Abstract
It is assumed that the retinoid X receptor (RXR) acts as a silent partner to the vitamin D receptor (VDR) with its only function to increase affinity of VDR/RXR to its DNA recognition site. In this study, we show that the RXR ligand 9-cis-retinoic acid (9-cis-RA) induces recruitment of coactivators by the DNA-bound heterodimer and potentiates vitamin D-dependent transcriptional responses. The presence of 9-cis-RA increases induction of cyp24 transcripts and differentiation of colon cancer cells by vitamin D, confers significant agonistic activity to a VDR ligand with very low agonistic activity and can even restore transcriptional activity of an AF-2 mutant VDR that causes hereditary rickets. This study shows that, in VDR/RXR heterodimers, allosteric communication triggered by the RXR ligand has a previously unrecognized role in vitamin D signalling, with important physiological and therapeutic implications.
Keywords: vitamin D receptor, retinoid X receptor, heterodimer, coactivators
Introduction
Vitamin D (1α,25-dihydroxyvitamin D3, calcitriol) has a key role in calcium homeostasis, has suppressive effects on the immune system, promotes cell differentiation and inhibits proliferation of transformed cells. This has led to the search of vitamin D analogues with therapeutic effects but without calcemic activity. Their actions are mediated by binding to the nuclear vitamin D receptor (VDR) that regulates gene expression by binding to vitamin D responsive elements (VDREs), which are typically composed of a direct repeat of the sequence PuGGT/GTCA spaced by three nucleotides (DR3; Aranda & Pascual, 2001).
Stimulation of transcription by nuclear receptors is mediated through recruitment of coactivators. Three copies of the LXXLL motif, where X denotes any amino acid, contained in the receptor-interacting domain of the p160 coactivators, mediate association with the receptors (Heery et al, 1997). This motif is also present in the DRIP205 subunit of TRAP/DRIP (thyroid receptor-associated protein/vitamin D receptor interacting protein), also known as the Mediator complex. Ligand binding triggers a conformational change in the receptor that creates a surface for coactivator binding. Repositioning of the ligand-dependent transcriptional activation function (AF-2) located at the carboxy-terminal helix 12 (H12) of the ligand-binding domain (LBD), together with residues in H3, H4 and H5, forms a hydrophobic groove that accommodates this motif (Gronemeyer et al, 2004). A conserved glutamic acid in H12 (E420) and an invariable lysine (K246) in H3 of the VDR contact directly with the LXXLL motif and form a charge clamp that stabilizes binding (Vanhooke et al, 2004). Accordingly, mutations in these residues render a VDR unable to mediate vitamin D-dependent transactivation (Jimenez-Lara & Aranda, 1999).
Heterodimerization of VDR, as well as other nuclear receptors, with the retinoid X receptors (RXRs) increases DNA binding and transcriptional activity (Gronemeyer et al, 2004). Permissive heterodimers can be independently activated by an RXR ligand (9-cis-retinoic acid (9-cis-RA)), by an agonist of its partner receptor or by both ligands in a synergistic manner. However, in nonpermissive heterodimers, the ligand-induced transcriptional activities of RXR are suppressed, and it was even proposed that formation of the heterodimer could preclude binding of the ligand to RXR, although this hypothesis has been recently challenged (Germain et al, 2002; Castillo et al, 2004). Some heterodimers show conditional permissivity. In conditional heterodimers, such as retinoic acid receptor (RAR)/RXR, a full response occurs only in the presence of an RXR ligand. Statistical coupling analysis led to the identification of a network of residues in the LBD crucial for allosteric function, as their mutation can convert a permissive heterodimer into a conditional one and even discriminate between different ligands of a given receptor (Shulman et al, 2004). In this analysis, the VDR/RXR heterodimer has been considered to be a strict nonpermissive heterodimer, which cannot be activated by the RXR ligand either in the presence or absence of the VDR agonist, although an allosteric modification of unliganded RXR by liganded VDR has been proposed (Bettoun et al, 2003).
