Just as other parasites and extra-chromosomal elements have evolved to co-exist with their hosts, now it seems that a small circular plasmid, called the 2-micron circle (2μ, after the contour length of its DNA), has co-opted the chromosome segregation machinery of the budding yeast to ensure its own propagation. 2μ is an endogenous plasmid that confers no apparent selective advantage to the host. However, 2μ harbours two distinct mechanisms to ensure its safe passage at each cell division. The first is to increase its copy number per cell, by an ingenious strategy in which an internal cross-over midway through replication results in ‘chasing polymerases' that increase the copy number (Futcher, 1986). The second—revealed in a study by Hajra and colleagues, and published in the 11 September issue of the Journal of Cell Biology—is to incorporate a histone H3 variant (Cse4 in yeast, CENP-A in mammals) and hitch a ride on the mitotic spindle (Fig 1; Hajra et al, 2006).
Figure 1.
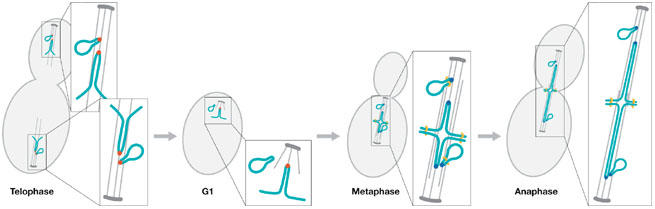
Chromosome and 2-micron circle segregation during mitosis. The centromeres on yeast chromosomes and the stable (STB) locus on the 2-micron circle (2μ) are organized around a specialized histone H3 variant Cse4 (red dot; telophase). In G1, Cse4 is observed at kinetochores but not on 2μ. Coincident with S phase, new Cse4 is incorporated on sister kinetochores and 2μ (blue dots; metaphase). Attachment to the mitotic spindle is required for stable Cse4 assembly on 2μ in metaphase. Cse4-mediated 2μ association with the mitotic spindle is responsible for segregation to the daughter cell at anaphase. 2μ remains associated with the spindle until the next cell cycle.
Eukaryotic chromosome segregation mechanisms are microtubule-based and rely on a random search and capture process that ensures all chromosomes are attached to the spindle. A powerful signalling cascade—the spindle checkpoint—prevents the onset of anaphase until all chromosomes are attached and bi-orientated (Maiato et al, 2004). The kinetochore is the protein machine responsible for linking DNA to microtubules and comprises at least 70 components organized into several discrete sub-domains that are built at the centromere locus (Meraldi et al, 2006). The excitement of discovering a hitchhiker is in its potential to reveal the key interactions responsible for hooking DNA to the segregation apparatus.
At the core of every eukaryotic kinetochore is a histone H3 variant—such as human CENP-A—that together with centromere DNA constitutes a unique nucleosome. This histone variant is highly conserved throughout the eukaryotes (Malik & Henikoff, 2003). It is crucial that Cse4/CENP-A is not deposited elsewhere in the genome because potential genomic catastrophes would result if two or more kinetochores assembled on one chromosome.
Although we are starting to understand the essential and complex proteolytic mechanisms that degrade any unincorporated Cse4 (Collins et al, 2004), it is not clear what initiates the deposition of Cse4 at the centromere. 2μ has commandeered this mechanism and incorporates a Cse4-containing nucleosome at the stable (STB) partitioning locus within the plasmid. Thus, even more surprising than 2μ hitching a ride on the spindle is that 2μ has circumvented powerful degradation mechanisms that remove excess Cse4. How does 2μ ‘trick' Cse4 into loading onto the plasmid, does this seed the deposition of additional kinetochore components, and importantly, to which component is 2μ hitching?
The stable assembly of Cse4 onto 2μ is independent of the requirements for stable kinetochore assembly. Cse4 is recruited to the STB locus through one of the site-specific binding proteins REP1, which is a transcriptional repressor of FLP—the recombinase that promotes the high copy number of 2μ in each cell (Som et al, 1988). How did 60 extra Cse4 nucleosomes go unnoticed? Although the chromatin immunoprecipitation experiments of Hajra and colleagues (Hajra et al, 2006), showing that Cse4 is bound to 2μ, do not address the actual number of plasmids incorporating Cse4 per cell, two other studies have exploited quantitative fluorescence microscopy to address such questions (Joglekar et al, 2006; Wu & Pollard, 2005). It will be important in the future to determine how many Cse4 nucleosomes exist in these cells. Whatever the fraction of 2μ circles that incorporate Cse4, we should first ask how Cse4 is recruited to 2μ, and second, how this promotes association between 2μ and the mitotic spindle.
