LIKE; ta-siRNA, trans-acting short interfering RNA.
The data show the relative increase in the expression of each gene in the serrate-1 mutant compared with wild type.
The microarray data were verified for selected genes using real-time reverse transcription–PCR (RT–PCR) (supplementary Table 2 online). The misregulation of multiple miRNA targets in se-1 suggested that SE is a general regulator of miRNA-based silencing.
SERRATE regulates microRNA accumulation
We used northern blotting to compare the abundance of miRNAs in wild type and se-1. Three miRNAs (miR156, miR165 and miR167), the target genes of which showed increased expression in the microarray experiments, showed reduced levels of accumulation in se-1 compared with wild type (Fig 1A). To assess whether se-1 has any effect on specific miRNAs or more generally on all miRNAs, we examined the accumulation of other miRNAs—miR163, miR164, miR168, miR171 and miR-JAW—because most of these were shown to be expressed in leaves. The levels of all miRNAs studied were reduced in se-1 (Fig 1A).
Figure 1.
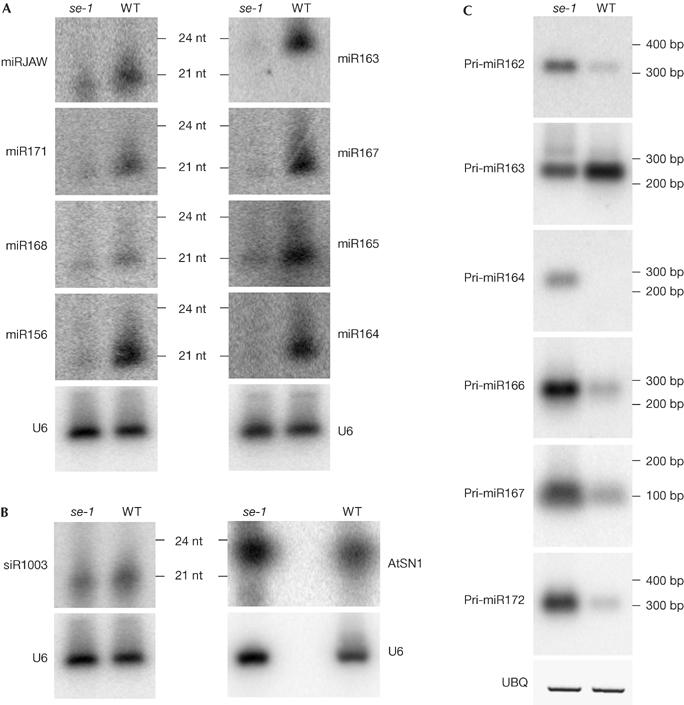
serrate-1 decreases the accumulation of mature microRNAs and increases the accumulation of primary microRNA transcripts. (A) Northern blotting of total RNA from wild-type and serrate-1 (se-1) seedlings. For all microRNAs (miRNAs) tested (miRJAW, miR171, miR168, miR156, miR163, miR167, miR165, miR164), the se-1 mutant showed a reduced level of miRNA accumulation compared with wild type. (B) Accumulation of short interfering RNA (siRNA) in se-1 compared with wild type. The se-1 mutation had little effect on the accumulation of these siRNAs. The corresponding U6 loading control is shown below each blot. (C) Reverse transcription–PCR assay comparing levels of primary miRNAs (pri-miRNAs) in wild type and se-1. PCR products were blotted and hybridized with random-primed probes. The UBIQUITIN (UBQ) loading control is shown below the blots.
In addition to miRNAs, Arabidopsis produces endogenous siRNAs (Llave et al, 2002a; Xie et al, 2004). The se-1 mutation had no effect on the abundance of AtSN1 siRNA, derived from the short interspersed repeated sequence retroelement AtSN1 (Fig 1B). The accumulation of siRNA 1003 was slightly reduced in se-1, but the reduction was negligible compared with the effects on miRNA accumulation. Thus, SE seems to be required for the accumulation of miRNAs in general, but has little effect on the accumulation of the siRNAs tested.