In this study, we have re-examined the role of the RXR ligand in transcription by VDR/RXR. We find that this heterodimer can recruit coactivators in response to either vitamin D or 9-cis-RA, and that both cooperate to stimulate the activity of VDRE reporters to increase transcription of the cyp24 gene or to promote differentiation of colon carcinoma cells. Furthermore, binding of the RXR agonist causes association with coactivators and transcriptional stimulation by a VDR ligand previously defined as a partial antagonist or even by an inactive AF-2 VDR mutant. These results show that the RXR agonist has a previously unrecognized role in signalling by VDR, with important physiological and pharmacological implications.
Results and Discussion
VDR/RXR can act as a conditional heterodimer
It is assumed that the RXR ligand cannot autonomously induce transcription from VDR/RXR. However, in 293-T cells transfected with a 4xVDRE reporter, 9-cis-RA caused a consistent increase (about threefold) of reporter activity and was able to cooperate with vitamin D, which in these cells caused a weak stimulation (Fig 1A). On expression of the heterodimer, vitamin D caused a strong dose-dependent transactivation, and 9-cis-RA caused a further induction (Fig 1B). Similar results were obtained with constructs containing single VDREs (DR3T, DR3G and IP9), or with the VDRE-containing cyp24 promoter (supplementary Fig 1 online).
Figure 1.
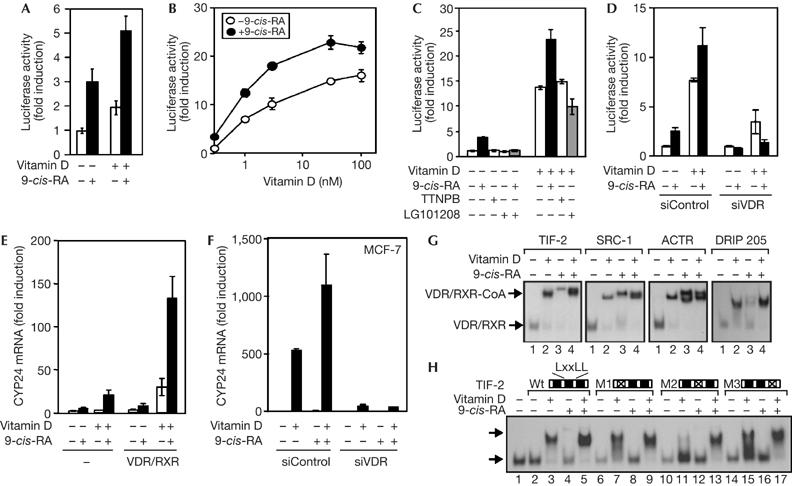
The retinoid X receptor ligand stimulates transcriptional activity and coactivator recruitment by vitamin D receptor/retinoid X receptor. (A) 293-T cells were transfected with the 4xVDRE reporter plasmid and incubated with vitamin D (3 nM) and/or 9-cis-RA. In (B), the plasmid was co-transfected with VDR and RXR and the cells were incubated with 9-cis-RA alone or in combination with the indicated concentrations of vitamin D. (C) Cells were transfected with the heterodimer and treated with vitamin D (3 nM) and/or 9-cis-RA, TTNPB or LG101208. (D) Reporter activity determined in cells transfected with the receptors in the presence of control siRNA (siControl) or VDR siRNA (siVDR). (E) 293-T cells were transfected with the heterodimer or an empty vector and the levels of cyp24 transcripts were determined in cells treated for 4 h with vitamin D and/or 9-cis-RA. (F) cyp24 transcripts in MCF-7 cells treated for 12 h with the ligands. (G) Electrophoretic mobility-shift assays with in vitro-translated VDR and RXR and the p160 coactivators (TIF-2, SRC-1, ACTR), or DRIP205 fused to glutathione S-transferase. Mobilities of heterodimers and complexes with the coactivator (CoA) are shown by arrows. (H) Association of VDR/RXR with wild-type His-tagged TIF-2 or with mutants in the first (M1), second (M2) or third (M3) LXXLL motifs. 9-cis-RA, 9-cis-retinoic acid; RXR, retinoid X receptor; siRNA, short interfering RNA; VDR, vitamin D receptor.
