Abstract
The dimorphic fungus Candida albicans produces farnesol as a quorum-sensing molecule that regulates cellular morphology. The biosynthetic origin of farnesol has been resolved by treating these cells with zaragozic acid B, a potent inhibitor of squalene synthase in the sterol biosynthetic pathway. Treatment with zaragozic acid B leads to an eightfold increase in the amount of farnesol produced by C. albicans. Furthermore, C. albicans cell extracts contain enzymatic activity to convert [3H]farnesyl pyrophosphate to [3H]farnesol. Many common antifungal antibiotics (e.g., zaragozic acids, azoles, and allylamines) target steps in sterol biosynthesis. We suggest that the fungicidal activity of zaragozic acid derives in large part from the accumulation of farnesol that accompanies the inhibition of sterol biosynthesis.
Candida albicans is one of the most commonly isolated fungal pathogens of humans. It is a common cause of nosocomial infections and is the fourth most common cause of all bloodstream infections (6) as well as 31% of all urinary tract infections in intensive care units (4). It is a member of the body's normal microbial flora, and it is a medically important opportunistic pathogen—especially for immunocompromised individuals. Candida bloodstream infections, i.e., candidemia, are also widespread due to chemotherapy, extensive use of antibiotics, indwelling intravenous catheters, and other surgical and medical manipulations (6). The mortality for candidemia is in excess of 30%, irrespective of treatment (6), partially because of a shortage of effective antifungal antibiotics. Furthermore, those antifungals that do exist are often, for unknown reasons, very strain dependent and dosage dependent (11). Because of its medical importance, C. albicans has also become a model system for fungal molecular biology.
C. albicans can grow as hyphae, pseudohyphae, or budding yeasts, and the availability of these multiple, interconvertible morphologies is of great benefit to the organism's pathogenic lifestyle. Indeed, monomorphic mutants are typically avirulent (7). Because of its importance in pathogenicity, yeast-mycelial dimorphism in C. albicans has been of great interest for a long time (10). In this regard, we showed that C. albicans produces (E,E)-farnesol as an extracellular quorum-sensing molecule which, when it has accumulated above a threshold level, prevents the yeast-to-mycelium conversion and causes the culture to grow as actively budding yeasts without influencing cellular growth rates (5). Farnesol has also been found to prevent biofilm formation by C. albicans (12). Thus, farnesol's synthesis and mode of action are of interest because (i) it is the first eukaryotic quorum-sensing molecule identified, and (ii) it provides a novel target for the development of antifungal drugs intended to prevent mycelial growth or biofilm production in C. albicans. The present study shows that C. albicans synthesizes farnesol from farnesyl pyrophosphate (FPP), a well-known intermediate in the highly conserved sterol biosynthetic pathway. Furthermore, our studies show that compounds that block the sterol pathway beyond FPP, such as the zaragozic acids, cause an increase of up to eightfold in intracellular and extracellular farnesol levels. Significantly, many clinically useful antifungal antibiotics target the ergosterol biosynthetic pathway. Examples include the allylamines (e.g., terbinafine) and the azoles (e.g., fluconazole, ketoconazole, and itraconazole). Our results suggest that farnesol accumulation plays a role in both the antifungal activity exhibited by drugs that target sterol biosynthesis and some of the idiosyncrasies exhibited by those drugs, e.g., difficulties in obtaining precise MICs (11).