MicroRNA cleavage products are reduced in serrate-1
The effects of se-1 on the accumulation of miRNA target gene cleavage products for three miRNA target genes, PHB, NAC1 and ARF8, were analysed by detection of exposed 5′ RNA ends (Llave et al, 2002b). For all three genes, cleavage products were less abundant in se-1 than in wild type (Fig 2). These results confirmed that the reduced accumulation of miRNAs in se-1 results in less cleavage directed by miRNAs in se-1 compared with wild type.
Figure 2.
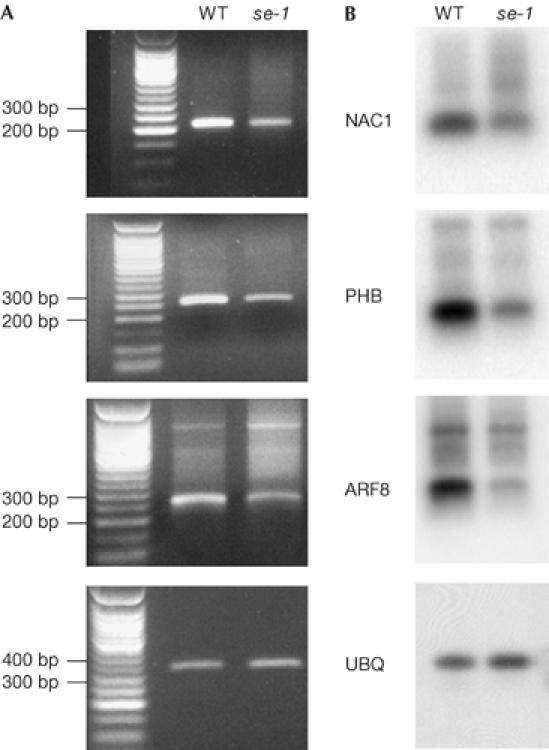
serrate-1 decreases the accumulation of microRNA cleavage products. (A) Agarose gels showing the nested PCR products of PHB, NAC1 and ARF8 microRNA (miRNA) cleavage products. (B) Southern blot analysis of the PHB, NAC1 and ARF8 miRNA cleavage products hybridized with a probe corresponding to the 5′ rapid amplification of cloned ends nested PCR products. The UBIQUITIN (UBQ) loading control is shown below. ARF, AUXIN RESPONSE FACTOR; NAC, for NAM, ATAF1-2, CUC2; PHB, PHABULOSA; se-1, serrate-1; WT, wild type.
Primary microRNAs accumulate in serrate-1
To investigate whether SE might be involved in miRNA biogenesis, we examined the levels of pri-miRNA transcripts in se-1. Grigg et al (2005) showed that miR165 and miR166 primary transcripts were elevated in se-3 compared with wild type, which was confirmed for se-1 (Fig 1C). We also examined the levels of pri-miR162, pri-miR163, pri-miR164, pri-miR167 and pri-miR172 (Fig 1C); all, except pri-miR163, accumulated in se-1. These results indicate that SE is involved in the processing of most pri-miRNA transcripts in miRNA biogenesis.
SERRATE interacts with HYL1
It has been suggested that the HYL1 protein is part of a macromolecular complex (Han et al, 2004) and interacts with DCL1 (Hiraguri et al, 2005; Kurihara et al, 2006) to process pri-miRNA in the nucleus. SE is localized in the nucleus (supplementary Fig 2 online), as are DCL1 and HYL1 (Hiraguri et al, 2005). We used a yeast two-hybrid system to test for interaction of SE with HYL1. SE interacted with HYL1 in yeast (supplementary Fig 3 online). To determine which domains are important for protein–protein interactions of HYL1 and SE, truncated HYL1 and SE fragments were used. Both dsRNA-binding motifs of HYL1 interacted with SE in yeast, whereas the full-length SE protein was required for interaction with HYL1. These studies indicate that SE and HYL1 might interact in vivo as well and that SE is another basic component of the miRNA processing complex in plants.
ago2 and ago3 do not rescue the serrate-1 phenotypes
To determine whether the phenotypic effects of se-1 work through direct effects of SE on AGO2 and AGO3 genes, we created double se-1 ago2 and se-1 ago3 mutants. If the phenotypes of the se-1 mutation were caused by the increase in AGO2 and AGO3 transcripts, we predicted suppression of the se-1 phenotype in the double mutants.