In contrast to 9-cis-RA, the RAR-specific ligand TTNPB was unable to induce basal activity or to cooperate with vitamin D, excluding the possibility that activation by 9-cis-RA could be mediated by cryptic binding of RAR/RXR (Fig 1C). In addition, the RXR antagonist LG101208 abolished induction by 9-cis-RA and slightly decreased transactivation by vitamin D, showing that binding to RXR mediates the effect of the retinoid. Finally, short interfering RNA (siRNA) knockdown of VDR not only affected the response to vitamin D but also abolished induction by 9-cis-RA and the cooperation of both ligands (Fig 1D), showing that the action of 9-cis-RA was mediated by VDR/RXR. The same was observed with the cyp24 reporter (supplementary Fig 2A online).
The RXR agonist also induced transcription from an endogenous gene. cyp24 transcripts were induced synergistically by 9-cis-RA and vitamin D in 293-T cells (Fig 1E). The effect of vitamin D was weaker than that of 9-cis-RA in cells expressing endogenous receptors. However, this situation was reversed on expression of VDR/RXR, when the action of vitamin D was strongly enhanced and the effect of the RXR ligand was only weakly induced. The synergistic effect of both ligands to induce cyp24 gene transcription was also observed in MCF-7 cells, in which endogenous receptors mediated a strong increase by vitamin D that was further induced by 9-cis-RA. This effect was also abolished after knockdown of VDR by siRNA (Fig 1F), showing that VDR/RXR mediates induction of cyp24 transcripts by these ligands. These results show that the VDR/RXR heterodimer cannot be considered as nonpermissive and that it rather behaves as a conditionally permissive heterodimer.
Activation by 9-cis-RA suggests that its binding to RXR can result in recruitment of coactivators by the DNA-bound VDR/RXR. Fig 1G shows that both 9-cis-RA and vitamin D cause association of p160 and DRIP205 coactivators with the receptors, demonstrating that dimerization with VDR does not preclude binding of ligand to RXR. Similar results were obtained with the heterodimers of RXR with RAR (Germain et al, 2002) or the thyroid hormone receptor (Castillo et al, 2004). The ternary complex of the coactivators with the heterodimer had a slower mobility with 9-cis-RA than vitamin D, showing that different conformations of the complex were obtained depending on the ligand. In general, recruitment was enhanced when both ligands were combined, suggesting that they cooperate to recruit the coactivator. This was also observed with various natural VDREs (supplementary Fig 3 online). The use of a mutant coactivator allows a clear detection of the effect of the RXR ligand. Mutation of LXXLL motifs I and III (M1 and M3) had little effect, whereas mutation of motif II (M2) essentially abolished recruitment of transcriptional intermediary factor 2 (TIF-2) by vitamin D. However, both ligands acted synergistically and promoted a strong interaction with the M2 mutant (Fig 1H). These results show that RXR does not act as a silent partner for VDR with the only function of increasing binding to the VDRE.
Role of RXR ligand in coactivators recruitment
In Fig 2, binding of p160 coactivators was analysed in response to 9-cis-RA, vitamin D and the vitamin D analogue ZK159222. This compound binds to VDR with high affinity but has very low agonistic activity, antagonizing transactivation by vitamin D (Herdick et al, 2000; Ochiai et al, 2005). In contrast to vitamin D (lane 3), ZK159222 was unable to cause coactivators recruitment (lane 5). However, robust binding was found when this ligand was combined with 9-cis-RA (lane 6). Modelling of the structure of VDR bound to ZK159222 shows that its side chain displaces H12, preventing normal association with coactivators (Tocchini-Valentini et al, 2004). Our finding that 9-cis-RA restores this association suggests that binding of ligand to RXR changes the conformation of its partner, repositioning H12 to an agonist location.
Figure 2.
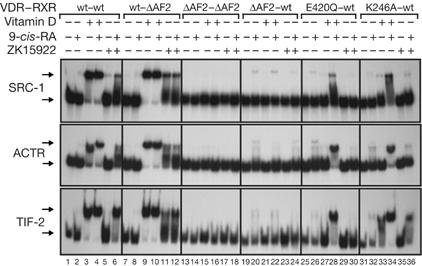
Recruitment of coactivators by vitamin D receptor mutants and ZK159222 in the presence of 9-cis-retinoic acid. Electrophoretic mobility-shift assays were performed with SRC-1, ACTR and TIF-2. As indicated, wild-type (wt) VDR and RXR, receptors lacking H12 (ΔAF-2) and the mutants VDR E420Q and K246A were used. Experiments were performed in the presence and absence of the indicated ligands. 9-cis-RA, 9-cis-retinoic acid; RXR, retinoid X receptor; VDR, vitamin D receptor.