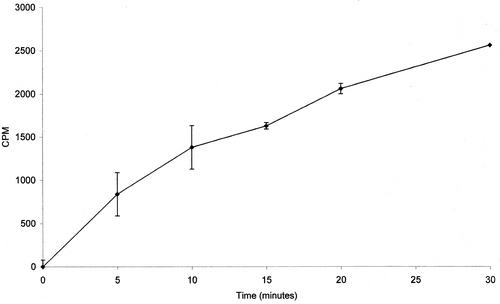
The conversion of FPP to farnesol was shown by incubating [1-3H] (E,E)-FPP with a C. albicans cell homogenate in a modification of the allylpyrophosphatase assay described by Bansal and Vaidya (1) for rat liver enzymes. C. albicans A72 was grown overnight in yeast extract-peptone-dextrose broth (8). One milliliter of culture (5 × 108 cells/ml) was placed in a 1.5-ml microfuge tube, and the cells were collected by centrifugation at 13,000 rpm (unless otherwise stated, all centrifugation was performed with an Eppendorf 5415D centrifuge). The pellet was resuspended in 100 μl of 0.1 M citrate buffer (Na+ salt) at pH 5.5, 0.45% Triton X-100, 5 mM EDTA, 25 μM pepstatin A, 25 μM leupeptin, and 0.5 mM phenylmethylsulfonyl fluoride. The two chelating agents (5 mM EDTA and 100 mM citrate) inactivate the metal ion-dependent squalene synthase for which FPP could also act as substrate (1). This expectation that squalene would not be produced was confirmed by thin-layer chromatography (TLC) assay of the product. The buffered cell suspension was transferred to a 0.5-ml microfuge tube, and 0.45-mm acid-washed glass beads (0.4 g) were added to just below the meniscus. The tubes were vortexed at top speed at 4°C for 6 min. The cell extract was separated from the beads by forming a hole in the bottom with a hot 25-gauge needle, placing the 0.5-ml tube in a 1.5-ml tube, and collecting the buffered cell extract by centrifugation at 13,000 rpm. This cell extract was diluted 1:20 with fresh citrate-EDTA buffer so that each assay contained ca. 0.5 mg of protein in 100 μl of solution mixture. The FPP stock contained (per 100 μl) 2 μg of (E,E)-FPP (Sigma, St. Louis, Mo.) and 0.09 μg of [3H](E,E)-FPP (American Radiolabeled Chemicals, Inc., St. Louis, Mo.) in distilled water. To remove any non-FPP impurities present, 15-μl portions of the FPP stock were spotted on a glass-backed silica TLC plate and developed by using a mobile phase of ethyl acetate-hexane solution (1:4). The FPP, being hydrophilic, will not move from the baseline. The baseline area was scraped and added directly to the cell extract. The final concentration of FPP was 0.45 μCi/0.31 μg/100 μl. The reaction was run at 37°C by using an Eppendorf Thermostat 5320 heating block. To stop the reaction, 0.5 ml of 100% hexane was added. The tubes were vortexed for 2 min and, to achieve a clean phase separation, centrifuged at 13,000 rpm for 1 min. Samples (200 μl) were removed from the top layer, placed in 2 ml of scintillation fluid, and counted in a Beckman LS1701 scintillation counter. TLC (ethyl acetate-hexane, 1:4) confirmed that the radioactivity detected was due to labeled farnesol. Counts per minute were measured at Rf values from 0 to 0.1 (FPP Rf = 0), 0.35 to 0.55 (farnesol Rf = 0.45), and 0.65 to 1 (squalene Rf = 0.87). No radioactivity was detected at Rf from 0 to 0.1 or 0.65 to 1. Furthermore, autoradiograms of the TLC plates confirmed that the FPP was converted to farnesol. All of the radioactivity that had left the origin was confined to a single spot whose migration coincided exactly with that of authentic [1-3H](E,E)-farnesol (American Radiolabeled Chemicals, Inc.). Figure 1 shows a time course for the conversion of FPP to farnesol by C. albicans cell extracts. The reaction was linear over the first 30 min, and ca. 8% of the FPP had been converted to farnesol at that time. No farnesol was made by heated (95°C for 10 min) cell extracts. Thus, C. albicans possesses the enzymatic machinery to convert FPP to farnesol.
FIG. 1.
Time course for conversion of FPP to farnesol by C. albicans cell extracts. Each point is the average of at least two separate experiments.
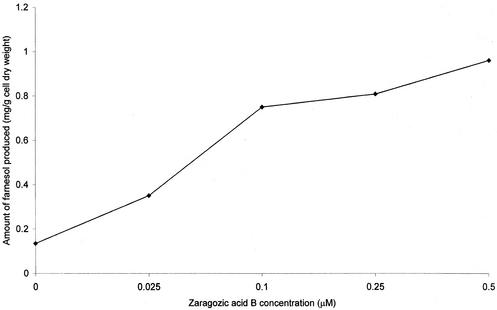
Recognition that farnesol is made from FPP suggests that cell perturbations that elevate the FPP pool size should also elevate farnesol production levels. As a case in point: treatment of mice (15), rats, or dogs (3) with zaragozic acid, a potent inhibitor of squalene synthase, leads to greatly increased production of farnesoic acid and the farnesol-derived dicarboxylic acids as excretion products in urine. C. albicans A72 was grown for 24 h at 30°C in 100 ml of glucose-phosphate-proline (5) with 0 to 0.5 μM zaragozic acid B, generously supplied by Merck. The zaragozic acid B stock (1 mM in water) was wrapped in aluminum foil and stored at 4°C. The cells were harvested by centrifugation in a Beckman J-21C centrifuge at 15,300 × g for 5 min. The cell pellets were washed once in distilled water, and dry weights were determined by using preweighed planchets and baking the samples in an oven at 180°C for 1 h. The farnesol-containing supernatants were filter sterilized (0.22-μm-pore-size Whatman filters) and extracted with 1/4 volume ethyl acetate. The ethyl acetate was removed under reduced pressure (Yamato RE-47) with mild heat (30°C), whereupon the dried samples were resuspended in 1 ml of 20% ethyl acetate-hexane and transferred to a 2-ml vial. The solvent was again removed under reduced pressure at 30°C, and then the samples were resuspended in 50 μl of 20% ethyl acetate-hexane solution. One microliter was used for farnesol determination by gas chromatography-mass spectroscopy as previously described by Hornby et al. (5). The resulting dose-response curve is shown in Fig. 2. The levels of farnesol excreted increased eightfold in a roughly linear, dose-dependent fashion, reaching a maximum of ca. 1 mg of farnesol per g of cell dry weight at a 0.5 μM concentration of zaragozic acid B. By using a broth dilution method based on growth within 18 h in glucose-phosphate-proline medium (5), the MIC of zaragozic acid B for C. albicans A72 was ca. 0.5 μM. The levels of intracellular farnesol also increased in a proportional manner (data not shown).