We used lines with a T-DNA insertion in the AGO2 gene (SALK_003380) and a dSpm insertion in AGO3. In both mutants, we detected shorter transcripts of the respective genes compared with wild type. Neither ago2 nor ago3 showed any obvious phenotypic abnormalities. Double mutants between se-1 and ago2 or ago3 did not suppress the se-1 phenotype. These data indicate that the upregulation of AGO2 or AGO3 in se-1 does not cause the se-1 phenotype.
A strong serrate allele is embryonic lethal
As the se-1 allele is predicted to confer only a partial loss of function, we characterized a new se allele to assess the role of SE in plant development. The SALK_059424 line carries a T-DNA insertion in the fifth intron of SE (se-4). We were unable to identify plants homozygous for this insertion. When se-4 heterozygotes were crossed with se-1, 50% of the progeny had se mutant phenotypes, including aerial rosettes, serrated leaves and adaxial leaf curling. To determine whether se-4 affected embryo viability, siliques of plants heterozygous for se-4 were examined. At later stages of development, 25% of seeds were paler in colour than wild-type seeds. Wild-type and mutant embryos at the heart, torpedo and cotyledon stages of development were examined using Nomarski optics (Fig 3). Developmental stages of mutant embryos were established by comparison with wild-type embryos in the same silique. In wild type, the globular-stage embryo consists of an embryo proper and the suspensor (West & Harada, 1993). Abnormal development and cell divisions of se-4 mutant embryos were first visible at the heart stage, compared with wild-type embryos (compare Fig 3A and D). From the heart stage onwards, cotyledon primordia could not be recognized by cell shape or arrangement in se-4 embryos (Fig 3D–F). Irregular cell divisions continued throughout the abnormal embryo during the torpedo stage (Fig 3E), to produce irregularly shaped mature embryos (Fig 3F). Twenty-five per cent of the embryos in siliques of heterozygotes were ‘mutant'. These data show that the strong se-4 allele confers lethality, when homozygous, under the growth conditions we used. Characterization of the se-3 allele (Grigg et al, 2005) showed that it also confers embryonic lethality under some environmental conditions. This underlines the importance of the SE gene product to plant development, and in turn emphasizes the significance of miRNA regulation to all stages of plant development.
Figure 3.
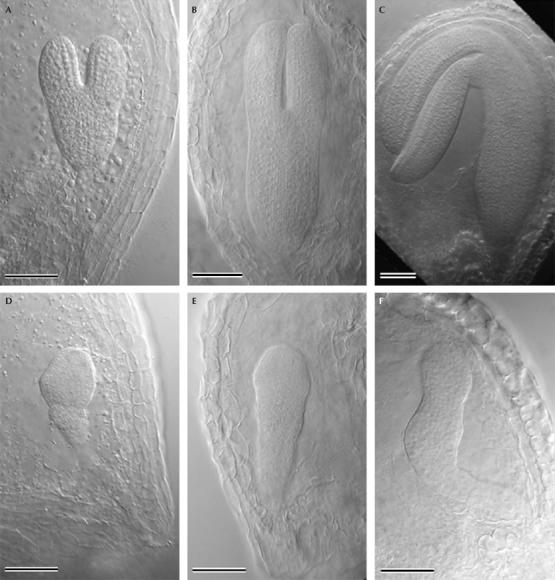
The serrate-4 mutant allele confers embryonic lethality. The development of wild-type embryos (A–C) and serrate-4 (se-4) mutant embryos (D–F) is shown. Each column depicts embryos from seeds of the same age. Scale bars, 50 μm. (A) Wild-type, heart-stage embryo with cotyledon primordia. (B) Wild-type, torpedo-stage embryos showing elongating cotyledons. (C) Wild-type, curled cotyledon-stage embryo. (D) se-4, heart-stage embryo. (E) se-4, torpedo-stage embryo. (F) se-4, curled cotyledon-stage embryo.