Electrophoretic mobility-shift assays (EMSAs) were also performed with receptors lacking H12 (ΔAF2). Deletion of H12 in RXR did not affect vitamin D-dependent coactivator binding, but had a paradoxical effect on the action of ZK159222, which was able to induce recruitment even in the absence of 9-cis-RA (lane 11), although synergism was not observed (lane 12). Deletion of the AF-2 domains of both receptors totally abolished coactivator recruitment (lanes 13–18), and deletion of VDR AF-2 allowed recruitment by 9-cis-RA, but blocked coactivators association by vitamin D, as well as the synergism between the RXR ligand and ZK159222 (lanes 19–24). The inactive VDR mutants E420Q and K246A were also used. Remarkably, the E420 mutation has been detected in families with hereditary vitamin D-resistant rickets without alopecia (Malloy et al, 2002). As expected, mutation E420Q inhibited recruitment by vitamin D (lane 27). However, with the combination of 9-cis-RA and vitamin D, a strong association of the coactivator to the defective heterodimer was found (lane 28). This synergism was not observed when 9-cis-RA and ZK159222 were combined (lane 30). Similar results were obtained with the H3 mutant K264A, as vitamin D alone did not recruit coactivators, but robust binding was found when both ligands were combined (lane 34). These results indicate that the RXR ligand alters the structure of the heterodimer, allowing formation of productive coactivator binding surfaces even when residues that interact with the LXXLL motif are mutated. To gain further insight into the structural requirements for coactivators recruitment by the heterodimer, point mutants on the coactivator binding surface of both receptors were used (supplementary Fig 4 online). Mutations in H3 and H4 of VDR affected binding by vitamin D but did not reduce the response to 9-cis-RA, whereas equivalent mutations in RXR were able to abolish recruitment by this ligand. Furthermore, point mutations in RXR H12 had a stronger effect than deletion of this helix—they not only inhibited the response to 9-cis-RA, but also markedly reduced binding of coactivators by vitamin D.
RXR agonist restores activation by a mutant VDR
The finding that the RXR ligand causes coactivator recruitment by an inactive AF-2 VDR mutant suggests that transcriptional activity of this receptor could be restored in the presence of 9-cis-RA. This is indeed illustrated in Fig 3. Vitamin D strongly increased reporter activity on expression of the native receptor, whereas the E420 mutation prevented the ligand-dependent response. However, strong transactivation by the AF-2 mutant was observed when vitamin D was combined with 9-cis-RA (Fig 3A). A marked synergistic effect of both ligands on endogenous cyp24 messenger RNA induction was also obtained. Vitamin D alone was essentially inactive to augment cyp24 transcripts in cells expressing the E420Q mutant, whereas a significant increase was found in the presence of both ligands (Fig 3B). These results are compatible with the ‘in vitro' coactivator association to this AF-2-defective heterodimer observed in the presence of both agonists, and suggest that the use of rexinoids could have a therapeutic effect in patients with the E420 point mutation. In addition, the fact that these patients do not present with alopecia could be due to residual transcriptional activity; It could also be a consequence of activation of the heterodimer by endogenous RXR ligands, the concentration of which could be locally regulated.
Figure 3.
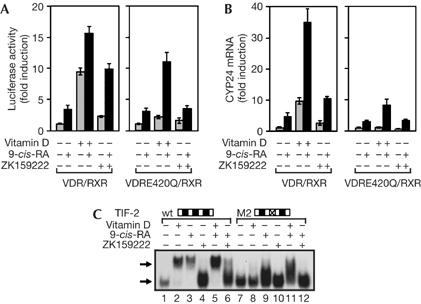
9-cis-retinoic acid restores the transcriptional activity of mutant vitamin D receptor/retinoid X receptor heterodimers and confers agonistic activity to ZK159222. (A) Reporter activity in 293-T cells expressing wild-type VDR/RXR or a heterodimer of RXR with the E420Q VDR mutant. (B) cyp24 messenger RNA levels measured in the same groups after 4 h incubation with vitamin D, ZK159222 and/or 9-cis-RA, as indicated. (C) Electrophoretic mobility-shift assays with the native VDR/RXR heterodimer and either wild-type (wt) TIF-2 or the mutant in the second LXXLL motif (M2). 9-cis-RA, 9-cis-retinoic acid; RXR, retinoid X receptor; VDR, vitamin D receptor.