FIG. 2.
Increased farnesol production by zaragozic acid B-treated cells. Each point is the average of at least two separate experiments agreeing within ±10%.
It is reasonable to expect that farnesol accumulation will contribute to the antifungal activity of sterol-blocking drugs such as zaragozic acid. As an example, 25 μM farnesol inhibits growth of the budding yeast Saccharomyces cerevisiae (9). The inhibitory effect of farnesol is thought to act by arresting the cell cycle (9) and inducing reactive oxygen species in the yeast mitochondria (8). Also, in higher organisms, excess farnesol has been reported to inhibit Ca2+ channels (13) and induce apoptosis (16). However, for C. albicans, concentrations of up to 250 μM farnesol in one study (5) and 300 μM in another study (12) caused no inhibition of growth rate by itself. It is possible that farnesol becomes more inhibitory when ergosterol synthesis is blocked. Odds (11) suggested that some of the inhibitory effects of the azoles are caused by their insertion into the membrane of ergosterol-deprived cells. Due to farnesol's lipophilicity, it is likely that it would do the same.
Our basic thesis is that farnesol accumulation contributes to the antifungal activity of zaragozic acid and may be a common theme for other antibiotics that block sterol biosynthesis. That is, the overall mode of action of these inhibitors is not based simply upon their ability to deplete fungal cells of ergosterol (11). This idea is supported by several previous observations and comments. Taylor et al. (14) showed that the azoles were still inhibitory toward sterol auxotrophic mutants of S. cerevisiae. Odds (11) also states, “most azoles are able to prevent or greatly perturb hyphal growth of C. albicans. The drugs retard or annul the initial outgrowth of germ tubes and entirely prevent hyphal branching, thus leading to cultures of largely or entirely yeast-form cells, even on media that normally support development of long hyphae.” When we combine the accumulation of farnesol in response to sterol inhibition (Fig. 2) and farnesol's known ability to block hyphal development in C. albicans (5), these observations are the expected result.
The remainder of our discussion will concern fungal MICs. Sterol biosynthetic inhibitors such as the azole antifungals “tend not to give clear MIC end points: they cause partial inhibition of fungal growth over a wide range of concentrations, sometimes even giving sigmoid dose-response curves” (11). Odds also documents the wide variability of MIC results for the azoles, often greater than 100-fold, as being due to variations in the inoculum size, pH and composition of the growth medium, incubation time, cation concentration, and incubation temperature (11). Additionally, we observed variability in determining MICs for zaragozic acid B depending on whether the experiments used 25- or 250-ml Erlenmeyer flasks. Prior to this study, there have been no reports of MICs for the zaragozic acids against whole cells of C. albicans. Earlier studies have shown MICs for these inhibitors against S. cerevisiae and Ki values for the target enzyme, squalene synthase (2). A possible reason for this is the ineffectiveness of the zaragozic acids in a clinical setting. These compounds readily bind to serum components, rendering them unavailable in the bloodstream (2). At the time of their discovery, it was thought surprising that the zaragozic acids were fungicidal rather than fungistatic (2). We now suggest that the fungicidal nature of zaragozic acid derives in part from the accumulation of farnesol, which accompanies the inhibition of ergosterol biosynthesis. Furthermore, we predict that other antifungals that block carbon flow through the sterol biosynthetic pathway will cause C. albicans to secrete elevated levels of farnesol, which might also contribute to their toxicity.