Discussion
se-1 shares phenotypes with miRNA pathway mutants
The phenotype of se-1 shares many similarities with developmental defects in general miRNA pathway mutants (dcl1, hen1 and hyl1). Similar to se-1, all three mutants reduce miRNA accumulation and increase uncleaved target mRNA levels (Park et al, 2002; Boutet et al, 2003; Vazquez et al, 2004). The weak sin1-2 allele shows that DCL1 activity is essential very early on in diploid maternal cells for normal embryogenesis. A homozygous sin1 maternal plant gives rise to a few viable seed irrespective of the genotype of the embryo, and seedlings derived from crosses with sin1-2/sin1-2 show various morphological abnormalities, including seedlings with zero, one or three cotyledons, or a funnel-shaped cotyledon (Ray et al, 1996). The only other locus with a similar mutant phenotype in Arabidopsis is SE; progeny of selfed se-1 plants often have either a single fused cotyledon or extra cotyledons (Prigge & Wagner, 2001). The strong se-4 allele is embryonic lethal as are dcl1 null alleles (Schwartz et al, 1994).
The Arabidopsis HYL1 gene encodes a nuclear dsRNA-binding protein and the hyl1 mutation is pleiotropic. hyl1 mutant plants show reduced sensitivity to exogenous cytokinin and auxin and are hypersensitive to germination inhibition by ABA (Lu & Fedoroff, 2000). Hypersensitivity to ABA has also been shown for se-1 (Bezerra et al, 2004).
Mutations in HASTY (HST) share phenotypic similarities with se-1, with delayed leaf initiation, accelerated vegetative phase change and aberrant phyllotaxy in the inflorescence (Clarke et al, 1999; Prigge & Wagner, 2001; Bollman et al, 2003). HST probably exports miRNAs from the nucleus (Park et al, 2005).
SERRATE does not work through AGO2 or AGO3
Similar to AGO1, AGO2 has been identified as a target of miRNA gene silencing (miR403; Allen et al, 2005). This suggests that the upregulation of AGO2 in se-1 is caused by the reduced accumulation of its miRNA. Double mutant analysis showed that the se-1 phenotype is not due to upregulation of AGO2 or AGO3 expression alone, although it is possible that AGO2 and AGO3 act redundantly, as they are closely related structurally. In this model, SE might regulate the transcription of AGO2 and AGO3 and their upregulation might then disturb the miRNA biogenesis pathway (as shown for AGO1; Vaucheret et al, 2004). However, triple mutants of se-1, ago2 and ago3 would be necessary to test the effect of both on the se-1 phenotype, and as AGO2 and AGO3 are very closely linked (only 3.2 kb apart), it would be very difficult to test this effectively.
SERRATE is a regulator of miRNA-based gene regulation
Microarray analysis showed misexpression of 20 mRNAs that are targets of miRNAs or ta-siRNAs in the se-1 mutant; eight mature miRNAs have been tested and all were decreased in se-1, whereas the levels of six pri-miRNAs were increased compared with wild type. In total, we tested pri-miRNA, mature miRNA or miRNA target mRNA levels of 19 different miRNAs and showed that all were affected by se-1.