Recruitment of coactivators by ZK159222 in the presence of 9-cis-RA also suggested that this compound could have transcriptional activity under these conditions. Fig 3A shows that the VDRE reporter was indeed synergistically stimulated by these ligands in cells expressing native receptors. This synergism was also observed when cyp24 transcripts were quantified (Fig 3B). These results show the importance of the occupancy of RXR on VDR signalling, as the presence of the RXR ligand can convert ZK159222 into a rather strong agonist. Interestingly, the response obtained with the combination of ZK159222 and 9-cis-RA was at least as strong as that observed with vitamin D alone in the absence of the RXR ligand. The cooperation between both ligands could have important pharmacological implications and must be taken into account when novel antagonist analogues are developed.
In contrast to vitamin D, ZK159222 was unable to cooperate with 9-cis-RA to stimulate either promoter activity (Fig 3A) or endogenous cyp24 mRNA levels (Fig 3B) in cells expressing the AF-2 VDR mutant. This is also in accordance with the lack of coactivators recruitment observed in EMSAs. These assays showed the ability of this compound to recruit coactivators to the native heterodimer in the presence of 9-cis-RA, and this can be observed again in Fig 3C with the native TIF-2. However, vitamin D, but not ZK159222, cooperated with the RXR ligand when the second LXXLL motif of the coactivator was mutated. These results show that 9-cis-RA can overcome the effect of an AF-2 VDR mutation, compensate the effect of a deleterious mutation in the coactivator or even allow transcriptional activity by ZK159222, but the system does not tolerate more than one of these changes probably because stable coactivator binding to the receptors cannot be achieved under these conditions.
RXR ligands and differentiation of colon carcinoma cells
High concentrations of vitamin D promote differentiation of SW480-ADH colon carcinoma cells, and induction of E-cadherin gene transcription seems to have a key role in this response (Palmer et al, 2001). As shown in Fig 4A, 9-cis-RA (1 μM) or a low dose of vitamin D (3 nM) alone were unable to increase E-cadherin levels. However, a strong synergistic effect was found when both compounds were combined. This effect was also observed with the combination of the RXR ligand and ZK159222, showing again that this analogue presents a strong agonistic effect in the presence of 9-cis-RA. The combination of vitamin D with a low concentration of 9-cis-RA (10 nM) also synergistically increased E-cadherin expression (supplementary Fig 5 online).
Figure 4.
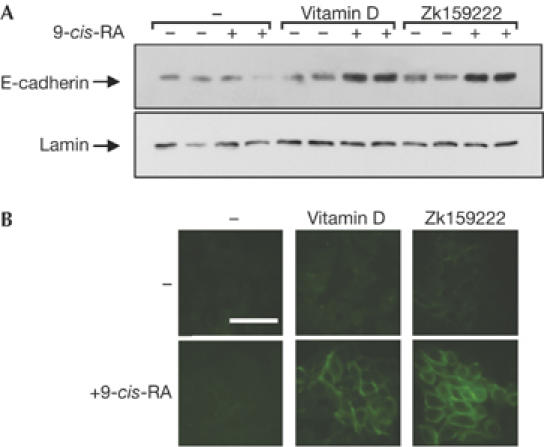
Cooperation of vitamin D receptor and retinoid X receptor ligands on differentiation of colon carcinoma cells. (A) E-cadherin levels in SW480-ADH cells treated with the VDR ligands in the presence and absence of 1 μM 9-cis-RA. The membrane was reprobed with an anti-lamin antibody. (B) After the same treatments, differentiation was tested by immunofluorescence with E-cadherin. Scale bar, 100 μm. 9-cis-RA, 9-cis-retinoic acid. RXR, retinoid X receptor; VDR, vitamin D receptor.