Cse4 incorporation onto 2μ requires intact microtubules. Hajra and colleagues (Hajra et al, 2006) show by using chromatin immunoprecipitation that, in cells treated with the microtubule poison nocodazole, Cse4 no longer binds to the STB locus. The interpretation is that Cse4 loading or stable association on 2μ is tension-dependent, in contrast to the interaction of Cse4 with the centromere, to which it remains bound after microtubule depolymerization. One possibility is that centromeres remain under tension through higher-order loop domains in the chromosome that cannot be relieved by microtubule depolymerization. Alternatively, Cse4 has a higher affinity for the centromere and is more resistant to various cellular perturbations. Furthermore, it seems unlikely that microtubules provide any specific information for loading Cse4 onto 2μ, or participate in the DNA-binding properties of Cse4. So what might be the role of tension in Cse4 binding to 2μ?
With respect to this role, it is relevant that Cse4 also promotes cohesin loading along 2μ. Why does a high-copy plasmid need cohesin? If the strategy for survival (in this case propagation) is to increase copy number, there is no obvious reason to link the replicated strands. In fact, as the mechanism of replication is ‘chasing polymerases', there are approximately 60 copies generated per division. How does cohesin function in this mechanism and, importantly, is it crucial for associations between strands, or perhaps higher-order interactions between 2μ and the yeast chromosomes?
Without a genuine kinetochore at 2μ it is unlikely that these circles interact with kinetochore microtubule plus-ends. The yeast mitotic spindle comprises approximately 16 kinetochore microtubules (indicative of one microtubule per chromosome in budding yeast; O'Toole et al, 1999). There is some additional carrying capacity, as the cell can tolerate 3–4 extra-chromosomal centromeres, but no more (Futcher & Carbon, 1986). Cohesin might also promote interactions between 2μ and the chromosome. As cohesin binds along the length of chromosomal arms, the structure of kinetochores on the spindle must be considered to understand how 2μ is restricted to this location. The 16 chromosomal centromeres form a cluster of kinetochores within the spindle (Pearson et al, 2001). There is a region of increased centromeric cohesion spanning approximately 40 kb of pericentric chromatin (about threefold greater in centromere-flanking chromatin relative to chromosome arms (Blat & Kleckner, 1999; Glynn et al, 2004)). One possibility is that the increased concentration of cohesin near centromeres is sufficient to shift the binding equilibrium between 2μ and chromosomal cohesin predominantly to pericentric regions. 2μ has been localized near kinetochores by fluorescence microscopy and this might reflect these types of interaction (Mehta et al, 2005).
If the entire pericentric domain is considered as the functional centromere, an alternative structure for 2μ to exploit is revealed. Cse4 has been proposed to promote the folding of centromere DNA into a cruciform structure, also known as a modified Holliday junction (Bloom et al, 2006). If 2μ adopts a Holliday junction-like cruciform on cohesin recruitment, this structure might reinforce the binding affinity of Cse4 by bending the arms tightly around the unique nucleosome at the STB locus. This combination of DNA secondary structure and cohesin oligomerization would link 2μ to the spindle. Interestingly, this idea provides an explanation for the role of tension in Cse4 binding. It is not that microtubules or forces from microtubules change the affinity of Cse4 for DNA, but rather that microtubules are required for the structure of cohesin around the spindle, which in turn is required for 2μ to ‘piggyback'. After the loss of cohesin structure, oligomers of cohesin can no longer form between 2μ and the chromosome, the cruciform structure is lost and the affinity between Cse4 and 2μ is reduced. Alternatively, tension itself might reinforce the cruciform structure of the DNA, which in turn results in increased affinity of Cse4 for the STB. Thus by recruiting cohesin, 2μ ensures itself a place in the spindle.
After segregation, Hajra and colleagues (Hajra et al, 2006) show that the Cse4-containing histone octamer is completely remodelled. Rather than using any Cse4 from the previous cycle, the ‘old' Cse4-containing nucleosome is jettisoned at the end of mitosis, and ‘new' Cse4 nucleosomes are assembled at the STB locus in the next cycle (Fig 1). This ‘use once and discard' strategy for the Cse4 nucleosome mimics the behaviour of the Cse4 nucleosome at the centromere (Pearson et al, 2004). Whether the forces exerted on the centromere deform the Cse4 nucleosome such that it is more economical to discard it, or the behaviour of Cse4 in 2μ reveals an alternative reason for this strategy remains to be seen. The lifetime of Cse4 is shorter in 2μ relative to the centromere. Interestingly, Cse4 is released shortly after mitotic exit. This reflects the finding that Cse4 stability in 2μ is tension-dependent, unlike the chromosome. Thus, there are apparently structural requirements for Cse4 deposition and assembly beyond simply sequence-binding interactions.