Our results differ from those of Grigg et al (2005), who proposed that SE functions specifically in miRNA-mediated repression of PHB/PHV gene expression. Our data show that genes targeted by miRNA-based regulation in 10-day-old seedlings are upregulated in se-1 in association with reduced miRNA levels. Consequently, we propose that SE is a general regulator of miRNA-based gene silencing. In humans, it has been shown that single C2H2 zinc-fingers can bind specifically to RNA (Friesen & Darby, 2001). One possibility is that SE binds to miRNA and acts together with DCL1 and HYL1 to process miRNAs. Supporting this, pri-miRNA transcripts are elevated in se-1, as also observed in hyl1-2 and dcl1-7 mutants (Kurihara et al, 2006). In addition, SE interacts with HYL1 in yeast, indicating that both proteins might interact in vivo as well and that SE is a basic component of the miRNA processing complex in plants.
Speculation
Analysis of SE gene expression has shown that SE is not constitutively expressed, but restricted to specific tissue types and developmental stages (Prigge & Wagner, 2001). This leads us to propose that the presence or absence of SE activity might therefore promote or limit miRNA production. In support of this idea, overexpression of SE causes variable phenotypes both within and between overexpression lines, showing that the level of expression of SE greatly influences development. A significant portion of overexpression lines showed seedling lethality (Prigge & Wagner, 2001). Other phenotypes involved sterility, an increased rate of leaf production, inflorescence phyllotaxy defects, adaxial leaf curling, and disorganized flowers with variable numbers of floral organs and radially symmetric filaments. Consequently, SE activity defines a new level of potential developmental regulation: general, post-transcriptional regulation of miRNA levels.
Methods
Plant material. Arabidopsis thaliana (Col-4 and se-1) was grown under 16 h light and 8 h dark conditions at 20°C for 10 days.
Microarray analysis. Affymetrix GeneChip (ATH1) array expression profiling was carried out according to the Affymetrix Expression Analysis Technical Manual II. Microarray data are available on the ArrayExpress database under accession number E-MEXP-838.
Real-time reverse transcription–PCR. Total RNA was isolated from the aerial parts of wild-type and se-1 seedlings using TRI reagent (Sigma, St Louis, MO, USA), and poly(A)+RNA was purified using the Oligotex mRNA Mini Kit (Qiagen, Hilden, Germany). Real-time RT–PCR using first-strand complementary DNA was performed with a DNA Engine Opticon® 2 Continuous Fluorescence Detector (MJ Research, Waltham, MA, USA).
Northern blotting. For analysis of miRNAs and siRNA 1003, total RNA was separated on 8% denaturing polyacrylamide gels. The AtSN1 blot was generated by using low-molecular-weight RNA.
5′ rapid amplification of cloned ends. Target cleavage products were detected using a modified 5′ rapid amplification of cloned ends protocol (Llave et al, 2002b).
Detection of pri-miRNAs. cDNA synthesis from wild-type and se-1 seedlings was performed as described for real-time RT–PCR. UBIQUITIN5 was amplified as a control for cDNA synthesis and amplification. Pri-miR166 PCR primers have been described by Juarez et al (2004), and primer sequences for UBIQUITIN5, pri-miR162, pri-miR163, pri-miR164, pri-miR167 and pri-miR172 can be found as supplementary information online.
Yeast two-hybrid assay. A yeast two-hybrid assay was performed using the Matchmaker GAL4 two-hybrid system (Clontech, Mountain View, CA, USA) for detecting protein–protein interactions in yeast.
Differential interference contrast microscopy of cleared seeds.. Seeds were removed from siliques, cleared for 16 h in Hoyer's solution (7.5 g gum Arabic, 100 g chloral hydrate, 5 ml glycerol in 30 ml water) and examined using a Nikon Microphot-SA microscope equipped with Nomarski optics.
Further information about the mutant lines, sample preparation, primer sequences, reactions and data analysis can be found in supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Table S1
Supplementary Methods
Acknowledgments
We thank J. Hadfield for expression profiling, D. Baulcombe for the ago2 line and small RNA probes, J. Rothe for help with crosses and R. Sablowski and M. Byrne for comments on the manuscript. Seeds of T-DNA insertion lines were supplied by the Arabidopsis Biological Resource Center. This work was funded by the European Community's Human Potential Program HPRN-CT-2002-00267 (DAGOLIGN).