Differentiation of SW480-ADH cells is characterized by the acquisition of an epithelial-like phenotype with formation of compact islands with high E-cadherin expression at the cell membrane. In agreement with the E-cadherin levels, this process was not induced by any of the ligands individually, but the combination of the VDR ligands with the RXR ligand clearly induced differentiation (Fig 4B). Therefore, the role of the RXR ligand is also manifested in a complex response such as differentiation, showing its physiological importance in VDR-dependent responses.
Methods
Plasmids. The multimerized DR3 (4xVDRE) AGGTCAtgaAGGACA is cloned in pGL3. Other reporters are described in supplementary Fig 1 online. Human RXRα and VDR are cloned in pSG5. Point mutations were obtained by PCR with PfuTurbo DNA polymerase (Stratagene, La Jolla, CA, USA). Glutathione S-transferase (GST)–activator of retinoic acid receptor (ACTR), GST–TIF-2, GST–steroid receptor coactivator 1 (SRC-1) and GST–DRIP205 code for the nuclear receptor-interacting domains of these proteins. These plasmids, as well as the His-tagged nuclear receptor-interacting domain of TIF-2 and mutants in the LXXLL motif I (M1), motif II (M2) and motif III (M3), have been described previously (Castillo et al, 2004).
Transfections. 293-T cells, grown in 24-well plates, were transfected with 40 ng of reporter using calcium phosphate, plated in medium containing hormone-stripped serum and treated after an overnight incubation. When appropriate, the reporter was co-transfected with RXR (12.5 ng) and wild-type or mutant VDR (12.5 ng) or empty vectors. Luciferase activity was determined after 36 h of treatment with 3 nM vitamin D, 3 nM ZK159222 and/or 1 μM 9-cis-RA, TTNPB or LG101208. Experiments were performed with triplicate cultures and each experiment was repeated at least three times. Data are represented as mean±s.d. For siRNA experiments, 33 nM of a control or VDR siRNAs was transfected with Lipofectamine 2000. Transfected cells were incubated for 48 h before treatment. The efficiency of knockdown was determined by western blot (supplementary Fig 2 online).
RNA extraction. Total RNA was extracted using Tri Reagent (Sigma-Aldrich Química SA, Madrid, Spain). cyp24 mRNA levels were analysed by quantitative reverse transcriptase–PCR using the primers 5′-GGCAACAGTTCTGGGTGAAT-3′ and 5′-TATTTGCGGACAATCCAACA-3′. Values were corrected by glyceraldehyde-3-phosphate dehydrogenase expression determined with the primers 5′-ACAGTCCATGCCATCACTGCC-3′ and 5′-CTAGCTGACCTCCTTGACCTG-3′.
Gel retardation assays. EMSAs were performed as described previously (Castillo et al, 2004), with the single DR3 5′-AGGTCAAGGAGGTCA-3′ and 2.5 μl of in vitro-translated receptors (TNT Quick, Promega, Madison, WI, USA), in the presence and absence of 400–600 ng of GST-fused coactivators or 450 ng of His-tagged TIF-2. Vitamin D (1 μM), ZK159222 (1 μM) or 9-cis-RA (10 μM) was present in the assays as indicated. Sequences of other VDREs are shown in supplementary Fig 2 online.
Western blotting and immunostaining. SW480-ADH cells were grown as described previously (Palmer et al, 2001) and treated with vitamin D (3 nM), ZK159222 (30 nM) or 9-cis-RA (1 μM or 10 nM) for 48 h. Proteins (20 μg) were transferred to Immobilon P and probed sequentially with E-cadherin (BD Transduction Laboratories, BD Biosciences, San José, CA, USA, 1:1,000) and Lamin B1 (Santa Cruz Biotechnology, Heidelberg, Germany, 1:2,000) antibodies. For immunostaining, cells were incubated with a 1:100 dilution of the primary and secondary antibodies as described previously (Palmer et al, 2004).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary Figures
Acknowledgments
We thank A. Muñoz for SW480-ADH cells. This work was supported by grants BFU2004 03165 from the Ministerio de Educación y Ciencia, Fondo de Investigaciones Sanitarias C03/10 and SAL/0911/2004 from the Comunidad Autónoma de Madrid and CRESCENDO (FP-018652) from the European Union. A.I.C. was supported by an Ernst Schering Research Foundation Fellowship.