Acknowledgments
We thank Sara Basiaga for her assistance with gas chromatography-mass spectroscopy and Merck and Co., Inc., Rahway, N.J., for the zaragozic acid B used in this study.
This work was supported by grants from the National Science Foundation (grant number MCB-0110999) and the University of Nebraska Tobacco Settlement Biomedical Research Enhancement Fund.
REFERENCES
- 1.Bansal, V. S., and S. Vaidya. 1994. Characterization of two distinct allyl pyrophosphatase activities from rat liver microsomes. Arch. Biochem. Biophys. 315:393-399. [DOI] [PubMed] [Google Scholar]
- 2.Bergstrom, J. D., C. Dufresne, G. F. Bills, M. Nallin-Omstead, and K. Byrne. 1995. Discovery, biosynthesis, and mechanism of action of the zaragozic acids: potent inhibitors of squalene synthase. Annu. Rev. Microbiol. 49:607-639. [DOI] [PubMed] [Google Scholar]
- 3.Bostedor, R. G., J. D. Karkas, B. H. Arison, V. S. Bansal, S. Vaidya, J. I. Germershausen, M. M. Kurtz, and J. D. Bergstrom. 1997. Farnesol-derived dicarboxylic acids in the urine of animals treated with zaragozic acid A or with farnesol. J. Biol. Chem. 272:9197-9203. [DOI] [PubMed] [Google Scholar]
- 4.Filler, S. G., and B. J. Kullberg. 2002. Deep-seated Candidal infections, p. 341-348. In R. A. Calderone (ed.), Candida and candidiasis. ASM Press, Washington, D.C.
- 5.Hornby, J. M., E. C. Jensen, A. D. Lisec, J. J. Tasto, B. Jahnke, R. Shoemaker, P. D. Dussault, and K. W. Nickerson. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kullberg, B. J., and S. G. Filler. 2002. Candidemia, p. 327-340. In R. A. Calderone (ed.), Candida and candidiasis. ASM Press, Washington, D.C.
- 7.Lo, H. J., J. R. Köhler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939-949. [DOI] [PubMed] [Google Scholar]
- 8.Machida, K., T. Tanaka, K.-I. Fujita, and M. Tanaguchi. 1998. Farnesol-induced generation of reactive oxygen species via indirect inhibition of the mitochondrial electron transport chain in the yeast Saccharomyces cerevisiae. J. Bacteriol. 180:4460-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machida, K., T. Tanaka, Y. Yano. S. Otani, and M. Taniguchi. 1999. Farnesol-induced growth inhibition in Saccharomyces cerevisiae by a cell cycle mechanism. Microbiology 145:293-299. [DOI] [PubMed] [Google Scholar]
- 10.Nickerson, W. J. 1947. Biology of pathogenic fungi. Chronica Botanica Company, Waltham, Mass.
- 11.Odds, F. C. 1988. Candida and candidosis, 2nd ed. Bailliere Tindall, London, England.
- 12.Ramage, G., S. P. Saville, B. L. Wickes, and J. L. Lopez-Ribot. 2002. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 68:5459-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roullet, J.-B., U. C. Luft, H. Xue, J. Chapman, R. Bychkov, C. M. Roullet, F. C. Luft, H. Haller, and D. A. McCarron. 1997. Farnesol inhibits L-type Ca2+ channels in vascular smooth muscle cells. J. Biol. Chem. 272:32240-32246. [DOI] [PubMed] [Google Scholar]
- 14.Taylor, F. R., R. J. Rodriguez, and L. W. Parks. 1983. Relationship between antifungal activity and inhibition of sterol biosynthesis in miconazole, clotrimazole, and 15-azasterol. Antimicrob. Agents Chemother. 23:515-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaidya, S., R. Bostedor, M. M. Kurtz, J. D. Bergstrom, and V. S. Bansal. 1998. Massive production of farnesol-derived dicarboxylic acids in mice treated with squalene synthase inhibitor zaragozic acid B. Arch. Biochem. Biophys. 355:84-92. [DOI] [PubMed] [Google Scholar]
- 16.Voziyan, P. A., J. S. Haug, and G. Melnykovych. 1995. Mechanism of farnesol cytotoxicity: further evidence for the role of PKC-dependent signal transduction in farnesol induced apoptotic cell death. Biochem. Biophys. Res. Commun. 212:479-486. [DOI] [PubMed] [Google Scholar]