References
- Allen E, Xie Z, Gustafson AM, Carrington JC (2005) microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121: 207–221 [DOI] [PubMed] [Google Scholar]
- Bezerra IC, Michaels SD, Schomburg FM, Amasino RM (2004) Lesions in the mRNA cap-binding gene ABA HYPERSENSITIVE 1 suppress FRIGIDA-mediated delayed flowering in Arabidopsis. Plant J 40: 112–119 [DOI] [PubMed] [Google Scholar]
- Bollman KM, Aukerman MJ, Park MY, Hunter C, Berardini TZ, Poethig RS (2003) HASTY, the Arabidopsis ortholog of exportin 5/MSN5, regulates phase change and morphogenesis. Development 130: 1493–1504 [DOI] [PubMed] [Google Scholar]
- Boutet S, Vazquez F, Liu J, Beclin C, Fagard M, Gratias A, Morel J-B, Crete P, Chen X, Vaucheret H (2003) Arabidopsis HEN1: a genetic link between endogenous miRNA controlling development and siRNA controlling transgene silencing and virus resistance. Curr Biol 13: 843–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XM (2004) A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303: 2022–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JH, Tack D, Findlay K, Van Montagu M, Van Lijsebettens M (1999) The SERRATE locus controls the formation of the early juvenile leaves and phase length in Arabidopsis. Plant J 20: 493–501 [DOI] [PubMed] [Google Scholar]
- Fahlgren N, Montgomery TA, Howell MD, Allen E, Dvorak SK, Alexander AL, Carrington JC (2006) Regulation of AUXIN RESPONSE FACTOR3 by TAS3 ta-siRNA affects developmental timing and patterning in Arabidopsis. Curr Biol 16: 939–944 [DOI] [PubMed] [Google Scholar]
- Friesen WJ, Darby MK (2001) Specific RNA binding by a single C2H2 zinc finger. J Biol Chem 276: 1968–1973 [DOI] [PubMed] [Google Scholar]
- Grigg SP, Canales C, Hay A, Tsiantis M (2005) SERRATE coordinates shoot meristem function and leaf axial patterning in Arabidopsis. Nature 437: 1022–1026 [DOI] [PubMed] [Google Scholar]
- Guo H-S, Xie Q, Fei J-F, Chua N-H (2005) MicroRNA directs mRNA cleavage of the transcription factor NAC1 to downregulate auxin signals for Arabidopsis lateral root development. Plant Cell 17: 1376–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M-H, Goud S, Song L, Fedoroff N (2004) The Arabidopsis double-stranded RNA-binding protein HYL1 plays a role in microRNA-mediated gene regulation. Proc Natl Acad Sci USA 101: 1093–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraguri A, Itoh R, Kondo N, Nomura Y, Aizawa D, Murai Y, Koiwa H, Seki M, Shinozaki K, Fukuhara T (2005) Specific interactions between Dicer-like proteins and HYL1/DRB-family dsRNA-binding proteins in Arabidopsis thaliana. Plant Mol Biol 57: 173–188 [DOI] [PubMed] [Google Scholar]
- Juarez MT, Kui JS, Thomas J, Heller BA, Timmermans MCP (2004) microRNA-mediated repression of rolled leaf1 specifies maize leaf polarity. Nature 428: 84–88 [DOI] [PubMed] [Google Scholar]
- Kidner CA, Martienssen RA (2005) The developmental role of microRNA in plants. Curr Opin Plant Biol 8: 38–44 [DOI] [PubMed] [Google Scholar]
- Kim J et al. (2005) microRNA-directed cleavage of ATHB15 mRNA regulates vascular development in Arabidopsis inflorescence stems. Plant J 42: 84–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Choi DS, Kende H (2003) The AtGRF family of putative transcription factors is involved in leaf and cotyledon growth in Arabidopsis. Plant J 36: 94–104 [DOI] [PubMed] [Google Scholar]