References
- Aranda A, Pascual A (2001) Nuclear hormone receptors and gene expression. Physiol Rev 81: 1269–1304 [DOI] [PubMed] [Google Scholar]
- Bettoun DJ, Burris TP, Houck KA, Buck DW II, Stayrook KR, Khafila B, Lu J, Chin WW, Nagpal S (2003) Retinoid X receptor is a non-silent major contributor to vitamin D receptor-mediated transcriptional activation. Mol Endocrinol 17: 2320–2328 [DOI] [PubMed] [Google Scholar]
- Castillo AI, Sánchez-Martínez R, Moreno JL, Martínez-Iglesias OA, Palacios D, Aranda A (2004) A permissive retinoid X receptor/thyroid hormone receptor heterodimer allows stimulation of prolactin gene transcription by thyroid hormone and 9-cis-retinoic acid. Mol Cell Biol 24: 502–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain P, Iyer J, Zechel C, Gronemeyer H (2002) Co-regulator recruitment and the mechanism of retinoic acid receptor synergy. Nature 415: 187–192 [DOI] [PubMed] [Google Scholar]
- Gronemeyer H, Gustafsson JA, Laudet V (2004) Principles for modulation of the nuclear receptor superfamily. Nat Rev 3: 950–964 [DOI] [PubMed] [Google Scholar]
- Heery DM, Kalkhoven E, Hoare S, Parker MG (1997) A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387: 733–736 [DOI] [PubMed] [Google Scholar]
- Herdick M, Steinmeyer A, Carlberg C (2000) Antagonistic action of a 25-carboxylic ester analogue of 1α,25-dihydroxyvitamin D3 is mediated by a lack of ligand-induced vitamin D receptor interaction with coactivators. J Biol Chem 275: 16506–16512 [DOI] [PubMed] [Google Scholar]
- Jimenez-Lara AM, Aranda A (1999) Lysine 246 of the vitamin D receptor is crucial for ligand-independent interaction with coactivators and transcriptional activity. J Biol Chem 274: 13503–13510 [DOI] [PubMed] [Google Scholar]
- Malloy PJ, Xu R, Peng L, Feldman D (2002) A novel mutation in helix 12 of the vitamin D receptor impairs coactivator interaction and causes hereditary 1,25-dihydroxyvitamin D-resistant rickets without alopecia. Mol Endocrinol 16: 2538–2546 [DOI] [PubMed] [Google Scholar]
- Ochiai E, Miura D, Eguchi H, Ohara S, Takenouchi K, Azuma Y, Kamimura T, Norman AW, Ishizuka S (2005) Molecular mechanism of the vitamin D antagonistic actions of (23S)-25-dehydro-1a-hydroxyvitamin D3-23,26-lactone depends on the primary structure of the carboxy-terminal region of the vitamin D receptor. Mol Endocrinol 19: 1147–1157 [DOI] [PubMed] [Google Scholar]
- Palmer HG et al. (2001) Vitamin D3 promotes the differentiation of colon cancer carcinoma cells by the induction of E-cadherin and the inhibition of β-catenin signaling. J Cell Biol 154: 369–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman AI, Larson C, Mangelsdorf DJ, Ranganathan R (2004) Structural determinants of allosteric ligand activation in RXR heterodimers. Cell 116: 417–429 [DOI] [PubMed] [Google Scholar]
- Tocchini-Valentini G, Rochel N, Wurtz JM, Moras D (2004) Crystal structures of the vitamin D nuclear receptor liganded with the vitamin D side chain analogues calcipotriol and seocalcitol, receptor agonists of clinical importance. Insights into a structural basis for the switching of calcipotriol to a receptor antagonist by further side chain modifications. J Med Chem 47: 1956–1961 [DOI] [PubMed] [Google Scholar]
- Vanhooke JL, Benning MM, Bauer CB, Pike JW, DeLuca HF (2004) Molecular structure of the rat vitamin D receptor ligand binding domain complexed with 2-carbon-substituted vitamin D3 hormone analogues and a LXXLL-containing coactivator peptide. Biochemistry 43: 4101–4110 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Figures