What are the minimal requirements for segregation? Faithful segregation might not require the ability to interact with the plus-end of a microtubule as with eukaryotic chromosomes, but rather the ability to hitch a ride on the spindle. This would include, but perhaps not be restricted to, microtubules, chromosomes, cohesin, or kinetochore components. It is not clear why 2μ also exploits recombination to increase its copy number. Perhaps it is the combination of these processes that contributes to the fidelity and perpetuation of 2μ. Either mechanism alone might not provide the requisite fidelity typical of chromosome segregation (error rate of 1/100,000 divisions). Between its high copy number and association with the spindle, 2μ ensures itself a place in every daughter cell, at every division.
The main lesson learned from this new study is the incredible ingenuity in nature. It is through this conservation of function that we are able to unravel key features of segregation. Apparently, recruitment of Cse4 and cohesin is sufficient to ensure propagation. It is unlikely that these elements could work alone, that is, independent of chromosomes and kinetochores. However, perhaps the 2μ strategy for self-perpetuation will be found elsewhere. 2μ might also provide us with instructions on how to design stable delivery vectors in other systems.

References
- Blat Y, Kleckner N (1999) Cohesins bind to preferential sites along yeast chromosome III, with differential regulation along arms versus the centric region. Cell 98: 249–259 [DOI] [PubMed] [Google Scholar]
- Bloom K, Sharma S, Dokholyan NV (2006) The path of DNA in the kinetochore. Curr Biol 16: R276–R278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins KA, Furuyama S, Biggins S (2004) Proteolysis contributes to the exclusive centromere localization of the yeast Cse4/CENP-A histone H3 variant. Curr Biol 14: 1968–1972 [DOI] [PubMed] [Google Scholar]
- Futcher AB (1986) Copy number amplification of the 2μ circle plasmid of Saccharomyces cerevisiae. J Theor Biol 119: 197–204 [DOI] [PubMed] [Google Scholar]
- Futcher B, Carbon J (1986) Toxic effects of excess cloned centromeres. Mol Cell Biol 6: 2213–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn EF, Megee PC, Yu HG, Mistrot C, Unal E, Koshland DE, DeRisi JL, Gerton JL (2004) Genome-wide mapping of the cohesin complex in the yeast Saccharomyces cerevisiae. PLoS Biol 2: E259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra S, Ghosh SK, Jayaram M (2006) The centromere-specific histone variant Cse4p (CENP-A) is essential for functional chromatin architecture at the yeast 2μ circle partitioning locus and promotes equal plasmid segregation. J Cell Biol (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joglekar AP, Bouck DC, Molk JN, Bloom KS, Salmon ED (2006) Molecular architecture of a kinetochore–microtubule attachment site. Nat Cell Biol 8: 581–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiato H, DeLuca J, Salmon ED, Earnshaw WC (2004) The dynamic kinetochore–microtubule interface. J Cell Sci 117: 5461–5477 [DOI] [PubMed] [Google Scholar]
- Malik HS, Henikoff S (2003) Phylogenomics of the nucleosome. Nat Struct Biol 10: 882–891 [DOI] [PubMed] [Google Scholar]
- Mehta S, Yang XM, Jayaram M, Velmurugan S (2005) A novel role for the mitotic spindle during DNA segregation in yeast: promoting 2μ plasmid–cohesin association. Mol Cell Biol 25: 4283–4298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi P, McAinsh AD, Rheinbay E, Sorger PK (2006) Phylogenetic and structural analysis of centromeric DNA and kinetochore proteins. Genome Biol 7: R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole ET, Winey M, McIntosh JR (1999) High-voltage electron tomography of spindle pole bodies and early mitotic spindles in the yeast Saccharomyces cerevisiae. Mol Biol Cell 10: 2017–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CG, Maddox PS, Salmon ED, Bloom K (2001) Budding yeast chromosome structure and dynamics during mitosis. J Cell Biol 152: 1255–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CG, Yeh E, Gardner M, Odde D, Salmon ED, Bloom K (2004) Stable kinetochore–microtubule attachment constrains centromere positioning in metaphase. Curr Biol 14: 1962–1967 [DOI] [PubMed] [Google Scholar]
- Som T, Armstrong KA, Volkert FC, Broach JR (1988) Autoregulation of 2μ circle gene expression provides a model for maintenance of stable plasmid copy levels. Cell 52: 27–37 [DOI] [PubMed] [Google Scholar]
- Wu JQ, Pollard TD (2005) Counting cytokinesis proteins globally and locally in fission yeast. Science 310: 310–314 [DOI] [PubMed] [Google Scholar]