- Kurihara Y, Watanabe Y (2004) Arabidopsis micro-RNA biogenesis through Dicer-like 1 protein functions. Proc Natl Acad Sci USA 101: 12753–12758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara Y, Takashi Y, Watanabe Y (2006) The interaction between DCL1 and HYL1 is important for efficient and precise processing of pri-miRNA in plant microRNA biogenesis. RNA 12: 206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauter N, Kampani A, Carlson S, Goebel M, Moose SP (2005) microRNA172 down-regulates glossy15 to promote vegetative phase change in maize. Proc Natl Acad Sci USA 102: 9412–9417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llave C, Kasschau KD, Rector MA, Carrington JC (2002a) Endogenous and silencing-associated small RNAs in plants. Plant Cell 14: 1605–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llave C, Xie ZX, Kasschau KD, Carrington JC (2002b) Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science 297: 2053–2056 [DOI] [PubMed] [Google Scholar]
- Lu C, Fedoroff N (2000) A mutation in the Arabidopsis HYL1 gene encoding a dsRNA binding protein affects responses to abscisic acid, auxin, and cytokinin. Plant Cell 12: 2351–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G, Tuschl T (2004) Mechanisms of gene silencing by double-stranded RNA. Nature 431: 343–349 [DOI] [PubMed] [Google Scholar]
- Palatnik JF, Allen E, Wu XL, Schommer C, Schwab R, Carrington JC, Weigel D (2003) Control of leaf morphogenesis by microRNAs. Nature 425: 257–263 [DOI] [PubMed] [Google Scholar]
- Park MY, Wu G, Gonzalez-Sulser A, Vaucheret H, Poethig RS (2005) Nuclear processing and export of microRNAs in Arabidopsis. Proc Natl Acad Sci USA 102: 3691–3696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W, Li J, Song R, Messing J, Chen X (2002) CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr Biol 12: 1484–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekker I, Alvarez JP, Eshed Y (2005) Auxin response factors mediate Arabidopsis organ asymmetry via modulation of KANADI activity. Plant Cell 17: 2899–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge MJ, Wagner DR (2001) The Arabidopsis SERRATE gene encodes a zinc-finger protein required for normal shoot development. Plant Cell 13: 1263–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Golden T, Ray A (1996) Maternal effects of the short integument mutation on embryo development in Arabidopsis. Dev Biol 180: 365–369 [DOI] [PubMed] [Google Scholar]
- Schwartz BW, Yeung EC, Meinke DW (1994) Disruption of morphogenesis and transformation of the suspensor in abnormal suspensor mutants of Arabidopsis. Development 120: 3235–3245 [DOI] [PubMed] [Google Scholar]
- Vaucheret H, Vazquez F, Crete P, Bartel DP (2004) The action of ARGONAUTE1 in the miRNA pathway and its regulation by the miRNA pathway are crucial for plant development. Genes Dev 18: 1187–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez F, Gasciolli V, Crete P, Vaucheret H (2004) The nuclear dsRNA binding protein HYL1 is required for microRNA accumulation and plant development, but not posttranscriptional transgene silencing. Curr Biol 14: 346–351 [DOI] [PubMed] [Google Scholar]
- West M, Harada JJ (1993) Embryogenesis in higher plants: an overview. Plant Cell 5: 1361–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie ZX, Johansen LK, Gustafson AM, Kasschau KD, Lellis AD, Zilberman D, Jacobsen SE, Carrington JC (2004) Genetic and functional diversification of small RNA pathways in plants. PLoS Biol 2: 642–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Yang ZY, Li JJ, Minakhina S, Yang MC, Padgett RW, Steward R, Chen XM (2005) Methylation as a crucial step in plant microRNA biogenesis. Science 307: 932–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1
Supplementary Methods


